Stuck in Our Teeth? Crystal Structure of a New Copper Amalgam, Cu3Hg
Abstract
:1. Introduction
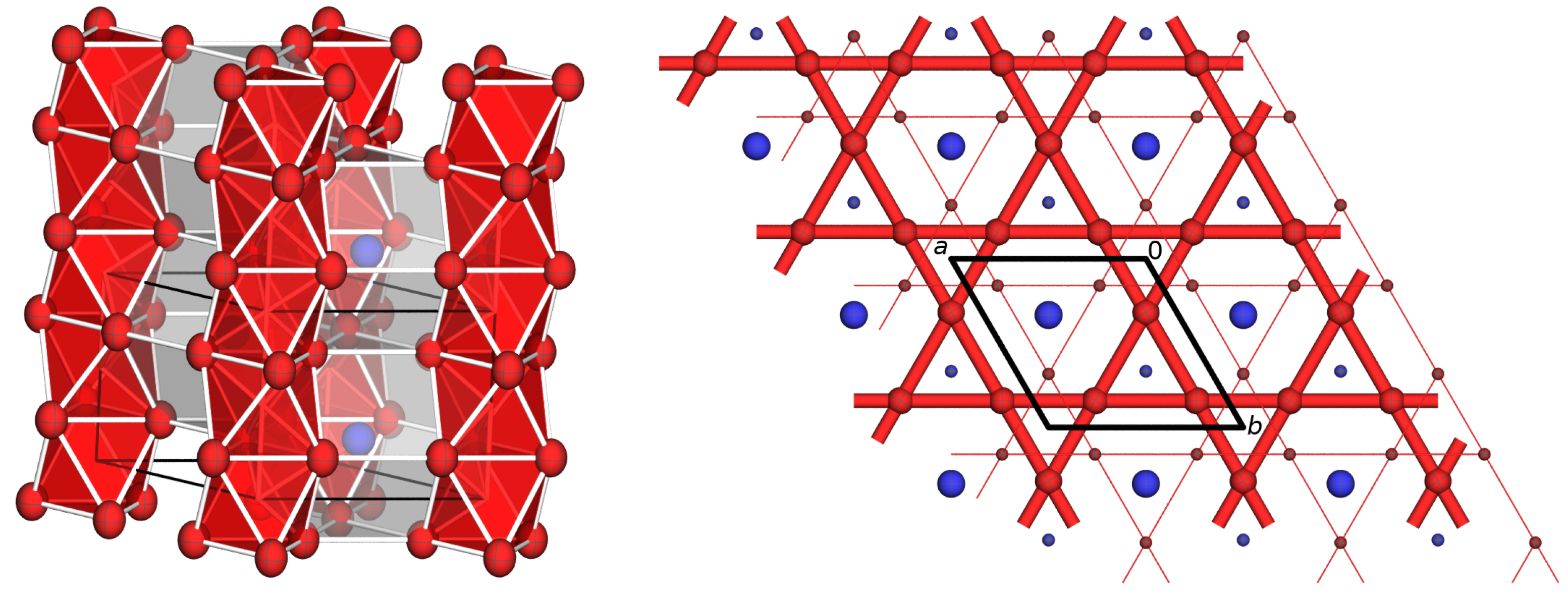
2. Results
3. Discussion
4. Materials and Methods
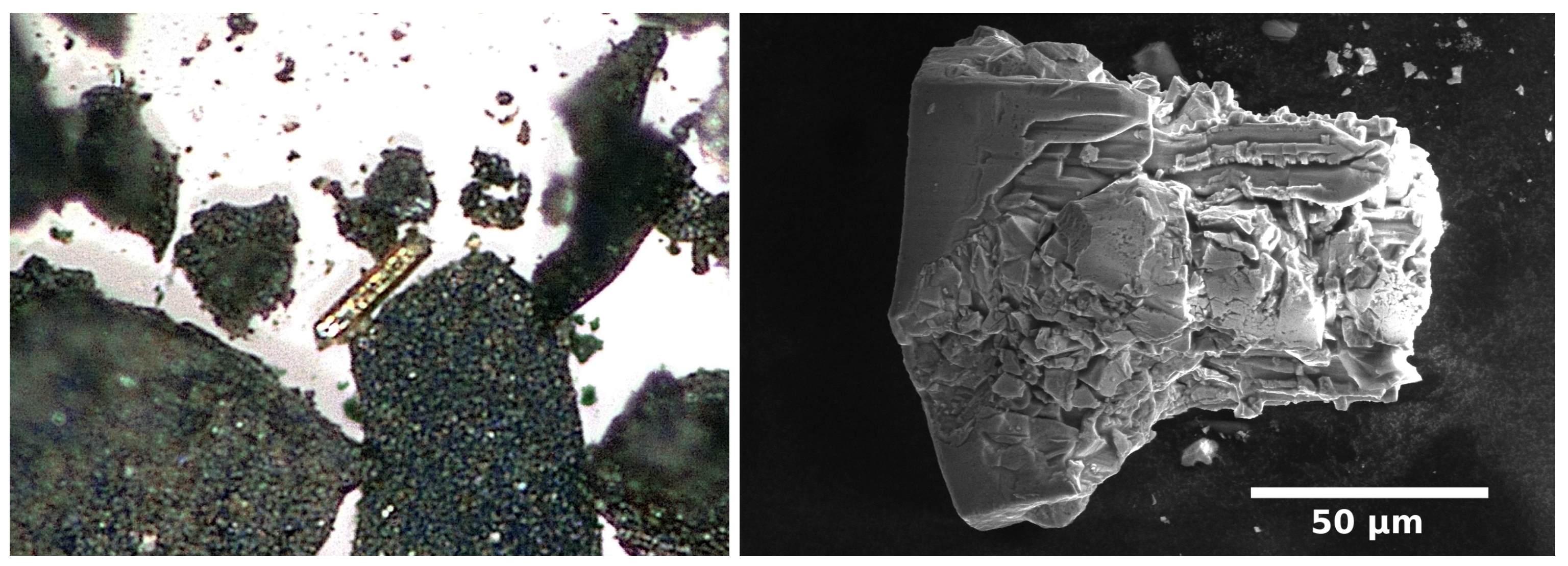
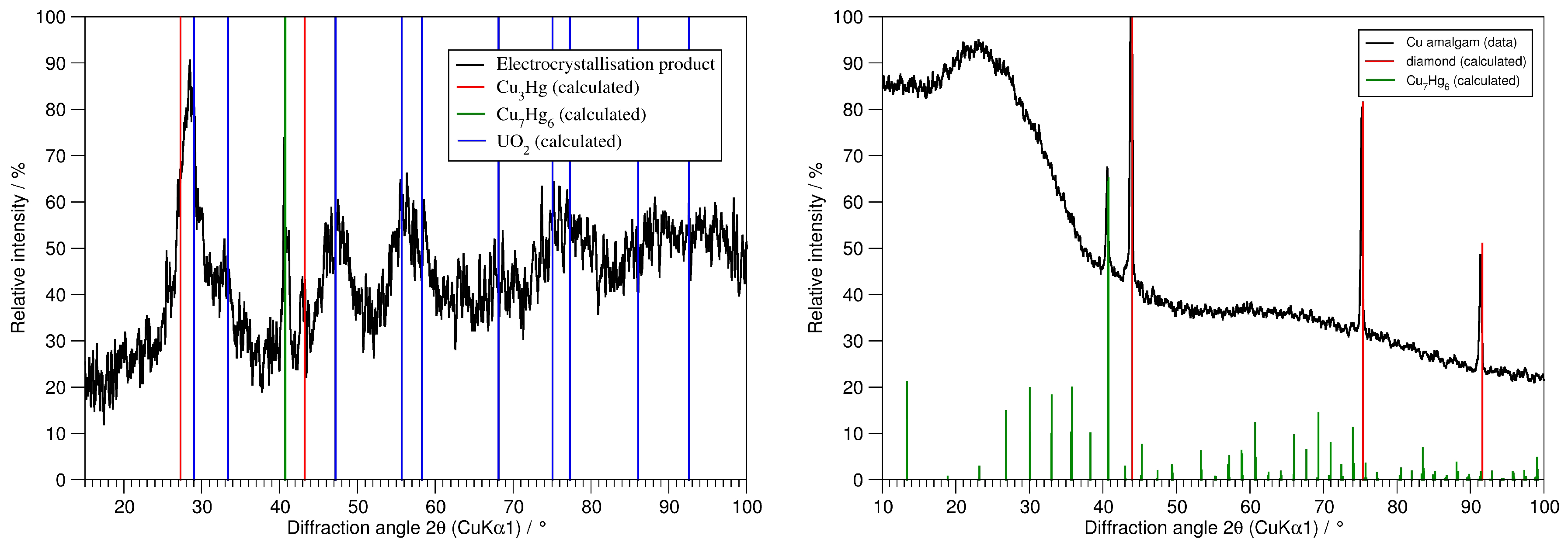
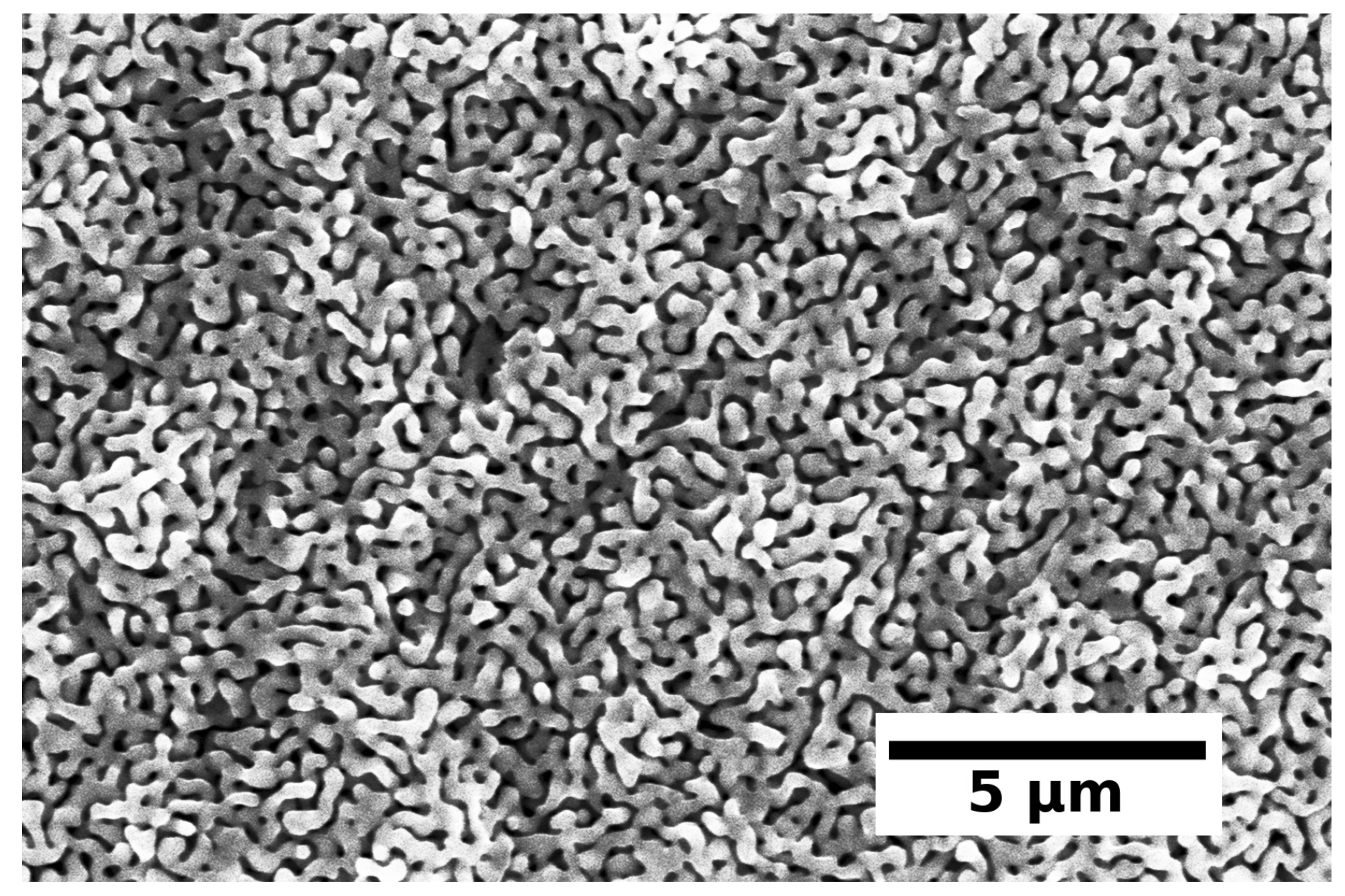
4.1. Synthesis of Copper Amalgams
4.2. Single Crystal Investigations
4.3. Powder Diffractometry
4.4. EDX Spectroscopy
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bharti, R.; Wadhwani, K.K.; Tikku, A.P.; Chandra, A. Dental amalgam: An update. J. Conserv. Dent. 2010, 13, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, R.L.; Powers, J.M. Craig’s Restorative Dental Materials, 13rd ed.; Mosby: St. Louis, MI, USA, 2011; ISBN 978-0323081085. [Google Scholar]
- Bonsor, S.; Pearson, G. A Clinical Guide to Applied Dental Materials, 1st ed.; Churchill Livingstone: London, UK, 2012; ISBN 978-0702031588. [Google Scholar]
- Fairhurst, C.W.; Cohen, J.B. The crystal structures of two compounds found in dental amalgam: Ag2Hg3 and Ag3Sn. Acta Crystallogr. 1972, B28, 371–378. [Google Scholar] [CrossRef]
- Rossi, P.J.; Zotov, N.; Mittemeijer, E.J. Redetermination of the crystal structure of the Ag3Sn intermetallic compound. Z. Kristallogr. 2016, 231, 1–9. [Google Scholar] [CrossRef]
- Che, G.C.; Ellner, M.; Schubert, K. The hP1-type phases in alloys of cadmium, mercury, and indium with tin. J. Mater. Sci. 1991, 26, 2417–2420. [Google Scholar] [CrossRef]
- King, H.W.; Massalski, T.B. Lattice spacing relationships and the electronic structure of h.c.p. ζ-phases based on silver. Philos. Mag. 1961, 6, 669–682. [Google Scholar] [CrossRef]
- Larsson, A.K.; Stenberg, L.; Lidin, S. The superstructure of the domain-twinned η’-Cu6Sn5. Acta Crystallogr. 1994, B50, 636–643. [Google Scholar] [CrossRef]
- Watanabe, Y.; Fujinaga, Y.; Iwasaki, H. Lattice modulation in the long-period superstructure of Cu3Sn. Acta Crystallogr. 1983, B39, 306–311. [Google Scholar] [CrossRef]
- Xiahan, S.; Kui, D.; Hengqiang, Y. An ordered structure of Cu3Sn in Cu-Sn alloy investigated by transmission electron microscopy. J. Alloys Compd. 2009, 469, 129–136. [Google Scholar] [CrossRef]
- Mitchell, R.J.; Okabe, T. Setting reactions in dental amalgam. Part 1: Phases and microstructures between one hour and one week. Crit. Rev. Oral Biol. Med. 1996, 7, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.J.; Okabe, T. Setting reactions in dental amalgam. Part 2: The kinetics of amalgamation. Crit. Rev. Oral Biol. Med. 1996, 7, 23–25. [Google Scholar] [CrossRef]
- Beech, D.R. High copper alloys for dental amalgam. Int. Dent. J. 1982, 32, 240–251. [Google Scholar] [PubMed]
- Städtler, P. Dental amalgam. I: Conventional and non-gamma-2 amalgams. Int. J. Clin. Pharmacol. Ther. Toxicol. 1991, 29, 161–163. [Google Scholar] [PubMed]
- Chu, H.-T. The use of amalgam as filling material in dentistry in ancient china. Chin. Med. J. 1958, 76, 553–555. [Google Scholar] [PubMed]
- Czarnetzki, A.; Ehrhardt, S. Re-dating the chinese amalgam filling of teeth in Europe. Int. J. Anthropol. 1990, 5, 325–332. [Google Scholar]
- Newman, S.M. Amalgam alternatives: What can compete? J. Am. Dent. Assoc. 1991, 122, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Massalski, T.B.; Okamoto, H.; Subramanian, P.R. Binary Alloy Phase Diagrams; ASM International: Materials Park, OH, USA, 1990; ISBN 978-0871704030. [Google Scholar]
- Lindahl, T.; Westman, S. The structure of the rhombohedral gamma brass like phase in the copper-mercury system. Acta Chem. Scand. 1969, 23, 1181–1190. [Google Scholar] [CrossRef]
- Bernhardt, H.J.; Schmetzer, K. Belendorffite, a new copper amalgam dimorphous with kolymite. Neues Jahrb. Mineral. Monatsh. 1992, 1992, 18–21. [Google Scholar]
- Markova, E.A.; Chernitsova, N.M.; Borodaev, N.M.; Yu, S.; Dubakina, L.S.; Yushko-Zakharova, O.E. The new mineral kolymite, Cu7Hg6. Int. Geol. Rev. 1982, 24, 233–237. [Google Scholar] [CrossRef]
- Carnasciali, M.M.; Costa, G.A. CuxHgy: A puzzling compound. J. Alloys Compd. 2001, 317–318, 491–496. [Google Scholar] [CrossRef]
- Hoch, C.; Simon, A. Cs2Hg27, the mercury-richest amalgam with close relationship to the Bergman phases. Z. Anorg. Allg. Chem. 2008, 634, 853–856. [Google Scholar] [CrossRef]
- Biehl, E.; Deiseroth, H.J. Darstellung, Strukturchemie und Magnetismus der Amalgame MHg11 (M: K, Rb, Ba, Sr). Z. Anorg. Allg. Chem. 1999, 625, 1073–1080. [Google Scholar] [CrossRef]
- Hoch, C.; Simon, A. Tetramethylammoniumamalgam [N(CH3)4]Hg8. Z. Anorg. Allg. Chem. 2006, 632, 2288–2294. [Google Scholar] [CrossRef]
- Tambornino, F.; Hoch, C. Bad metal behaviour in the new Hg-rich amalgam KHg6 with polar metallic bonding. J. Alloys Compd. 2015, 818, 299–304. [Google Scholar] [CrossRef]
- Lahiri, S.K.; Angilello, J.; Natan, M. Precise lattice parameter determination of PtHg4. J. Appl. Crystallogr. 1982, 15, 100–101. [Google Scholar] [CrossRef]
- Biehl, E.; Deiseroth, H.J. K2Hg7 und Rb2Hg7, zwei Vertreter eines neuen Strukturtyps binärer intermetallischer Verbindungen. Z. Anorg. Allg. Chem. 1999, 625, 1337–1342. [Google Scholar] [CrossRef]
- Tambornino, F. Electrolytic Synthesis and Structural Chemistry of Intermetallic Phases with Polar Metal-Metal Bonding. Ph.D. Thesis, LMU, München, Germany, 2016. [Google Scholar]
- Merlo, F.; Fornasini, M.L. Crystal structure of the R11Hg45 compounds (R = La, Ce, Pr, Nd, Sm, Gd, U). J. Less-Common Met. 1976, 44, 259–265. [Google Scholar] [CrossRef]
- Iandelli, A.; Palenzona, A. Su alcuni composti intermetallici dell’europio con zinco, cadmio e mercurio. Atti Accad. Nazl. Lincei Rend. Cl. Sci. Fis. Mater. Nat. 1964, 37, 165–168. [Google Scholar]
- Hoch, C.; Simon, A. Na11Hg52: Komplexität in einem polaren Metall. Angew. Chem. 2012, 124, 3316–3319. [Google Scholar] [CrossRef]
- Hoch, C.; Simon, A. Na11Hg52: Complexity in a polar metal. Angew. Chem. Int. Ed. 2012, 51, 3262–3265. [Google Scholar] [CrossRef] [PubMed]
- Stoe & Cie. X-SHAPE v. 2.07; Stoe & Cie: Darmstadt, Germany, 2005. [Google Scholar]
- Stoe & Cie. X-RED v. 1.31; Stoe & Cie: Darmstadt, Germany, 2005. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Gelato, L.M.; Parthé, E. Structure Tidy—A computer program to standardize crystal structure data. J. Appl. Crystallogr. 1987, 20, 139–143. [Google Scholar] [CrossRef]
- Rahlfs, P. Die Kristallstruktur des Ni3Sn (Mg3Cd-Typ = Überstruktur der hexagonal dichtesten Kugelpackung). Metallwirtschaft 1937, 16, 343–345. [Google Scholar]
- Lyubimtsev, A.L.; Baranov, A.I.; Fischer, A.; Kloo, L.; Popovkin, B.A. The structures and bonding of Ni3Sn. J. Alloys Compd. 2002, 340, 167–172. [Google Scholar] [CrossRef]
- Lihl, F.; Krinbauer, H. Untersuchung binärer metallischer Systeme mit Hilfe des Amalgamverfahrens. Das System Nickel-Zinn. Monatsh. Chem. 1955, 86, 745–751. [Google Scholar] [CrossRef]
- Watanabe, Y.; Murakumi, Y.; Kachi, S. Martensitic and massive transformations and phase diagram in Ni3−xMxSn (M = Cu, Mn) alloys. J. Jpn. Inst. Met. 1981, 45, 551–558. [Google Scholar] [CrossRef]
- Mantina, M.; Chamberlin, A.C.; Valero, R.; Cramer, C.J.; Truhlar, D.G. Consistent van der Waals radii for the whole main group. J. Phys. Chem. 2009, 113, 5806–5812. [Google Scholar] [CrossRef] [PubMed]
- Köhler, J.; Whangbo, M.-H. Late transition metal anions acting as p-metal elements. Solid State Sci. 2008, 10, 444–449. [Google Scholar] [CrossRef]
- Barrett, C.S. The structure of mercury at low temperature. Acta Crystallogr. 1957, 10, 58–60. [Google Scholar] [CrossRef]
- Deng, S.; Simon, A.; Köhler, J. Supraleitung und chemische Bindung in Quecksilber. Angew. Chem. 1998, 110, 664–666. [Google Scholar] [CrossRef]
- Deng, S.; Simon, A.; Köhler, J. Superconductivity and chemical bonding in mercury. Angew. Chem. Int. Ed. 1998, 37, 640–643. [Google Scholar] [CrossRef]
- Zintl, E.; Schneider, A. Röntgenanalyse der Lithium-Amalgame. Z. Elektrochem. Angew. Phys. Chem. 1935, 41, 771–774. [Google Scholar] [CrossRef]
- Bruzzone, G.; Merlo, F. The strontium-mercury system. J. Less-Common Met. 1974, 35, 153–157. [Google Scholar] [CrossRef]
- Bruzzone, G.; Ruggiero, A.F. Struttura di alcuni composti intermetallici dell’ittrio—I. Composti con Cu, Ag, Au, Zn, Cd, Hg. Atti Accad. Nazl. Lincei Rend. Cl. Sci. Fis. Mater. Nat. 1962, 33, 312–314. [Google Scholar]
- Laube, E.; Nowotny, H.N. Die Kristallstrukturen von ScHg, ScHg3, YCd, YHg und YHg3. Monatsh. Chem. 1963, 94, 851–858. [Google Scholar] [CrossRef]
- Bruzzone, G.; Merlo, F. The lanthanum-mercury system. J. Less-Common Met. 1976, 44, 259–265. [Google Scholar] [CrossRef]
- Olcese, G.L. Sul comportamento magnetico del cerio nei composti intermetallici. II. I sistemi Ce-Zn, Ce-Cd Ce-Hg. Atti Accad. Nazl. Lincei Rend. Cl. Sci. Fis. Mater. Nat. 1963, 35, 48–52. [Google Scholar]
- Palenzona, A. MX3 intermetallic phase of the rare earths with Hg, In, Tl, Pb. J. Less-Common Met. 1966, 10, 290–292. [Google Scholar] [CrossRef]
- Ferro, R. The crystal structures of ThHg3, ThIn3, ThTl3, ThSn3 and ThPb3. Acta Crystallogr. 1958, 11, 737–738. [Google Scholar] [CrossRef]
- Rundle, R.E.; Wilson, A.S. The structures of some metal compounds of uranium. Acta Crystallogr. 1949, 2, 148–150. [Google Scholar] [CrossRef]
- Tambornino, F.; Hoch, C. The Hg-richest europium amalgam, Eu10Hg55. Z. Anorg. Allg. Chem. 2015, 641, 537–542. [Google Scholar] [CrossRef]
- Tambornino, F.; Sappl, J.; Pultar, F.; Cong, T.M.; Hübner, S.; Giftthaler, T.; Hoch, C. Electrocrystallization—A synthetic method for intermetallic phases with polar metal-metal bonding. Inorg. Chem. 2016, 55, 11551–11559. [Google Scholar] [CrossRef] [PubMed]
- Stoe & Cie. X-AREA v. 1.39; Stoe & Cie: Darmstadt, Germany, 2006. [Google Scholar]
| Composition | Hg/A Ratio x | Decomposition Temperature (C) |
|---|---|---|
| CsHg | 13.5 | 12 [23] |
| KHg | 11.0 | 70 [24] |
| RbHg | 11.0 | 70 [24] |
| CaHg | 11.0 | 84 |
| SrHg | 11.0 | 63 [24] |
| BaHg | 11.0 | 162 [24] |
| (N(CH))Hg | 8.0 | 9 [25] |
| RbHg | 6.67 | 132 |
| CsHg | 6.67 | 158 |
| KHg | 6.0 | 170 [26] |
| BaHg | 6.0 | 410 |
| BaHg | 5.15 | 505 |
| KHg | 4.43 | 187 |
| RbHg | 4.43 | 162 |
| AHg | 4.09 | 145–720 |
| PtHg | 4.0 | ≥200 [27] |
| RbHg | 3.8 | 193 |
| CsHg | 3.8 | 164 |
| KHg | 3.67 | 195 |
| AHg | 3.64 | 157–480 |
| KHg | 3.5 | 202 [28] |
| RbHg | 3.5 | 197 [28] |
| Composition | CuHg |
| Crystal system | hexagonal |
| Space group | P/mmc, (No. 194) |
| Lattice parameters, a, c (Å) | 5.408(4), 4.390(3) |
| Unit cell volume, V (Å) | 111.18(13) |
| No. of formula units, Z | 2 |
| Density (X-ray), (g cm) | 11.235 |
| Diffractometer | STOE IPDS 1, Ag radiation |
| Data collection temperature, T (K) | 295(2) |
| Absorption coefficient, (mm) | 48.62 |
| Diffraction angle range, () | 5.02–23.53 |
| Index range | −7 ≤ h, k ≤ 7, −6 ≤ l ≤ 6 |
| No. of collected reflections | 2154 |
| No. of independent reflections | 72 |
| No. of independent reflections (I ≥ 2(I)) | 71 |
| 0.2198 | |
| 0.0485 | |
| Structure factor, F(000) | 322.8 |
| Corrections | Lorentz, polarization, absorption effects |
| Absorption correction | numerical [34,35] |
| Structure solution | direct methods [36] |
| Structure refinement | full-matrix least-squares on [36] |
| No. of lest-squares parameters | 9 |
| GooF | 1.180 |
| R values (I ≥ 2(I)) | R1 = 0.0345, wR2 = 0.0757 |
| R values (all data) | R1 = 0.0368, wR2 = 0.0773 |
| Residual (e) max/min (eÅ) | +1.698/−1.419 |
| Extinction coefficient | 0.047(4) |
| Atom | Occupation Factor | Wyckoff Letter | x | y | z | |
|---|---|---|---|---|---|---|
| Hg1 | 0.89(5) | 2d | 0.0160(7) | |||
| Cu1 | 0.11(5) | 2d | 0.0160(7) | |||
| Cu2 | 1 | 6h | 0.1589(3) | 2x | 0.0201(18) |
| Atom | ||||||
|---|---|---|---|---|---|---|
| Hg1 | 0.0156(7) | =U | 0.0168(8) | 0 | 0 | 0.0078(4) |
| Cu1 | 0.0156(7) | =U | 0.0168(8) | 0 | 0 | 0.0078(4) |
| Cu2 | 0.0195(19) | 0.024(2) | 0.019(2) | 0 | 0 | 0.0118(10) |
| Atom 1 | Atom 2 | Distance |
|---|---|---|
| Hg1 | Cu2 | 2.7048(19) (6×) |
| Cu2 | 2.736(2) (6×) | |
| Cu2 | Cu2 | 2.578(5) (2×) |
| Cu2 | 2.6521(19) (4×) |
| Atom | Atom-% Detected | Atom-% Calculated |
|---|---|---|
| Cu | 73.6(2) | 77.7 |
| Hg | 26.4(2) | 22.3 |
| Phase AHg | r [pm] | r/r | c/a |
|---|---|---|---|
| LiHg [29,47] | 152 | 1.01 | 0.768 |
| CaHg [31] | 197 | 1.30 | 0.757 |
| SrHg [31,48] | 215 | 1.42 | 0.741 |
| ScHg [29,49] | 160 | 1.06 | 0.748 |
| YHg [29,49,50] | 181 | 1.20 | 0.744 |
| LaHg [29,51] | 187 | 1.24 | 0.729 |
| CeHg [52] | 182 | 1.21 | 0.731 |
| EuHg [31] | 180 | 1.19 | 0.747 |
| GdHg [29,53] | 180 | 1.19 | 0.742 |
| TbHg [53] | 177 | 1.17 | 0.748 |
| DyHg [29,53] | 178 | 1.18 | 0.746 |
| HoHg [29,53] | 176 | 1.17 | 0.747 |
| ErHg [29,53] | 176 | 1.17 | 0.748 |
| TmHg [29,53] | 176 | 1.17 | 0.748 |
| YbHg [53] | 176 | 1.17 | 0.761 |
| LuHg [29,53] | 174 | 1.15 | 0.750 |
| ThHg [54] | 179 | 1.19 | 0.730 |
| UHg [55] | 156 | 1.03 | 0.735 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sappl, J.; Freund, R.; Hoch, C. Stuck in Our Teeth? Crystal Structure of a New Copper Amalgam, Cu3Hg. Crystals 2017, 7, 352. https://doi.org/10.3390/cryst7120352
Sappl J, Freund R, Hoch C. Stuck in Our Teeth? Crystal Structure of a New Copper Amalgam, Cu3Hg. Crystals. 2017; 7(12):352. https://doi.org/10.3390/cryst7120352
Chicago/Turabian StyleSappl, Jonathan, Ralph Freund, and Constantin Hoch. 2017. "Stuck in Our Teeth? Crystal Structure of a New Copper Amalgam, Cu3Hg" Crystals 7, no. 12: 352. https://doi.org/10.3390/cryst7120352





