Chalcogenide Quaternary Cu2FeSnS4 Nanocrystals for Solar Cells: Explosive Character of Mechanochemical Synthesis and Environmental Challenge
Abstract
:1. Introduction
2. Methods
3. Results and Discussion
3.1. Explosive Character of Synthesis
3.2. Structural Properties
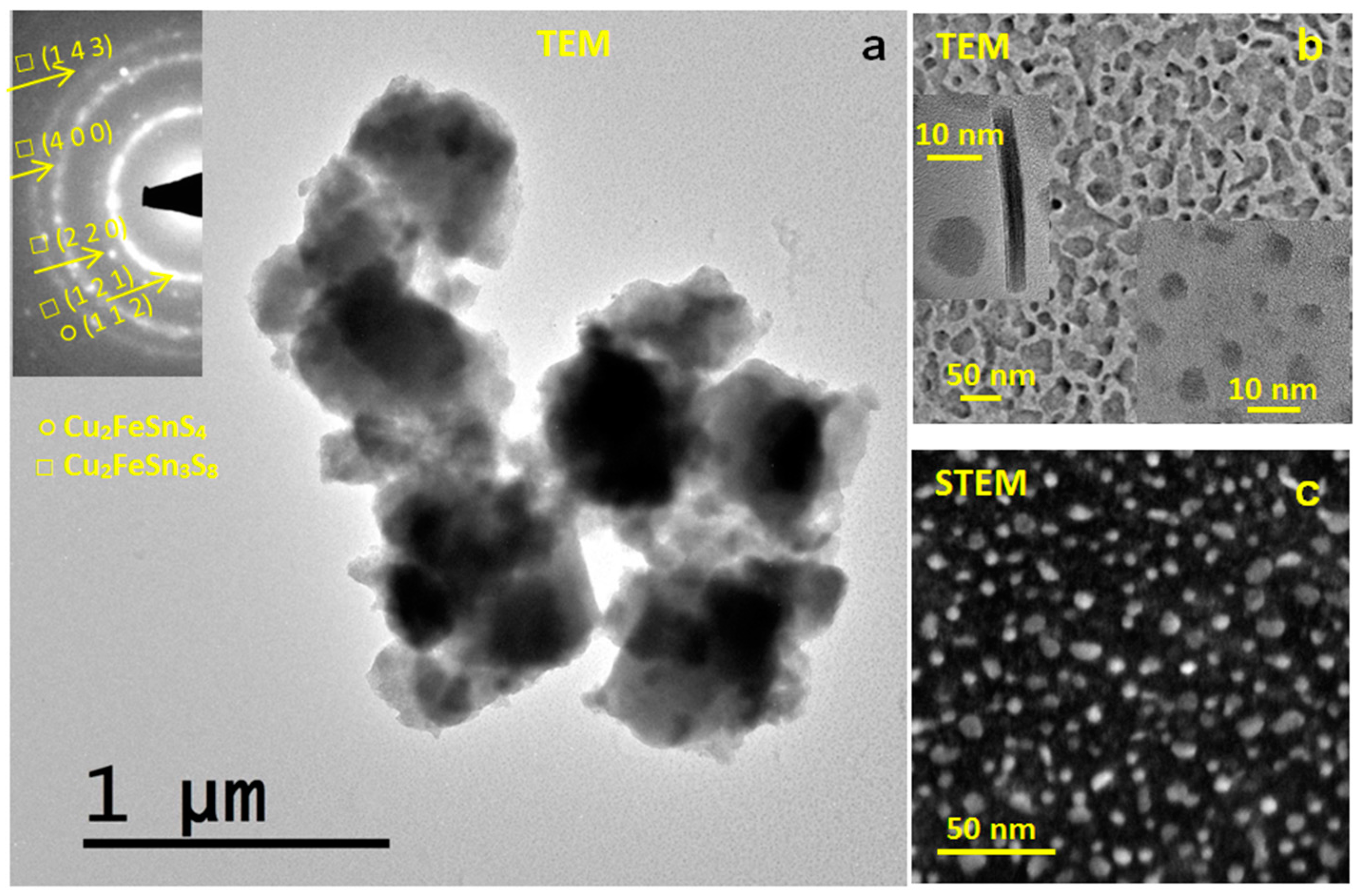
3.3. Microstructural Properties
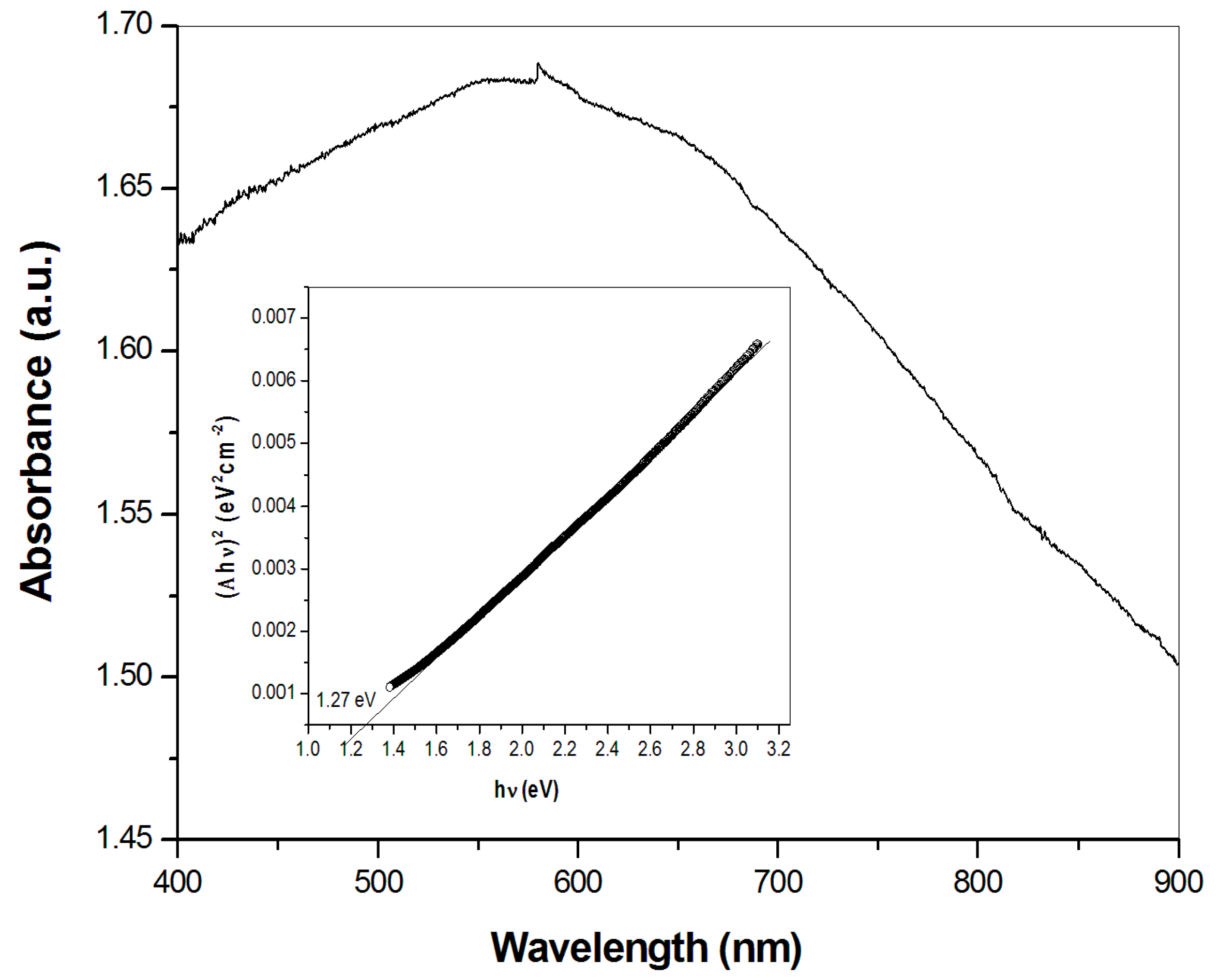
3.4. Optical Properties
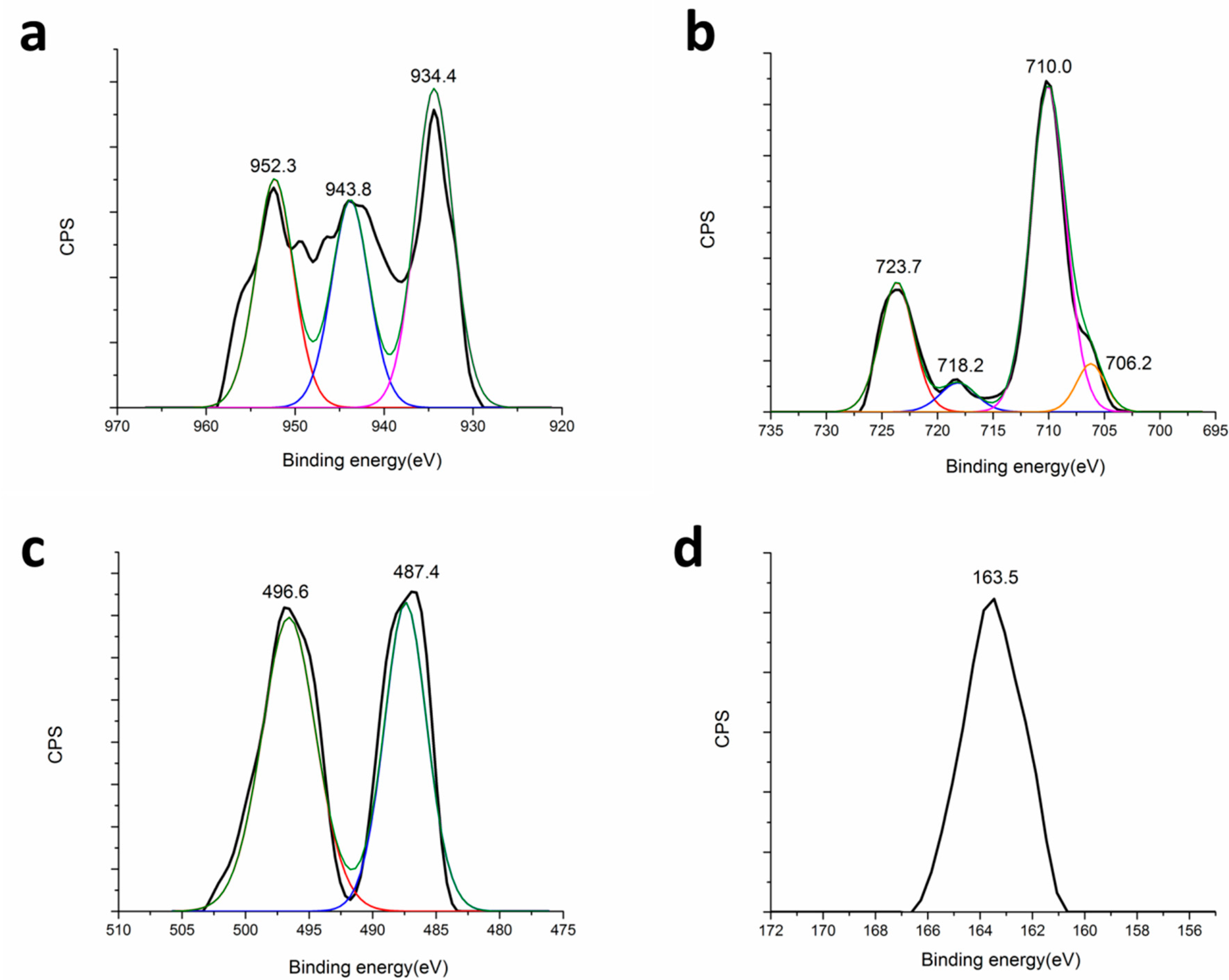
3.5. Surface Properties
3.6. Photocatalytic Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Polman, A.; Knight, M.; Garnett, E.C.; Ehrler, B.; Sinke, W.C. Photovoltaic materials: Present efficiencies and future challenges. Science 2016, 352, aad4424. [Google Scholar] [CrossRef] [PubMed]
- Delbos, S. Kesterite thin films for photovoltaics: A review. EPJ Photovolt. 2012, 3, 35004. [Google Scholar] [CrossRef]
- Hughes, E. The Next Generation. Available online: https://www.chemistryworld.com/feature/the-next-generation/1010134.article (accessed on 30 October 2017).
- Guo, Q.; Ford, G.M.; Yang, W.C.; Walker, B.C.; Stach, E.A.; Hillhouse, H.W.; Agrawal, R. Fabrication of 7.2% efficient CZTSSe solar cells using CZTS nanocrystals. J. Am. Chem. Soc. 2010, 132, 17384–17386. [Google Scholar] [CrossRef] [PubMed]
- Song, X.B.; Ji, X.; Li, M.; Lin, W.D.; Luo, X.; Zhang, H. A review on development prospect of CZTS based thin film solar cells. Int. J. Photoenergy 2014, 2014, 613173. [Google Scholar] [CrossRef]
- Scragg, J.J. Copper Zinc Tin Sulfide Thin Films for Photovoltaics; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Siebentritt, S.; Schorr, S. Kesterites — A challenging material for solar cells. Prog. Photovolt. 2012, 20, 512–519. [Google Scholar] [CrossRef]
- Shin, B.; Gunawan, O.; Zhu, Y.; Bojarczuk, N.A.; Chey, S.J.; Guha, S. Thin film solar cell with 8.4% power conversion efficiency using an earth-abundant Cu2ZnSnS4 absorber. Prog. Photovolt. 2013, 21, 72–76. [Google Scholar] [CrossRef]
- Hamanaka, Y.; Oyaizu, W.; Kawase, M.; Kuzuya, T. Synthesis of highly non-stoichiometric Cu2ZnSnS4 nanoparticles with tunable bandgaps. J. Nanopart. Res. 2016, 19, 9. [Google Scholar] [CrossRef]
- Regulacio, M.D.; Ye, C.; Lim, S.H.; Bosman, M.; Ye, E.; Chen, S.D.; Xu, Q.H.; Han, M.Y. Colloidal nanocrystals of wurtzite-type Cu2ZnSnS4: Facile noninjection synthesis and formation mechanism. Chem. Eur. J. 2012, 18, 3127–3131. [Google Scholar] [CrossRef]
- Regulacio, M.D.; Han, M.Y. Multinary I-III-VI2 and I2-II-IV-VI4 semiconductor nanostructures for photocatalytic applications. Acc. Chem. Res. 2016, 49, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.M.; Regulacio, M.D.; Ye, C.; Lim, S.H.; Lua, S.K.; Xu, Q.H.; Dong, Z.; Xu, A.W.; Han, M.Y. Colloidal nanocrystals of orthorombic Cu2ZnGeS4: Phase-controlled synthesis, formation mechanism and photocatalytic behavior. Nanoscale 2015, 7, 3247–3253. [Google Scholar] [CrossRef]
- Baláž, P. Mechanochemistry in Nanoscience and Minerals Engineering; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Baláž, P.; Achimovičová, M.; Baláž, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutková, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Du, B.; Bonini, N.; Weber, C.; Yan, H.; Reece, M.J. Theory-guided synthesis of an eco-friendly low-cost copper based sulfide thermoelectric material. J. Phys. Chem. C 2016, 120, 27135–27140. [Google Scholar] [CrossRef]
- Du, B.L.; Zhang, R.Z.; Chen, K.; Mahajan, A.; Reece, M.J. The impact of lone-pair electrons on the lattice thermal conductivity of the thermoelectric compound CuSbS2. J. Mater. Chem. A 2017, 5, 3249–3259. [Google Scholar] [CrossRef]
- Zhang, R.Z.; Chen, K.; Du, B.; Reece, M.J. Screening for Cu-S based thermoelectric materials using crystal structure features. J. Mater. Chem. A 2017, 5, 5013–5019. [Google Scholar] [CrossRef]
- Barbier, T.; Rollin-Martinet, S.; Lemoine, P.; Gascoin, F.; Kaltzoglou, A.; Vaqueiro, P.; Powell, A.V.; Guilmeau, E. Thermoelectric materials: A new rapid synthesis process for nontoxic and high-performance tetrahedrite compounds. J. Am. Ceram. Soc. 2016, 99, 51–56. [Google Scholar] [CrossRef]
- Kumar, V.P.; Paradis-Fortin, L.; Lemoine, P.; Caignaert, V.; Raveau, B.; Malaman, B.; Le Caër, G.; Cordier, S.; Guilmeau, E. Designing a thermoelectric copper-rich sulfide from a naturnal mineral: Synthetic germanite Cu22Fe8Ge4S32. Inorg. Chem. 2017, 56, 13376–13381. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.P.; Barbier, T.; Caignaert, V.; Raveau, B.; Daou, R.; Malaman, B.; Le Caër, G.; Lemoine, P.; Guilmeau, E. Copper hyper-stoichiometry: The key for the optimization of thermoelectric properties in stannoidite Cu8+xFe3-xSn2S12. J. Phys. Chem. C 2017, 121, 16454–16461. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, H. Cu2ZnSnS4 synthesized through a green and economic process. J. Alloys Compd. 2011, 509, 9627–9630. [Google Scholar] [CrossRef]
- Gao, F.; Yamazoe, S.; Maeda, T.; Nakanishi, K.; Wada, T. Structural and optical properties of in-free Cu2ZnSn(S,Se)4 solar cell materials. Jpn. J. Appl. Phys. 2012, 51, 10NC29. [Google Scholar] [CrossRef]
- Mokurala, K.; Bhargava, P.; Mallick, S. Single step synthesis of chalcogenide nanoparticles Cu2ZnSnS4, Cu2FeSnS4 by thermal decomposition of metal precursors. Mater. Chem. Phys. 2014, 147, 371–374. [Google Scholar] [CrossRef]
- Park, B.I.; Hwang, Y.; Lee, S.Y.; Lee, J.S.; Park, J.K.; Jeong, J.; Kim, J.Y.; Kim, B.; Cho, S.H.; Lee, D.K. Solvent-free synthesis of Cu2ZnSnS4 nanocrystals: A facile, green, up-scalable route for low cost photovoltaic cells. Nanoscale 2014, 6, 11703–11711. [Google Scholar] [CrossRef] [PubMed]
- Shyju, T.S.; Anandhi, S.; Suriakarthick, R.; Gopalakrishnan, R.; Kuppusami, P. Mechanosynthesis, deposition and characterization of CZTS and CZTSe materials for solar cell applications. J. Solid State Chem. 2015, 227, 165–177. [Google Scholar] [CrossRef]
- Ritscher, A.; Just, J.; Dolotko, O.; Schorr, S.; Lerch, M. A mechanochemical route to single phase Cu2ZnSnS4 powder. J. Alloys Compd. 2016, 670, 289–296. [Google Scholar] [CrossRef]
- Pareek, D.; Balasubramaniam, K.R.; Sharma, P. Reaction pathway for synthesis of Cu2ZnSn(S/Se)4 via mechano-chemical route and annealing studies. J. Mater. Sci. Mater. Electron. 2016, 28, 1199–1210. [Google Scholar] [CrossRef]
- Quennet, M.; Ritscher, A.; Lerch, M.; Paulus, B. The order-disorder transition in Cu2ZnSnS4: A theoretical and experimental study. J. Solid State Chem. 2017, 250, 140–144. [Google Scholar] [CrossRef]
- Liu, C.Q.; Wen, B.; Wang, N.; Liu, S.M.; Wang, H.L.; Jiang, W.W.; Ding, W.Y.; Xu, S.C.; Chai, W.P. Phase evolution and sintering behaviors of Cu2ZnSnS4 powders synthesized by mechanochemical process with different milling parameters. J. Alloys Compd. 2017, 708, 428–436. [Google Scholar] [CrossRef]
- Baláž, P.; Baláž, M.; Sayagués, M.J.; Škorvánek, I.; Zorkovská, A.; Dutková, E.; Briančin, J.; Kováč, J., Jr.; Kováč, J.; Shpotyuk, Y. Mechanochemical solvent-free synthesis of quaternary semiconductor Cu-Fe-Sn-S nanocrystals. Nanoscale Res. Lett. 2017, 12, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Baláž, P.; Baláž, M.; Zorkovská, A.; Škorvánek, I.; Bujňáková, Z.; Trajić, J. Kinetics of solid-state synthesis of quaternary Cu2ZnSnS4 (stannite) nanocrystals for solar energy applications. Acta Phys. Pol. A 2017, 131, 1153–1155. [Google Scholar] [CrossRef]
- Kostova, N.G.; Achimovičová, M.; Eliyas, A.; Stoyanova, V.; Shopska, M.; Velinov, N.; Baláž, P. Structural and photocatalytic properties of mechanochemically synthesized nanosized ferrite. Nanosci. Nanotechnol. 2012, 12, 52–55. [Google Scholar]
- Baláž, M.; Zorkovská, A.; Urakaev, F.; Baláž, P.; Briančin, J.; Bujňáková, Z.; Achimovičová, M.; Gock, E. Ultrafast mechanochemical synthesis of copper sulfides. RSC Adv. 2016, 6, 87836–87842. [Google Scholar] [CrossRef]
- Guan, H.; Shen, H.L.; Jiao, B.X.; Wang, X. Structural and optical properties of Cu2ZnSnS4 thin film synthesized via a simple chemical method. Mater. Sci. Semicond. Process. 2014, 25, 159–162. [Google Scholar] [CrossRef]
- Zhou, B.B.; Yan, X.N.; Li, P.; Yang, L.B.; Yu, D.Y. Raman spectroscopy as a superior tool to understand the synthetic pathway of Cu2ZnSnS4 nanoparticles. Eur. J. Inorg. Chem. 2015, 2015, 2690–2694. [Google Scholar] [CrossRef]
- Baláž, P. Extractive Metallurgy of Activated Minerals; Elsevier: Amsterdam, Netherland, 2000. [Google Scholar]
- Ai, L.H.; Jiang, J. Self-sacrificial templating synthesis of porous quaternary Cu-Fe-Sn-S semiconductor nanotubes via microwave irradiation. Nanotechnology 2012, 23, 495601. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, X.Y.; Huang, J.; Cao, M.; Chen, S.Y.; Shen, Y.; Wang, L.J. Solution-based synthesis and characterization of Cu2FeSnS4 nanocrystals. Mater. Chem. Phys. 2012, 133, 688–691. [Google Scholar] [CrossRef]
- Riha, S.C.; Parkinson, B.A.; Prieto, A.L. Solution-based synthesis and characterization of Cu2ZnSnS4 nanocrystals. J. Am. Chem. Soc. 2009, 131, 12054–12055. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.B.; Tang, Z.L.; Zhang, Z.T.; Shen, W. Preparation of LiFePO4 with inverse opal structure and its satisfactory electrochemical properties. Mater. Res. Bull. 2005, 40, 2039–2046. [Google Scholar] [CrossRef]
- Zhong, H.Z.; Zhou, Y.; Ye, M.F.; He, Y.J.; Ye, J.P.; He, C.; Yang, C.H.; Li, Y.F. Controlled synthesis and optical properties of colloidal ternary chalcogenide CuInS2 nanocrystals. Chem. Mater. 2008, 20, 6434–6443. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, W.; Tan, R.Q.; Song, W.J.; Chen, J.M. Solvothermal synthesis of highly crystallized quaternary chalcogenide Cu2FeSnS4 particles. Mater. Lett. 2013, 102, 39–42. [Google Scholar] [CrossRef]
- Wang, W.; Shen, H.L.; Yao, H.Y.; Li, J.Z. Preparation and properties of Cu2FeSnS4 nanocrystals by ultrasound-assisted microwave irradiation. Mater. Lett. 2014, 125, 183–186. [Google Scholar] [CrossRef]
- Meng, X.K.; Deng, H.M.; Sun, L.; Yang, P.X.; Chu, J.H. Sulfurization temperature dependence of the structural transition in Cu2FeSnS4-based thin films. Mater. Lett. 2015, 161, 427–430. [Google Scholar] [CrossRef]
- Kush, P.; Deori, K.; Kumar, A.; Deka, S. Efficient hydrogen/oxygen evolution and photocatalytic dye degradation and reduction of aqueous Cr(VI) by surfactant free hydrophilic Cu2ZnSnS4 nanoparticles. J. Mater. Chem. A 2015, 3, 8098–8106. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Zhang, P.G.; Lin, Y.L.; Ashalley, E.; Ji, H.N.; Wu, J.; Li, H.D.; Wang, Z.M. Microwave fabrication of Cu2ZnSnS4 nanoparticle and its visible light photocatalytic properties. Nanoscale Res. Lett. 2014, 9, 477. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.Z.; Faraz, M.; Munjal, S.; Kumar, V.; Khare, N. Highly dispersible and uniform size Cu2ZnSnS4 nanoparticles for photocatalytic application. Adv. Powder Technol. 2017, 28, 2402–2409. [Google Scholar] [CrossRef]
- Phaltane, S.A.; Vanalakar, S.A.; Bhat, T.S.; Patil, P.S.; Sartale, S.D.; Kadam, L.D. Photocatalytic degradation of methylene blue by hydrothermally synthesized CZTS nanoparticles. J. Mater. Sci. Mater. Electron. 2017, 28, 8186–8191. [Google Scholar] [CrossRef]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baláž, P.; Baláž, M.; Sayagués, M.J.; Eliyas, A.; Kostova, N.G.; Kaňuchová, M.; Dutková, E.; Zorkovská, A. Chalcogenide Quaternary Cu2FeSnS4 Nanocrystals for Solar Cells: Explosive Character of Mechanochemical Synthesis and Environmental Challenge. Crystals 2017, 7, 367. https://doi.org/10.3390/cryst7120367
Baláž P, Baláž M, Sayagués MJ, Eliyas A, Kostova NG, Kaňuchová M, Dutková E, Zorkovská A. Chalcogenide Quaternary Cu2FeSnS4 Nanocrystals for Solar Cells: Explosive Character of Mechanochemical Synthesis and Environmental Challenge. Crystals. 2017; 7(12):367. https://doi.org/10.3390/cryst7120367
Chicago/Turabian StyleBaláž, Peter, Matej Baláž, María J. Sayagués, Alexander Eliyas, Nina G. Kostova, Mária Kaňuchová, Erika Dutková, and Anna Zorkovská. 2017. "Chalcogenide Quaternary Cu2FeSnS4 Nanocrystals for Solar Cells: Explosive Character of Mechanochemical Synthesis and Environmental Challenge" Crystals 7, no. 12: 367. https://doi.org/10.3390/cryst7120367





