Dispersability of Carbon Nanotubes in Biopolymer-Based Fluids
Abstract
:1. Introduction
2. Physico-Chemical Properties of CNT Dispersions


- (a)
- Surface area per unit volume;
- (b)
- Different solubility in a given medium;
- (c)
- Possible bundling or clustering, and;
- (d)
- Elasticity, which is in direct proportion to L/D.
3. Dispersions of CNTS in Protein Solutions
3.1. Generalities
3.2. Dispersions in Protein-Based Gels

3.3. Dispersions of CNTs in DNA and RNA
3.3.1. Phase Behavior


3.3.2. Formation of Nematic Liquid Crystalline Phases


4. Conclusions
Acknowledgments
Appendix
A.1. Materials
A.2. Methods
Conflicts of Interest
References
- Feynmn, R. There’s plenty of room at the bottom. Caltech Eng. Sci. 1960, 23, 22–36. [Google Scholar]
- French, R.H.; Parsegian, V.A.; Podgornik, R.; Rajter, R.F.; Jagota, A.; Luo, J.; Asthagiri, D.; Chandury, M.K.; Chiang, Y.-M.; Granick, S.; et al. Long range interactions in nanoscale science. Rev. Mod. Phys. 2010, 82, 1887–1944. [Google Scholar] [CrossRef]
- Saito, R.; Dresselhaus, G.; Dresselhaus, M.S. Physical Properties of Carbon Nanotubes; Imperial College Press: London, UK, 1998. [Google Scholar]
- Baugham, R.H.; Zakhidov, A.A.; de Heer, W.A. Carbon nanotubes: The route towards applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Zhou, O.; Shimoda, H.; Gao, B.; Oh, S.; Fleming, L.; Yue, G.Z. Materials science of carbon nanotubes: Fabrication, integration, and properties of macroscopic structures of carbon nanotubes. Acc. Chem. Res. 2002, 35, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.T.; Gun’ko, Y.K. Recent advances in research on carbon nanotube-polymer composites. Adv. Mater. 2010, 22, 1672–1688. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, E.; Nesheva, D.; Krusteva, E.; Dobreva, T.; Kotsilkova, R. Rheological and electrical properties of epoxy nanocomposites filled with multiwalled carbon nanotubes. Nanosci. Nanotechnol. 2009, 9, 40–43. [Google Scholar]
- Talyzin, A.V.; Anoshkin, I.V.; Krasheninnikov, A.V.; Nieminen, R.M.; Nasibulin, A.G.; Jiang, H.; Kauppinen, E.I. Synthesis of Graphene Nanoribbons Encapsulated in Single-Walled Carbon Nanotubes. Nano Lett. 2011, 11, 4352–4356. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.P.; Fu, K.; Lin, Y.; Huang, W. Functionalized carbon nanotubes: Properties and applications. Acc. Chem. Res. 2002, 35, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Mazzucchelli, S.; Montenegro, J.M.; Galbiati, E.; Corsi, F.; Parak, W.J.; Prosperi, D. Protein Oriented Ligation on Nanoparticles Exploiting O6-Alkylguanine-DNA Transferase (SNAP) Genetically Encoded Fusion. Small 2012, 8, 1492–1497. [Google Scholar] [CrossRef] [PubMed]
- Moore, V.C.; Strano, M.S.; Haroz, E.H.; Hauge, R.H.; Smalley, R.E.; Schmidt, J.; Talmon, Y. Individually suspended single-walled carbon nanotubes in various surfactants. Nano Lett. 2003, 3, 1379–1382. [Google Scholar] [CrossRef]
- O’Connell, M.J.; Boul, P.; Ericson, L.M.; Huffman, C.; Wang, Y.; Haroz, E.; Kuper, C.; Tour, J.; Ausman, K.D.; Smalley, R.E. Reversible water-solubilization of single-walled carbon nanotubes by polymer wrapping. Chem. Phys. Lett. 2001, 342, 265–271. [Google Scholar] [CrossRef]
- Bomboi, F.; Bonincontro, A.; la Mesa, C.; Tardani, F. Interactions between single-walled carbon nanotubes and lysozyme. J. Colloid Interface Sci. 2011, 355, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, G.R.; Dalton, A.B.; Johnson, P.A.; Razal, J.; Chen, J.; Giordano, G.M.; Munoz, E.; Musselman, J.H.; Baugham, R.H.; Draper, R.K. Controlled assembly of carbon nanotubes by designed amphiphilic peptide helices. J. Am. Chem. Soc. 2003, 125, 1770–1777. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yang, X.; Zhao, S.; Zhang, M.; Hu, X.; Wang, J.; Zhao, H. Fast and selective recognizes polysaccharide by surface molecularly imprinted film coated onto aldehyde-modified magnetic nanoparticles. Analyst 2013, 138, 6653–6661. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.S.P.; Sahoo, B.; Behera, B.; Maiti, T.K. Nanoparticle and polysaccharide conjugate: A potential candidate vaccine to improve immunological stimuli. Intern. J. Biol. Macromol. 2015, 72, 1254–1264. [Google Scholar] [CrossRef]
- Cathcart, H.; Quinn, S.; Nicolosi, V.; Kelly, J.M.; Blau, W.J.; Coleman, J.N. Spontaneous debuldling of single-walled carbon nanotubes in DNA-based dispersions. J. Phys. Chem. C 2007, 111, 66–74. [Google Scholar] [CrossRef]
- Boxus, T.; Touillaux, R.; Dive, G.; Marchand-Brynaert, J. Synthesis and evaluation of RGD peptidomimetics aimed at surface bioderivatization of polymer substrates. Bioorg. Med. Chem. 1998, 6, 1577–1595. [Google Scholar] [CrossRef] [PubMed]
- Rivas, G.A.; Rubianes, M.D.; Pedano, M.L.; Ferreyra, N.F.; Luque, G.; Miscoria, S.A. Carbon Nanotubes: A New Alternative for Electrochemical Sensors; Nunez, M., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2006; pp. 1–46. [Google Scholar]
- Shin, S.; Yoon, S.; Kim, Y.; Lee, C. Effect of particle parameters on the deposition characteristics of a hard/soft-particles composite in kinetic spraying. Surface Coat. Technol. 2006, 201, 3457–3461. [Google Scholar] [CrossRef]
- Samuel, R.E.; Shukla, A.; Paik, D.H.; Wang, M.X.; Fang, J.C.; Schmidt, D.J.; Hammond, P.T. Osteoconductive protamine-based polyelectrolyte multilayer functionalized surfaces. Biomaterials 2011, 32, 7491–7502. [Google Scholar] [CrossRef] [PubMed]
- Roach, P.; Farrar, D.; Perry, C.C. Surface Tailoring for Controlled Protein Adsorption: Effect of Topography at the Nanometer Scale and Chemistry. J. Am. Chem. Soc. 2006, 128, 3939–3945. [Google Scholar] [CrossRef] [PubMed]
- Ojamaee, L.; Aulin, C.; Pedersen, H.; Kaell, P.-O. IR and quantum-chemical studies of carboxylic acid and glycine adsorption on rutile TiO2 nanoparticles. J. Colloid Interface Sci. 2006, 296, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Verboekend, D.; Milina, M.; Perez-Ramirez, J. Hierarchical Silicoaluminophosphates by Postsynthetic Modification: Influence of Topology, Composition, and Silicon Distribution. Chem. Mater. 2014, 26, 4552–4562. [Google Scholar] [CrossRef]
- Liu, J.; Cui, L.; Wang, L.; Ni, X.; Zhang, S.; Jin, Y. Alkaline-acid treated mordenite and beta zeolites featuring mesoporous dimensional uniformity. Mater. Lett. 2014, 132, 78–81. [Google Scholar] [CrossRef]
- Creti, A.; Epifani, M.; Taurino, A.; Catalano, M.; Casino, F.; Lomascolo, M.; Milanese, M.; de Risi, A. Optical absorption measurements at high temperature (500 °C) of oxide nanoparticles for application as gas-based nanofluid in solar thermal collector systems. Adv. Mater. Res. 2013, 773, 80–86. [Google Scholar] [CrossRef]
- Di Crescenzo, A.; Demurtas, D.; Renzetti, A.; Siani, G.; de Maria, P.; Meneghetti, M.; Prato, M.; Fontana, A. Disaggregation of single-walled carbon nanotubes (SWNTs) promoted by the ionic liquid-based surfactant 1-hexadecyl-3-vinyl-imidazolium bromide in aqueous solution. Soft Matter 2009, 5, 62–66. [Google Scholar] [CrossRef]
- Van Blaaderen, A. Materials science: Colloids get complex. Nature 2006, 439, 545–546. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.-F.; Yuan, G.; Li, C.; Liu, W.-H.; Mimura, H. Modulation of the Work Function of Capped Single-Walled Carbon Nanotube by Alkali-Metal Adsorption: A Theoretical Study. J. Phys. Chem. C 2011, 115, 8928–8933. [Google Scholar] [CrossRef]
- Lystvet, S.M.; Volden, S.; Halskau, O.; Glomm, W.R. Immobilization onto gold nanoparticles alters alpha-lactalbumin interaction with pure and mixed phospholipid monolayers. Soft Matter 2011, 7, 11501–11509. [Google Scholar] [CrossRef]
- Badaire, S.; Zakri, C.; Maugey, M.; Derré, A.; Barisci, J.N.; Wallace, G.; Poulin, P. Liquid crystals of DNA-stabilized carbon nanotubes. Adv. Mater. 2005, 17, 1673–1676. [Google Scholar] [CrossRef]
- Karachevtsev, M.V.; Gladchenko, G.O.; Plokhotnichenko, A.M.; Leontiev, V.S.; Karachevtsev, V.A. Adsorption of Biopolymers on SWCNT: Ordered Poly(rC) and Disordered Poly(rI). J. Phys. Chem. B 2013, 117, 2636–2644. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Battigelli, A.; Menard-Moyon, C.; Bianco, A. Carbon nanomaterials as new tools for immunotherapeutic applications. J. Mater. Chem. B Mater. Biol. Med. 2014, 2, 6144–6156. [Google Scholar] [CrossRef]
- Lee, H.S. Classification of Mass-Produced Carbon Nanotubes and Their Physico-Chemical Properties. In Syntheses and Applications of Carbon Nanotubes and Their Composites; Suzuki, S., Ed.; InTech: Rijeka, Croatia, 2013; pp. 39–53. [Google Scholar]
- Weidenkaff, A.; Ebbinghaus, S.G.; Mauron, Ph.; Reller, A.; Zhang, Y.; Zuttel, A. Metal nanoparticles for the production of carbon nanotube composite materials by decomposition of different carbon sources. Mater. Sci. Eng. C 2002, 19, 119–123. [Google Scholar] [CrossRef]
- Charlier, J.C. Defects in carbon nanotubes. Acc. Chem. Res. 2002, 35, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Berne, B.J.; Pecora, R. Dynamic Light Scattering: With Applications to Chemistry, Biology and Physics; Courier Dover Publications: New York, NY, USA, 2013. [Google Scholar]
- Tardani, F.; la Mesa, C. Elasticity of dispersions based on carbon nanotubes dissolved in a lyotropic nematic solvent. J. Phys. Chem. C 2011, 115, 9424–9431. [Google Scholar] [CrossRef]
- Onsager, L. The effects of shapes on the interaction of colloidal particles. Ann. N.Y. Acad. Sci. 1949, 51, 627–659. [Google Scholar] [CrossRef]
- Matsuyama, A. Theory of binary mixtures of a rodlike polymer and a liquid crystal. J. Chem. Phys. 2010, 132, 214902:1–214902:10. [Google Scholar]
- Binder, K.; Mognetti, B.; Paul, W.; Virnau, P.; Yelash, L. Computer simulations and coarse-grained molecular models predicting the equation of state of polymer solutions. Adv. Polym. Sci. 2011, 238, 329–387. [Google Scholar]
- Van den Pol, E.; Thies-Weesie, D.M.E.; Petukhov, A.V.; Vroege, G.J.; Kvashnina, K. Influence of polydispersity on the phase behavior of colloidal goethite. J. Chem. Phys. 2008, 129, 164715:1–164715:8. [Google Scholar] [CrossRef]
- Stroobants, A.; Lekkerkerker, H.N.W.; Odiik, T. Effect of electrostatic interaction on the liquid crystal phase transition in solutions of rodlike polyelectrolytes. Macromolecules 1986, 19, 2232–2238. [Google Scholar] [CrossRef]
- Feigin, R.I.; Napper, D.H. Depletion stabilization and depletion flocculation. J. Colloid Interface Sci. 1980, 75, 525–541. [Google Scholar] [CrossRef]
- De Gennes, P.G. Polymer solutions near an interface. Adsorption and depletion layers. Macromolecules 1981, 14, 1637–1644. [Google Scholar] [CrossRef]
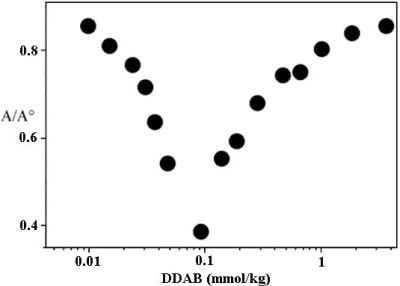
- Tardani, F.; la Mesa, C. Attempts to control depletion in the surfactant-assisted stabilization of single-walled carbon nanotubes. Colloids Surf. A 2014, 443, 123–128. [Google Scholar] [CrossRef]
- Li-In-On, F.K.R.; Vincent, B.; Waite, F.A. Stability of sterically stabilized dispersions at high polymer concentrations. ACS Symp. Ser. 1975, 9, 165–172. [Google Scholar]
- Brash, J.L.; Horbett, T.A. Proteins at Interfaces: An Overview. ACS Symp. Ser. 1995, 602, 1–23. [Google Scholar]
- Karchemsky, F.; Drug, E.; Mashiach-Farkash, E.; Fadeev, L.; Wolfson, H.J.; Gozin, M.; Regev, O. Diameter-selective dispersion of carbon nanotubes by beta-lactoglobulin whey protein. Colloids Surf. B 2013, 112, 16–22. [Google Scholar] [CrossRef]
- Hirano, A.; Tanaka, T.; Kataura, H.; Kameda, T. Arginine Side Chains as a Dispersant for Individual Single-Wall Carbon Nanotubes. Chem. Eur. J. 2014, 20, 4922–4930. [Google Scholar] [CrossRef] [PubMed]
- Bomboi, F.; Tardani, F.; Gazzoli, D.; Bonincontro, A.; La Mesa, C. Lysozyme binds onto functionalized carbon nanotubes. Colloids Surf. B 2013, 108, 16–22. [Google Scholar] [CrossRef]
- Moridi, Z.; Mottaghitalab, V. Recent Advances of Carbon Nanotube/Biopolymers Nanocomposites: A Technical Review. In Modern Trends in Chemistry and Chemical Engineering; Apple Academic Press: Waretown, NJ, USA, 2011; pp. 104–119. [Google Scholar]
- Van der Sman, R.G.M. Thermodynamics of meat proteins. Food Hydrocoll. 2012, 27, 529–535. [Google Scholar] [CrossRef]
- Chodankar, S.; Aswal, V.K.; Kohlbrecher, J.; Vavrin, R.; Wagh, A.G. Small-angle neutron scattering study of structure and kinetics of temperature-induced protein gelation. Phys. Rev. E 2009, 79. [Google Scholar] [CrossRef]
- Tardani, F.; la Mesa, C. Effects of single-walled carbon nanotubes on lysozyme gelation. Colloids Surf. B 2014, 121, 165–170. [Google Scholar] [CrossRef]
- Tardani, F.; Strobbia, P.; Scipioni, A.; la Mesa, C. Encapsulating carbon nanotubes in aqueous ds-DNA anisotropic phases: Shear orientation and rheological properties. RSC Adv. 2013, 3, 25917–25923. [Google Scholar] [CrossRef]
- Tardani, F.; la Mesa, C.; Poulin, P.; Maugey, M. Phase Behavior of DNA-Based Dispersions containing Carbon Nanotubes: Effects of Added Polymers and Ionic Strength on Excluded Volume. J. Phys. Chem. C 2012, 116, 9888–9894. [Google Scholar] [CrossRef]
- Tardani, F.; Sennato, S. Phase behavior of DNA-stabilized carbon nanotubes dispersions: Association with oppositely-charged additives. J. Phys. Chem. C 2014, 118, 9268–9274. [Google Scholar] [CrossRef]
- Goddard, E.D. Polymer-Surfactant Interactions; Part II. Polymers and Surfactants of Opposite Charge. In Interactions of Surfactants with Polymers and Proteins; Goddard, E.D., Ananthapadmanabhan, K.P., Eds.; CRC Press: Boca Raton, FL, USA, 1993; Chapter IV; pp. 171–201. [Google Scholar]
- Rill, R.L.; Strzelecka, T.E.; Davidson, M.W.; van Winkle, D.H. Ordered phases in concentrated DNA solutions. Phys. A Stat. Mech. Appl. 1991, 176, 87–116. [Google Scholar] [CrossRef]
- Bergfeldt, K.; Piculell, L. Segregation and Association in Mixed Polymer Solutions from Flory-Huggins Model Calculations. J. Phys. Chem. 1996, 100, 3680–3687. [Google Scholar] [CrossRef]
- Marrucci, G. Rheology of liquid crystalline polymers. Pure Appl. Chem. 1985, 57, 1545–1552. [Google Scholar] [CrossRef]
- Marrucci, G. Rheology of Nematic Polymers. In Liquid Crystallinity in Polymers; Ciferri, A., Ed.; Wiley-VCH: New York, NY, USA, 1991; pp. 395–422. [Google Scholar]
- Tardani, F.; Pucci, C.; la Mesa, C. Confining ss-DNA/carbon nanotube complexes in ordered droplets. Soft Matter 2014, 10, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Tardani, F.; la Mesa, C. Titration of ss-DNA/CNT complexes. Unpublished work. 2015. [Google Scholar]
- Svensek, D.; Veble, G.; Podgornik, R. Confined nematic polymers: Order and packing in a nematic drop. Phys. Rev. E 2010, 82, 011708:1–011708:14. [Google Scholar] [CrossRef]
- Davis, V.A.; Parra-Vasquez, A.N.G.; Green, M.J.; Raj, P.K.; Behabtu, N.; Prieto, V.; Booker, R.D.; Schmidt, J.; Kesselman, E.; Zhou, W.; et al. True Solutions of Single-Walled Carbon Nanotubes for Assembly into Macroscopic Materials. Nat. Nanotechnol. 2009, 4, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Scalia, G.; von Buhler, C.; Hagele, C.; Roth, S.; Giesselmann, F.; Lagerwall, J.P.F. Spontaneous Macroscopic Carbon nanotube Alignment via Colloidal Suspension in Hexagonal Columnar Lyotropic Liquid Crystals. Soft Matter 2008, 4, 570–576. [Google Scholar] [CrossRef]
- Lagerwall, J.P.F.; Scalia, G.; Haluska, M.; Dettlaff-Weglikowska, U.; Roth, S.; Giesselmann, F. Nanotube Alignment Using Lyotropic Liquid Crystals. Adv. Mater. 2007, 19, 359–364. [Google Scholar] [CrossRef]
- Ao, G.; Nepal, D.; Aono, M.; Davis, V.A. Cholesteric and Nematic Liquid Crystalline Phase Behavior of Double Stranded DNA Stabilized Single-Walled Carbon Nanotube Dispersions. ACS Nano 2011, 5, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Sanchez, M.; Simmons, T.J.; Vidal, M.A. Liquid Crystal Behavior of Single Wall Carbon Nanotubes. Carbon 2010, 48, 3531–3542. [Google Scholar] [CrossRef]
- Puech, N.; Blanc, C.; Grelet, E.; Zamora-Ledezma, C.; Maugey, M.; Zakri, C.; Anglaret, E.; Poulin, P. Highly Ordered Carbon Nanotube Nematic Liquid Crystals. J. Phys. Chem. C 2011, 115, 3272–3278. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Boncheva, M. Beyond molecules: Self-assembly of meso-scopic and macroscopic components. Proc. Nat. Acad. Sci. USA 2002, 99, 4769–4774. [Google Scholar] [CrossRef] [PubMed]
- Glotzer, S.C.; Solomon, M.J. Anisotropy of building blocks and their assembly into complex structures. Nat. Mater. 2007, 6, 557–562. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tardani, F.; Mesa, C.L. Dispersability of Carbon Nanotubes in Biopolymer-Based Fluids. Crystals 2015, 5, 74-90. https://doi.org/10.3390/cryst5010074
Tardani F, Mesa CL. Dispersability of Carbon Nanotubes in Biopolymer-Based Fluids. Crystals. 2015; 5(1):74-90. https://doi.org/10.3390/cryst5010074
Chicago/Turabian StyleTardani, Franco, and Camillo La Mesa. 2015. "Dispersability of Carbon Nanotubes in Biopolymer-Based Fluids" Crystals 5, no. 1: 74-90. https://doi.org/10.3390/cryst5010074