Calcined Solution-Based PVP Influence on ZnO Semiconductor Nanoparticle Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural, Morphological, Phase Composition, and Optical Features Following Calcination at Various Temperatures
2.2. Structural, Morphological, Phase Composition, and Optical Features in Relation to PVP Concentration
2.2.1. Structural Analysis
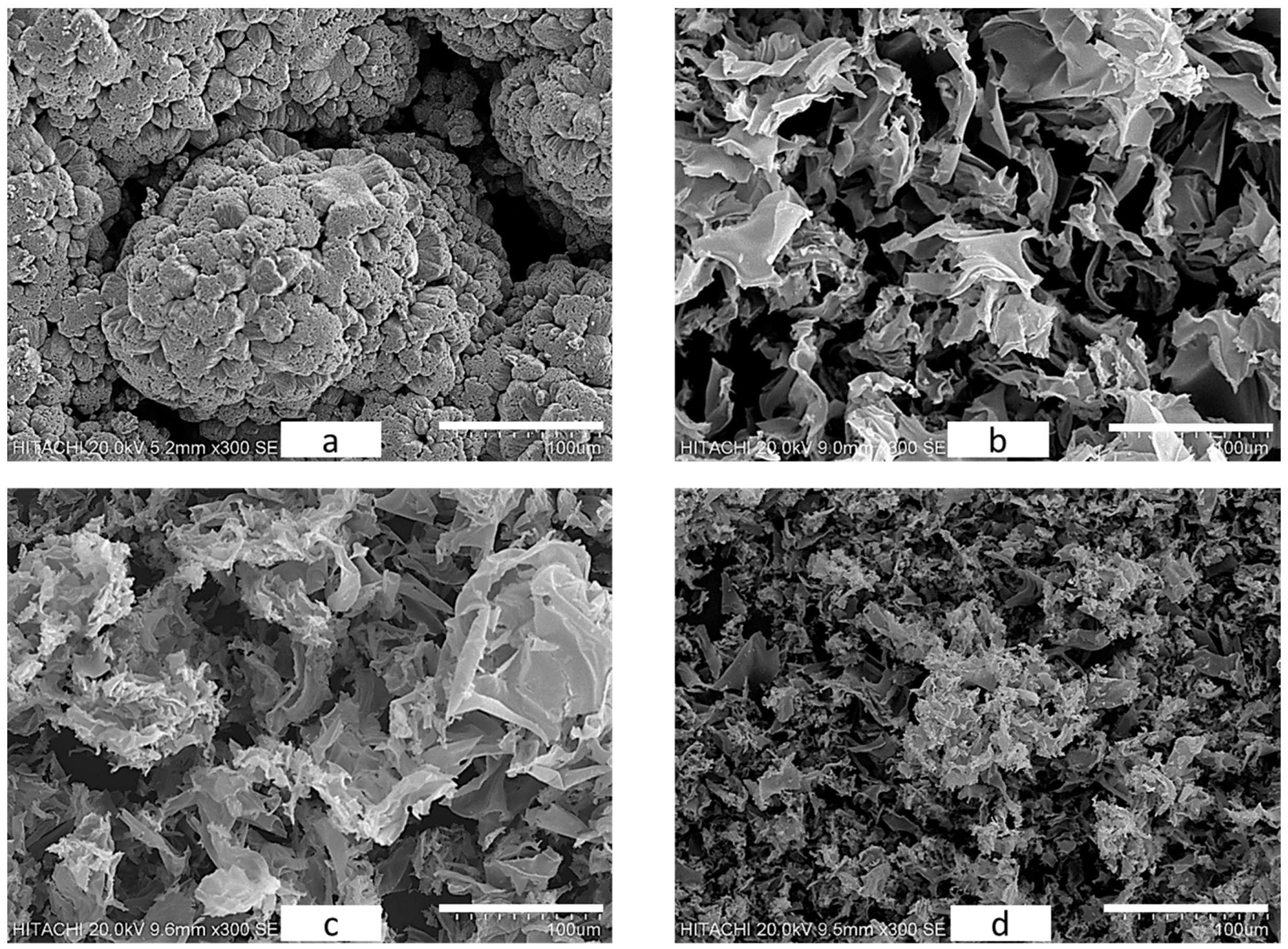
2.2.2. Surface Morphology Analysis
2.2.3. TEM Analysis
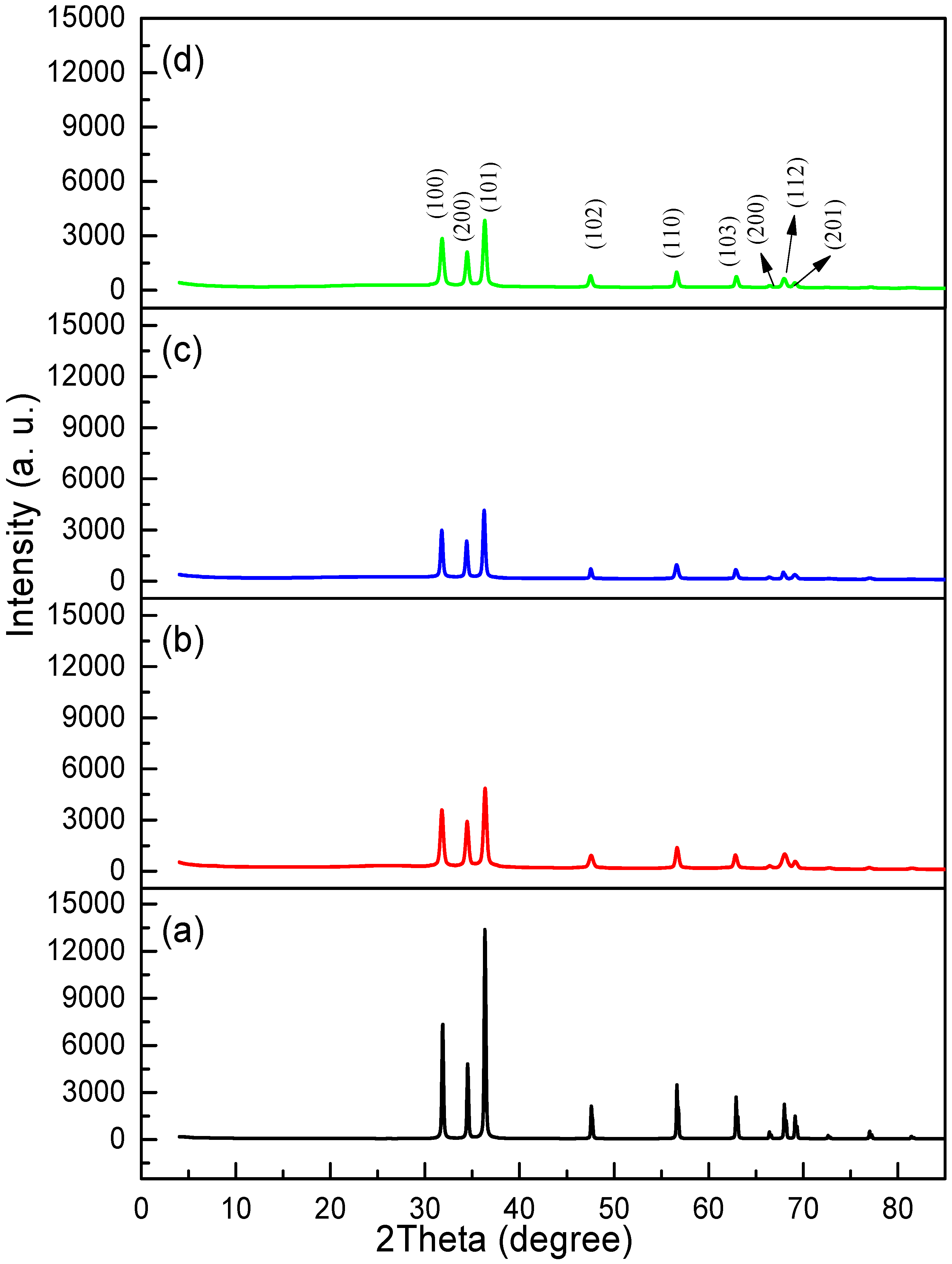
2.2.4. Phase Composition Analysis
2.2.5. UV-VIS Spectrophotometer Analysis
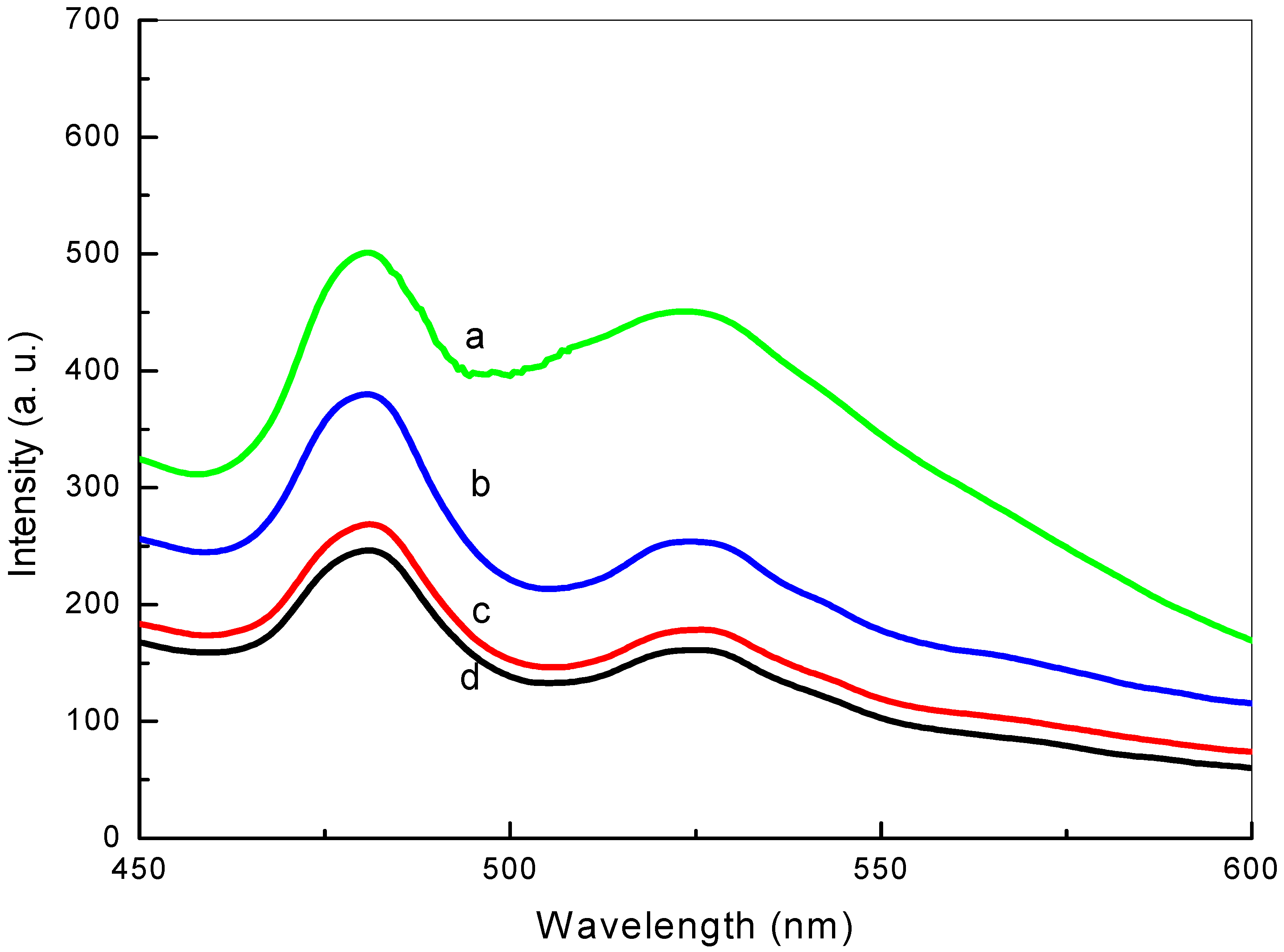
2.2.6. Photoluminescence Analysis
3. Materials and Methods
3.1. Materials
3.2. Synthesis of the Nanoparticles
3.3. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pyun, J.; Matyjaszewski, K. Synthesis of Nanocomposite Organic/Inorganic Hybrid Materials Using Controlled/“Living” Radical Polymerization. Chem. Mater. 2001, 13, 3436–3448. [Google Scholar] [CrossRef]
- Schottner, G. Hybrid Sol-Gel-Derived Polymers: Applications of Multifunctional Materials. Chem. Mater. 2001, 13, 3422–3435. [Google Scholar] [CrossRef]
- Wang, Z.; Pinnavaia, T.J. Hybrid Organic-Inorganic Nanocomposites: Exfoliation of Magadiite Nanolayers in an Elastomeric Epoxy Polymer. Chem. Mater. 1998, 10, 1820–1826. [Google Scholar] [CrossRef]
- Jia, X.; Li, Y.; Cheng, Q.; Zhang, S.; Zhang, B. Preparation and Properties of Poly (Vinyl Alcohol)/Silica Nanocomposites Derived From Copolymerization of Vinyl Silica Nanoparticles and Vinyl Acetate. Eur. Polym. J. 2007, 43, 1123–1131. [Google Scholar] [CrossRef]
- Hau, S.K.; Yip, H.-L.; Baek, N.S.; Zou, J.; O’Malley, K.; Jen, A.K.-Y. Air-Stable Inverted Flexible Polymer Solar Cells Using Zinc Oxide Nanoparticles as an Electron Selective Layer. Appl. Phys. Lett. 2008, 92, 253301. [Google Scholar] [CrossRef]
- Boucle, J.; Ravirajan, P.; Nelson, J. Hybrid Polymer–Metal Oxide Thin Films for Photovoltaic Applications. J. Mater. Chem. 2007, 17, 3141–3153. [Google Scholar] [CrossRef]
- Sanvicens, N.; Marco, M.P. Multifunctional Nanoparticles—Properties and Prospects for Their Use in Human Medicine. Trends Biotechnol. 2008, 26, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Neoh, K.G.; Kang, E.T. Functionalization of Inorganic Nanoparticles with Polymers for Stealth Biomedical Applications. Polym. Chem. 2011, 2, 747–759. [Google Scholar] [CrossRef]
- Tong, W.; Song, X.; Gao, C. Layer-by-Layer Assembly of Microcapsules and Their Biomedical Applications. Chem. Soc. Rev. 2012, 41, 6103–6124. [Google Scholar] [CrossRef] [PubMed]
- Krasia-Christoforou, T. Organic–Inorganic Polymer Hybrids: Synthetic Strategies and Applications. In Hybrid and Hierarchical Composite Materials; Springer: Berlin, Germany, 2015; pp. 11–63. [Google Scholar]
- Niederberger, M.; Pinna, N. Metal Oxide Nanoparticles in Organic Solvents: Synthesis, Formation, Assembly and Application; Springer: Berlin, Germany, 2009. [Google Scholar]
- Oskam, G. Metal Oxide Nanoparticles: Synthesis, Characterization and Application. J. Sol-Gel Sci. Technol. 2006, 37, 161–164. [Google Scholar] [CrossRef]
- Rozenberg, B.; Tenne, R. Polymer-Assisted Fabrication of Nanoparticles and Nanocomposites. Prog. Polym. Sci. 2008, 33, 40–112. [Google Scholar] [CrossRef]
- Kumar, A.P.; Depan, D.; Tomer, N.S.; Singh, R.P. Nanoscale Particles for Polymer Degradation fnd Stabilization—Trends and Future Perspectives. Prog. Polym. Sci. 2009, 34, 479–515. [Google Scholar] [CrossRef]
- Al-Hada, N.M.; Saion, E.B.; Shaari, A.H.; Kamarudin, M.A.; Flaifel, M.H.; Ahmad, S.H.; Gene, A. A facile Thermal-Treatment Route to Synthesize the Semiconductor CdO Nanoparticles and Effect of Calcination. Mater. Sci. Semicond. Process. 2014, 26, 460–466. [Google Scholar] [CrossRef]
- Song, N.; Fan, H.; Tian, H. PVP Assisted in Situ Synthesis of Functionalized Graphene/ZnO (FGZnO) Nanohybrids With Enhanced Gas-Sensing Property. J. Mater. Sci. 2015, 50, 2229–2238. [Google Scholar] [CrossRef]
- Reetz, M.T.; Helbig, W. Size-Selective Synthesis of Nanostructured Transition Metal Clusters. J. Am. Chem. Soc. 1994, 116, 7401–7402. [Google Scholar] [CrossRef]
- Antonietti, M.; Goltner, C. Superstructures of Functional Colloids: Chemistry on the Nanometer Scale. Angew. Chem. Int. Ed. Engl. 1997, 36, 910–928. [Google Scholar] [CrossRef]
- Schmid, G.; Chi, L.F. Metal Clusters and Colloids. Adv. Mater. 1998, 10, 515–526. [Google Scholar] [CrossRef]
- Pei, L.W. Architectural Control of Metal Sulfide Nanocrystals and Polymer Composites. Ph.D. Thesis, Department of Chemistry, National University of Singapore, Singapore, 2006. [Google Scholar]
- Liu, T.; Burger, C.; Chu, B. Nanofabrication in Polymer Matrices. Prog. Polym. Sci. 2003, 28, 5–26. [Google Scholar] [CrossRef]
- Adachi, S. Properties of Semiconductor Alloys: Group-IV, III–V and II–VI Semiconductors; John Wiley & Sons: Hoboken, NJ, USA, 2009; Volume 28. [Google Scholar]
- Cardona, M.; Peter, Y.Y. Fundamentals of Semiconductors; Springer: Berlin, Germany, 2005. [Google Scholar]
- Gupta, T.K. Application of Zinc Oxide Varistors. J. Am. Ceram. Soc. 1990, 73, 1817–1840. [Google Scholar] [CrossRef]
- Jagadish, C.; Pearton, S.J. Zinc Oxide Bulk, Thin Films and Nanostructures: Processing, Properties, and Applications; Elsevier: Oxford, UK; Amsterdam, The Netherlands, 2011. [Google Scholar]
- Tian, H.; Fan, H.; Guo, H.; Song, N. Solution-Based Synthesis of ZnO/Carbon Nanostructures by Chemical Coupling for High Performance Gas Sensors. Sens. Actuators B: Chem. 2014, 195, 132–139. [Google Scholar] [CrossRef]
- Fang, J.; Fan, H.; Tian, H.; Dong, G. Morphology Control of ZnO Nanostructures for High Efficient Dye-Sensitized Solar Cells. Mater. Charact. 2015, 108, 51–57. [Google Scholar] [CrossRef]
- Li, H.; Jiao, S.; Bai, S.; Li, H.; Gao, S.; Wang, J.; Yu, Q.; Guo, F.; Zhao, L. Precursor-Controlled Synthesis of Different ZnO Nanostructures by the Hydrothermal Method. Phys. Status Solidi 2014, 211, 595–600. [Google Scholar] [CrossRef]
- Baruah, S.; Dutta, J. Hydrothermal Growth of ZnO Nanostructures. Sci. Technol. Adv. Mater. 2009, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Andiyana, Y.; Suyatma, N.E. Physico-Mechanical Properties of Starch-Based Nanocomposite Film Incorporated with Hydrothermally Synthesized Zinc Oxide Nanoparticles. Mater. Sci. Forum 2016, 872, 162–167. [Google Scholar] [CrossRef]
- Farhadi-Khouzani, M.; Fereshteh, Z.; Loghman-Estarki, M.R.; Razavi, R.S. Different Morphologies of ZnO Nanostructures via Polymeric Complex Sol-Gel Method: Synthesis and Characterization. J. Sol-Gel Sci. Technol. 2012, 64, 193–199. [Google Scholar] [CrossRef]
- Khan, M.F.; Ansari, A.H.; Hameedullah, M.; Ahmad, E.; Husain, F.M.; Zia, Q.; Baig, U.; Zaheer, M.R.; Alam, M.M.; Khan, A.M.; et al. Sol-Gel Synthesis of Thorn-Like ZnO Nanoparticles Endorsing Mechanical Stirring Effect and Their Antimicrobial Activities: Potential Role as Nano-Antibiotics. Sci. Rep. 2016, 6, 27689. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Synthesis of ZnO Nanostructures by Microwave Irradiation Using Albumen As a Template. J. Nanopart. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Chen, D.; Ai, S.; Liang, Z.; Wei, F. Preparation and Photocatalytic Properties of Zinc Oxide Nanoparticles by Microwave-Assisted Ball Milling. Ceram. Int. 2016, 42, 3692–3696. [Google Scholar] [CrossRef]
- Azlinda, A.; Eswar, K.; Husairi, F.; Khusaimi, Z.; Rusop, M. Optical Properties of Nano-Porous Structure ZnO Prepared by Catalytic-Immersion Method; IOP Conference Series, Materials Science and Engineering; IOP Publishing: Shah Alam, Malaysia, 2013; p. 012044. [Google Scholar]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Karthikeyan, D.; Lee, Y.R. Facile Synthesis of Zinc Oxide Nanoparticles Decorated Graphene Oxide Composite via Simple Solvothermal Route and Their Photocatalytic Activity on Methylene Blue Degradation. J. Photochem. Photobiol. B: Biol. 2016, 162, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, Y.; Kong, J.; Yu, M.; Jin, H. Preparation of Controlled ZnO Nanostructures and Their Optical Properties. J. Mater. Sci. Mater. Electron. 2016, 27, 7227–7232. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Zhang, L.; Yuan, J.; Yan, S.; Wang, C. Low-Temperature Synthesis of ZnO Nanoparticles by Solid-State Pyrolytic Reaction. Nanotechnology 2002, 14, 11. [Google Scholar] [CrossRef]
- Gene, S.A.; Saion, E.; Shaari, A.H.; Kamarudin, M.A.; Al-Hada, N.M.; Kharazmi, A. Structural, Optical, and Magnetic Characterization of Spinel Zinc Chromite Nanocrystallines Synthesised by Thermal Treatment Method. J. Nanomater. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Zakiyah, L.B.; Saion, E.; Al-Hada, N.M.; Gharibshahi, E.; Salem, A.; Soltani, N.; Gene, S. Up-Scalable Synthesis of Size-Controlled Copper Ferrite Nanocrystals by Thermal Treatment Method. Mater. Sci. Semicond. Process. 2015, 40, 564–569. [Google Scholar] [CrossRef]
- Hashem, M.; Saion, E.; Al-Hada, N.M.; Kamari, H.M.; Shaari, A.H.; Talib, Z.A.; Paiman, S.B.; Kamarudeen, M.A. Fabrication and Characterization of Semiconductor Nickel Oxide (NiO) Nanoparticles Manufactured Using A Facile Thermal Treatment. Results Phys. 2016, 6, 1024–1030. [Google Scholar] [CrossRef]
- Sekine, N.; Chou, C.-H.; Kwan, W.L.; Yang, Y. ZnO Nano-Ridge Structure and Its Application in Inverted Polymer Solar Cell. Org. Electron. 2009, 10, 1473–1477. [Google Scholar] [CrossRef]
- Ko, S.H.; Lee, D.; Kang, H.W.; Nam, K.H.; Yeo, J.Y.; Hong, S.J.; Grigoropoulos, C.P.; Sung, H.J. Nanoforest of Hydrothermally Grown Hierarchical ZnO Nanowires for A High Efficiency Dye-Sensitized Solar Cell. Nano Lett. 2011, 11, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Al-Hada, N.M.; Saion, E.; Kamari, H.M.; Flaifel, M.H.; Shaari, A.H.; Talib, Z.A.; Abdullahi, N.; Baqer, A.A.; Kharazmi, A. Structural, Morphological and Optical Behaviour of PVP Capped Binary (ZnOo)0.4(CdO)0.6 Nanoparticles Synthesised by A Facile Thermal Route. Mater. Sci. Semicond. Process. 2016, 53, 56–65. [Google Scholar] [CrossRef]
- Al-Hada, N.M.; Saion, E.; Talib, Z.A.; Shaari, A.H. The Impact of Polyvinylpyrrolidone on Properties of Cadmium Oxide Semiconductor Nanoparticles Manufactured by Heat Treatment Technique. Polymers 2016, 8, 113. [Google Scholar] [CrossRef]
- Al-Hada, N.M.; Saion, E.B.; Shaari, A.H.; Kamarudin, M.A.; Flaifel, M.H.; Ahmad, S.H.; Gene, S.A. A Facile Thermal-Treatment Route to Synthesize ZnO Nanosheets and Effect of Calcination Temperature. PLoS ONE 2014, 9, e103134. [Google Scholar] [CrossRef] [PubMed]
- Cunha, D.M.; Souza, F.L. Facile Synthetic Route For Producing One-Dimensional Zinc Oxide Nanoflowers and Characterization of Their Optical Properties. J. Alloys Compd. 2013, 577, 158–164. [Google Scholar] [CrossRef]
- Al-Hada, N.M.; Saion, E.; Shaari, A.; Kamarudin, M.; Gene, S.A. The Influence of Calcination Temperature on the Formation of Zinc Oxide Nanoparticles by Thermal-Treatment. Appl. Mech. Mater. 2014, 446, 181–184. [Google Scholar] [CrossRef]
- Jang, Y.J.; Simer, C.; Ohm, T. Comparison of Zinc Oxide Nanoparticles and Its Nano-Crystalline Particles on the Photocatalytic Degradation of Methylene Blue. Mater. Res. Bull. 2006, 41, 67–77. [Google Scholar] [CrossRef]
- Cullity, B. Elements of X-ray Diffraction, 2nd ed.; Adisson-Wesley Publishing: Wesley, NY, USA, 1978. [Google Scholar]
- Zhang, Q.; Park, K.; Cao, G. Synthesis of ZnO Aggregates and Their Application in Dye-Sensitized Solar Cells. Mater. Matters 2010, 5, 32–39. [Google Scholar]
- Hu, J.; Bando, Y.; Zhan, J.; Li, Y.; Sekiguchi, T. Two-Dimensional Micrometer-Sized Single-Crystalline ZnO Thin Nanosheets. Appl. Phys. Lett. 2003, 83, 4414–4416. [Google Scholar] [CrossRef]
- Xiao, Q.; Huang, S.; Zhang, J.; Xiao, C.; Tan, X. Sonochemical Synthesis of ZnO Nanosheet. J. Alloys Compd. 2008, 459, L18–L22. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Kong, J.; Jia, H.; Wang, Z. Synthesis of ZnO Nanosheets via Electrodeposition Method and Their Optical Properties, Growth Mechanism. Opt. Mater. 2015, 46, 179–185. [Google Scholar] [CrossRef]
- Umar, A.; Hahn, Y. ZnO Nanosheet Networks and Hexagonal Nanodiscs Grown on Silicon Substrate: Growth Mechanism and Structural and Optical Properties. Nanotechnology 2006, 17, 2174. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, H.; Zhao, B.; Bao, X. Size Control of ZnO Nanoparticles via Thermal Decomposition of Zinc Acetate Coated on Organic Additives. J. Cryst. Growth 2004, 263, 447–453. [Google Scholar] [CrossRef]
- Hsiao, I.-L.; Huang, Y.-J. Effects of Various Physicochemical Characteristics on the Toxicities of ZnO and Tio2 Nanoparticles toward Human Lung Epithelial Cells. Sci. Total Environ. 2011, 409, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, G.; Rajeshwari, S.; Venckatesh, R. Green Synthesis of Zinc Oxide Nanoparticles by Aloe Barbadensis Miller Leaf Extract: Structure and Optical Properties. Mater. Res. Bull. 2011, 46, 2560–2566. [Google Scholar] [CrossRef]
- Kubelka, P.; Munk, F. A Contribution to the Optics of Pigments. Z. Tech. Phys. 1931, 12, 593–599. [Google Scholar]
- Kubelka, P. New Contributions to the Optics of Intensely Light-Scattering Materials. Part I. J. Opt. Soc. Am. 1948, 38, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J. Absorption Near the Fundamental Edge. Semicond. Semimet. 1967, 3, 153–258. [Google Scholar]
- Torrent, J.; Barron, V. Encyclopedia of Surface and Colloid Science; Marcel Dekker Inc.: New York, NY, USA, 2002; pp. 1438–1446. [Google Scholar]
- Pankove, J.I. Optical Processes in Semiconductors; Prentice-Hall: Englewood Cliffs, NJ, USA, 1971. [Google Scholar]
- Aita, C.R.; Liu, Y.-L.; Kao, M.L.; Hansen, S.D. Optical Behavior of Sputter-Deposited Vanadium Pentoxide. J. Appl. Phys. 1986, 60, 749–753. [Google Scholar] [CrossRef]
- Yu, J.; Li, C.; Liu, S. Effect of PSS on morphology and optical properties of ZnO. J. Colloid Interface Sci. 2008, 326, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced Bioactivity of ZnO Nanoparticles—An Antimicrobial Study. Sci. Technol. Adv. Mater. 2008, 9, 035004. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-H.; Liu, C.-J.; Lv, Z.-H. Photoluminescence of ZnO Nanoparticles and Nanorods. Int. J. Light Electron Opt. 2016, 127, 1421–1423. [Google Scholar] [CrossRef]
- Shi, G.; Mo, C.; Cai, W.; Zhang, L. Photoluminescence of ZnO Nanoparticles in Alumina Membrane with Ordered Pore Arrays. Solid State Commun. 2000, 115, 253–256. [Google Scholar] [CrossRef]
- Fujihara, S.; Naito, H.; Kimura, T. Visible Photoluminescence of ZnO Nanoparticles Dispersed in Highly Transparent MgF2 Thin-Films via Sol-Gel Process. Thin Solid Films 2001, 389, 227–232. [Google Scholar] [CrossRef]
- Leprince-Wang, Y.; Yacoubi-Ouslim, A.; Wang, G. Structure Study of Electrodeposited ZnO Nanowires. Microelectron. J. 2005, 36, 625–628. [Google Scholar] [CrossRef]
- Cao, H.; Zhao, Y.; Ong, H.; Ho, S.; Dai, J.Y.; Wu, J.Y.; Chang, R.P. Ultraviolet Lasing in Resonators Formed by Scattering in Semiconductor Polycrystalline Films. Appl. Phys. Lett. 1998, 73, 3656–3658. [Google Scholar] [CrossRef]
- Liu, M.; Kitai, A.; Mascher, P. Point Defects and Luminescence Centres in Zinc Oxide and Zinc Oxide Doped with Manganese. J. Lumin. 1992, 54, 35–42. [Google Scholar] [CrossRef]
- Bylander, E. Surface Effects on the Low-Energy Cathodoluminescence of Zinc Oxide. J. Appl. Phys. 1978, 49, 1188–1195. [Google Scholar] [CrossRef]
- Ghosh, A.; Deshpande, N.; Gudage, Y.; Joshi, R.; Sagade, A.; Phase, D.; Sharma, R. Effect of Annealing on Structural and Optical Properties of Zinc Oxide Thin Film Deposited by Successive Ionic Layer Adsorption and Reaction Technique. J. Alloys Compd. 2009, 469, 56–60. [Google Scholar] [CrossRef]
- Dingle, R. Luminescent Transitions Associated with Divalent Copper Impurities and the Green Emission From Semiconducting Zinc Oxide. Phys. Rev. Lett. 1969, 23, 579. [Google Scholar] [CrossRef]
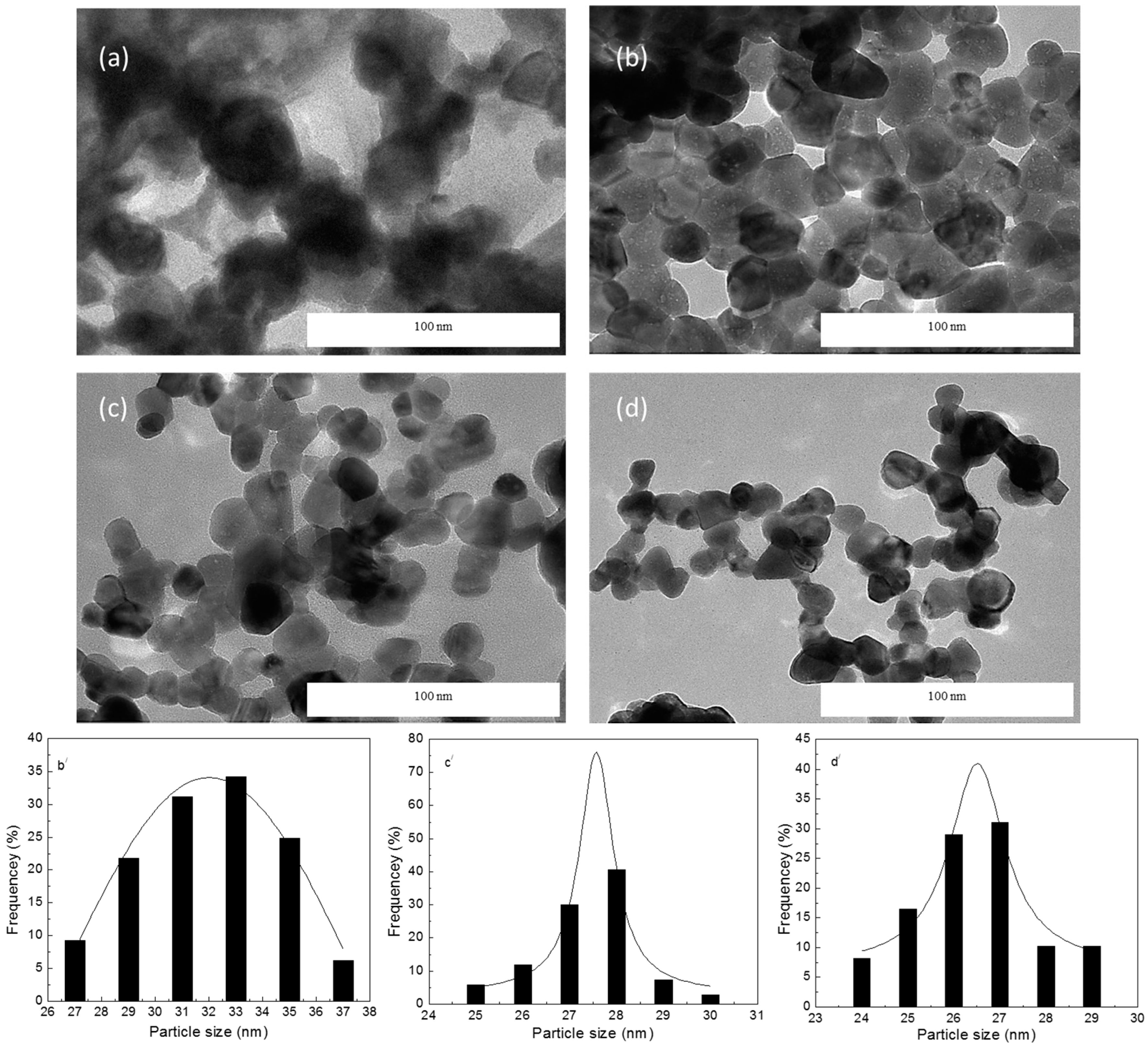

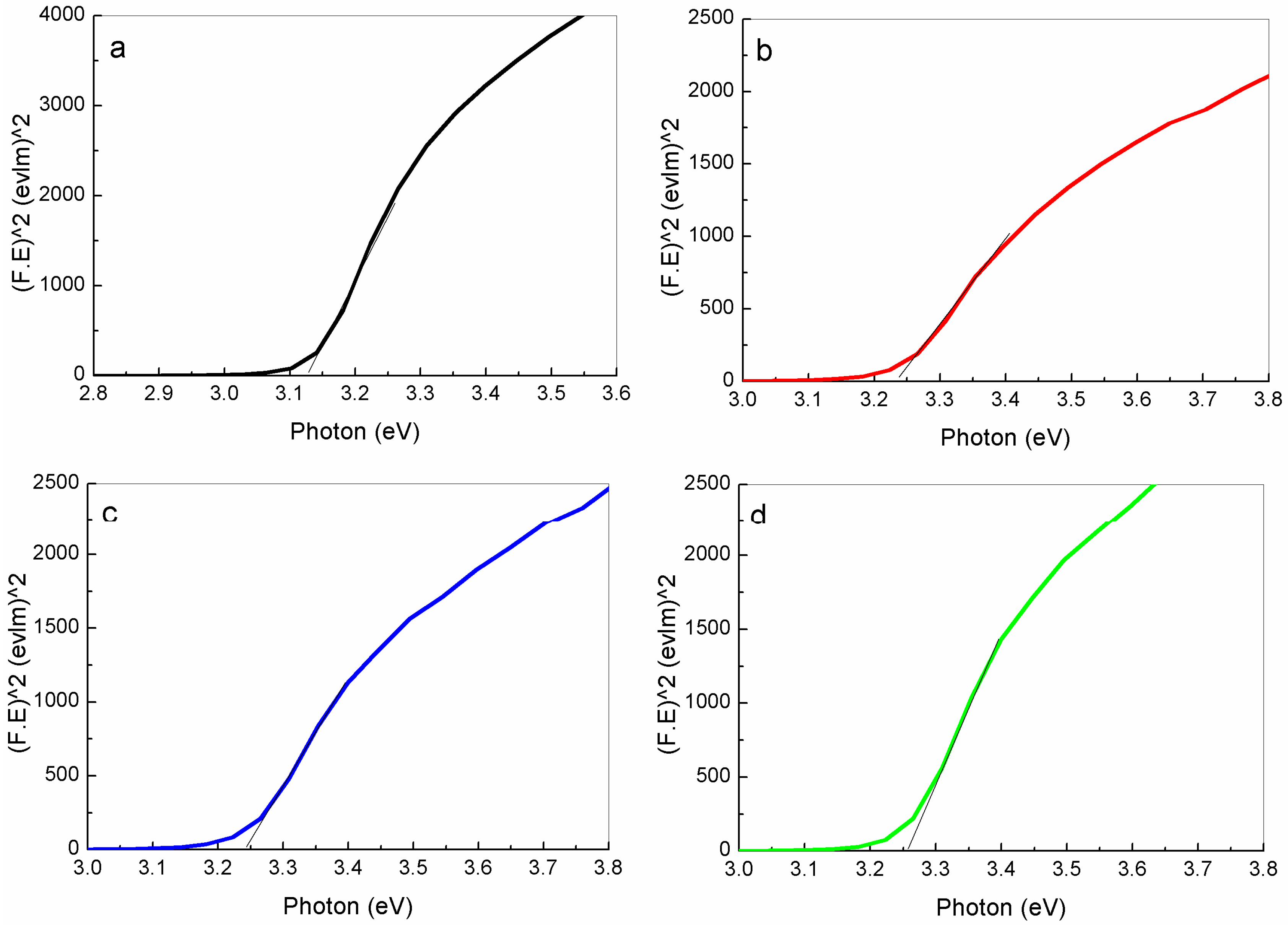
| ZnO NPs | PVP Concentration (g) | Crystalline Size XRD (nm) | Particle Size TEM (nm) | Energy Band Gap Eg (eV) |
|---|---|---|---|---|
| Sample 1 | 0 | - | - | 3.120 |
| Sample 2 | 3 | 31.8 | 32 ± 4 | 3.231 |
| Sample 3 | 4 | 27.5 | 27.5 ± 2 | 3.246 |
| Sample 4 | 5 | 25.7 | 26.5 ± 3 | 3.261 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamari, H.M.; Al-Hada, N.M.; Saion, E.; Shaari, A.H.; Talib, Z.A.; Flaifel, M.H.; Ahmed, A.A.A. Calcined Solution-Based PVP Influence on ZnO Semiconductor Nanoparticle Properties. Crystals 2017, 7, 2. https://doi.org/10.3390/cryst7020002
Kamari HM, Al-Hada NM, Saion E, Shaari AH, Talib ZA, Flaifel MH, Ahmed AAA. Calcined Solution-Based PVP Influence on ZnO Semiconductor Nanoparticle Properties. Crystals. 2017; 7(2):2. https://doi.org/10.3390/cryst7020002
Chicago/Turabian StyleKamari, Halimah Mohamed, Naif Mohammed Al-Hada, Elias Saion, Abdul Halim Shaari, Zainal Abidin Talib, Moayad Husein Flaifel, and Abdullah Ahmed Ali Ahmed. 2017. "Calcined Solution-Based PVP Influence on ZnO Semiconductor Nanoparticle Properties" Crystals 7, no. 2: 2. https://doi.org/10.3390/cryst7020002