Tetrel, Chalcogen, and Charge-Assisted Hydrogen Bonds in 2-((2-Carboxy-1-(substituted)-2-hydroxyethyl)thio) Pyridin-1-ium Chlorides
Abstract
:1. Introduction
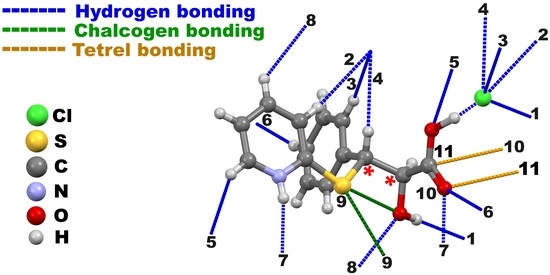
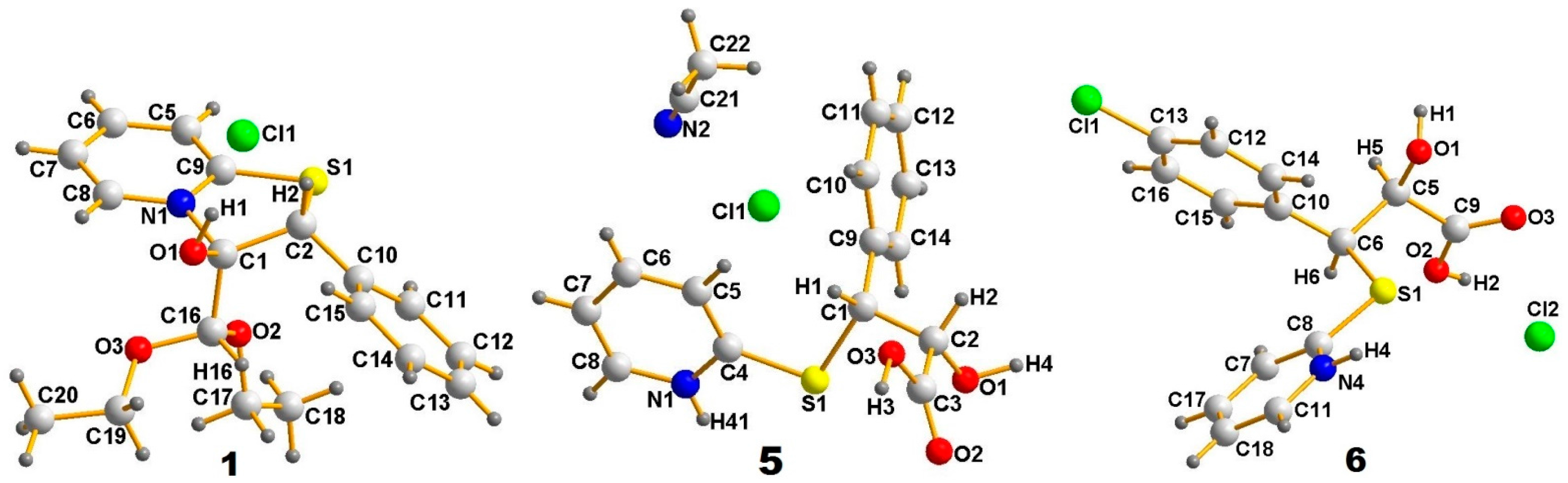
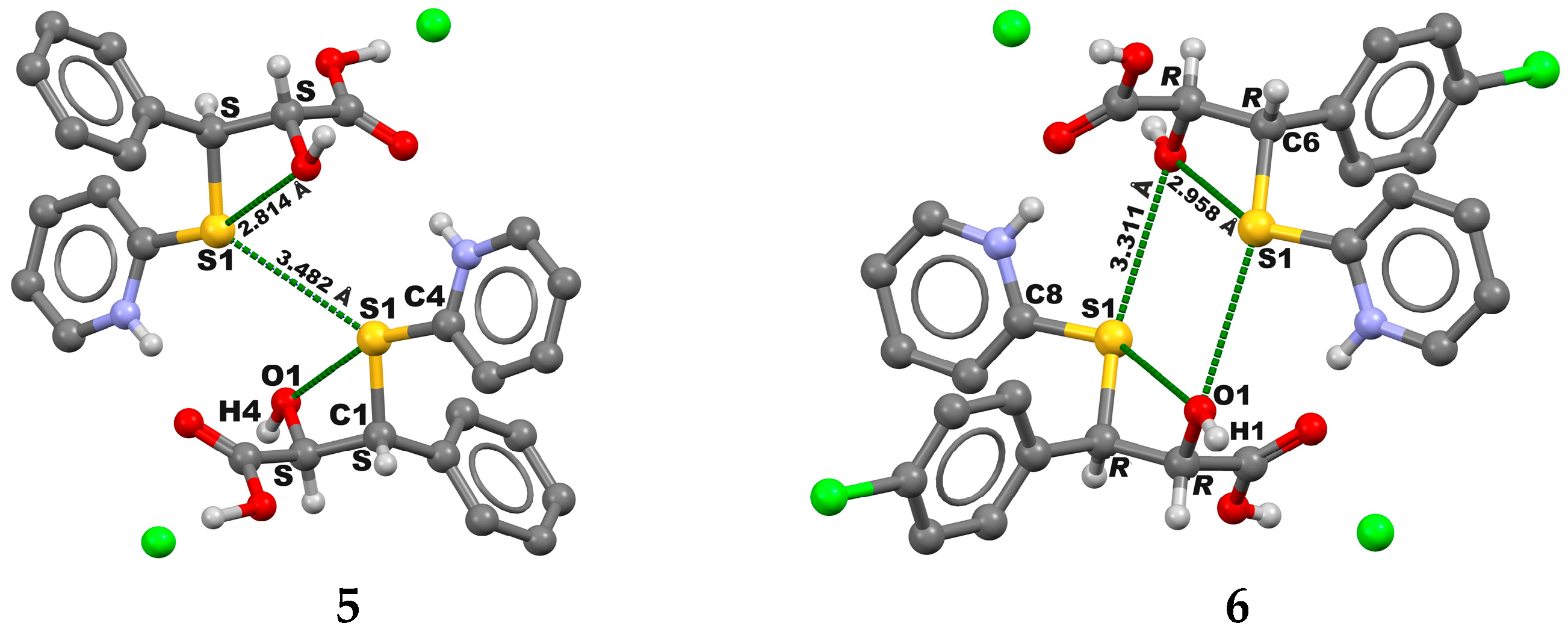
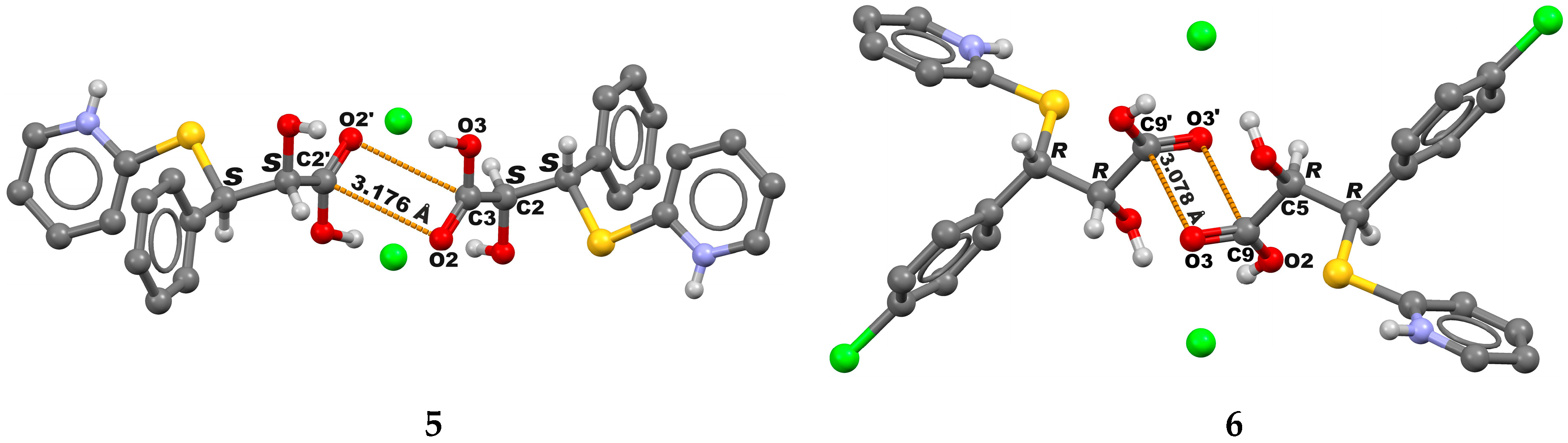
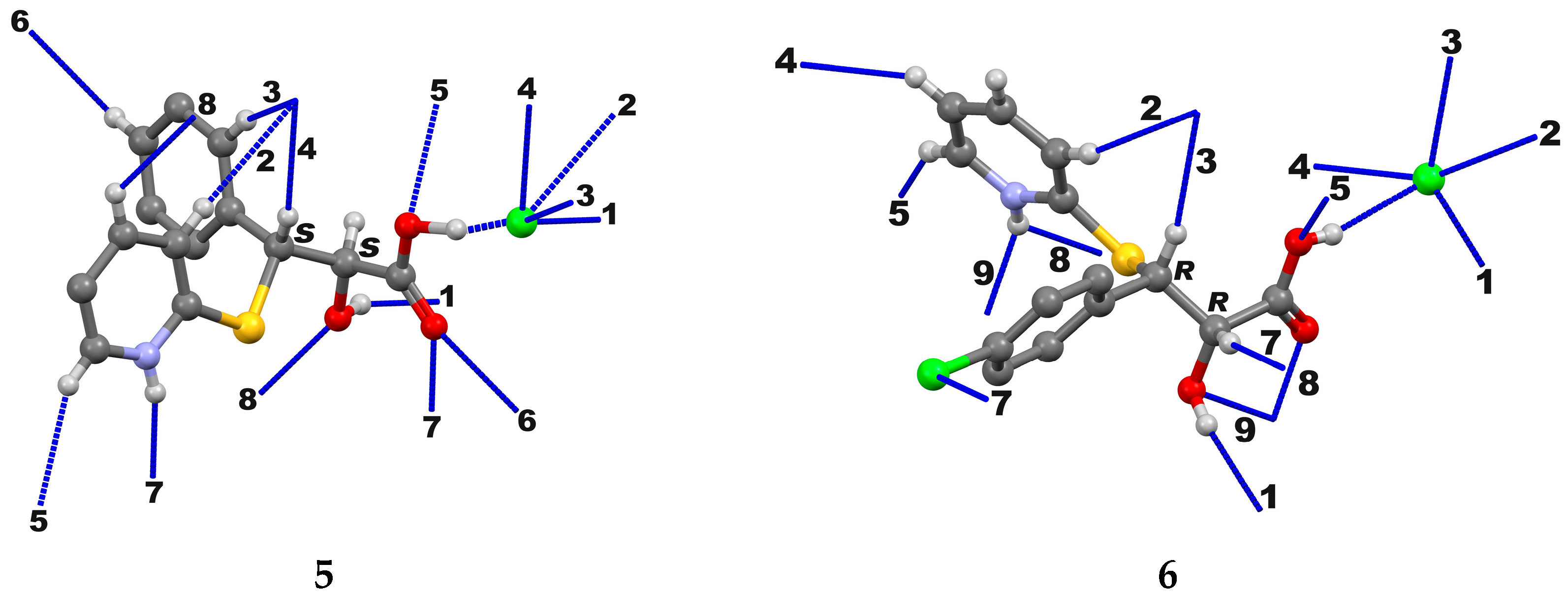
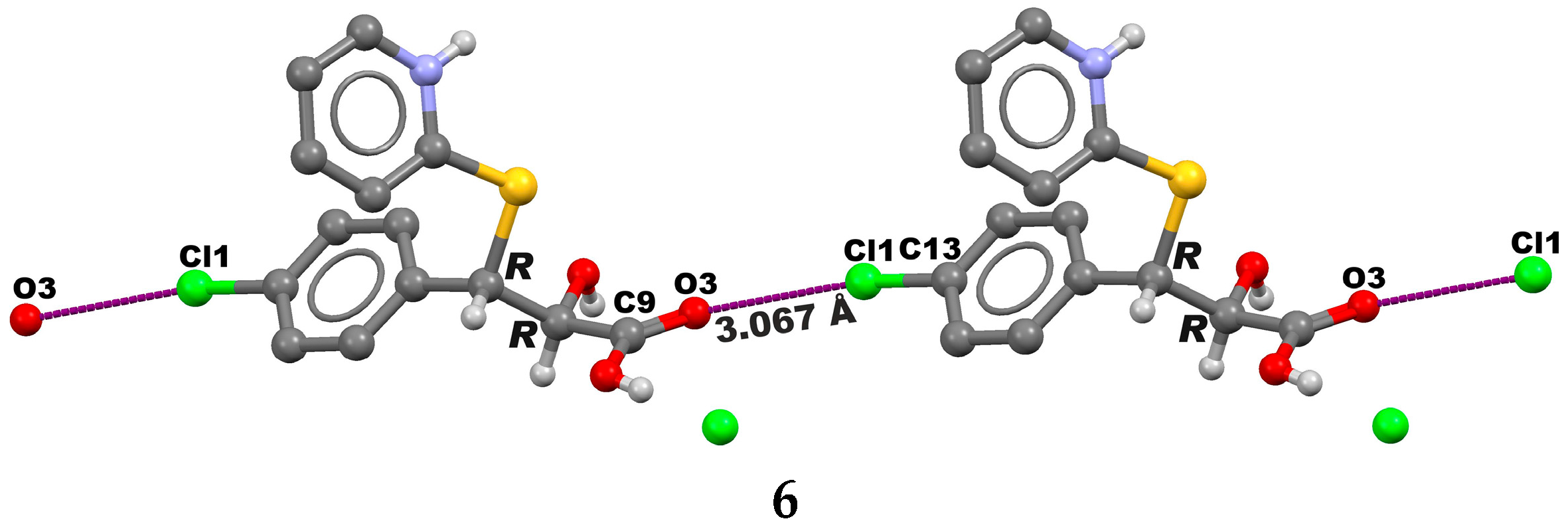
2. Results and Discussion
3. Conclusions
4. Experimental Section
4.1. Materials and Instrumentation
4.2. Synthesis of 1–8
4.2.1. Synthesis of 1–4
4.2.2. Synthesis of 5–8
4.3. X-ray Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Clark, T.; Murray, J.S.; Lane, P.; Politzer, P. Why are dimethyl sulfoxide and dimethyl sulfone such good solvents. J. Mol. Model. 2008, 14, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.S.; Lane, P.; Politzer, P.J. Expansion of the sigma-hole concept. J. Mol. Model. 2009, 15, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Peter, P.; Jane, S.M.; Timothy, C. Halogen bonding: An electrostatically-driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757. [Google Scholar]
- Murray, J.S.; Lane, P.; Clark, T.; Riley, K.E.; Politzer, P.; Politzer, P. Σ-holes, π-holes and electrostatically-driven interactions. J. Mol. Model. 2012, 18, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Peter, P.; Jane, S.M.; Timothy, C. Halogen bonding and other σ-hole interactions: a perspective. Phys. Chem. Chem. Phys. 2013, 15, 11178–11189. [Google Scholar]
- Gabriella, C.; Pierangelo, M.; Tullio, P.; Giuseppe, R.; Giancarlo, T. Naming Interactions from the Electrophilic Site. Cryst. Growth Des. 2014, 14, 2697–2702. [Google Scholar]
- Bauzá, A.; Frontera, A. Aerogen bonding interaction: A new supramolecular force? Angew. Chem. Int. Ed. 2015, 54, 7340–7343. [Google Scholar] [CrossRef] [PubMed]
- Maharramov, A.M.; Mahmudov, K.T.; Kopylovich, M.N.; Pombeiro, A.J.L. Non-Covalent Interactions in the Synthesis and Design of New Compounds; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; ISBN 978-1-119-10989-1. [Google Scholar]
- Mahmudov, K.T.; Kopylovich, M.N.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Non-covalent interactions in the synthesis of coordination compounds: Recent advances. Coord. Chem. Rev. 2017, 345, 54–72. [Google Scholar] [CrossRef]
- Mahmudov, K.T.; Kopylovich, M.N.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Chalcogen bonding in synthesis, catalysis and design of materials. Dalton Trans. 2017, 46, 10121–10138. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.V.; Jain, A.; Rodriguez, S.; Saez, J.A.; Vicent, C.; Peris, G. Stereoisomerization of β-Hydroxy-α-sulfenyl-γ-butyrolactones controlled by two concomitant 1,4-Type nonbonded sulfur-oxygen interactions as analyzed by X-ray crystallography. Org. Chem. 2010, 75, 5888–5894. [Google Scholar] [CrossRef] [PubMed]
- Menichetti, S.; Amorati, R.; Meoni, V.; Tofani, L.; Caminati, G.; Viglianisi, C. Role of noncovalent sulfur···oxygen interactions in phenoxyl radical stabilization: Synthesis of super tocopherol-like antioxidants. Org. Lett. 2016, 18, 5464–5467. [Google Scholar] [CrossRef] [PubMed]
- Garrett, G.E.; Carrera, E.I.; Seferos, D.S.; Taylor, M.S. Anion recognition by a bidentate chalcogen bond donor. Chem. Commun. 2016, 52, 9881–9884. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Fujii, H.; Yamashita, Y.; Kabuto, C.; Tanaka, S.; Harasawa, M.; Mukai, T.; Miyashi, T. Clathrate formation and molecular recognition by novel chalcogen-cyano interactions in tetracyanoquinodimethanes fused with thiadiazole and selenadiazole rings. J. Am. Chem. Soc. 1992, 114, 3034–3043. [Google Scholar] [CrossRef]
- Zhao, H.; Gabbaï, F. A bidentate Lewis acid with a telluronium ion as an anion-binding site. Nat. Chem. 2010, 2, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Mikherdov, A.S.; Kinzhalov, M.A.; Novikov, A.S.; Boyarskiy, V.P.; Boyarskaya, I.A.; Dar’in, D.V.; Starova, G.L.; Kukushkin, V.Y. Difference in energy between two distinct types of chalcogen bonds drives regioisomerization of binuclear (diaminocarbene)PdII complexes. J. Am. Chem. Soc. 2016, 138, 14129–14137. [Google Scholar] [CrossRef] [PubMed]
- Benz, S.; López-Andarias, J.; Mareda, J.; Sakai, N.; Matile, S. Catalysis with chalcogen bonds. Angew. Chem. Int. Ed. 2017, 56, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Robinson, E.R.T.; Fallan, C.; Simal, C.; Slawin, A.M.Z.; Smith, A.D. Anhydrides as α,β-unsaturated acyl ammonium precursors: isothiourea-promoted catalytic asymmetric annulation processes. Chem. Sci. 2013, 4, 2193–2200. [Google Scholar] [CrossRef] [Green Version]
- Fukata, Y.; Asano, K.; Matsubara, S. Facile net cycloaddition approach to optically active 1,5-benzothiazepines. J. Am. Chem. Soc. 2015, 137, 5320–5323. [Google Scholar] [CrossRef] [PubMed]
- Mahmudov, K.T.; Pombeiro, A.J.L. Resonance-Assisted hydrogen bonding as a driving force in synthesis and a synthon in the design of materials. Chem. Eur. J. 2016, 22, 16356–16398. [Google Scholar] [CrossRef] [PubMed]
- Gilli, G.; Gilli, P. The Nature of the Hydrogen Bond: Outline of a Comprehensive Hydrogen Bond Theory, 1st ed.; Oxford University Press: Oxford, UK, 2009; ISBN 978–0199558964. [Google Scholar]
- Ward, M.D. Design of crystalline molecular networks with charge-assisted hydrogen bonds. Chem. Commun. 2005, 47, 5838–5842. [Google Scholar] [CrossRef] [PubMed]
- Manna, D.; Mugesh, G. Regioselective deiodination of thyroxine by iodothyronine deiodinase mimics: an unusual mechanistic pathway involving cooperative chalcogen and halogen bonding. J. Am. Chem. Soc. 2012, 134, 4269–4279. [Google Scholar] [CrossRef]
- Guseinov, F.I.; Tagiev, S.S.; Moskva, B.B. Functional-substituted alpha-chloroxiranes. Synthesis and rearrangement. Rus. J. Org. Chem. 1995, 31, 1131–1133. [Google Scholar]
- Guseinov, F.I.; Yudina, N.A. New approaches to 2,5-disubstituted 4-formylthiazole synthesis. Chem. Het. Comp. 1998, 1, 124–129. [Google Scholar]
- Guseinov, F.I.; Burangulova, R.N.; Mukhamedzyanova, E.F.; Strunin, B.P.; Sinyashin, O.G.; Litvinov, I.A.; Gubaidullin, A.T. Synthesis and molecular structure of 3,7-dimethyl-2-[N-(4-methylpyridyl-2)-4-hydroxy-3-methyl-5-oxopyrrolen-3-yl-2]imidazo-[1,2-a]pyridine. Chem. Het. Comp. 2006, 7, 1089–1094. [Google Scholar] [CrossRef]
- Guseinov, F.I. Reactions of alpha-monochloro- and alpha,alpha-dichloro-beta-oxoaldehyde acetals with bases. Russ. Chem. Bull. 1998, 47, 663–665. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Thomas, S.P.; Jayatilaka, D.; Guru Row, T.N. S⋯O chalcogen bonding in sulfa drugs: insights from multipole charge density and X-ray wavefunction of acetazolamide. Phys. Chem. Chem. Phys. 2015, 17, 25411–25420. [Google Scholar] [CrossRef]
- SMART & SAINT Software Reference Manuals; Version 6.22; Bruker AXS Analytic X-ray Systems, Inc.: Madison, WI, USA, 2000.
- Sheldrick, G.M. SADABS, v. 2.10. Program for Empirical Absorption Correction of Area Detector Data. University of Gottingen: Gottingen, Germany, 2003. [Google Scholar]
- Sheldrick, G.M. SHELXTL V5.1, Software Reference Manual; Bruker AXS Inc.: Madison, WI, USA, 1997. [Google Scholar]

| 1 | 5 | 6 | |
|---|---|---|---|
| Empirical formula | C18H22ClNO3S | C16H17ClN2O3S | C14H13Cl2NO3S |
| fw | 367.87 | 352.84 | 346.21 |
| Temperature (K) | 295(2) | 295(2) | 297(2) |
| Crystal System | monoclinic | triclinic | triclinic |
| Space group | C 2/c | P-1 | P-1 |
| a (Å) | 21.1881(9) | 8.0318(6) | 7.8927(13) |
| b (Å) | 7.3063(2) | 9.4358(5) | 9.2432(14) |
| c (Å) | 24.64070(10) | 11.0398(7) | 12.1043(17) |
| α, ° | 90 | 79.935(5) | 74.695(11) |
| β, ° | 91.942(3) | 78.597(6) | 82.290(13) |
| γ, ° | 90 | 82.607(6) | 82.015(13) |
| V (Å3) | 3812.35(19) | 803.68(9) | 839.1(2) |
| Z | 8 | 2 | 2 |
| ρcalc (g cm−3) | 1.282 | 1.360 | 1.370 |
| μ(Mo Kα) (mm−1) | 2.923 | 3.413 | 4.720 |
| F (000) | 1552 | 343 | 356 |
| GOOF | 0.984 | 0.969 | 1.007 |
| R1a (I ≥ 2σ) | 0.0451 | 0.0846 | 0.0608 |
| wR2b (I ≥ 2σ) | 0.1145 | 0.2197 | 0.1664 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guseinov, F.I.; Pistsov, M.F.; Movsumzade, E.M.; Kustov, L.M.; Tafeenko, V.A.; Chernyshev, V.V.; Gurbanov, A.V.; Mahmudov, K.T.; Pombeiro, A.J.L. Tetrel, Chalcogen, and Charge-Assisted Hydrogen Bonds in 2-((2-Carboxy-1-(substituted)-2-hydroxyethyl)thio) Pyridin-1-ium Chlorides. Crystals 2017, 7, 327. https://doi.org/10.3390/cryst7110327
Guseinov FI, Pistsov MF, Movsumzade EM, Kustov LM, Tafeenko VA, Chernyshev VV, Gurbanov AV, Mahmudov KT, Pombeiro AJL. Tetrel, Chalcogen, and Charge-Assisted Hydrogen Bonds in 2-((2-Carboxy-1-(substituted)-2-hydroxyethyl)thio) Pyridin-1-ium Chlorides. Crystals. 2017; 7(11):327. https://doi.org/10.3390/cryst7110327
Chicago/Turabian StyleGuseinov, Firudin I., Mikhail F. Pistsov, Eldar M. Movsumzade, Leonid M. Kustov, Victor A. Tafeenko, Vladimir V. Chernyshev, Atash V. Gurbanov, Kamran T. Mahmudov, and Armando J. L. Pombeiro. 2017. "Tetrel, Chalcogen, and Charge-Assisted Hydrogen Bonds in 2-((2-Carboxy-1-(substituted)-2-hydroxyethyl)thio) Pyridin-1-ium Chlorides" Crystals 7, no. 11: 327. https://doi.org/10.3390/cryst7110327