Synthesis, Crystal Structure, Gas Absorption, and Separation Properties of a Novel Complex Based on Pr and a Three-Connected Ligand
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Synthesis of [Pr(TMTA)(H2O)2]·[DMF·2EtOH·4H2O] (1)
2.3. X-ray Crystallography
3. Results and Discussion
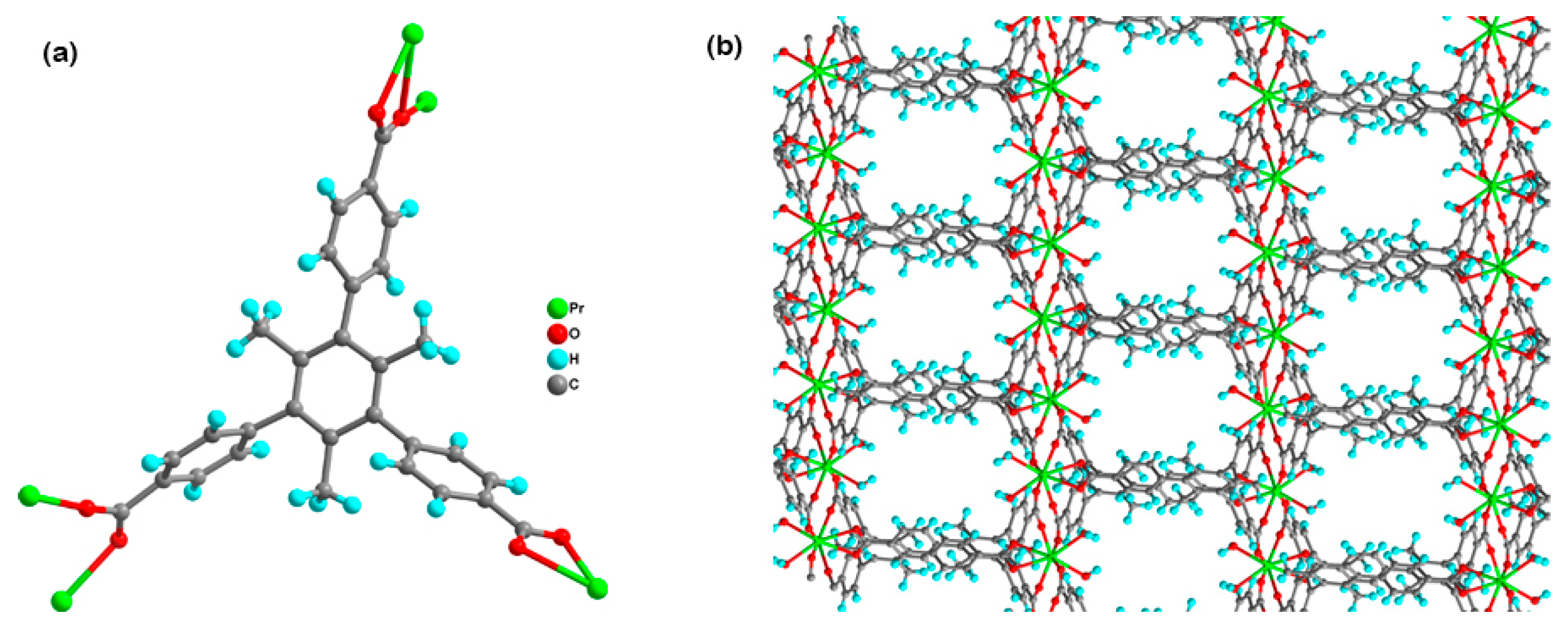
3.1. Crystal Structure of Complex 1
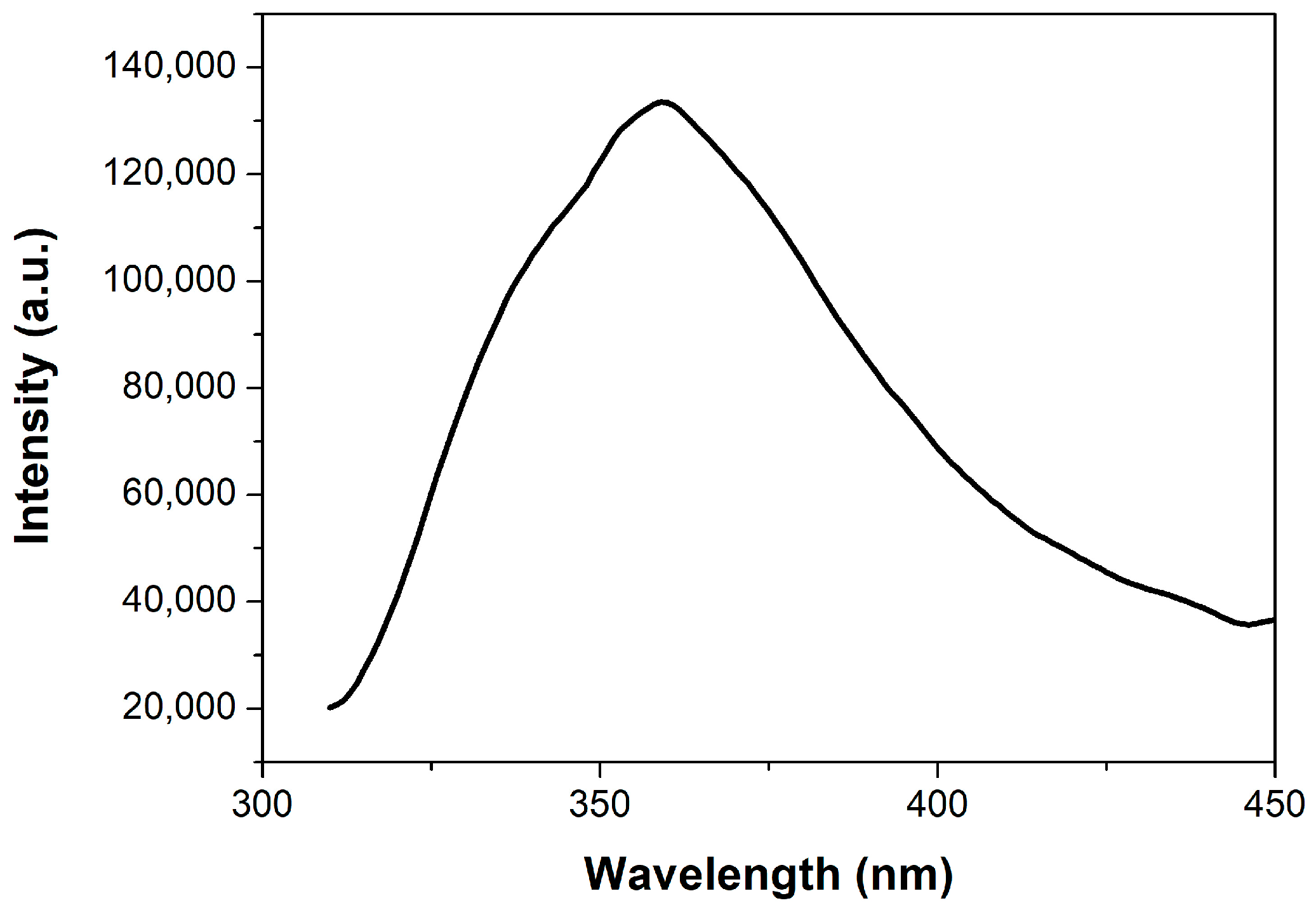
3.2. The Fluorescent Property
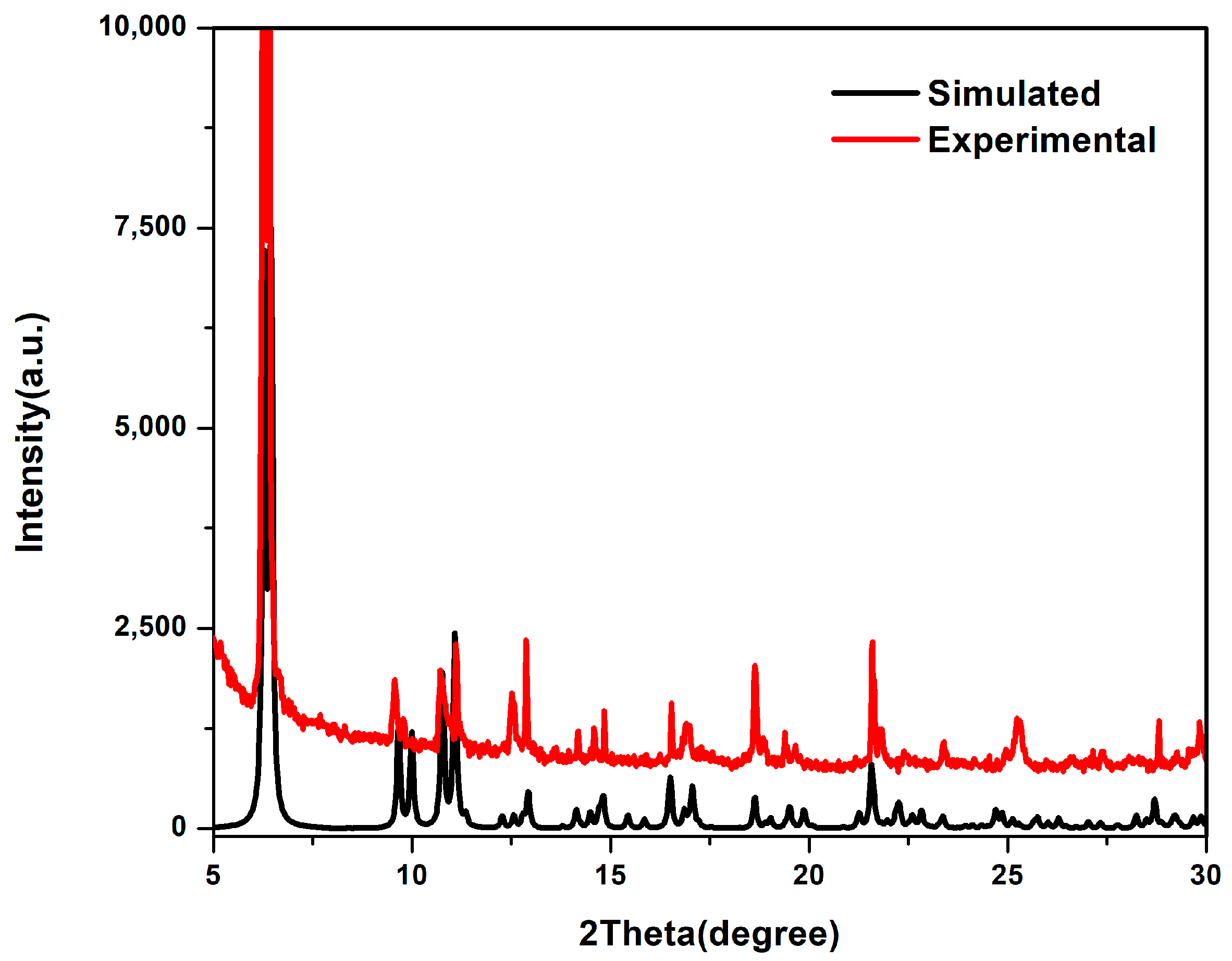
3.3. Powder X-ray Diffraction Analysis
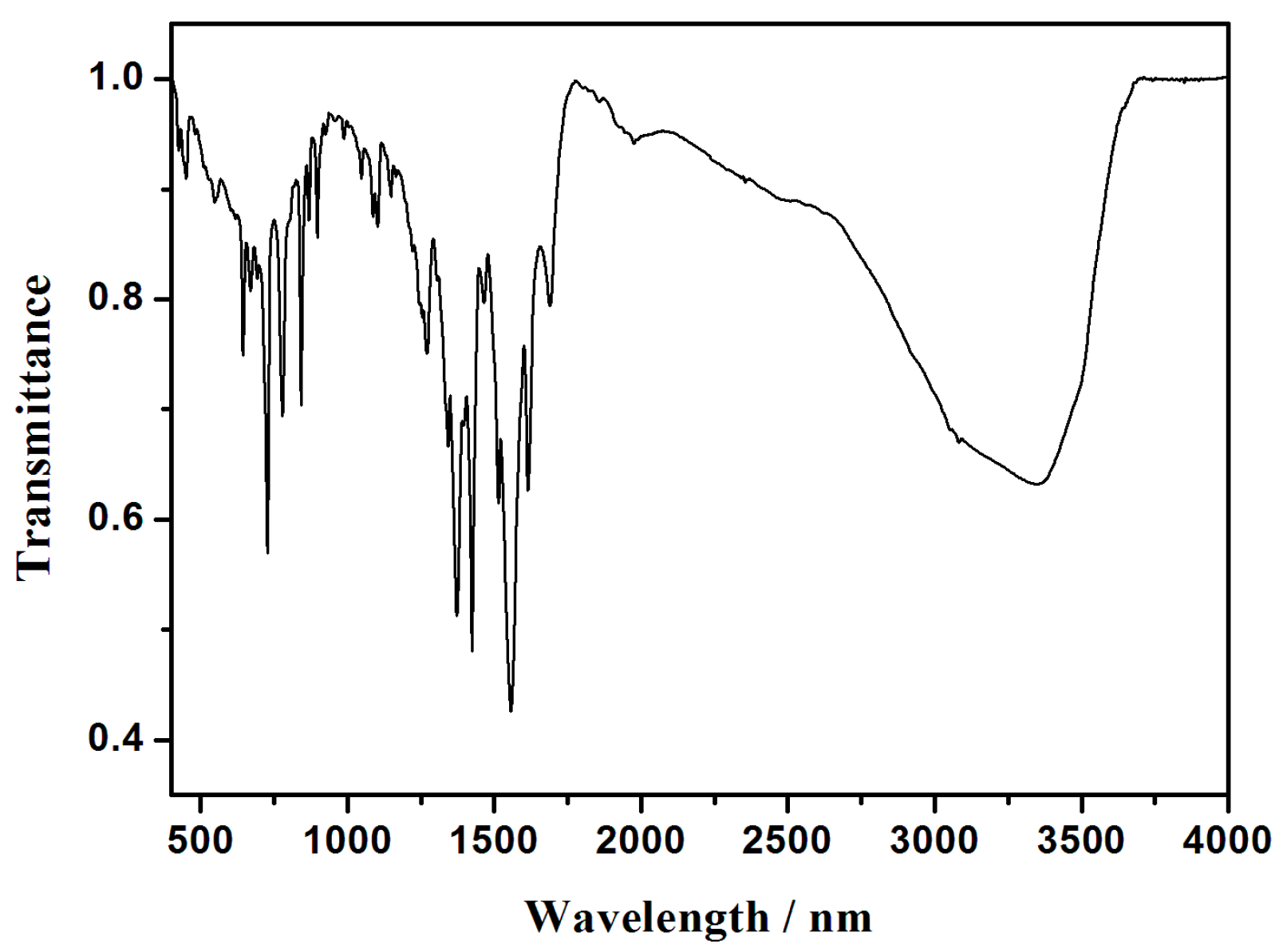
3.4. IR Spectra
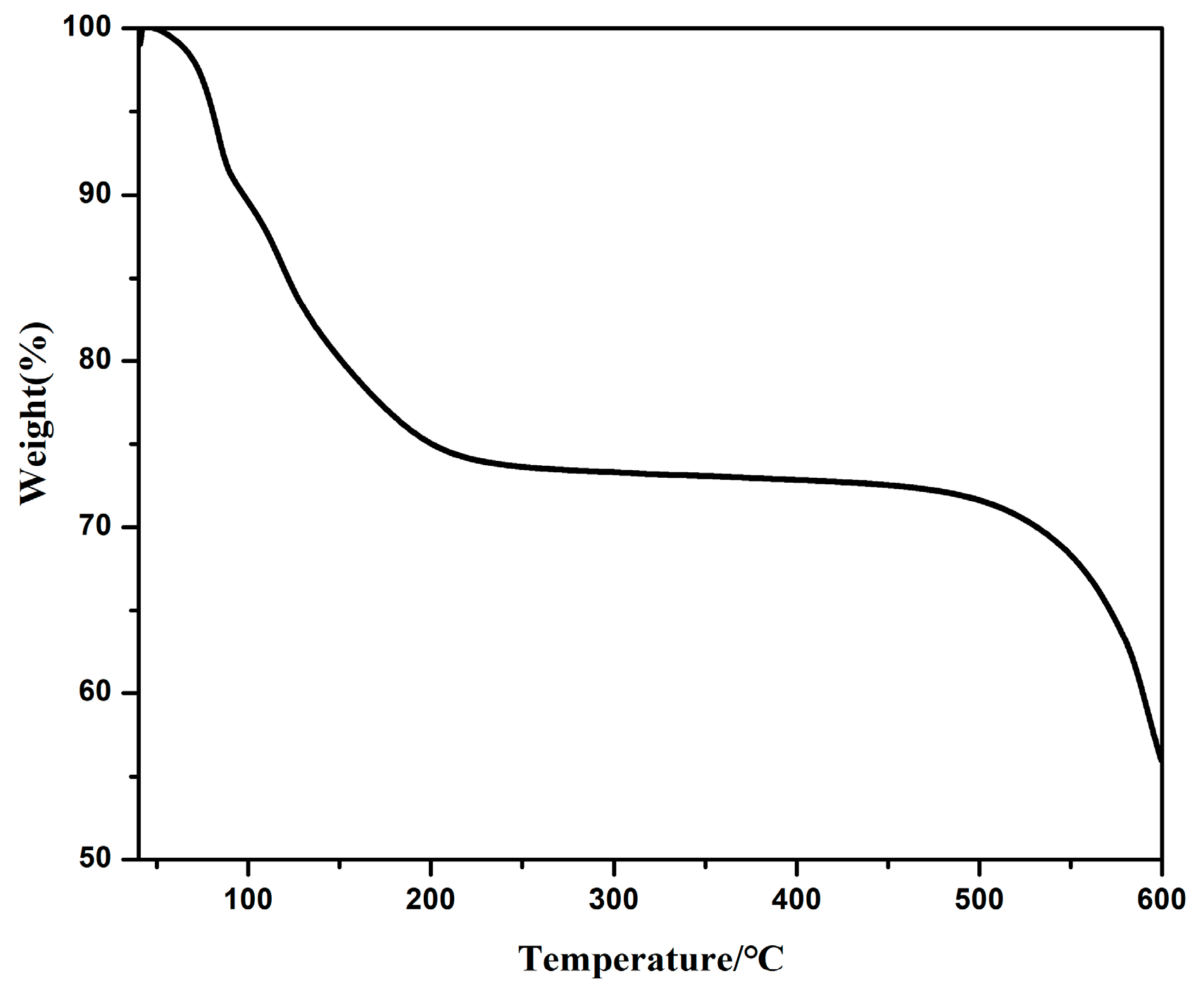
3.5. Thermogravimetric Analyses
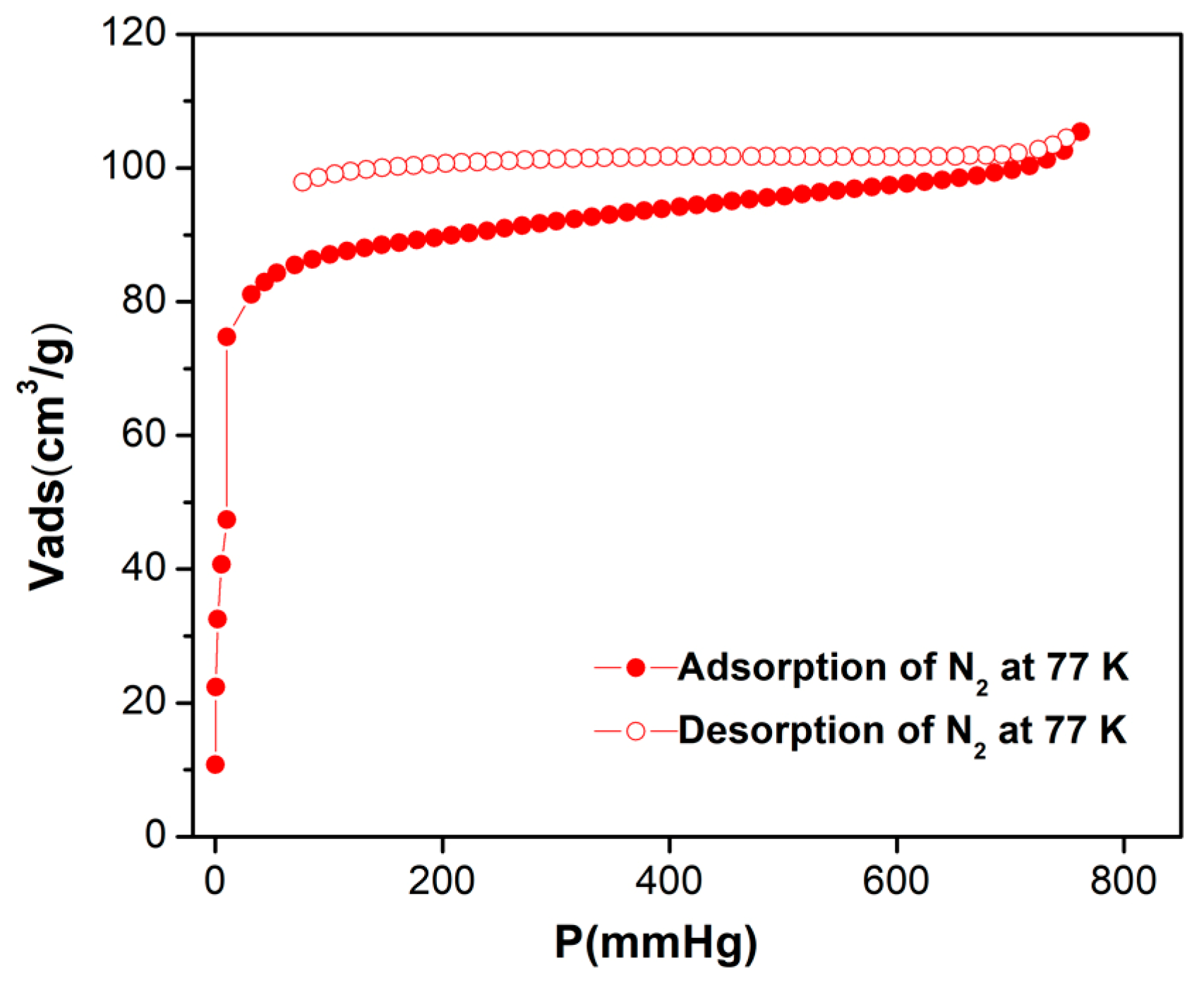
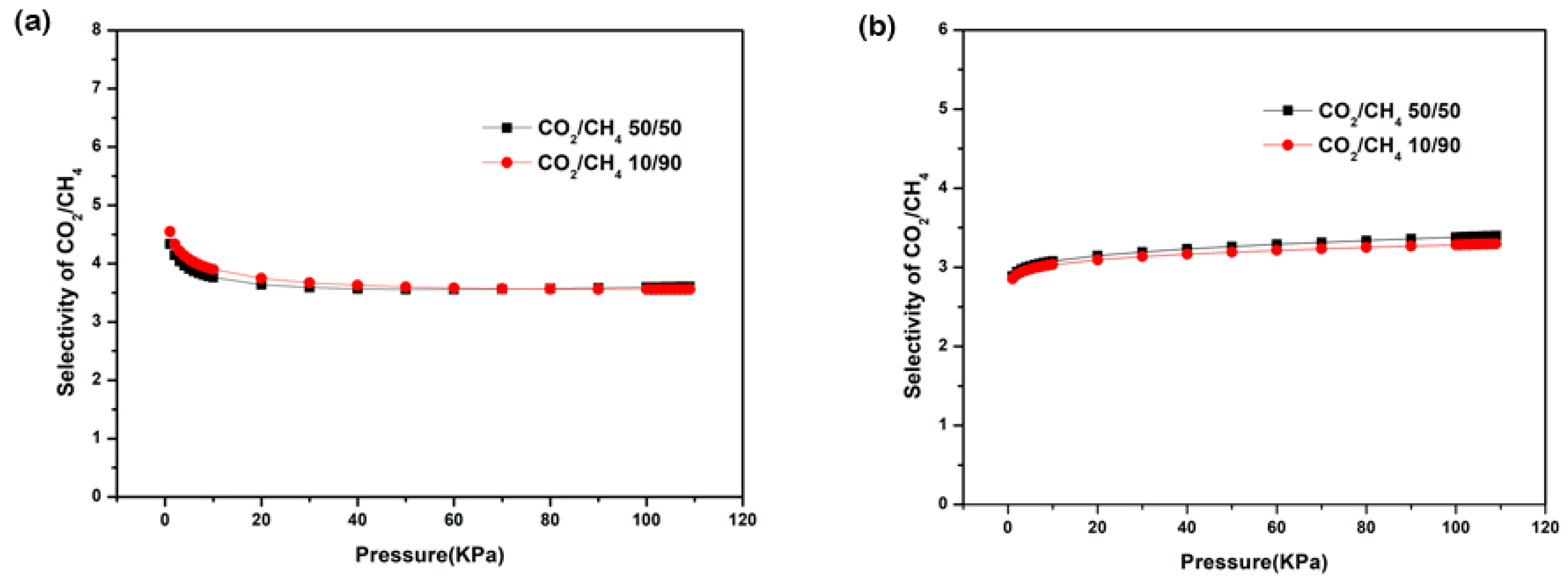
3.6. Gas Sorption and Separation Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Forgan, R.S.; Smaldone, R.A.; Gassensmith, J.J.; Furukawa, H.; Cordes, D.B.; Li, Q.; Wilmer, C.E.; Botros, Y.Y.; Snurr, R.Q.; Slawin, A.M.Z.; et al. Nanoporous carbohydrate metal-organic frameworks. J. Am. Chem. Soc. 2012, 134, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.-T.; Bu, J.T.; Li, Y.; Wu, T.; Zuo, F.; Feng, P.; Bu, X. Pore space partition and charge separation in cage-within-cage indium–organic frameworks with high CO2 uptake. J. Am. Chem. Soc. 2010, 132, 17062–17064. [Google Scholar] [CrossRef] [PubMed]
- Bloch, E.D.; Queen, W.L.; Krishna, R.; Zadrozny, J.M.; Brown, C.M.; Long, J.R. Hydrocarbon separations in a metal-organic framework with open iron(II) coordination sites. Science 2012, 335, 1606–1610. [Google Scholar] [CrossRef] [PubMed]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon dioxide capture in metal-organic frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef] [PubMed]
- Suh, M.P.; Park, H.J.; Prasad, T.K.; Lim, D.W. Hydrogen storage in metal-organic frameworks. Chem. Rev. 2012, 112, 782–835. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Sculley, J.; Zhou, H.-C. Metal-organic frameworks for separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.; Srirambalaji, R.; Kim, K. Homochiral metal-organic frameworks for asymmetric heterogeneous catalysis. Chem. Rev. 2012, 112, 1196–1231. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Abney, C.; Lin, W. Enantioselective catalysis with homochiral metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Ma, S.; Ke, Y.; Collins, D.J.; Zhou, H.-C. An interweaving MOF with high hydrogen uptake. J. Am. Chem. Soc. 2006, 128, 3896–3897. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Sun, D.; Ambrogio, M.; Fillinger, J.A.; Parkin, S.; Zhou, H.-C. Framework-catenation isomerism in metal–organic frameworks and its impact on hydrogen uptake. J. Am. Chem. Soc. 2007, 129, 1858–1859. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Wang, X.-S.; Yuan, D.; Zhou, H.-C. A coordinatively linked Yb metal-organic framework demonstrates high thermal stability and uncommon gas-adsorption selectivity. Angew. Chem. Int. Ed. 2008, 47, 4130–4133. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Yuan, D.; Wang, X.-S.; Zhou, H.-C. Microporous lanthanide metal-organic frameworks containing coordinatively linked interpenetration: Syntheses, gas adsorption studies, thermal stability analysis, and photoluminescence investigation. Inorg. Chem. 2009, 48, 2072–2077. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Yuan, D.; Chang, J.-S.; Zhou, H.-C. Investigation of gas adsorption performances and H2 affinities of porous metal-organic frameworks with different entatic metal centers. Inorg. Chem. 2009, 48, 5398–5402. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Eddaoudi, M.; Hyde, S.T.; O’Keeffe, M.; Yaghi, O.M. Interwoven metal-organic framework on a periodic minimal surface with extra-large pores. Science 2001, 291, 1021–1023. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chen, B.; Reineke, T.M.; Li, H.; Eddaoudi, M.; Moler, D.B.; O’Keeffe, M.; Yaghi, O.M. Assembly of metal–organic frameworks from large organic and inorganic secondary building units: New examples and simplifying principles for complex structures. J. Am. Chem. Soc. 2001, 123, 8239–8247. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.K.; Siberio-Perez, D.Y.; Kim, J.; Go, Y.; Eddaoudi, M.; Matzger, A.J.; O’Keeffe, M.; Yaghi, O.M. A route to high surface area, porosity and inclusion of large molecules in crystals. Nature 2004, 427, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Gedrich, K.; Senkovska, I.; Klein, N.; Stoeck, U.; Henschel, A.; Lohe, M.R.; Baburin, I.A.; Mueller, U.; Kaskel, S. A highly porous metal-organic framework with open nickel sites. Angew. Chem. Int. Ed. 2010, 49, 8489–8492. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lin, X.; Zhao, Y.; Zhao, Y.; Yan, D. Lanthanide metal-organic framework microrods: Colored optical waveguides and chiral polarized emission. Angew. Chem. Int. Ed. 2017, 56, 7853–7857. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal-organic frameworks. Chem Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Guo, N.; Chen, H.; Wang, H.; Yue, L.; Chen, X.; Ng, S.; Liu, X.; Ma, L.; Wang, L. A series of anionic host coordination polymers based on azoxybenzene carboxylate: Structures, luminescence and magnetic properties. Dalton Trans. 2017, 46, 14192–14200. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; He, H.; Dai, F.; Sun, D.; Ke, Y. Supramolecular isomerism in honeycomb metal–organic frameworks driven by CH … π interactions: Homochiral crystallization from an achiral ligand through chiral inducement. Inorg. Chem. 2010, 49, 8650–8652. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Dou, J.; Sun, D.; Cui, P.; Sun, D.; Wu, Q. A porous metal-organic framework (MOF) with unusual 2D→3D polycatenation based on honeycomb layers. Dalton Trans. 2012, 41, 1928–1930. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, F.; Zhang, L.; Sun, D.; Wang, R.; Ju, Z.; Yuan, D.; Sun, D. Achieving a rare breathing behavior in a polycatenated 2D to 3D net through a pillar-ligand extension strategy. Chem. Eur. J. 2014, 20, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Bruker. SMART, SAINT and SADABS; Bruker AXS Inc.: Madison, WI, USA, 1998. [Google Scholar]
- Sheldrick, G.M. SHELXS-97; Program for X-ray Crystal Structure Determination; University of Gottingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXL-97; Program for X-ray Crystal Structure Refinement; University of Gottingen: Göttingen, Germany, 1997. [Google Scholar]
- Spek, A.L. PLATON; A Multipurpose Crystallographic Tool; Utrecht University: Utrecht, The Netherlands, 2002. [Google Scholar]
- Zhang, L.; Guo, J.; Meng, Q.; Wang, R.; Sun, D. Syntheses, structures and characteristics of four metal–organic coordination polymers based on 5-hydroxyisophthalic acid and N-containing auxiliary ligands. CrystEngComm 2013, 15, 9578–9587. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; John Wiley & Sons: New York, NY, USA, 1986. [Google Scholar]
- Phan, A.; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.B.; O’Keeffe, M.; Yaghi, O.M. Synthesis, structure, and carbon dioxide capture properties of zeolitic imidazolate frameworks. Acc. Chem. Res. 2010, 43, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Nugent, P.; Belmabkhout, Y.; Burd, S.D.; Cairns, A.J.; Luebke, R.; Forrest, K.; Pham, T.; Ma, S.; Space, B.; Wojtas, L.; et al. Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation. Nature 2013, 495, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Yu, J.; Lu, W.; Sun, L.B.; Sculley, J.; Balbuena, P.B.; Zhou, H.C. Porous materials with pre-designed single-molecule traps for CO2 selective adsorption. Nat. Commun. 2013, 4, 1538–1544. [Google Scholar] [CrossRef] [PubMed]

| Empirical Formula | C30H25O8Pr |
|---|---|
| Formula weight | 654.41 |
| Temperature/K | 298.15 |
| Crystal system | monoclinic |
| Space group | P21/n |
| a/Å | 9.531(3) |
| b/Å | 16.417(5) |
| c/Å | 27.409(8) |
| α/° | 90.00 |
| β/° | 93.098(6) |
| γ/° | 90.00 |
| Volume/Å3 | 4282(2) |
| Z | 4 |
| ρcalcmg/mm3 | 1.015 |
| m/mm−1 | 1.169 |
| F(000) | 1312.0 |
| Index ranges | −10 ≤ h ≤ 10, 0 ≤ k ≤ 18, 0 ≤ l ≤ 30 |
| Reflections collected | 6198 |
| Independent reflections | 6198[R(int) = 0.1019] |
| Data/restraints/parameters | 6198/906/354 |
| Goodness-of-fit on F2 | 1.002 |
| Final R indexes [I >= 2σ (I)] | R1 = 0.1012, wR2 = 0.2613 |
| Final R indexes [all data] | R1 = 0.1277, wR2 = 0.2752 |
| Largest diff. peak/hole/e Å−3 | 5.28/−1.63 |
| Pr1-O1 | 2.390(8) | Pr1-O1w | 2.496(8) | Pr1-O2 1 | 2.384(8) |
| Pr1-O2w | 2.488(8) | Pr1-O3 2 | 2.535(8) | Pr1-O4 2 | 2.570(8) |
| Pr1-O5 3 | 2.445(8) | Pr1-O6 4 | 2.480(8) | Pr1-O6 3 | 2.967(8) |
| O1-Pr1-O1w | 77.9(3) | O1-Pr1-O2w | 78.6(3) | O1-Pr1-O3 1 | 76.5(3) |
| O1-Pr1-O4 1 | 124.3(3) | O1-Pr1-O5 2 | 155.4(3) | O1-Pr1-O6 2 | 138.1(3) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Zhang, M.; Wang, A.; Cai, Z. Synthesis, Crystal Structure, Gas Absorption, and Separation Properties of a Novel Complex Based on Pr and a Three-Connected Ligand. Crystals 2017, 7, 370. https://doi.org/10.3390/cryst7120370
Sun J, Zhang M, Wang A, Cai Z. Synthesis, Crystal Structure, Gas Absorption, and Separation Properties of a Novel Complex Based on Pr and a Three-Connected Ligand. Crystals. 2017; 7(12):370. https://doi.org/10.3390/cryst7120370
Chicago/Turabian StyleSun, Jie, Minghui Zhang, Aiyun Wang, and Ziwei Cai. 2017. "Synthesis, Crystal Structure, Gas Absorption, and Separation Properties of a Novel Complex Based on Pr and a Three-Connected Ligand" Crystals 7, no. 12: 370. https://doi.org/10.3390/cryst7120370





