High-Pressure Synthesis, Structure, and Magnetic Properties of Ge-Substituted Filled Skutterudite Compounds; LnxCo4Sb12−yGey, Ln = La, Ce, Pr, and Nd
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bauer, E.D.; Frederick, N.A.; Ho, P.-C.; Zapf, V.S.; Maple, M.B. Superconductivity and heavy fermion behavior in PrOs4Sb12. Phys. Rev. B 2002, 65, 1005061–1005064. [Google Scholar] [CrossRef]
- Shirotani, I.; Shimaya, Y.; Kihou, K.; Sekine, C.; Yagi, T. Systematic high-pressure synthesis of ne wfilled skutterudites with heavy lanthanide, LnFe4P12 (Ln = heavy lanthanide, including Y). J. Solid State Chem. 2003, 174, 32–34. [Google Scholar] [CrossRef]
- Sekine, C.; Uchiumi, T.; Shirotani, I.; Matsuhira, K.; Sakakibara, T.; Goto, T.; Yagi, T. Magnetic properties of the filled skutterudite-type structure compounds GdRu4P12 and TbRu4P12 synthesized under high pressure. Phys. Rev. B 2000, 62, 11581–11584. [Google Scholar] [CrossRef] [Green Version]
- Kihou, K.; Shirotani, I.; Shimaya, Y.; Sekine, C.; Yagi, T. High-pressure synthesis, electrical and magnetic properties of new filled skutterudites LnOs4P12 (Ln = Eu, Gd, Tb, Dy, Ho, Y). Mater. Res. Bull. 2004, 39, 317–325. [Google Scholar] [CrossRef]
- Shirotani, I.; Areseki, N.; Shimaya, Y.; Nakata, R.; Kihou, K.; Sekine, C.; Yagi, T. Electrical and magnetic properties of new filled skutterudites LnFe4P12 (Ln = Ho, Er, Tm and Yb) and YRu4P12 with heavy lanthanide (including Y) prepared at high pressure. J. Phys. Condens. Matter 2005, 17, 4383–4391. [Google Scholar] [CrossRef]
- Fukuoka, H.; Yamanaka, S. High-pressure synthesis, structure, and electrical property of iodine-filled skutterudite I0.9Rh4Sb12—First anion-filled skutterudite. Chem. Mater. 2010, 22, 47–51. [Google Scholar] [CrossRef]
- Li, X.; Xu, B.; Zhang, L.; Duan, F.; Yan, X.; Yang, J.; Tian, Y. Synthesis of iodine filled CoSb3 with extremely low thermal conductivity. J. Alloys Compd. 2014, 615, 177–180. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, B.; Li, X.; Duan, F.; Yan, X.; Tian, Y. Iodine-filled FexCo4−xSb12 polycrystals: Synthesis, structure, and thermoelectric properties. Mater. Lett. 2015, 139, 249–251. [Google Scholar] [CrossRef]
- Ortiz, B.R.; Crawford, C.M.; McKinney, R.W.; Parillab, P.A.; Toberer, E.S. Thermoelectric properties of bromine filled CoSb3 skutterudite. J. Mater. Chem. A 2016, 4, 8444–8450. [Google Scholar] [CrossRef]
- Nolas, G.S.; Slack, G.A.; Caillat, T.; Meisner, G.P. Raman scattering study of antimony-based skutterudites. J. Appl. Phys. 1996, 79, 2622–2626. [Google Scholar] [CrossRef]
- Nolas, G.S.; Slack, G.A.; Morelli, D.T.; Tritt, T.M.; Ehrlich, A.C. The effect of rare-earth filling on the lattice thermal conductivity of skutterudites. J. Appl. Phys. 1996, 79, 4002–4008. [Google Scholar] [CrossRef]
- Tritt, T.M.; Nolas, G.S.; Slack, G.A.; Ehrlich, A.C.; Gillespie, D.J.; Cohn, J.L. Low-temperature transport properties of the filled and unfilled IrSb3 skutterudite system. J. Appl. Phys. 1996, 79, 8412–8418. [Google Scholar] [CrossRef]
- Fukuoka, H.; Yamanaka, S. High-pressure synthesis and structure of new filled skutterudite compounds with Ge-substituted host network; LnRh4Sb9Ge3, Ln = La, Ce, Pr, and Nd. J. Alloys Compd. 2008, 461, 547–550. [Google Scholar] [CrossRef]
- Nolas, G.S.; Yang, J.; Takizawa, H. Transport properties of germanium-filled CoSb3. Appl. Phys. Lett. 2004, 84, 5210–5213. [Google Scholar] [CrossRef]
- Mori, H.; Anno, H.; Matsubara, K. Effect of Yb filling on thermoelectric properties of Ge-substituted CoSb3 skutterudites. Mater. Trans. 2005, 46, 1476–1480. [Google Scholar] [CrossRef]
- Izumi, F.; Momma, K. Three-dimensional visualization in powder diffraction. Solid State Phenom. 2007, 130, 15–20. [Google Scholar] [CrossRef]
- Schmidt, T.; Kliche, G.; Lutz, H.D. Structure refinement of skutterudite-type cobalt triantimonide, CoSb3. Acta Cryst. C 1987, C43, 1678–1679. [Google Scholar] [CrossRef]
- Errandonea, D.; Boehler, R.; Schwager, B.; Mezouar, M. Structural studies of gadolinium at high pressure and temperature. Phys. Rev. B 2007, 75, 014103. [Google Scholar] [CrossRef]
- Cunningham, N.C.; Qiu, W.; Hope, K.M.; Liermann, H.-P.; Vohra, Y.K. Symmetry lowering under high pressure: Structural evidence for f-shell delocalization in heavy rare earth metal terbium. Phys. Rev. B 2007, 76, 212101. [Google Scholar] [CrossRef]
- Fukuoka, H. High-pressure and properties of new filled skutterudite compounds using a Kawai-type cell. Rev. High Press. Sci. Technol. 2006, 16, 329–335. [Google Scholar] [CrossRef]
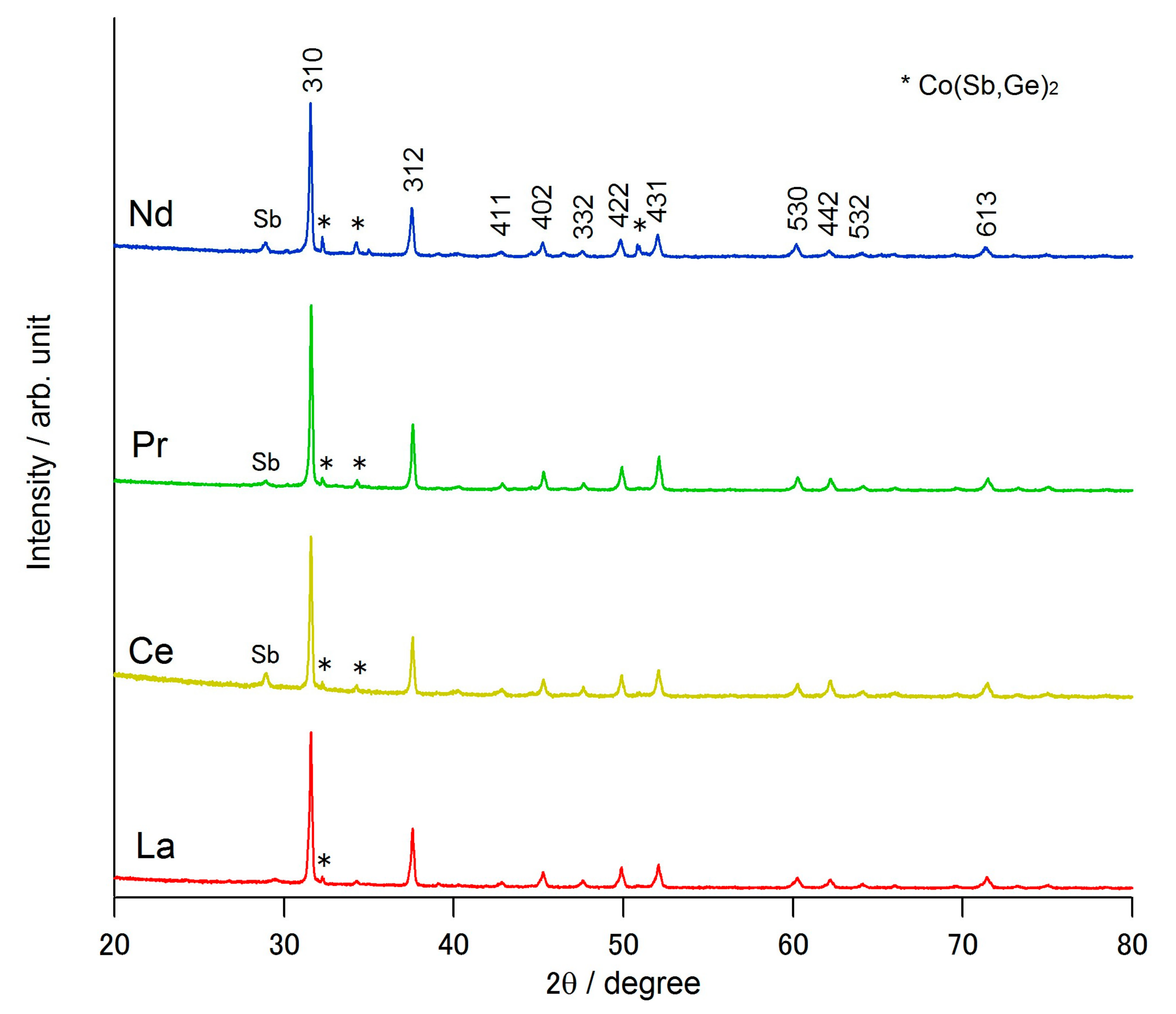
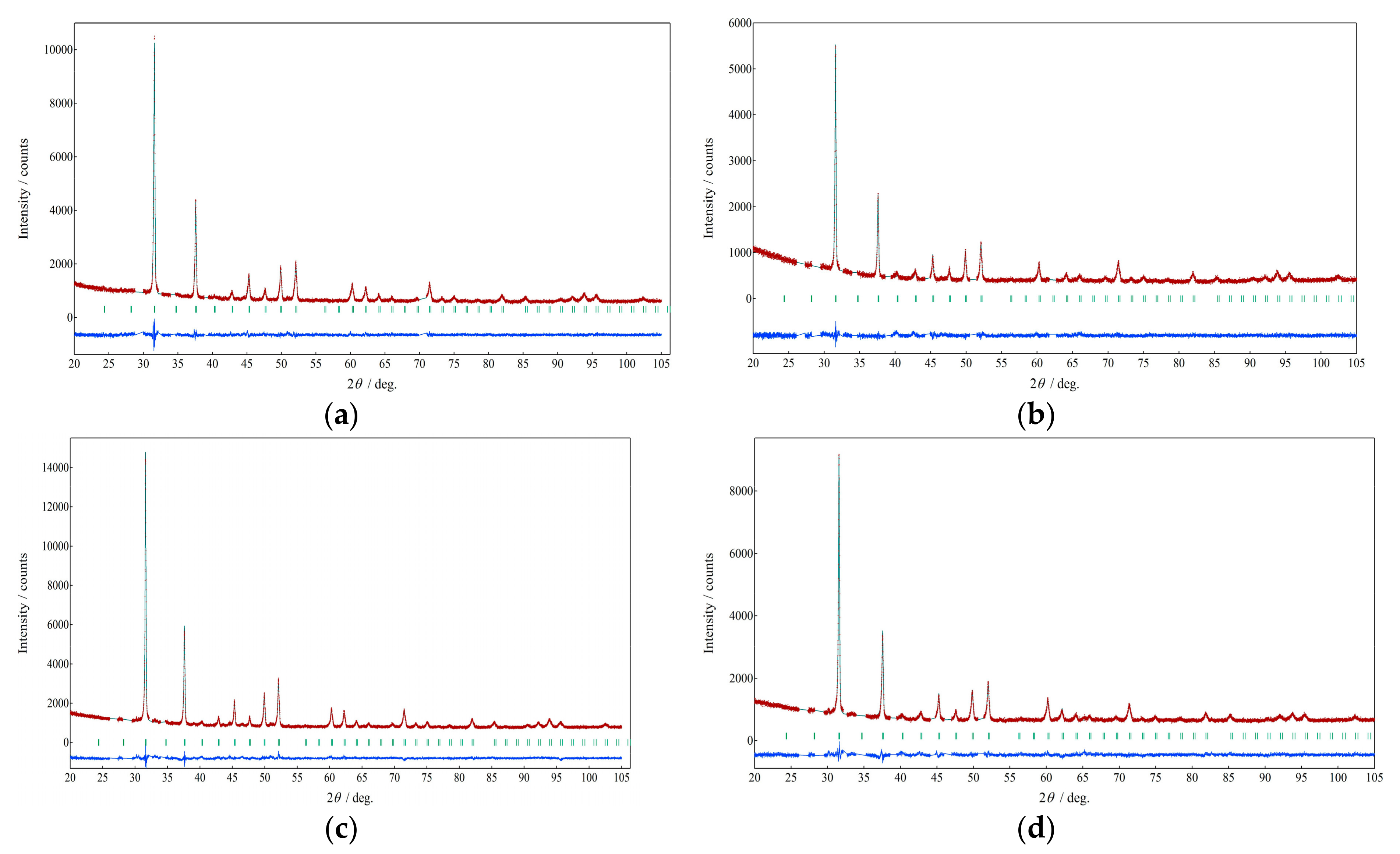
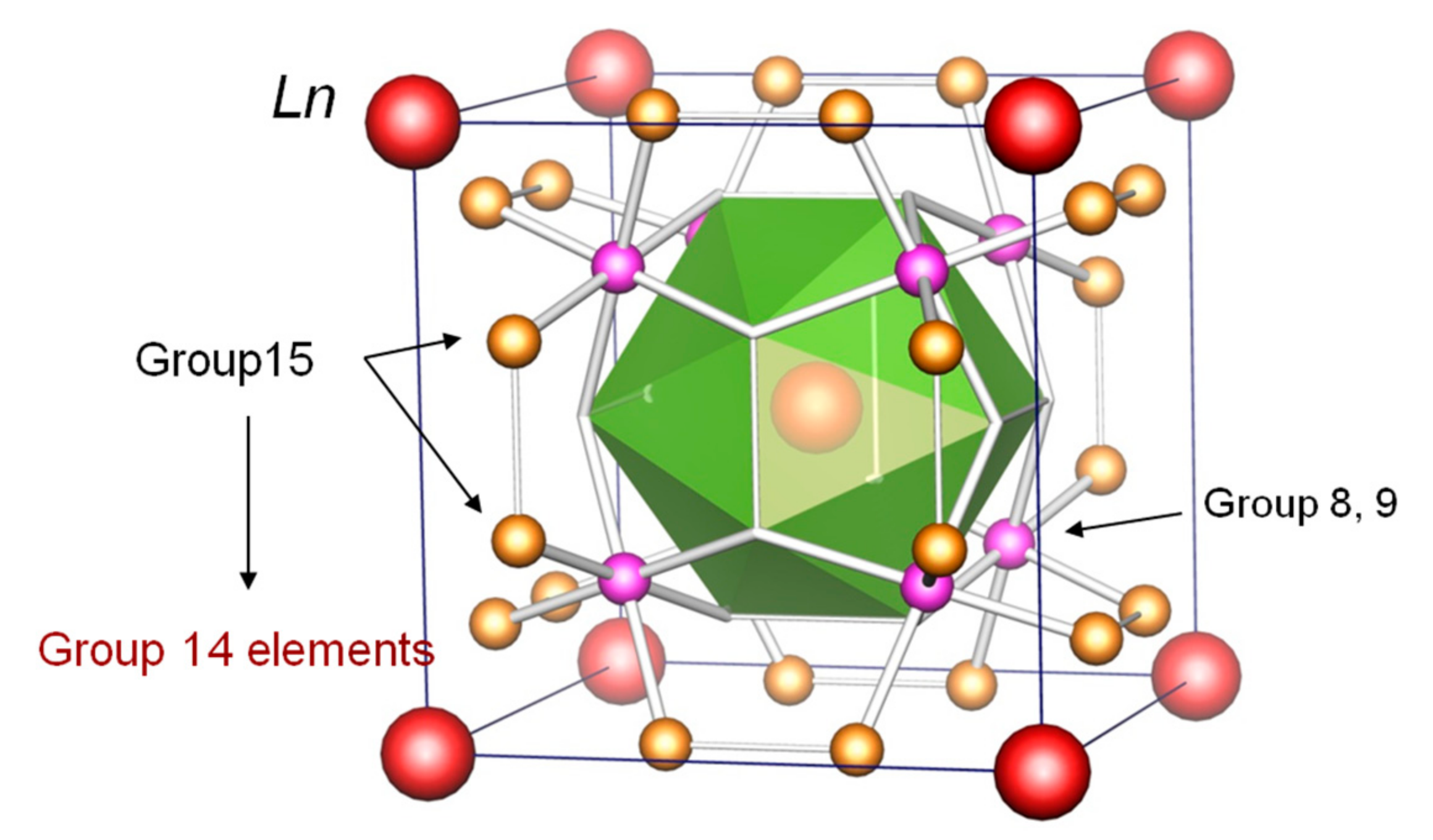
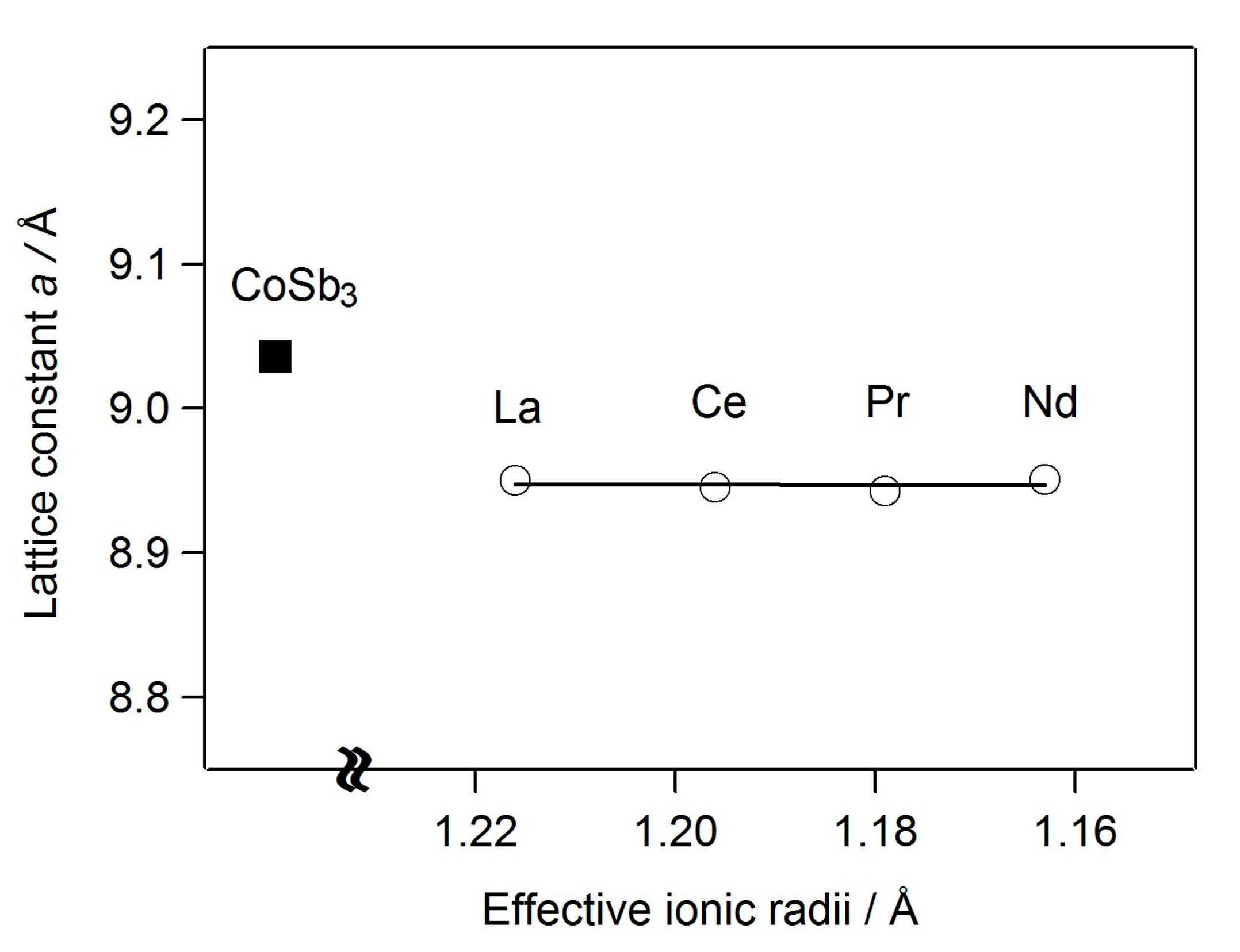
| Composition | La0.57Co4Sb10.1Ge2.38 | Ce0.99Co4Sb9.65Ge2.51 | Pr0.97Co4Sb9.52Ge2.61 | Nd0.87Co4Sb9.94Ge2.28 |
|---|---|---|---|---|
| Lattice constant (Å) | 8.9504 (3) | 8.94481 (6) | 8.9458 (3) | 8.9509 (4) |
| Formula | La0.57Co4Sb10.1Ge2.38 | Ce0.99Co4Sb9.65Ge2.51 | Pr0.97Co4Sb9.52Ge2.61 | Nd0.87Co4Sb9.94Ge2.28 |
|---|---|---|---|---|
| Space group | I m-3 (204) | I m-3 (204) | I m-3 (204) | I m-3 (204) |
| Lattice | ||||
| parameter (a/Å) | 8.9504 (3) | 8.94481 (6) | 8.9458 (3) | 8.9509 (4) |
| Unit cell | ||||
| volume V/Å3 | 717.02 (4) | 715.670 (9) | 715.90 (4) | 717.13 (6) |
| 2θ range/degree | 20–105 | 20–105 | 20–105 | 20–105 |
| Rwp/% | 4.23 | 5.03 | 4.04 | 4.35 |
| RP/% | 3.33 | 3.94 | 3.16 | 3.44 |
| Re/% | 3.59 | 4.46 | 3.20 | 3.56 |
| RB/% | 3.86 | 7.15 | 7.17 | 6.40 |
| RF/% | 3.04 | 3.31 | 3.95 | 4.41 |
| S | 1.18 | 1.13 | 1.26 | 1.22 |
| ocp | n * | x | y | z | B/Å2 | |
|---|---|---|---|---|---|---|
| La0.57Co4Sb10.1Ge2.38 | ||||||
| La | 0.704 (8) | 1.41 | 0 | 0 | 0 | overall |
| Co | 1 | 8 | 0.25 | 0.25 | 0.25 | 0.03 (5) |
| Sb | 0.72 (2) | 17.28 | 0 | 0.3396 (2) | 0.1599 (2) | |
| Ge | 0.28 | 6.72 | 0 | 0.3396 | 0.1599 | |
| Ce0.99Co4Sb9.65Ge2.51 | ||||||
| Ce | 0.90 (3) | 1.78 | 0 | 0 | 0 | 2.8 (5) |
| Co | 1 | 8 | 0.25 | 0.25 | 0.25 | 0.05 (4) |
| Sb | 0.85 (2) | 20.3 | 0 | 0.3384 (5) | 0.1611 (3) | 0.05 |
| Ge | 0.15 | 3.7 | 0 | 0.3384 | 0.1611 | 0.05 |
| Pr0.97Co4Sb9.52Ge2.61 | ||||||
| Pr | 0.94 (2) | 1.88 | 0 | 0 | 0 | 3.0 (4) |
| Co | 1 | 8 | 0.25 | 0.25 | 0.25 | 0.6 (3) |
| Sb | 0.77 (5) | 18.48 | 0 | 0.3408 (2) | 0.1581 (2) | 0.5 (1) |
| Ge | 0.23 | 5.52 | 0 | 0.3408 | 0.1581 | 0.5 |
| Nd0.87Co4Sb9.94Ge2.28 | ||||||
| Nd | 0.87 (3) | 1.74 | 0 | 0 | 0 | 5.6 (5) |
| Co | 1 | 8 | 0.25 | 0.25 | 0.25 | 0.2 (3) |
| Sb | 0.63 (4) | 15.2 | 0 | 0.3376 (2) | 0.1572 (2) | 0.3 (2) |
| Ge | 0.37 | 8.8 | 0 | 0.3376 | 0.1572 | 0.3 |
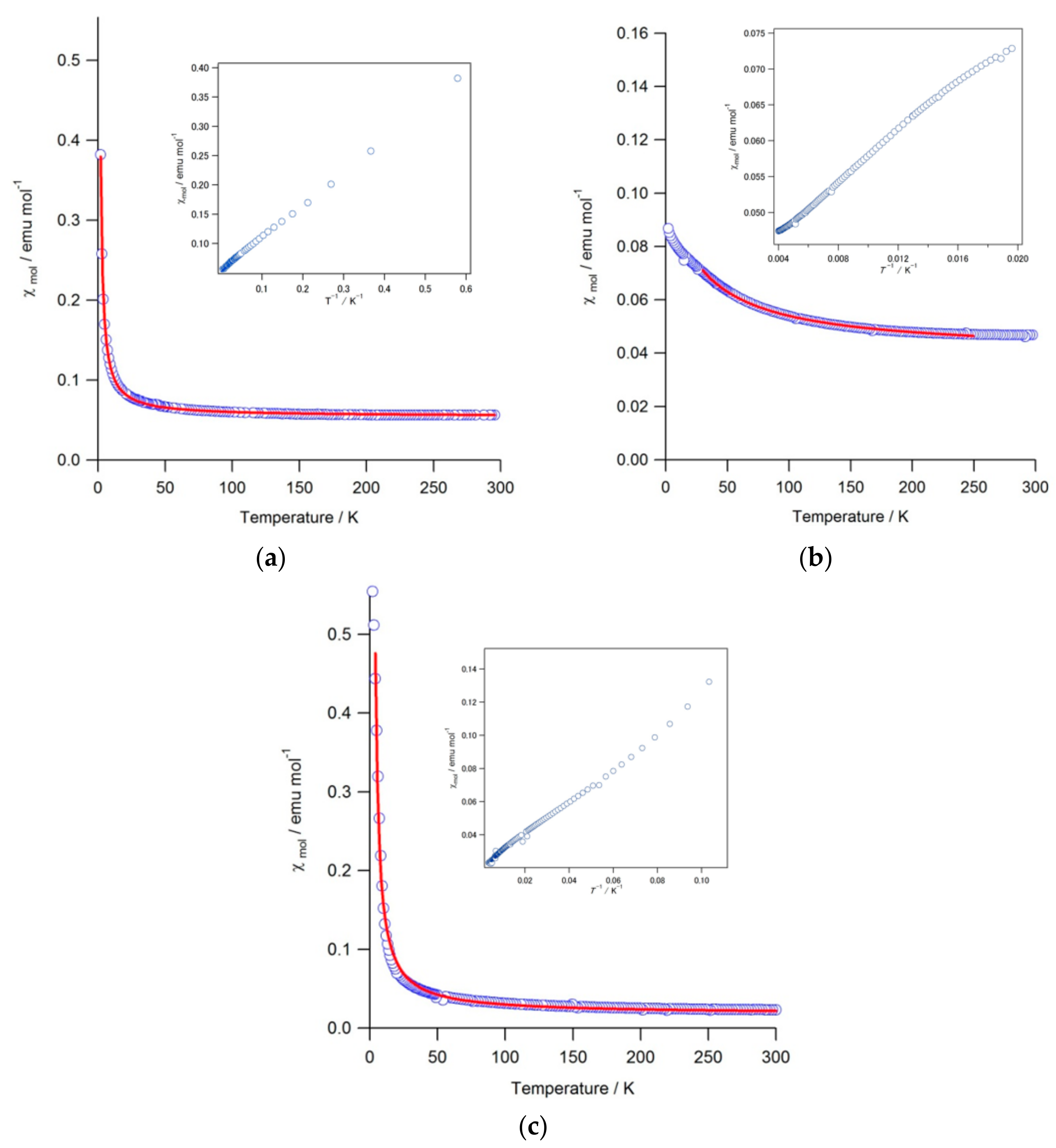
| Compound | C (emu mol−1 K−1) | θ (K) | χ0 (emu mol−1) | μeff (μB) | Temp. Range (K) |
|---|---|---|---|---|---|
| Ce0.99Co4Sb9.65Ge2.51 | 0.563 (3) | 0.27 (1) | 0.0544 (1) | 2.12 (2.54) | 2–295 |
| Pr0.97Co4Sb9.52Ge2.61 | 1.78 (4) | −27 (1) | 0.0401 (2) | 3.77 (3.58) | 30–250 |
| Nd0.87Co4Sb9.94Ge2.28 | 1.22 (3) | 1.33 (8) | 0.0175 (7) | 3.12 (3.62) | 4–300 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukuoka, H. High-Pressure Synthesis, Structure, and Magnetic Properties of Ge-Substituted Filled Skutterudite Compounds; LnxCo4Sb12−yGey, Ln = La, Ce, Pr, and Nd. Crystals 2017, 7, 381. https://doi.org/10.3390/cryst7120381
Fukuoka H. High-Pressure Synthesis, Structure, and Magnetic Properties of Ge-Substituted Filled Skutterudite Compounds; LnxCo4Sb12−yGey, Ln = La, Ce, Pr, and Nd. Crystals. 2017; 7(12):381. https://doi.org/10.3390/cryst7120381
Chicago/Turabian StyleFukuoka, Hiroshi. 2017. "High-Pressure Synthesis, Structure, and Magnetic Properties of Ge-Substituted Filled Skutterudite Compounds; LnxCo4Sb12−yGey, Ln = La, Ce, Pr, and Nd" Crystals 7, no. 12: 381. https://doi.org/10.3390/cryst7120381





