Reversed Crystal Growth of Calcite in Naturally Occurring Travertine Crust
Abstract
:1. Introduction
2. Results
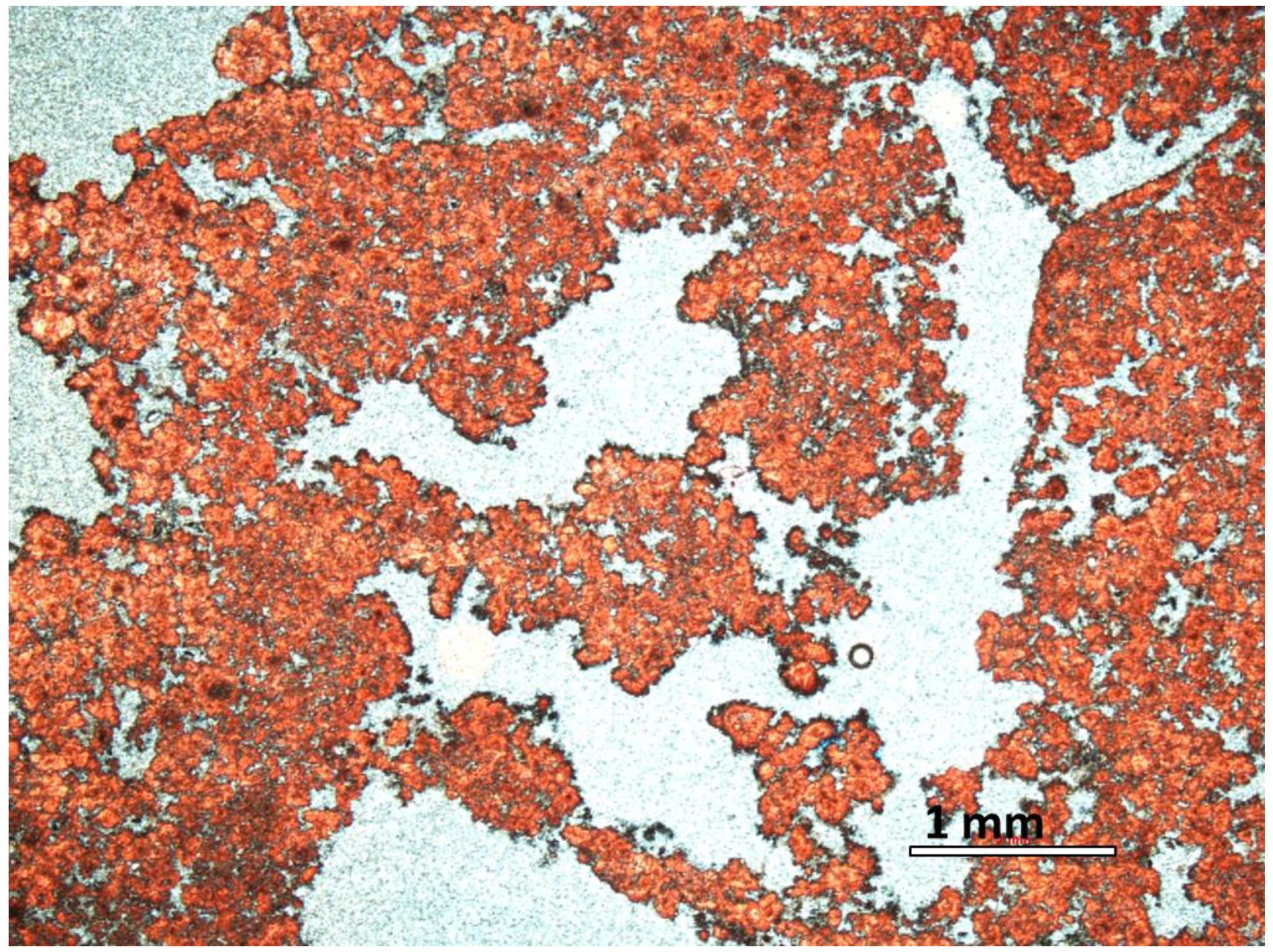
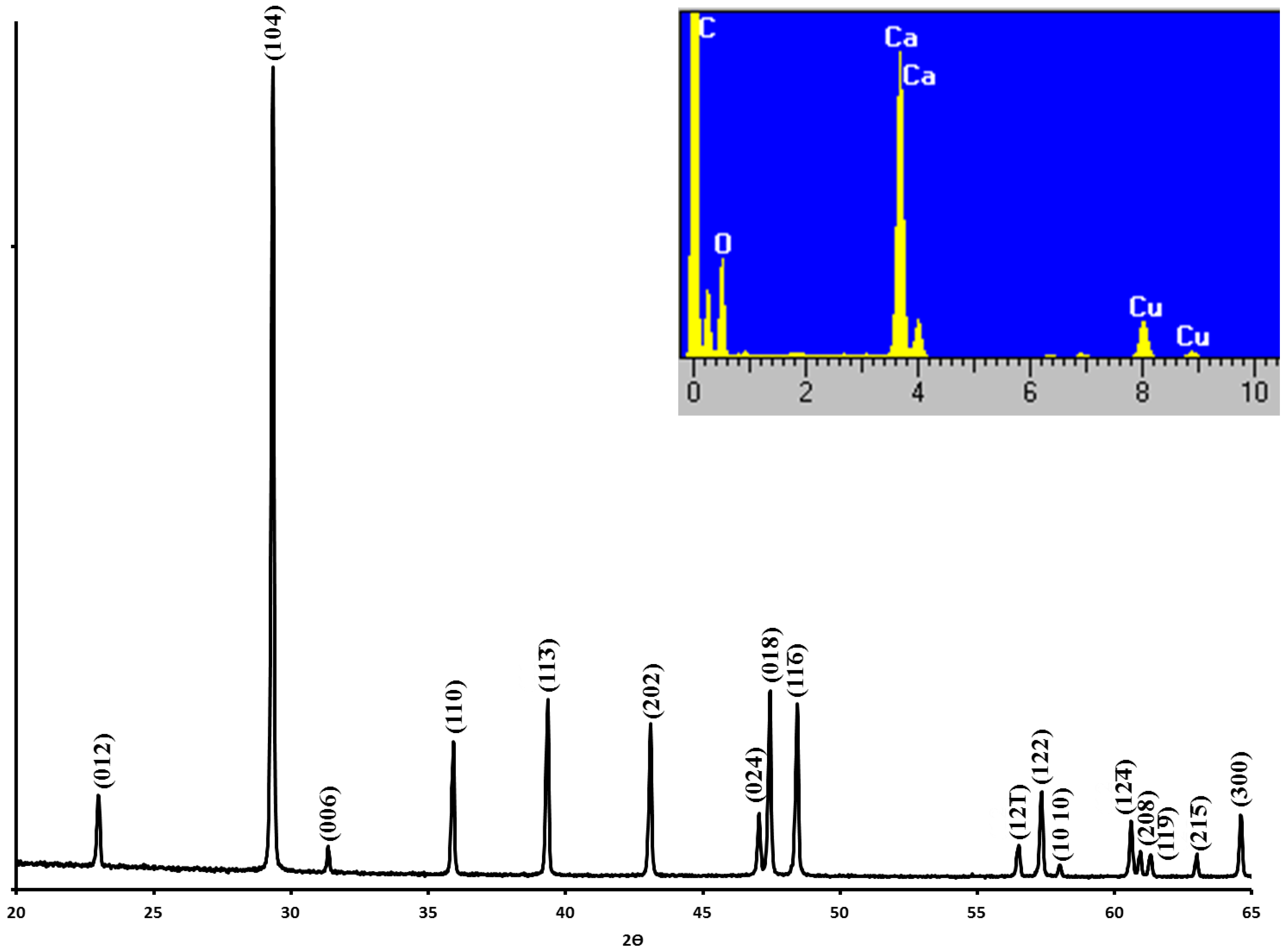
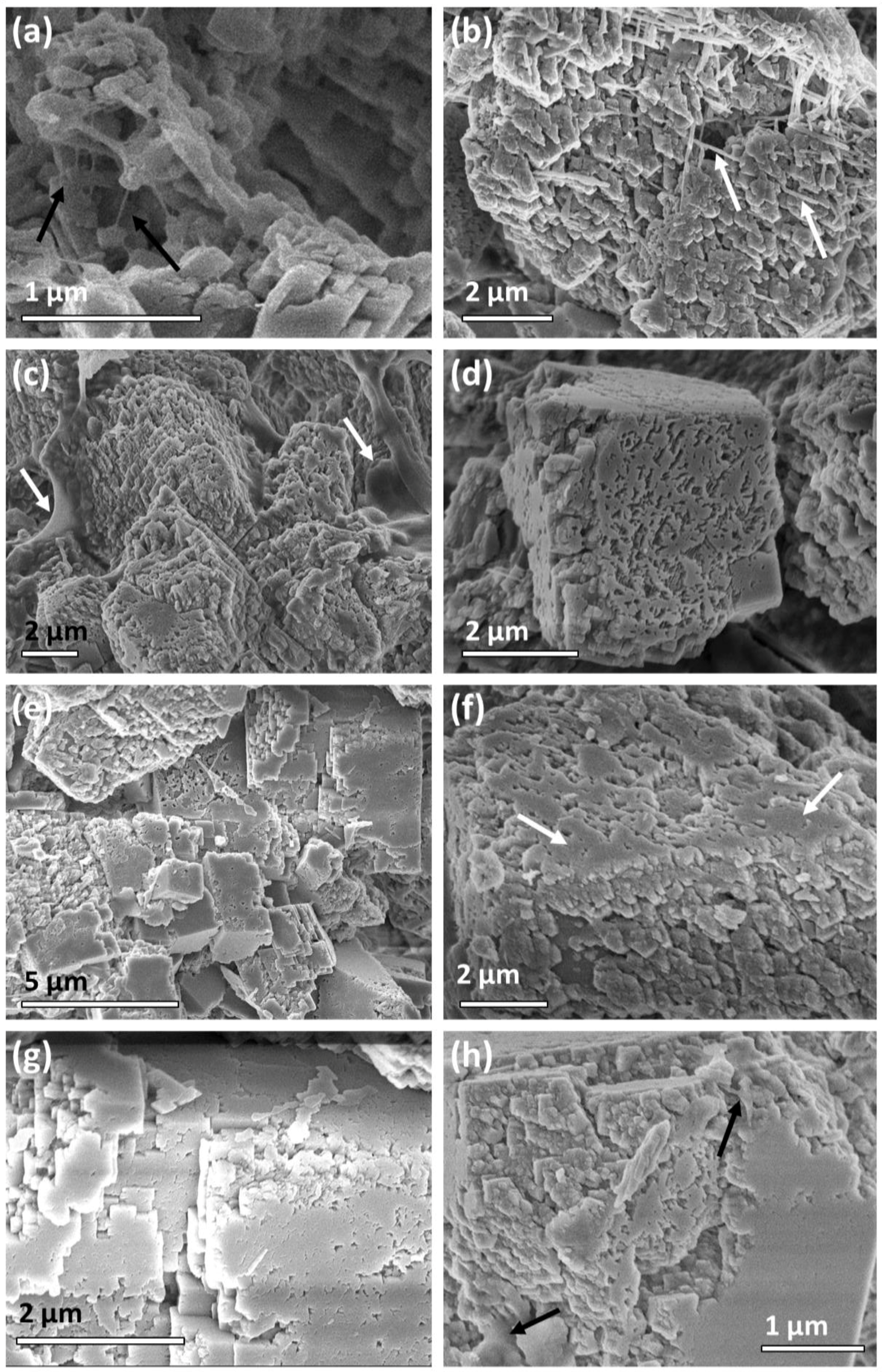
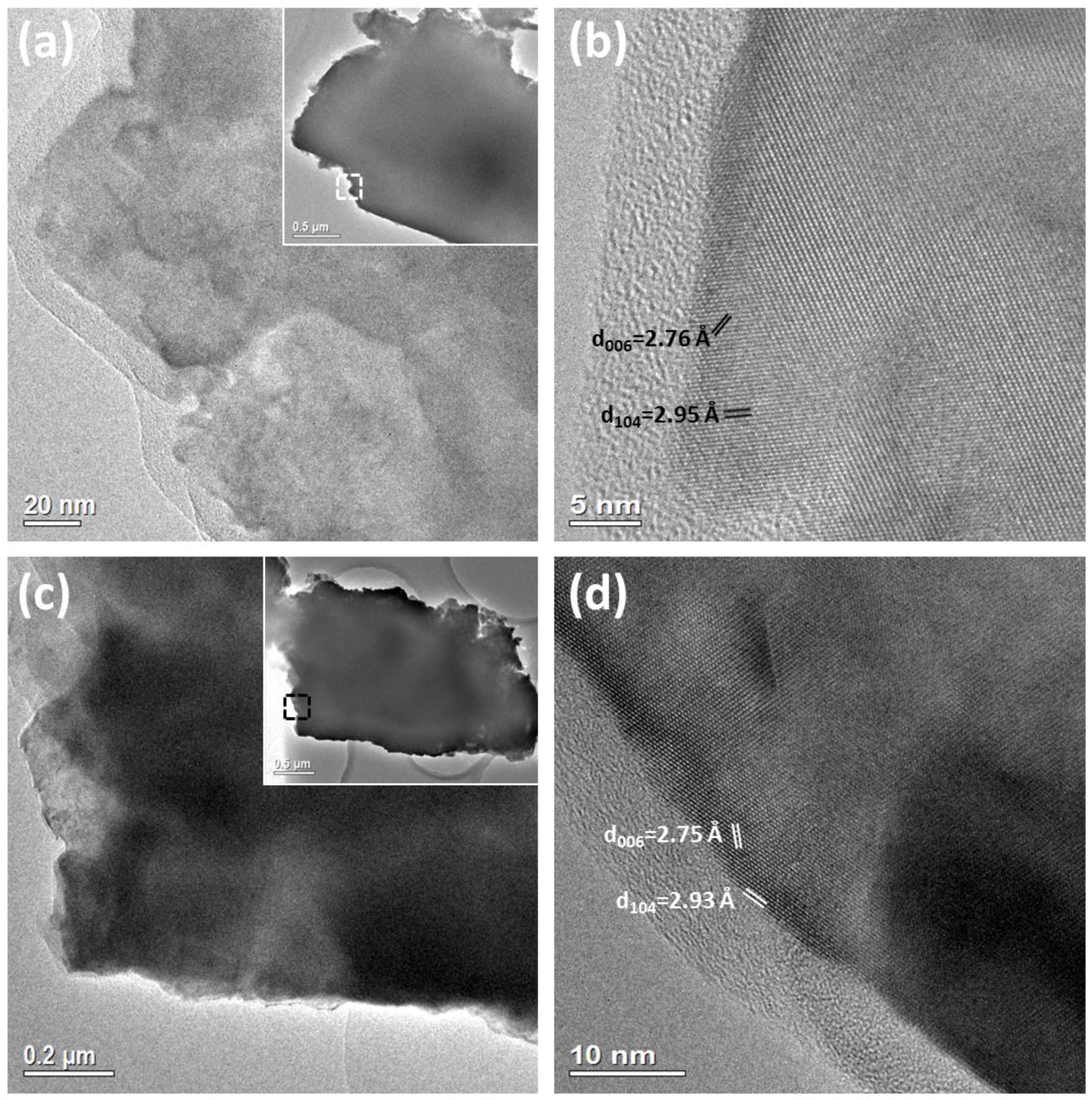
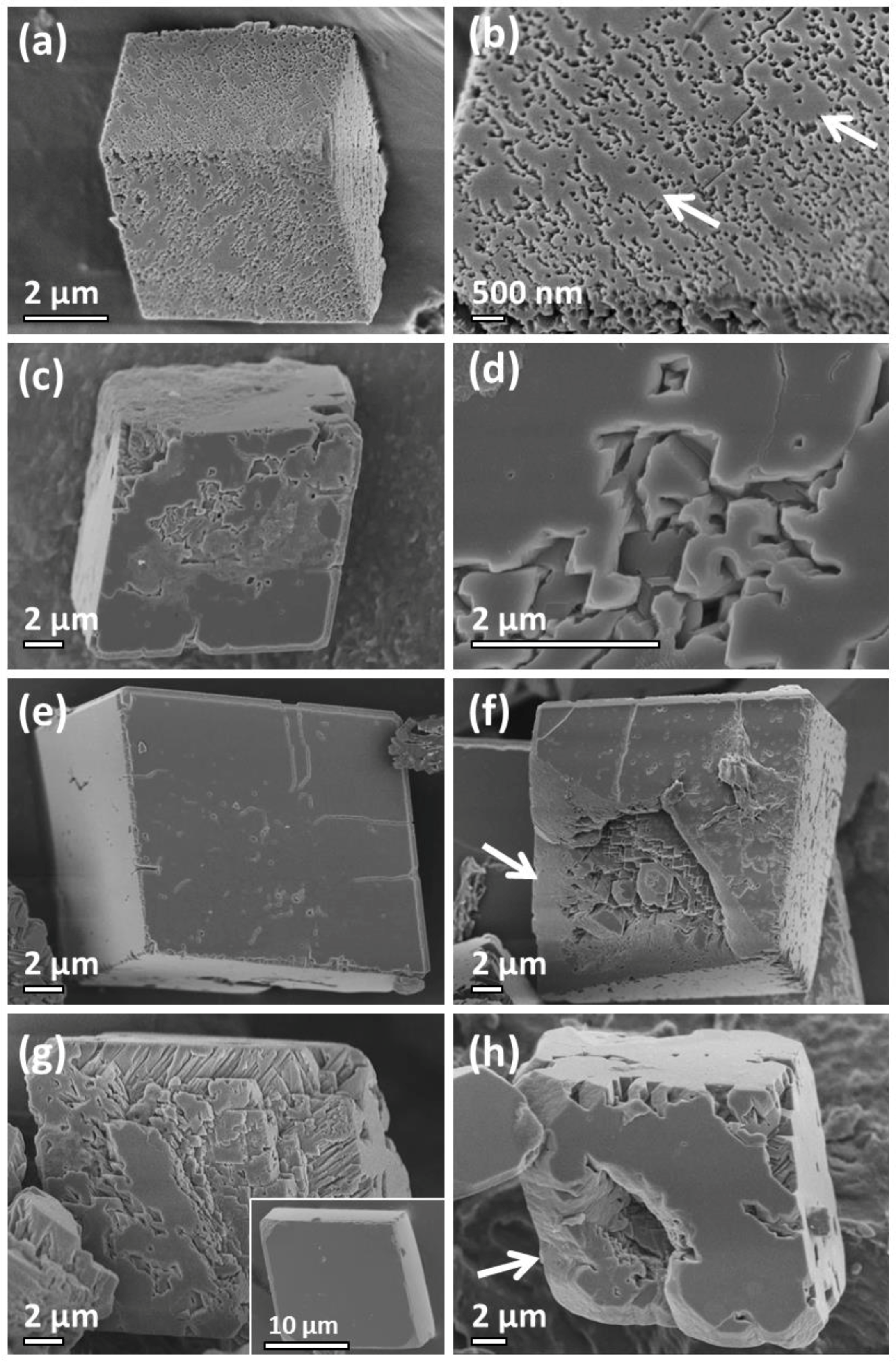
2.1. Travertine Calcite Deposits
2.2. Synthetic Calcite Grown in the Presence of Chitosan
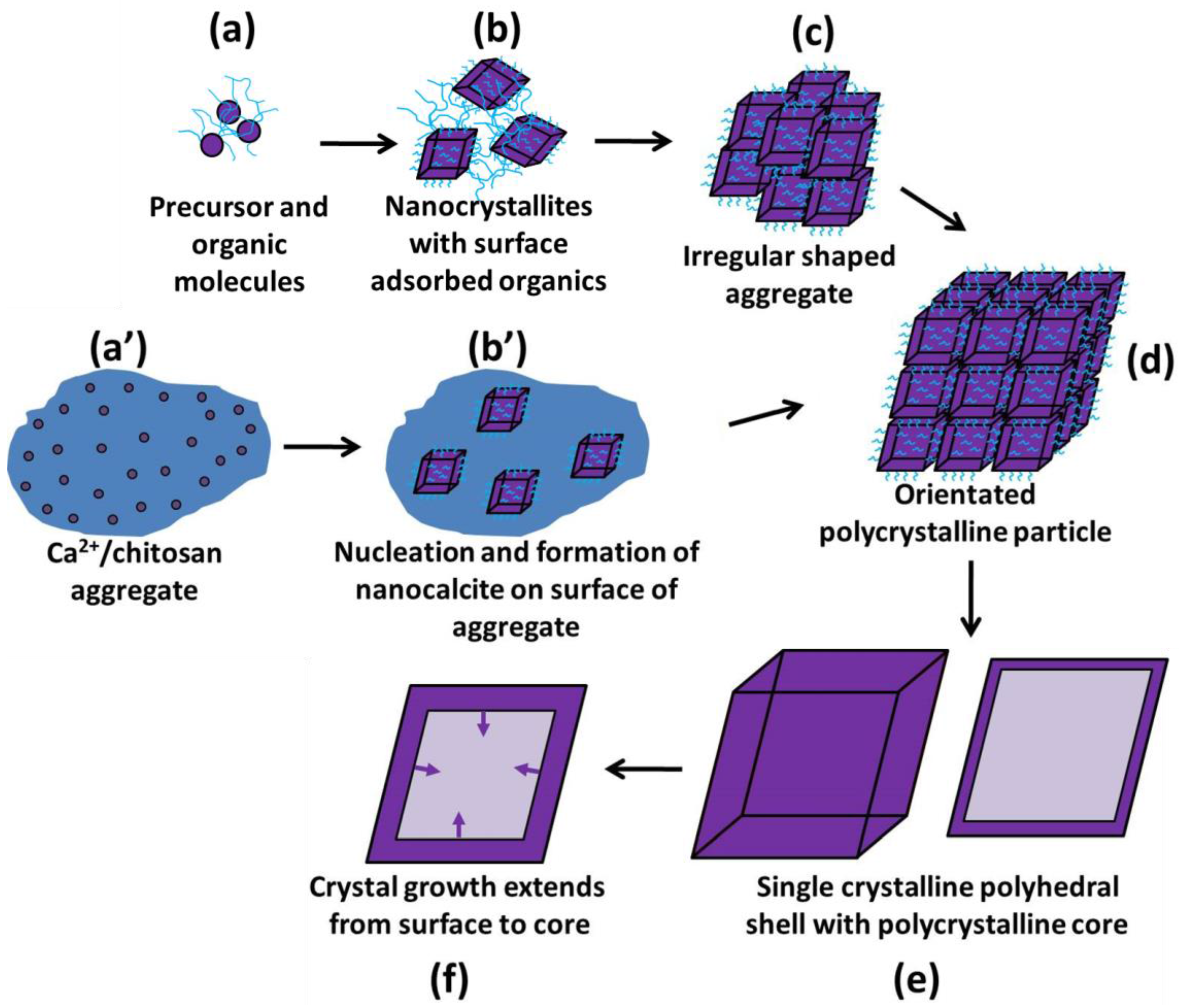
3. Discussion
Formation Mechanism
4. Materials and Methods
4.1. Collection of Travertine Specimen
4.2. Sample Characterization
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dickinson, S.R.; Henderson, G.E.; McGrath, K.M. Controlling the kinetic versus thermodynamic crystallisation of calcium carbonate. J. Cryst. Growth 2002, 244, 369–378. [Google Scholar] [CrossRef]
- Kawano, J.; Shimobayashi, N.; Miyake, A.; Kitamura, M. Precipitation diagram of calcium carbonate polymorphs: Its construction and significance. J. Phys. Condens. Matter 2009, 21, 425102. [Google Scholar] [CrossRef] [PubMed]
- Pentecost, A. Travertine; Springer: Berlin, Germany, 2005. [Google Scholar]
- Jones, B.; Renaut, R.W. Calcareous Spring Deposits in Continental Settings. In Developments in Sedimentology: Carbonates in Continental Settings: Facies, Environments and Processes; Elsevier: Amsterdam, The Netherlands, 2010; pp. 177–224. [Google Scholar]
- Capezzuoli, E.; Gandin, A.; Pedley, M. Decoding tufa and travertine (fresh water carbonates) in the sedimentary record: The state of the art. Sedimentology 2014, 61, 1–21. [Google Scholar] [CrossRef]
- Pedley, H.M. Classification and environmental models of cool freshwater tufas. Sediment. Geol. 1990, 68, 143–154. [Google Scholar] [CrossRef]
- Kim, J.-W.; Kogure, T.; Yang, K.; Kim, S.-T.; Jang, Y.-N.; Baik, H.-S.; Geesey, G. The characterisation of CaCO3 in a geothermal environment: A SEM/TEM-EELS study. Clays Clay Miner. 2012, 60, 484–495. [Google Scholar] [CrossRef]
- Coman, C.; Chiriac, C.M.; Robeson, M.S.; Ionescu, C.; Dragos, N.; Barbu-Tudoran, L.; Andrei, A.-Ş.; Banciu, H.L.; Sicora, C.; Podar, M. Structure, mineralogy, and microbial diversity of geothermal spring microbialites associated with a deep oil drilling in Romania. Front. Microbiol. 2015, 6, 253. [Google Scholar] [CrossRef] [PubMed]
- Best, J.L.; Fielding, C.R.; Jarvis, I.; Mozley, P. Sedimentology: Millenium Reviews—The Journal of the International Association of Sedimentologiests; Wiley-Blackwell: New York, NY, USA, 2009. [Google Scholar]
- Decho, A.W. Microbial exopolymer secretions in ocean environments: Their role(s) in food webs and marine processes. Oceanogr. Mar. Biol. Annu. Rev. 1990, 28, 73–153. [Google Scholar]
- Ruan, X.; Li, L.; Liu, J. Flocculating characteristic of activated sludge flocs: Interaction between Al3+ and extracellular polymeric substances. J. Environ. Sci. 2013, 25, 916–924. [Google Scholar] [CrossRef]
- Pentecost, A. Association of cyanobacteria with tufa deposits: Identity, enumeration and nature of the sheath material revealed by histochemistry. Geomicrobiol. J. 1985, 4, 285–298. [Google Scholar] [CrossRef]
- Plee, K.; Ariztegui, D.; Martini, R.; Davaud, E. Unravelling the microbial role in ooid formation—Results of an in situ experiment in modern freshwater Lake Geneva in Switzerland. Geobiology 2008, 6, 341–350. [Google Scholar] [CrossRef]
- Pedley, M. The morphology and function of thrombolitic calcite precipitating biofilms: A universal model derived from freshwater mesocosm experiments. Sedimentology 2014, 61, 22–40. [Google Scholar] [CrossRef]
- Riding, R. Microbial carbonates: The geological record of calcified bacterial-algal mats and biofilms. Sedimentology 2000, 47, 179–214. [Google Scholar] [CrossRef]
- Okumura, T.; Takashima, C.; Shiraishi, F.; Akmaluddin; Kano, A. Textural transition in an aragonite travertine formed under various flow conditions at Pancuran Pitu, Central Java, Indonesia. Sediment. Geol. 2012, 265, 195–209. [Google Scholar] [CrossRef]
- Jones, B.; Renaut, R.W. Noncrystallographic calcite dendrites from hot-spring deposits at Lake Bogoria, Kenya. J. Sediment. Res. 1995, 65, 154–169. [Google Scholar]
- Ritchie, A.W.; Watson, M.I.T.; Turnbull, R.; Lu, Z.Z.; Telfer, M.; Gano, J.E.; Self, K.; Greer, H.F.; Zhou, W.Z. Reversed crystal growth of rhombohedral calcite in the presence of chitosan and gum arabic. CrystEngComm 2013, 15, 10266–10271. [Google Scholar] [CrossRef]
- Xiao, J.; Zhu, Y.; Liu, Y.; Liu, H.; Zeng, Y.; Xu, F.; Wang, L. Vaterite selection by chitosan gel: An example of polymorph selection by morphology of biomacromolecules. Cryst. Growth Des. 2008, 8, 2887–2891. [Google Scholar] [CrossRef]
- Chen, A.; Luo, Z.; Akbulut, M. Ionic liquid mediated auto-templating assembly of CaCO3–chitosan hybrid nanoboxes and nanoframes. Chem. Commun. 2011, 47, 2312–2314. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Lin, H.-P.; Mou, C.-Y. Biomimetic formation of porous single-crystalline CaCO3 via nanocrystal aggregation. Adv. Mater. 2003, 15, 621–623. [Google Scholar] [CrossRef]
- Nindiyasari, F.; Ziegler, A.; Griesshaber, E.; Fernàndez-Díaz, L.; Huber, J.; Walther, P.; Schmahl, W.W. Effect of hydrogel matrices on calcite crystal growth morphology, aggregate formation, and co-orientation in biomimetic experiments and biomineralization environments. Cryst. Growth Des. 2015, 15, 2667–2685. [Google Scholar] [CrossRef]
- Li, H.; Xin, H.L.; Muller, D.A.; Estroff, L.A. Visualizing the 3D internal structure of calcite single crystals grown in agarose hydrogels. Science 2009, 326, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Nindiyasari, F.; Fernàndez-Díaz, L.; Griesshaber, E.; Astilleros, J.M.; Sànchez-Pastor, N.; Schmahl, W.W. Influence of gelatin hydrogel porosity on the crystallization of CaCO3. Cryst. Growth Des. 2014, 14, 1531–1542. [Google Scholar] [CrossRef]
- Tseng, Y.-H.; Lin, H.-Y.; Liu, M.-H.; Chen, Y.-F.; Mou, C.-Y. Biomimetic synthesis of nacrelike faceted mesocrystals of ZnO-gelatin composite. J. Phys. Chem. C 2009, 113, 18053–18061. [Google Scholar] [CrossRef]
- Greer, H.F.; Zhou, W.Z.; Liu, M.-H.; Tseng, Y.-H.; Mou, C.-Y. The origin of ZnO twin crystals in bio-inspired synthesis. CrystEngComm 2012, 14, 1247–1255. [Google Scholar] [CrossRef]
- Bauermann, L.P.; del Campo, A.; Bill, J.; Aldinger, F. Heterogeneous nucleation of ZnO using gelatin as the organic matrix. Chem. Mater. 2006, 18, 2016–2020. [Google Scholar] [CrossRef]
- Liu, M.-H.; Tseng, Y.-H.; Greer, H.F.; Zhou, W.Z.; Mou, C.-Y. Dipole field guided orientated attachment of nanocrystals to twin-brush ZnO mesocrystals. Chem. Eur. J. 2012, 18, 16104–16113. [Google Scholar] [CrossRef] [PubMed]
- Busch, S.; Dolhaine, H.; DuChesne, A.; Heinz, S.; Hochrein, O.; Laeri, F.; Podebrad, O.; Vietze, U.; Weiland, T.; Kniep, R. Biomimetic morphogenesis of fluorapatite-gelatin composites: Fractal growth, the question of intrinsic electric fields, core/shell assemblies, hollow spheres and reorganization of denatured collagen. Eur. J. Inorg. Chem. 1999, 1999, 1643–1653. [Google Scholar] [CrossRef]
- Simon, P.; Rosseeva, E.; Buder, J.; Carrillo-Cabrere, W.; Kniep, R. Embryonic states of fluorapatite–gelatine nanocomposites and their intrinsic electric-field-driven morphogenesis: The missing link on the way from atomistic simulations to pattern formation on the mesoscale. Adv. Funct. Mater. 2009, 19, 3596–3603. [Google Scholar] [CrossRef]
- Hernández-Hernández, A.; Rodríguez-Navarro, A.B.; Gómez-Morales, J.; Jiménez-Lopez, C.; Nys, Y.; García-Ruiz, J.M. Influence of model globular proteins with different isoelectric points on the precipitation of calcium carbonate. Cryst. Growth Des. 2008, 8, 1495–1502. [Google Scholar] [CrossRef]
- Wang, T.; Cölfen, H.; Antonietti, M. Nonclassical crystallization: mesocrystals and morphology change of CaCO3 crystals in the presence of a polyelectrolyte additive. J. Am. Chem. Soc. 2005, 127, 3246–3247. [Google Scholar] [CrossRef] [PubMed]
- Nan, Z.; Chen, X.; Yang, Q.; Wang, X.; Shi, Z.; Hou, W. Structure transition from aragonite to vaterite and calcite by the assistance of SDBS. J. Colloid Interface Sci. 2008, 325, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Seto, J.; Ma, Y.; Davis, S.A.; Meldrum, F.; Gourrier, A.; Kim, Y.-Y.; Schilde, U.; Sztucki, M.; Burghammer, M.; Maltsev, S.; Jäger, C.; Cölfen, H. Structure-property relationships of a biological mesocrystal in the adult sea urchin spine. Proc. Natl. Acad. Sci. USA 2012, 109, 3699–3704. [Google Scholar] [CrossRef] [PubMed]
- Schmahl, W.W.; Griesshaber, E.; Kelm, K.; Goetz, A.; Jordan, G.; Ball, A.; Xu, D.; Merkel, C.; Brand, U. Hierarchical structure of marine shell biomaterials: Biomechanical functionalization of calcite by brachiopods. Z. Kristollogr. 2012, 227, 793–804. [Google Scholar] [CrossRef]
- Qian, C.; Wang, R.; Cheng, L.; Wang, J. Theory of microbial carbonate precipitation and its application in restoration of cement-based materials defects. Chin. J. Chem. 2010, 28, 847–857. [Google Scholar] [CrossRef]
- Lian, B.; Hu, Q.; Chen, J.; Ji, J.; Teng, H.H. Carbonate biomineralization induced by soil bacterium Bacillus megaterium. Geochim. Cosmochim. Acta 2006, 70, 5522–5535. [Google Scholar] [CrossRef]
- Greer, H.F. Non-classical crystal growth of inorganic and organic materials. Mater. Sci. Technol. 2014, 30, 611–626. [Google Scholar] [CrossRef]
- Niederberger, M.; Cölfen, H. Oriented attachment and mesocrystals: Non-classical crystallization mechanisms based on nanoparticle assembly. Phys. Chem. Chem. Phys. 2006, 8, 3271–3287. [Google Scholar] [CrossRef] [PubMed]
- Volmer, M. Kinetik der Phasenbildung; Steinkopf: Leipzig, Germany, 1939. [Google Scholar]
- Bravais, A. Études Cristallographiques; Gauthier-Villars: Paris, France, 1866. [Google Scholar]
- Friedel, M.G. Études sur la loi de Bravais. Bull. Soc. Fr. Miner. Cristallogr. 1907, 30, 326–455. [Google Scholar]
- Donnay, J.D.H.; Harker, D. A new law of crystal morphology extending the law of Bravais. Am. Miner. 1937, 22, 446–467. [Google Scholar]
- Hartman, P.; Perdok, W.G. On the relations between structure and morphology of crystals. II. Acta Crystallogr. 1955, 8, 521–524. [Google Scholar] [CrossRef]
- Ostwald, W. Lehrbuch der Allgemeinen Chemie; Wilhelm Engelmann: Leipzig, Germany, 1896; Volume 2, Part 1. [Google Scholar]
- Boistelle, R.; Astier, J.P. Crystallization mechanisms in solution. J. Cryst. Growth 1988, 90, 14–30. [Google Scholar] [CrossRef]
- Ueda, S.; Koizuma, M. Crystallization of analcime solid solutions from aqueous solutions. Am. Miner. 1979, 64, 172–179. [Google Scholar]
- Chen, X.Y.; Qiao, M.H.; Xie, S.H.; Fan, K.N.; Zhou, W.Z.; He, H.Y. Self-construction of core−shell and hollow zeolite analcime icositetrahedra: A reversed crystal growth process via oriented aggregation of nanocrystallites and recrystallization from surface to core. J. Am. Chem. Soc. 2007, 129, 13305–13312. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, J.H.E.; Checa, A.G. The dynamics of nacre self-assembly. J. R. Soc. Interface 2007, 4, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Weiner, S.; Lowenstam, H. Organization of extracellularly mineralized tissues: A comparative study of biological crystal growth. CRC Crit. Rev. Biochem. 1986, 20, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Gilis, M.; Grauby, O.; Willenz, P.; Dubois, P.; Heresanu, V.; Baronnet, A. Biomineralization in living hypercalcified demosponges: Toward a shared mechanism? J. Struct. Biol. 2013, 183, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Finnemore, A.; Cunha, P.; Shean, T.; Vignolini, S.; Guldin, S.; Oyen, M.; Steiner, U. Biomimetic layer-by-layer assembly of artificial nacre. Nat. Comm. 2012, 3, 966. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.; Renaut, R.W. Cyclic development of large, complex, calcite dendrite crystals in the Clinton travertine, interior British Columbia, Canada. Sediment. Geol. 2008, 203, 17–35. [Google Scholar] [CrossRef]
- García-del-Cura, M.Á.; Benavente, D.; Martínez-Martínez, J.; Cueto, N. Sedimentary structures and physical properties of travertine and carbonate tufa building stone. Constr. Build. Mater. 2012, 28, 456–467. [Google Scholar] [CrossRef]
- Wang, L.; Tang, R.; Bonstein, T.; Orme, C.A.; Bush, P.J.; Nancollas, G.H. A new model for nanoscale enamel dissolution. J. Phys. Chem. B 2005, 109, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Estroff, L.A. Porous calcite single crystals grown from a hydrogel medium. CrystEngComm 2007, 9, 1153–1155. [Google Scholar] [CrossRef]
- Asenath-Smith, E.; Li, H.; Keene, E.C.; She, Z.W.; Estroff, L.A. Crystal growth of calcium carbonate in hydrogels as a model of biomineralisation. Adv. Funct. Mater. 2012, 22, 2891–2914. [Google Scholar] [CrossRef]
- Grassmann, O.; Müller, G.; Löbmann, P. Organic-Inorganic hybrid structure of calcite crystallline assemblies grown in a gelatin hydrogel matrix: Relevance to biomineralization. Chem. Mater. 2002, 14, 4530–4535. [Google Scholar] [CrossRef]
- Sheng, G.-P.; Yu, H.-Q.; Li, X.-Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fang, H.H.P. Characterization of electrostatic binding sites of extracellular polymers by linear programming analysis of titration data. Biotechnol. Bioeng. 2002, 80, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Guibaud, G.; van Hullebusch, E.; Bordas, F. Lead and cadmium biosorption by extracellular polymeric substances (EPS) extracted from activated sludges: pH-sorption edge tests and mathematical equilibrium modelling. Chemosphere 2006, 64, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Chen, Y.-P.; Guo, J.-S.; Shen, Y.; Yang, J.-X.; Fang, F.; Li, C.; Gao, X.; Wang, G.-X. Adsorption behavior of tightly bound extracellular polymeric substances on model organic surfaces under different pH and cations with surface plasmon resonance. Water Res. 2014, 57, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Sethmann, I.; Helbig, U.; Wörheide, G. Octocoral sclerite ultrastructures and experimental approach to underlying biomineralisation principles. CrystEngComm 2007, 9, 1262–1268. [Google Scholar] [CrossRef]
- Jones, B.; Renaut, R.W. Skeletal crystals of calcite and trona form hot-spring deposits in Keyna and New Zealand. J. Sediment. Res. 1996, 66, 265–274. [Google Scholar]
- Zhou, W.Z. Reversed crystal growth: Implications for crystal engineering. Adv. Mater. 2010, 22, 3086–3092. [Google Scholar] [CrossRef] [PubMed]
- Song, R.-Q.; Cölfen, H. Mesocrystals-ordered nanoparticle superstructures. Adv. Mater. 2010, 22, 1301–1330. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-Y.; Schenk, A.S.; Ihil, J.; Hetherington, N.B.J.; Tang, C.C.; Schmahl, W.W.; Griesshaber, E.; Hyett, G.; Meldrum, F.C. A critical analysis of calcium carbonate mesocrystals. Nat. Commun. 2014, 5, 4341. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Qi, L.; Ma, J. Well-defined star-shaped calcite crystals formed in agarose gels. Chem. Commun. 2003, 1180–1181. [Google Scholar] [CrossRef]
- Dupraz, C.; Reid, R.P.; Braissant, O.; Decho, A.W.; Norman, R.S.; Visscher, P.T. Processes of carbonate precipitation in modern microbial mats. Earth-Sci. Rev. 2009, 96, 141–162. [Google Scholar] [CrossRef]
- Greer, H.F.; Yu, F.J.; Zhou, W.Z. Early stages of non-classic crystal growth. Sci. China Chem. 2011, 54, 1867–1876. [Google Scholar] [CrossRef]
- Curie, P. Sur la formation des cristaux et sur les constants capillaires de leurdifférentes faces. Bull. Soc. Fr. Miner. Cristallogr. 1885, 8, 145–150. [Google Scholar]
- Wulff, G. Velocity of growth and dissolution of crystal faces. Z. Kristallogr. 1901, 34, 449–530. [Google Scholar]
- Yang, X.F.; Fu, J.X.; Jin, C.J.; Chen, J.A.; Liang, C.L.; Wu, M.M.; Zhou, W.Z. Formation mechanism of CaTiO3 hollow crystals with different microstructures. J. Am. Chem. Soc. 2010, 132, 14279–14287. [Google Scholar] [CrossRef] [PubMed]
- Greer, H.; Wheatley, P.S.; Ashbrook, S.E.; Morris, R.E.; Zhou, W.Z. Early stage reversed crystal growth of zeolite A and its phase transformation to sodalite. J. Am. Chem. Soc. 2009, 131, 17986–17992. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.F.; Li, D.; Zhang, X.Y.; Kong, C.H.; Yue, W.B.; Zhou, W.Z.; Wang, H.T. Cubes of zeolite A with an amorphous core. Angew. Chem. Int. Ed. 2008, 47, 8397–8399. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.L.; Andrés, J.; Mastelaro, V.R.; Varela, J.A.; Longo, E. On the reversed crystal growth of BaZrO3 decaoctahedron: Shape evolution and mechanism. CrystEngComm 2011, 13, 5818–5824. [Google Scholar] [CrossRef] [Green Version]
- Karn, A.; Kumar, M.; Singh, V.N.; Mehta, B.R.; Aravindan, S.; Singh, J.P. Growth of indium oxide and zinc-doped indium oxide nanostructures. Chem. Vap. Depos. 2012, 18, 295–301. [Google Scholar] [CrossRef]
- Sander, J.R.G.; Bučar, D.-K.; Baltrusaitis, J.; MacGillivray, L.R. Organic nanocrystals of the resorcinarene hexamer via sonochemistry: Evidence of reversed crystal growth involving hollow morphologies. J. Am. Chem. Soc. 2012, 134, 6900–6903. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.J.; Zhou, W.Z. Alloying and dealloying of CuPt bimetallic nanocrystals. Prog. Nat. Sci. Mater. 2013, 23, 331–337. [Google Scholar] [CrossRef]
- Zheng, C.M.; Greer, H.F.; Chiang, C.-Y.; Zhou, W.Z. Microstructural study of the formation mechanism of metal–organic framework MOF-5. CrystEngComm 2014, 16, 1064–1070. [Google Scholar] [CrossRef]
- Greer, H.F.; Liu, Y.H.; Greenaway, A.; Wright, P.A.; Zhou, W.Z. Synthesis and formation mechanism of textured MOF-5. Cryst. Growth Des. 2016, 16, 2104–2111. [Google Scholar] [CrossRef]
- Li, H.; Xin, H.L.; Kunitake, M.E.; Keene, E.C.; Muller, D.A.; Estroff, L.A. Calcite prisms from mollusk shells (Atrina Rigida): Swiss-cheese-like organic–inorganic single-crystal composites. Adv. Funct. Mater. 2011, 21, 2028–2034. [Google Scholar] [CrossRef]
- Thomas, J.M.; Midgley, P.A.; Ducati, C.; Leary, R.K. Nanoscale electron tomography and atomic scale high-resolution electron microscopy of nanoparticles and nanoclusters: A short survey. Prog. Nat. Sci. Mater. 2013, 23, 222–234. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greer, H.F.; Zhou, W.; Guo, L. Reversed Crystal Growth of Calcite in Naturally Occurring Travertine Crust. Crystals 2017, 7, 36. https://doi.org/10.3390/cryst7020036
Greer HF, Zhou W, Guo L. Reversed Crystal Growth of Calcite in Naturally Occurring Travertine Crust. Crystals. 2017; 7(2):36. https://doi.org/10.3390/cryst7020036
Chicago/Turabian StyleGreer, Heather F., Wuzong Zhou, and Li Guo. 2017. "Reversed Crystal Growth of Calcite in Naturally Occurring Travertine Crust" Crystals 7, no. 2: 36. https://doi.org/10.3390/cryst7020036





