Preparation of Potassium Dichromate Crystals from the Chromite Concentrate by Microwave Assisted Leaching
Abstract
:1. Introduction
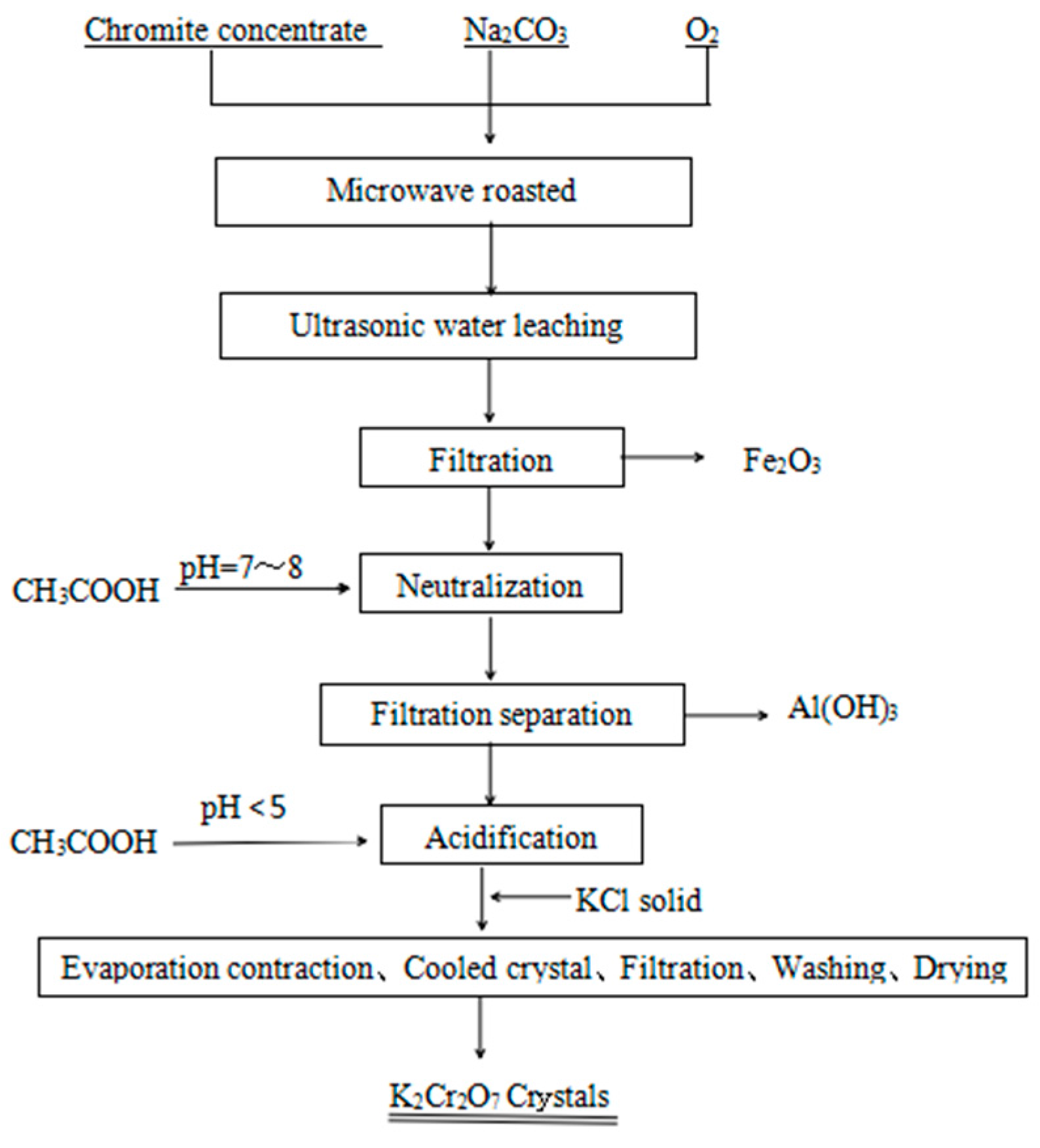
2. Experimental Work
2.1. Materials and Characterization
2.2. Dielectric Property Measurement System
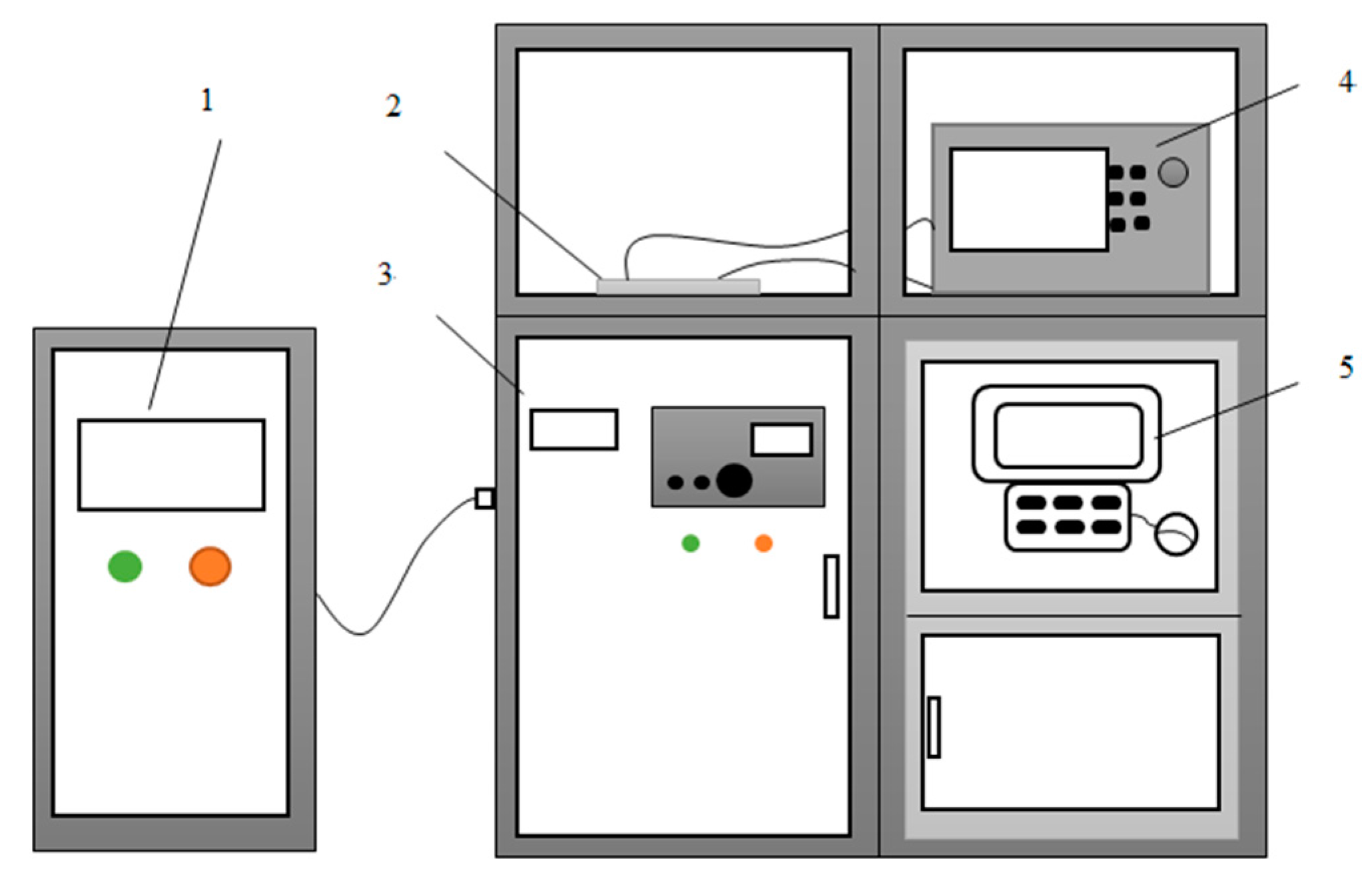
2.3. Experimental Setup and Procedure
2.4. Determination of Purity of the Resultant Product
3. Results and Discussion
3.1. The Absorption Properties of Raw Materials
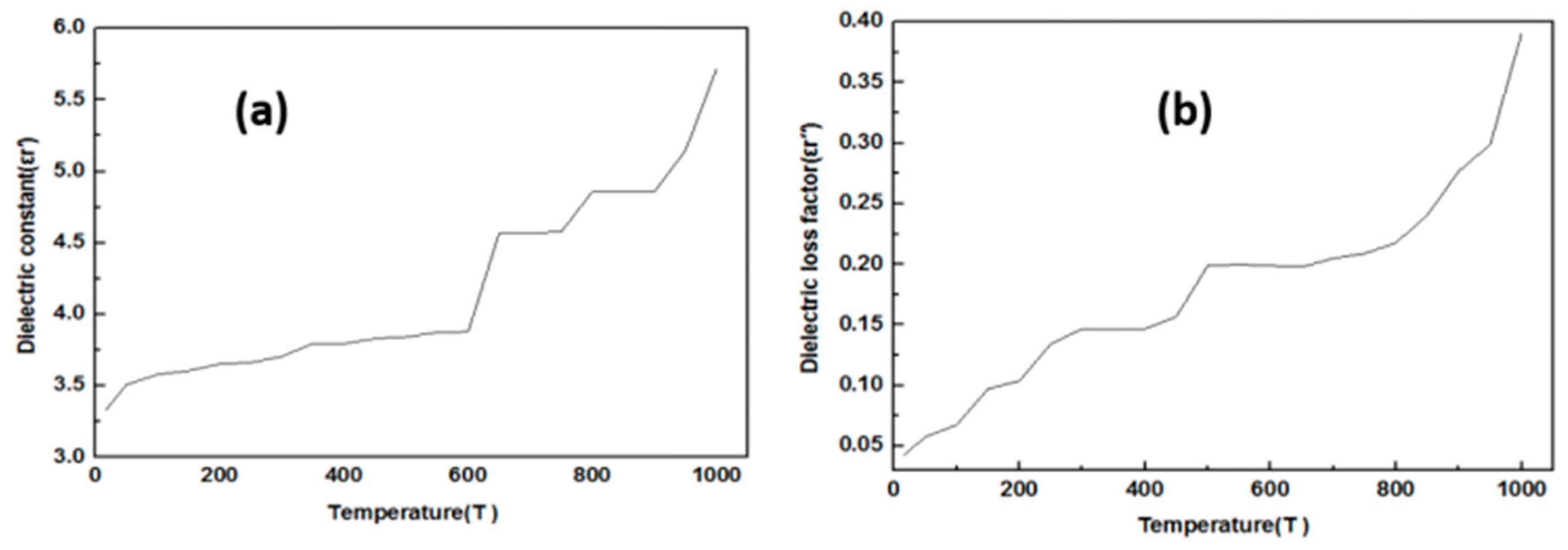
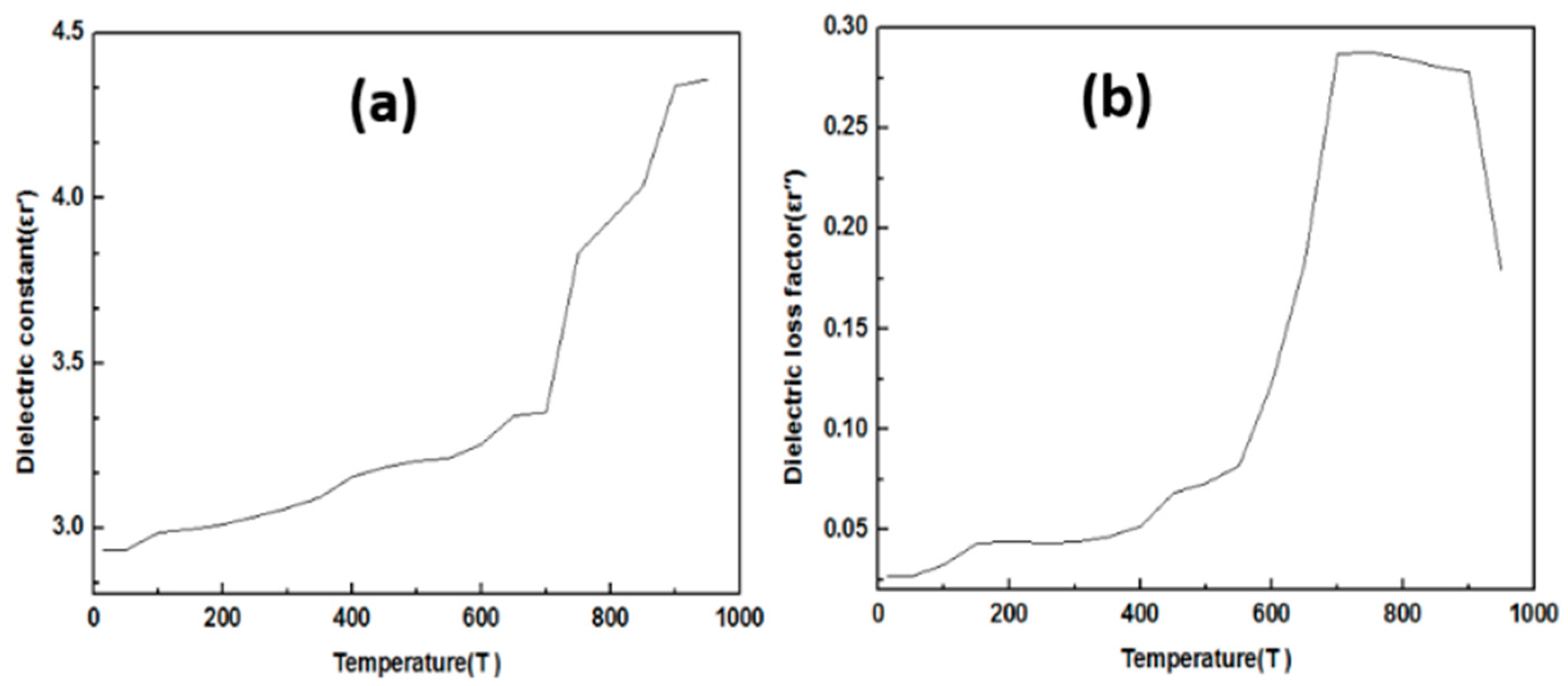
3.2. The Study Dielectric Constant and Dielectric Loss Factor of Raw Material
3.3. Characterization of the Roasted Material
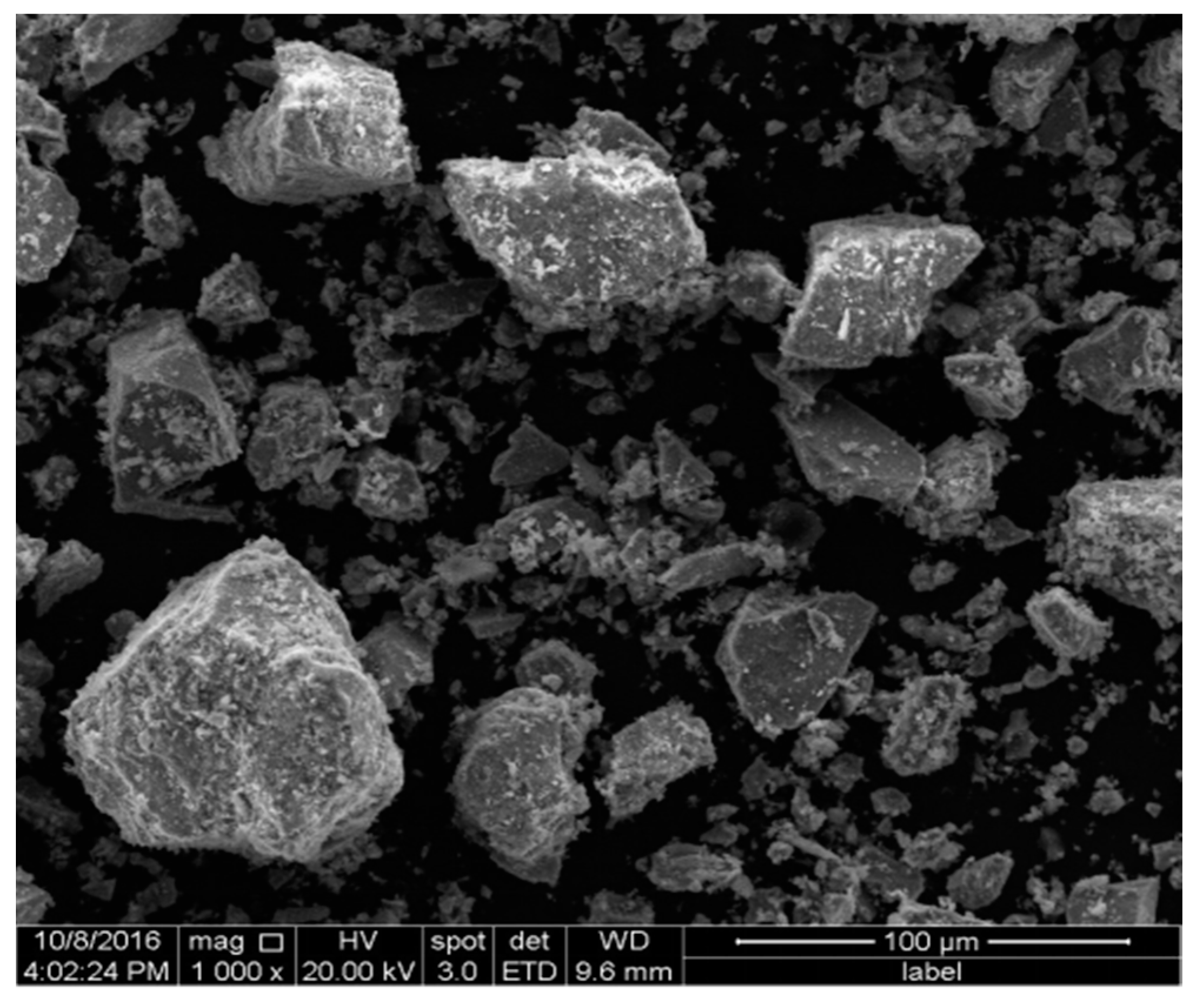
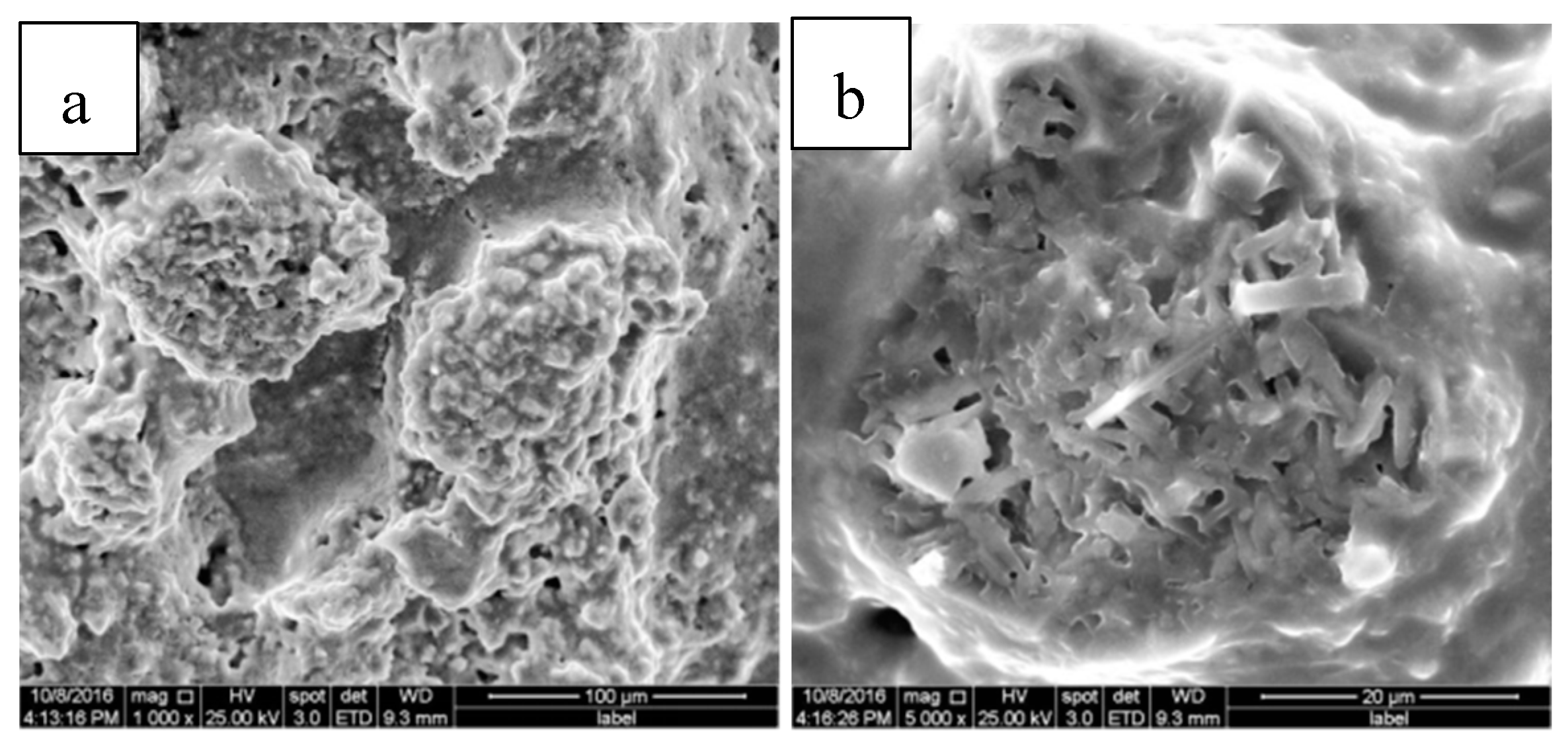
3.3.1. SEM Analysis
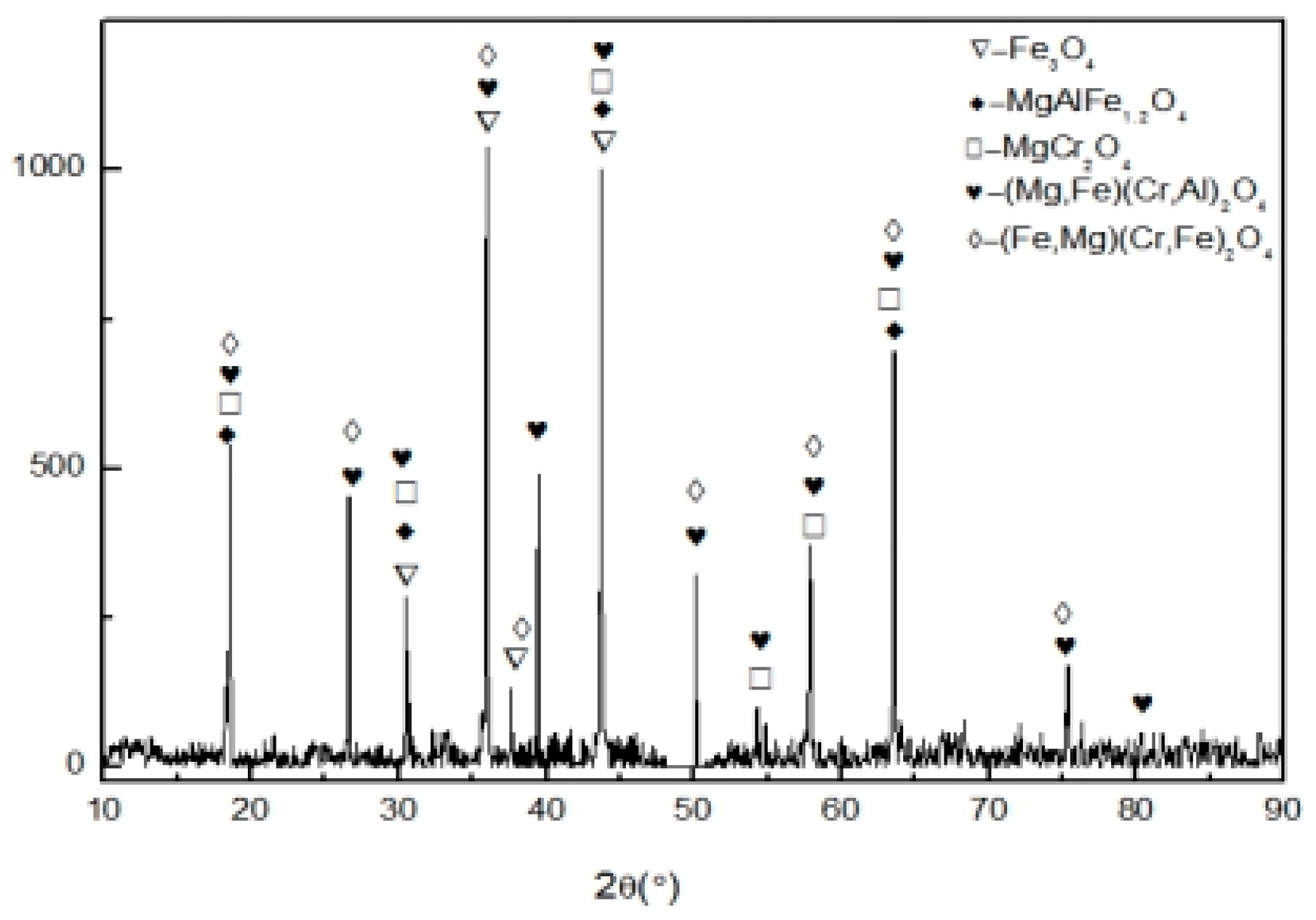
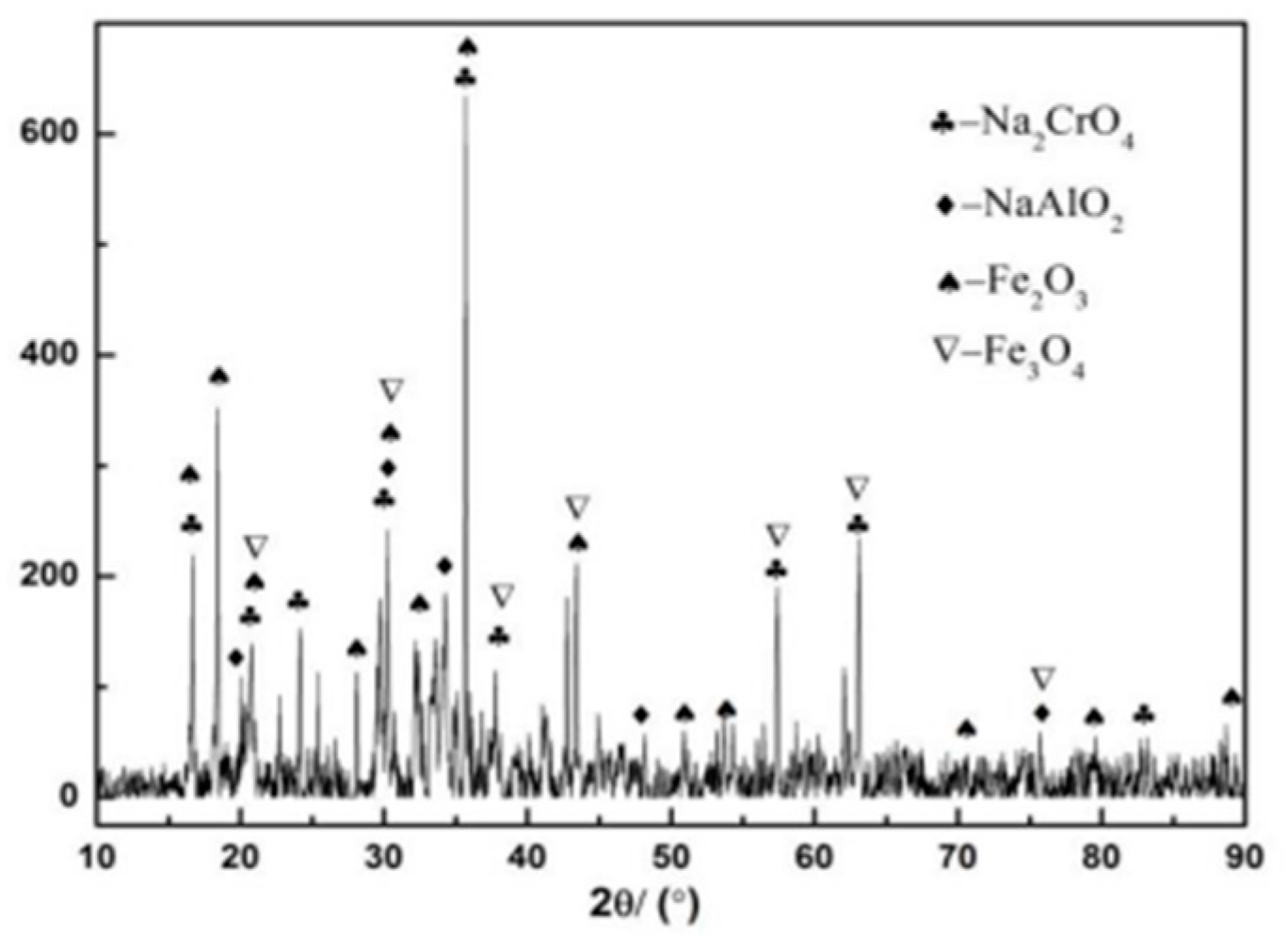
3.3.2. XRD Analysis
3.3.3. XRF Analysis
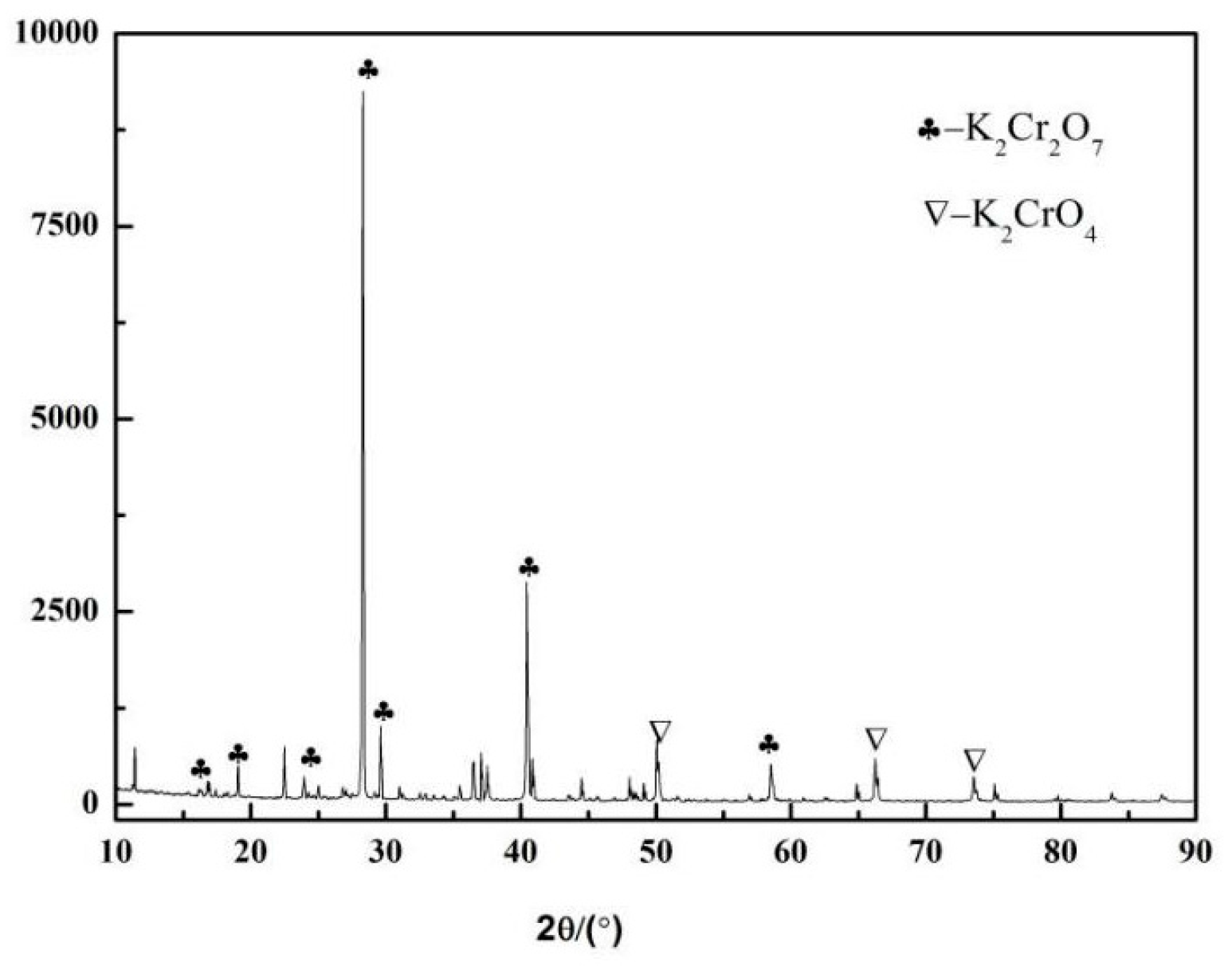
3.4. The Purity of the Product
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yu, W. Research progress in the field of metallurgy of vanadium titanium magnetite by microwave heating. Iron Steel Vanadium Titan. 2011, 32, 87–96. [Google Scholar]
- Tong, Z.; Bi, S.; Yang, Y. Present situation of study on microwave beating application in metallurgy. J. Mater. Metall. 2004, 3, 117–120. [Google Scholar]
- Zhang, S.; Peng, J.; Huang, M.; Sun, Y.; Zhang, L.; Fan, X.; Guo, S. Preparation of primary titanium materials from self-reduced pellet of titanium concentrate by microwave reduction. Chin. J. Rare Met. 2006, 30, 78–81. [Google Scholar]
- Gu, J.; Ao, N.; Liu, Y. The application and progress of microwave technology in mineral processing process. Min. Metall. 2002, 11, 139–146. [Google Scholar]
- Liu, Q.; Xiong, Y. Research on application mechanism of iron ore grinding by microwave. Metallurgy 1997, 3, 25–28. [Google Scholar]
- Zhou, X. Structure and microwave dielectric properties of Kunming ilmentite. Chem. Ind. 1992, 3, 7–8. [Google Scholar]
- Chen, J.; Li, N.; Wang, S. The heating performance of carbon containing chromite fines in microwave field. J. Beijing Univ. Sci. Technol. 2007, 29, 880–906. [Google Scholar]
- Chen, J. Application of Microwave Heating Reduction of Carbon Containing Pellet Melting; Iron & Steel Research Institute: Beijing, China, 2003; Volume 6, pp. 8–13. [Google Scholar]
- Jia, Z. Study on ore chromite pellet smelting high carbon ferrochrome. Zhejiang Metall. 1996, 2, 17–22. [Google Scholar]
- Bai, X.Y.; Wu, S.; Zhang, S.C. A new microwave heating system for processing ceramic materials. J. Microw. 1999, 2, 114. [Google Scholar]
- Alybakov, A.A.; Gubanova, V.A.; Kudabaev, K.; Tojchiev, N. Paramagnetic centres created by a thermal treatment in potassium dichromate crystals. Cryst. Res. Technol. 2010, 18, 68–70. [Google Scholar] [CrossRef]
- Dai, W.; Shu, L. Iron Alloy Metallurgy Engineering; Metallurgical Industry Press: Beijing, China, 1999. [Google Scholar]
- Shi, F.; Kongjin, Z.; Yalin, Q. New progress in mineral processing of chromium iron ore. In Proceedings of the China Mineral Processing Technology Exchange Meeting, Haikou, China, 13–17 November 2006. [Google Scholar]
- Liu, J. Mineral processing of low grade chrome ore. In Proceedings of the China Iron and Steel Annual Meeting, Beijing, China, 28–30 October 2005. [Google Scholar]
- Shi, F. Chromite mine of China and Volgadansk. Geo Exp. 1998, 2, 16–18. [Google Scholar]
- Medina-Campos, O.N.; Barrera, D.; Segoviano-Murillo, S.; Rocha, D.; Maldonado, P.D.; Mendoza-Patiño, N.; Pedraza-Chaverri, J. S-allylcysteine scavenges singlet oxygen and hypochlorous acid and protects LLC-PK1 cells of potassium dichromate-induced toxicity. Food Chem. Toxicol. 2007, 45, 2030–2039. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, O.V.; Kubrak, O.I.; Nykorak, M.Z.; Storey, K.B.; Lushchak, V.I. The effect of potassium dichromate on free radical processes in goldfish: Possible protective role of glutathione. Aquat. Toxicol. 2008, 87, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Reedijk, M.F.; Arsic, J.; Kaminski, D.; Van Enckevort, W.J.P.; Vlieg, E. Surface structure of potassium dichromate (KBC) crystals. Surf. Sci. 2003, 526, 133–140. [Google Scholar] [CrossRef]
- Thilak, T.; Basheer Ahamed, M.; Vinitha, G. Third order nonlinear optical properties of potassium dichromate single crystals by Z-scan technique. Optik 2013, 124, 4716–4720. [Google Scholar] [CrossRef]
- Andre, N.; Paut, O.; Arditti, J.; Fabre, P.; Bremond, V.; Alhmana, T.; Bellus, J.F.; Jouglard, J.; Camboulives, J. Intoxication grave au dichromate de potassium apres introduction nasale accidentelle. Arch. Pédiatrie 1998, 5, 145–148. [Google Scholar] [CrossRef]
- Qi, T.G.; Liu, N.; Li, X.B.; Peng, Z.H.; Liu, G.H.; Zhou, Q.S. Thermodynamics of chromite ore oxidative roasting process. J. Cent. Univ. Technol. 2011, 18, 83–88. [Google Scholar] [CrossRef]
- Li, J. Study on solid alkali prepared by the oxidation of potassium dichromate experimental. Chifeng Univ. 2005, 5, 25. [Google Scholar]
- Li, J. Effect of ultrasonic wave on leaching process. Metallurgy 2001, 30, 28–30. [Google Scholar]
- Xu, S.; Zhang, C.; Zhao, T. Application of ultrasonic in extraction process. HN Nonferrous Met. 1994, 11, 350–353. [Google Scholar]
- Timothy, M. Sonochemistry: Theory, Applications and Uses of Ultrasound in Chemistry; Ellis Horwood: New York, NY, USA, 1988. [Google Scholar]
- Mason, T.J. Advances in Sonochemistry; JAI Press Ltd.: London, UK, 1996; Volume 5. [Google Scholar]
- Vinatoru, M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001, 8, 303–313. [Google Scholar] [CrossRef]
- Chen, D.; Lázaro, F.; de Castro, L.D.; Valcárel, M. Direct spectrophotometric determination of total boron in soils with ultrasonic leaching in automatic flow systems. Anal. Chim. Acta 1989, 226, 221–227. [Google Scholar] [CrossRef]
- Yanan, L.; Kui, L.; Xiurong, S. Study on Producing Dichromate—Potassium by Chromium Containing Wastewaters. Chem. Ind. Guangzhou 2010, 38, 140–141. [Google Scholar]
- Zhang, H.; Xu, H.B.; Zhang, X.; Zhang, Y.; Zhang, Y. Pressure oxidative leaching of Indian chromite ore in concentrated NaOH solution. Hydrometallurgy 2014, 142, 47–55. [Google Scholar] [CrossRef]
- Looyenga, H. Dielectric constants of heterogeneous mixtures. Physica 1965, 31, 401–406. [Google Scholar] [CrossRef]
- Dick, B.G., Jr.; Overhauser, A.W. Theory of the Dielectric Constants of Alkali Halide Crystals. Phys. Rev. 1958, 112, 90–103. [Google Scholar] [CrossRef]
- Akerlof, G. Dielectric Constants of Some Organic Solvent-Water Mixtures at Various Temperatures. J. Am. Chem. Soc. 1932, 11, 4125–4139. [Google Scholar] [CrossRef]
- Dwyer, J.J.; Gittis, A.G.; Karp, D.A.; Lattman, E.E.; Spencer, D.S.; Stites, W.E.; Bertrand, G.-M.E. High Apparent Dielectric Constants in the Interior of a Protein Reflect Water Penetration. Biophys. J. 2000, 79, 1610–1620. [Google Scholar] [CrossRef]
- Uchino, K.; Nomura, S. Critical exponents of the dielectric constants in diffused-phase-transition crystals. Ferroelectrics 1982, 44, 55–61. [Google Scholar] [CrossRef]
- Ghodgaonkar, D.K.; Varadan, V.V. A free-space method for measurement of dielectric constants and loss tangents at microwave frequencies. IEEE Trans. Instrum. Meas. 2002, 38, 789–793. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L.; Peng, J.; Qu, W.; Liu, B.; Xia, H. Dielectric Properties and Microwave Heating Characteristics of Sodium Chloride at 2.45 GHz. High Temp. Mater. Process. 2013, 32, 587–596. [Google Scholar] [CrossRef]
- Huang, H.; Min, L. Study on chromite preparation of chromium oxide instead of potassium dichromate experimental. Shangrao Normal Univ. 2015, 35, 60–62. [Google Scholar]


| Cr2O3 | Fe | Al2O3 | MgO | H2O | SiO2 | CaO | P | S |
|---|---|---|---|---|---|---|---|---|
| 51.44 | 13.56 | 11.11 | 10.44 | 10.44 | 3.95 | 1.45 | 0.05 | 0.05 |
| O | Cr | Na | Fe | C | Mg | Al | Si | Ca |
|---|---|---|---|---|---|---|---|---|
| 65.71 | 33.27 | 45.95 | 6.20 | 5.11 | 2.42 | 1.89 | 0.83 | 0.29 |
| O | Cr | Na | Fe | C | Mg | Al | Si | Ca |
|---|---|---|---|---|---|---|---|---|
| 46.48 | 11.18 | 9.52 | 17.67 | 2.82 | 7.04 | 4.12 | 3.11 | 0.74 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Guo, S.; Peng, J.; Yu, D.; Zhang, L.; Dai, L. Preparation of Potassium Dichromate Crystals from the Chromite Concentrate by Microwave Assisted Leaching. Crystals 2017, 7, 312. https://doi.org/10.3390/cryst7100312
Liu H, Guo S, Peng J, Yu D, Zhang L, Dai L. Preparation of Potassium Dichromate Crystals from the Chromite Concentrate by Microwave Assisted Leaching. Crystals. 2017; 7(10):312. https://doi.org/10.3390/cryst7100312
Chicago/Turabian StyleLiu, Hua, Shenghui Guo, Jinhui Peng, Duan Yu, Libo Zhang, and Linqing Dai. 2017. "Preparation of Potassium Dichromate Crystals from the Chromite Concentrate by Microwave Assisted Leaching" Crystals 7, no. 10: 312. https://doi.org/10.3390/cryst7100312





