Agglomeration Control during Ultrasonic Crystallization of an Active Pharmaceutical Ingredient
Abstract
:1. Introduction
2. Materials and Methods
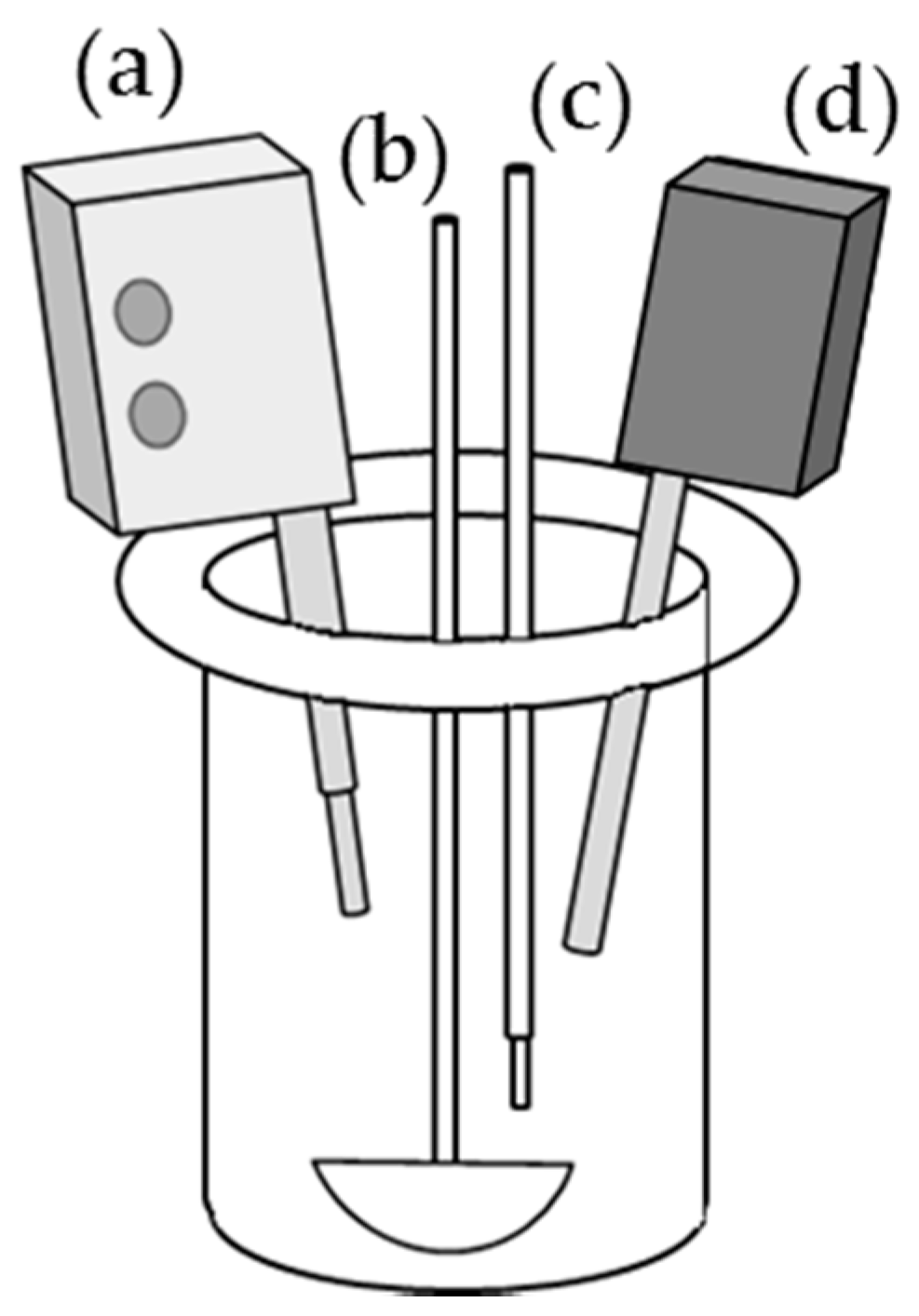
2.1. Experimental Setup
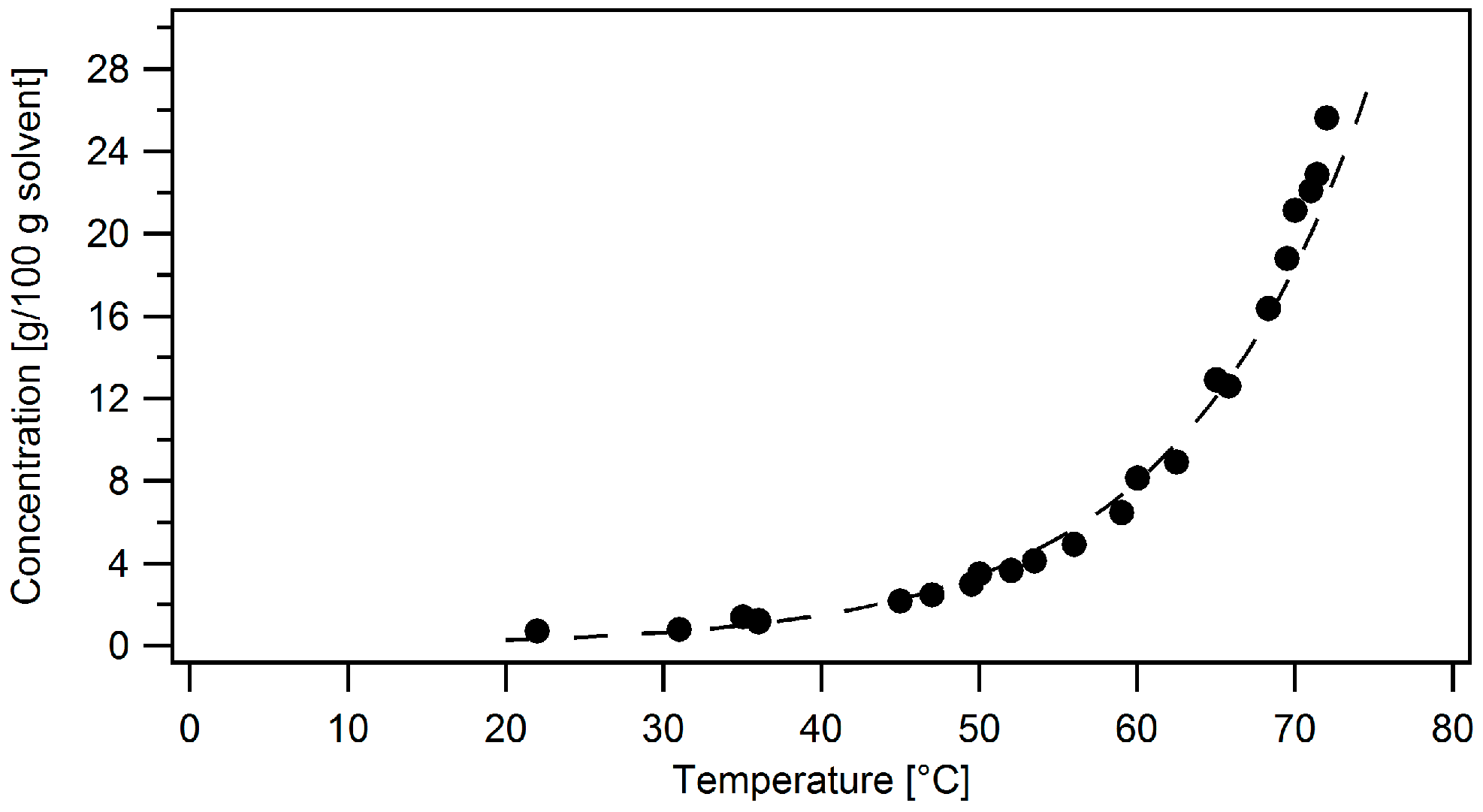
2.2. Experimental Procedure
3. Results and Discussion
3.1. Evaluation of the Conventional Crystallization Process
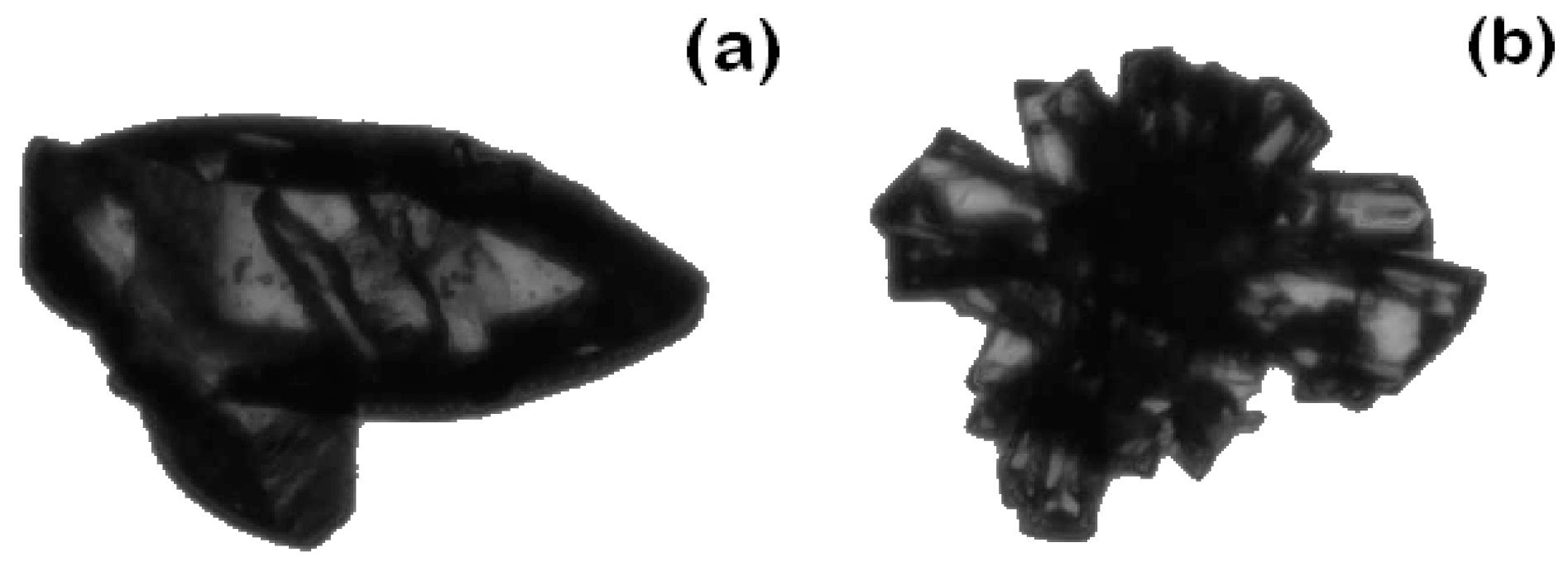
3.1.1. Effect of Seed Mass
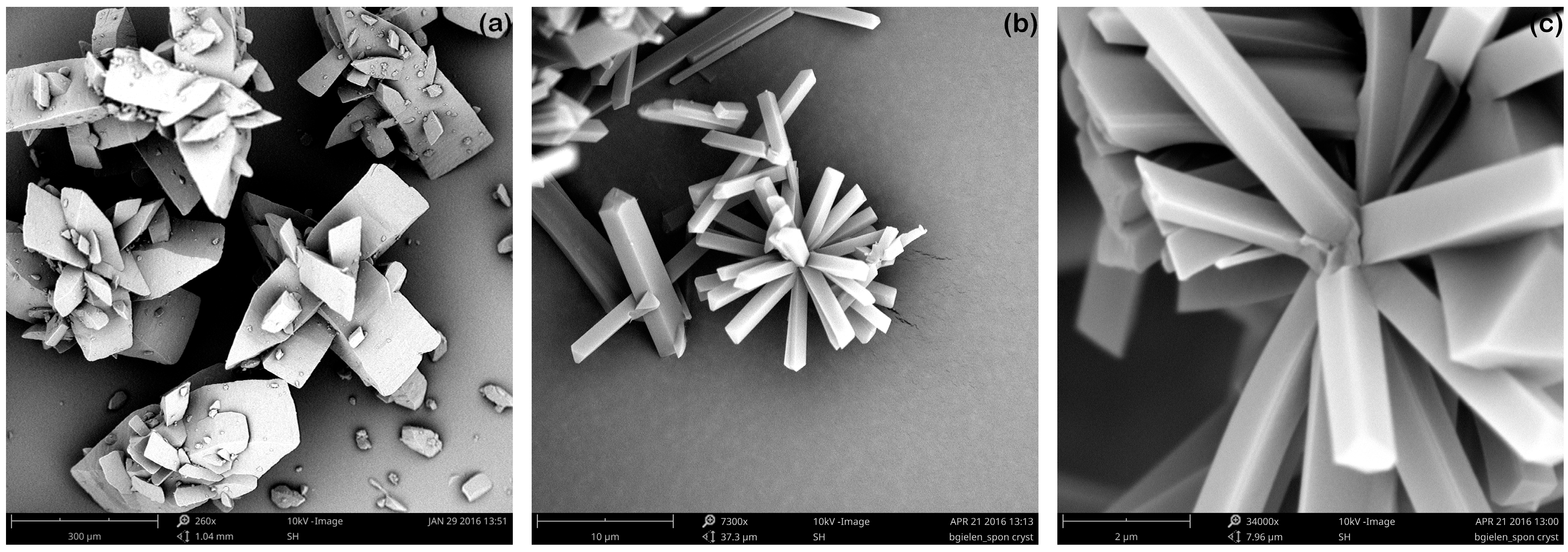
3.1.2. Effect of Stirring Rate
3.1.3. Prompt Generation of Nuclei
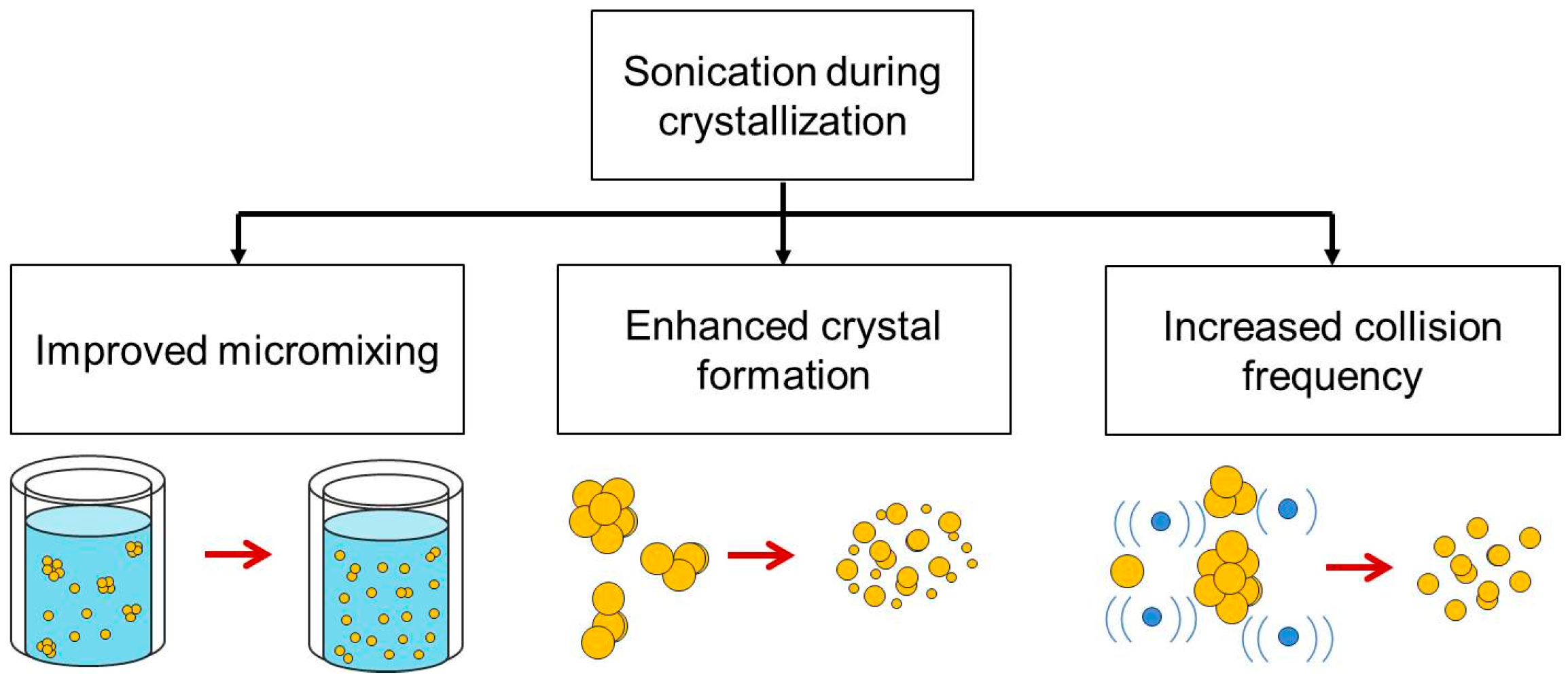
3.2. Implementation of Ultrasound in the Crystallization Process
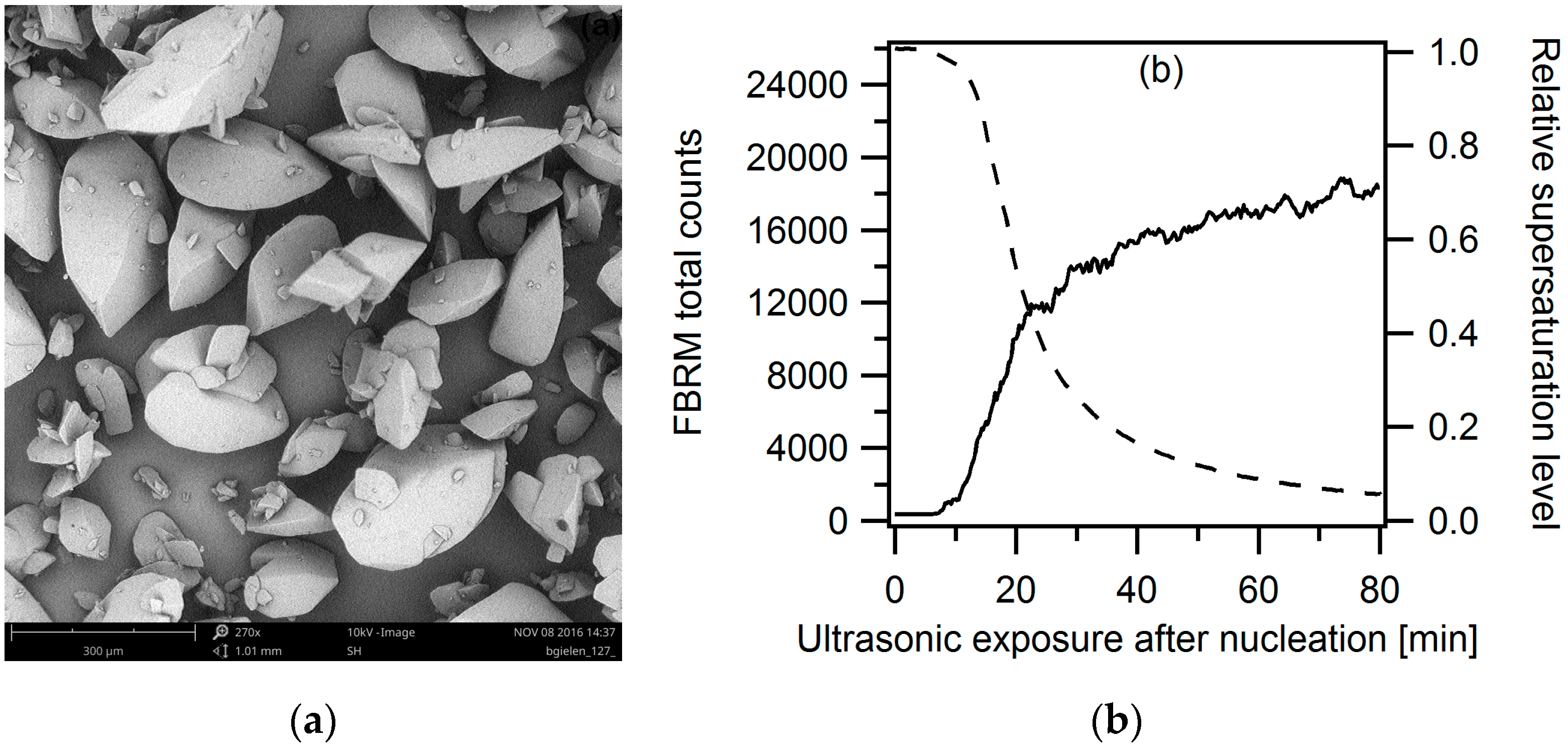
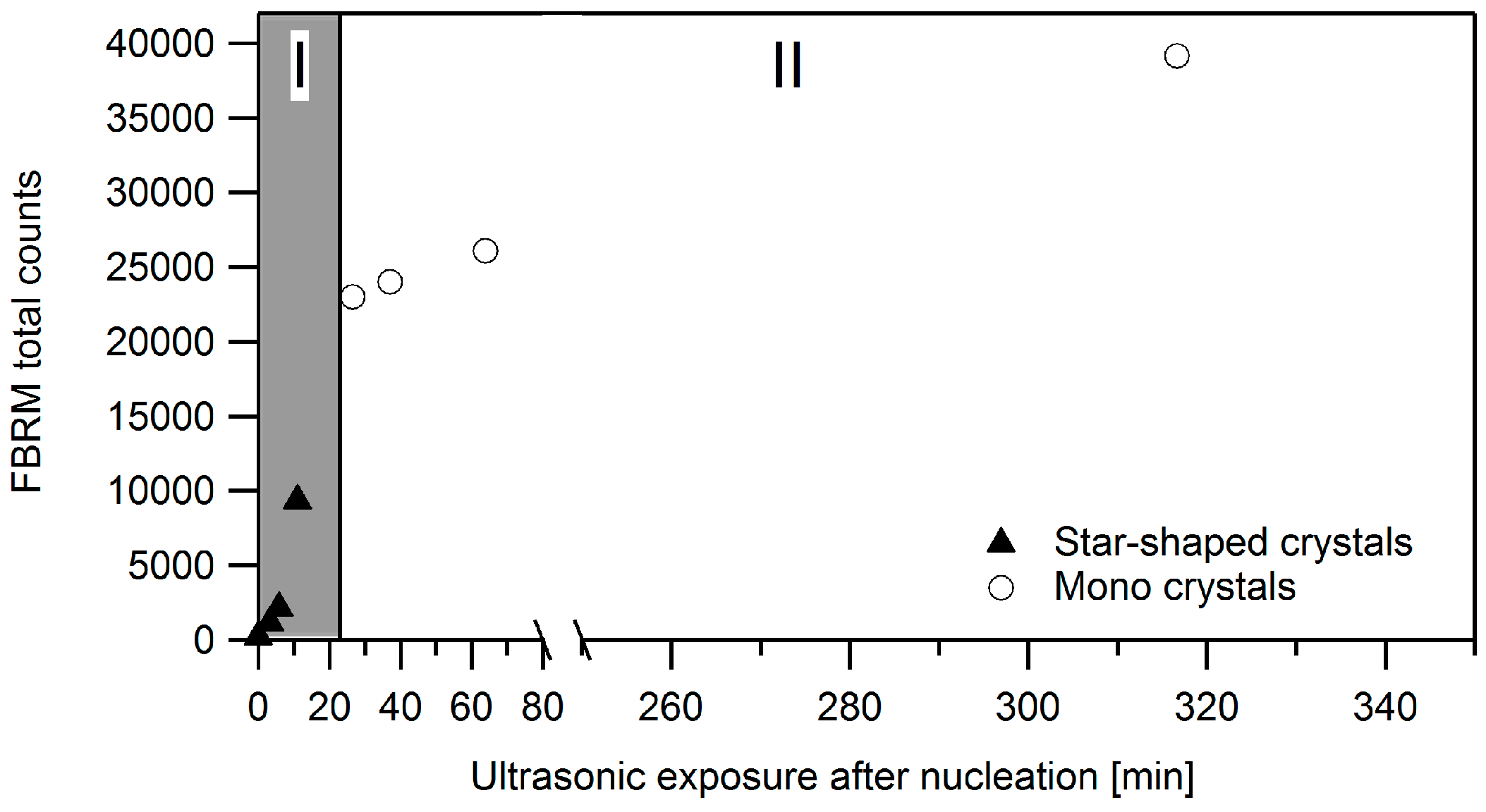
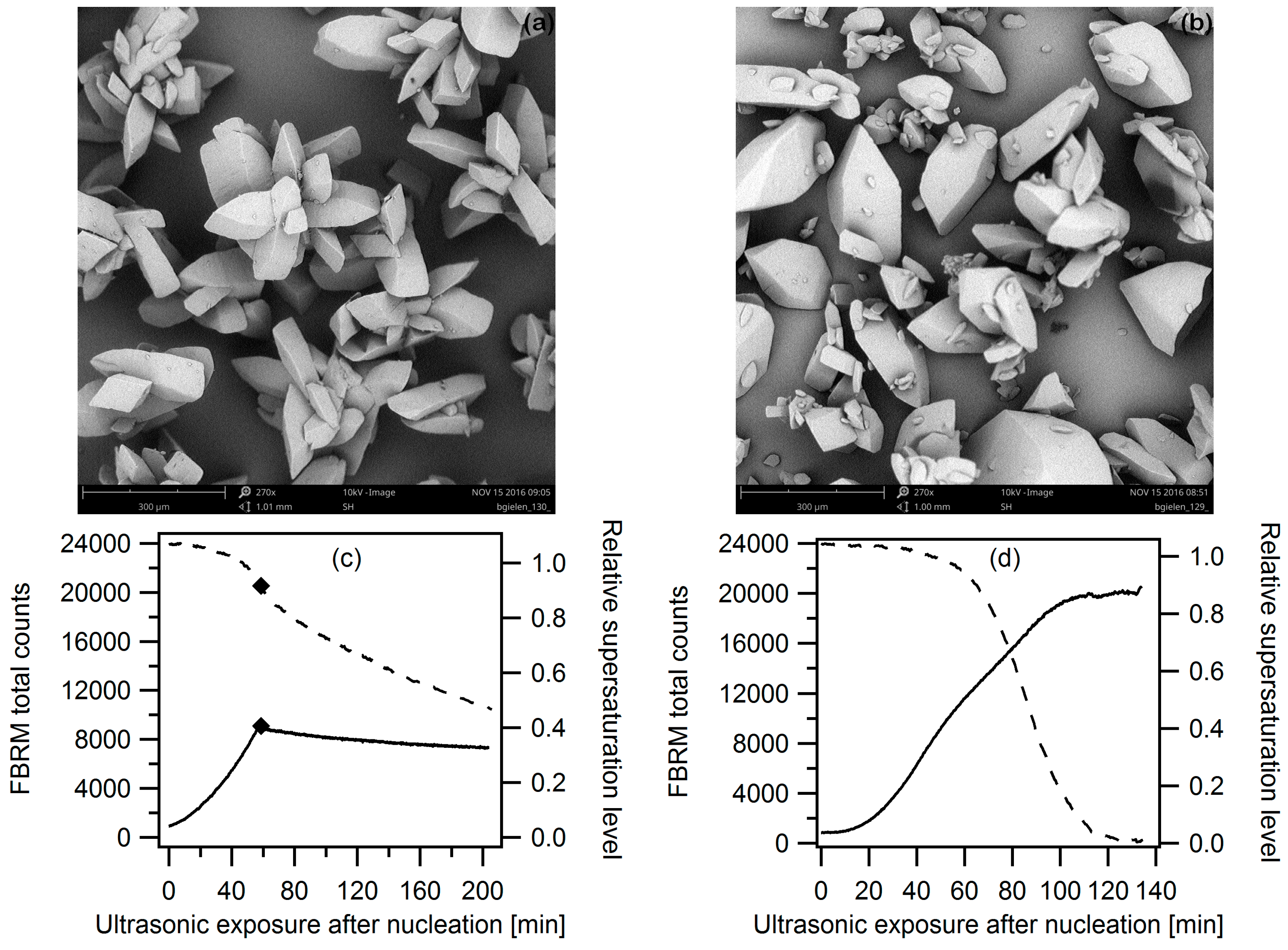
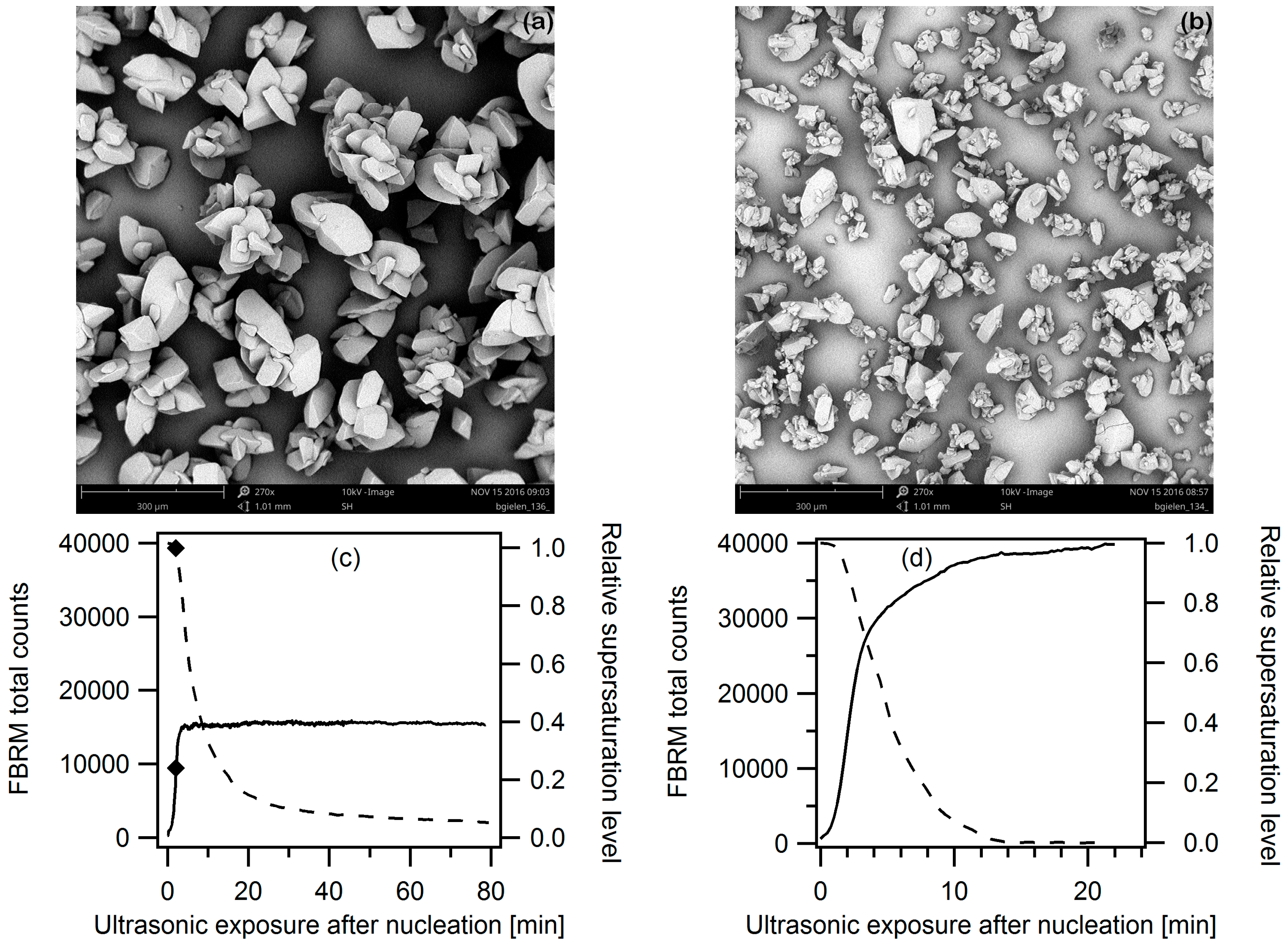
3.2.1. Ultrasound as Post-Treatment
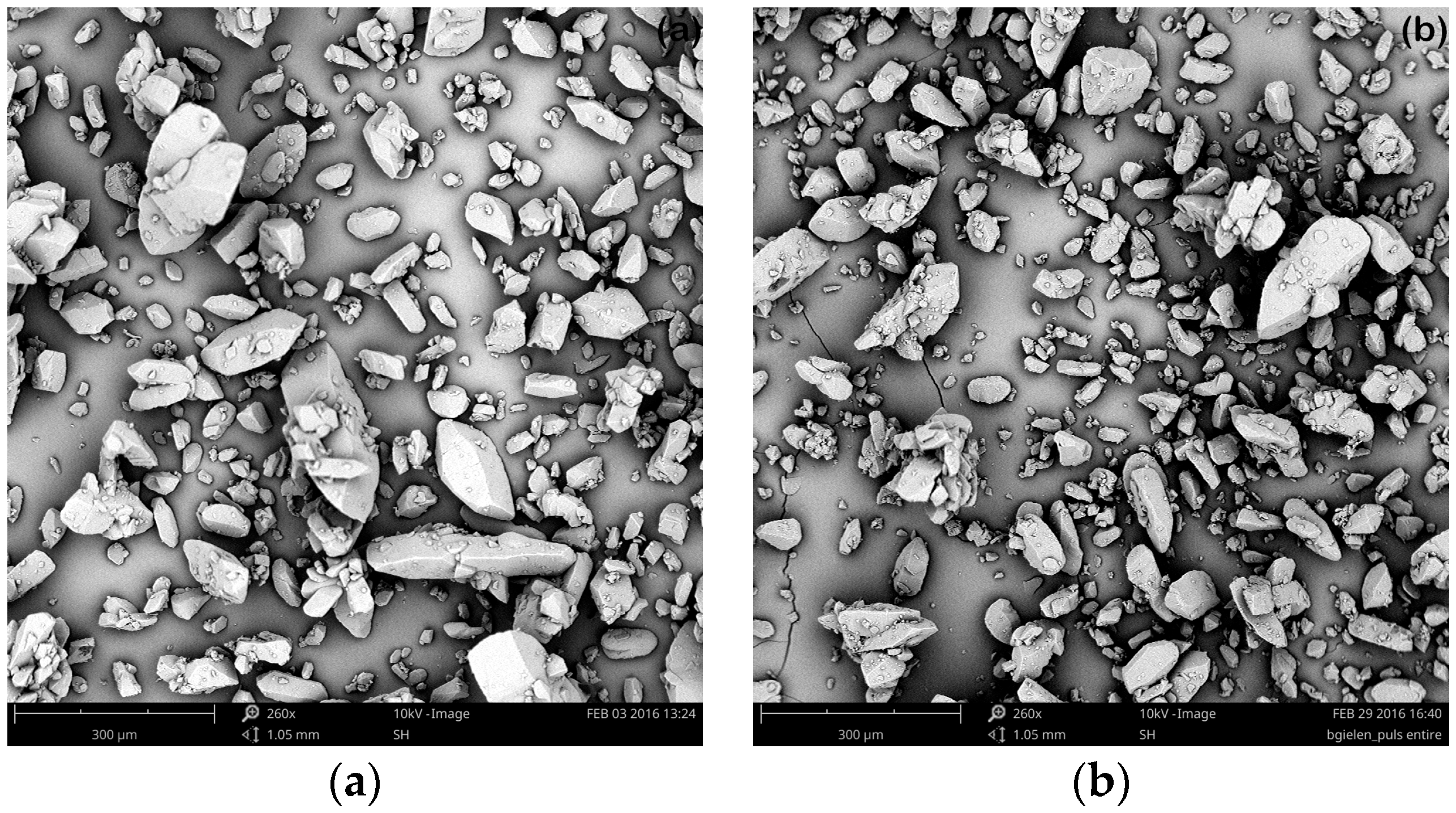
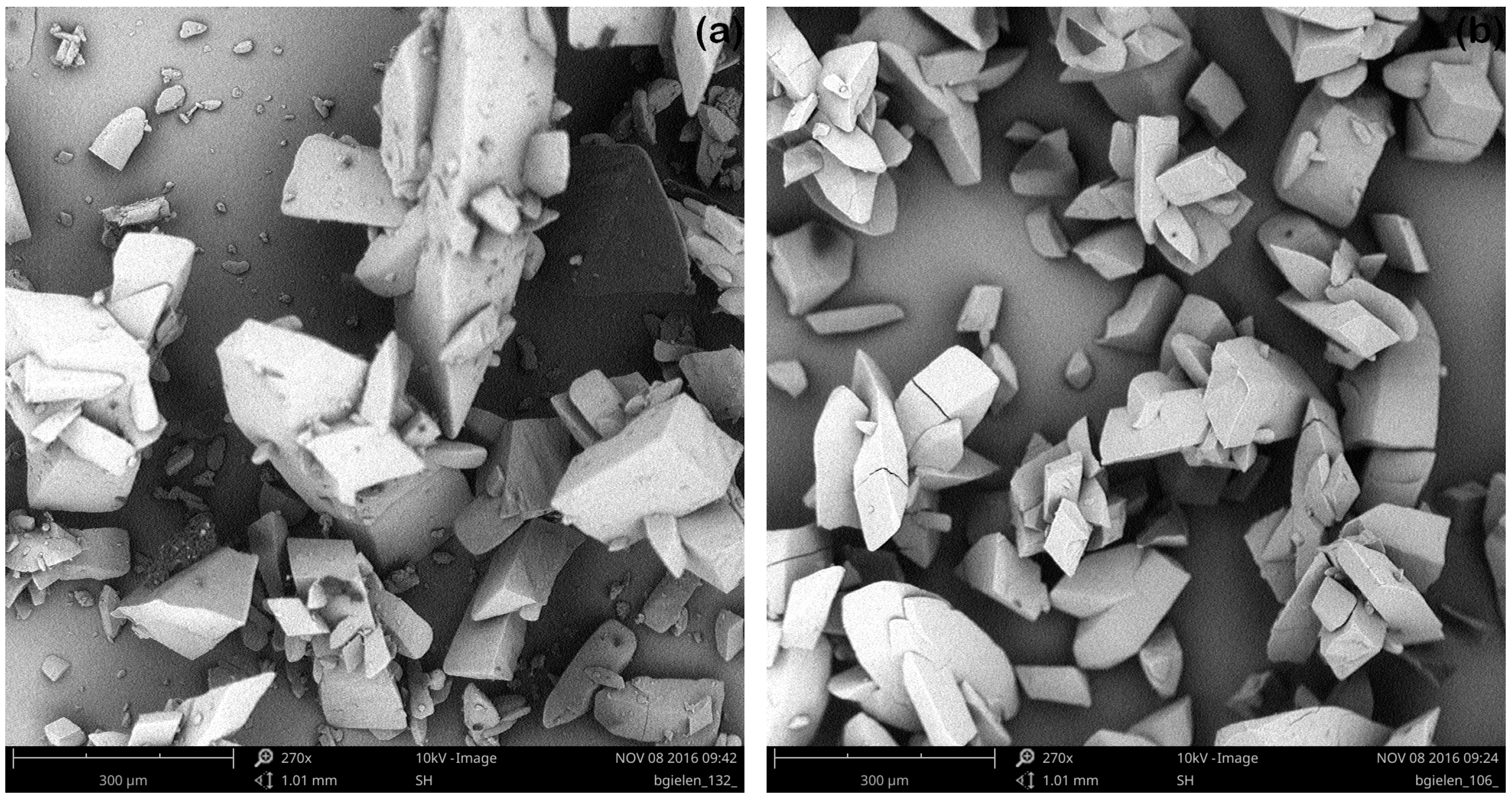
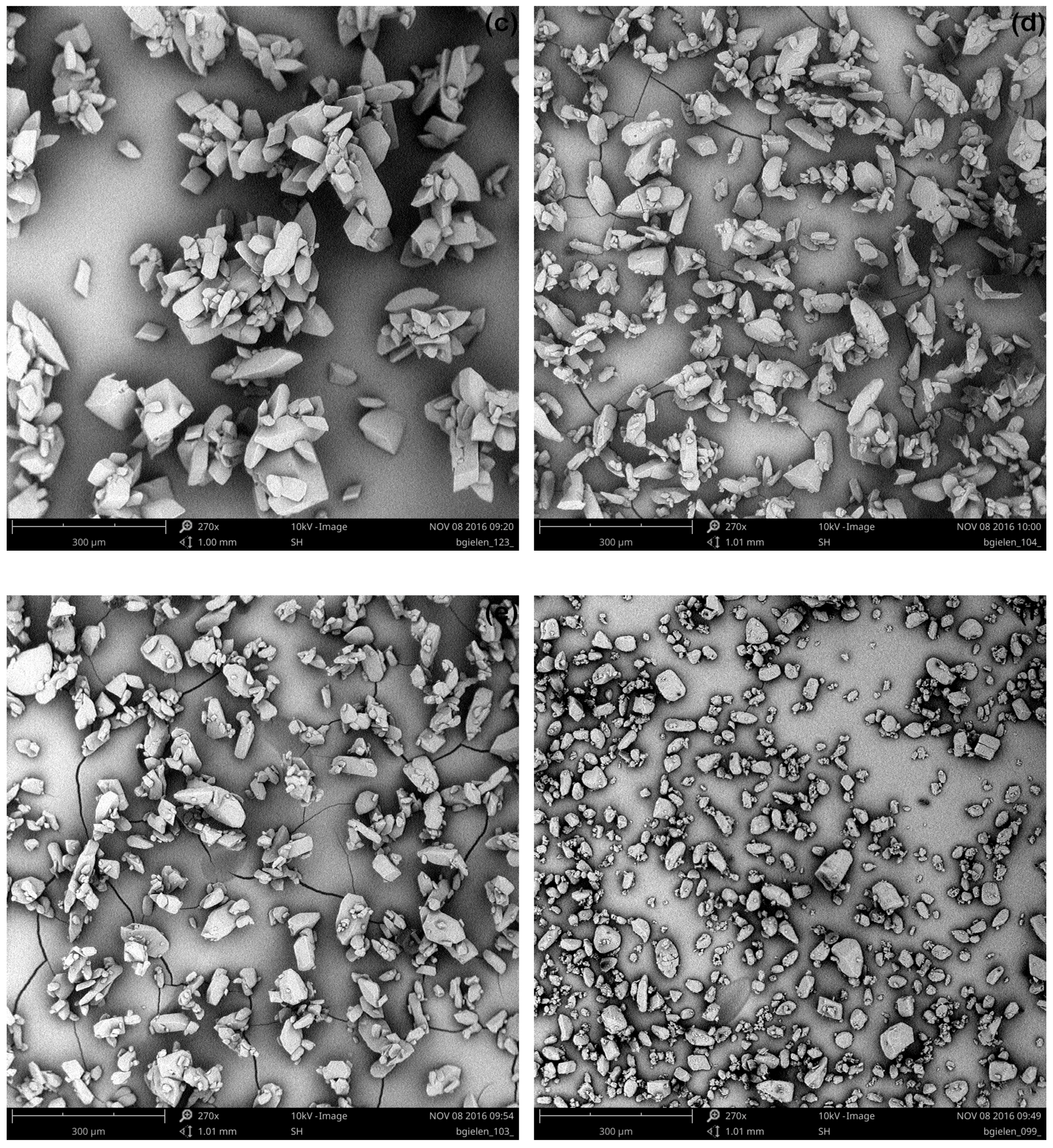
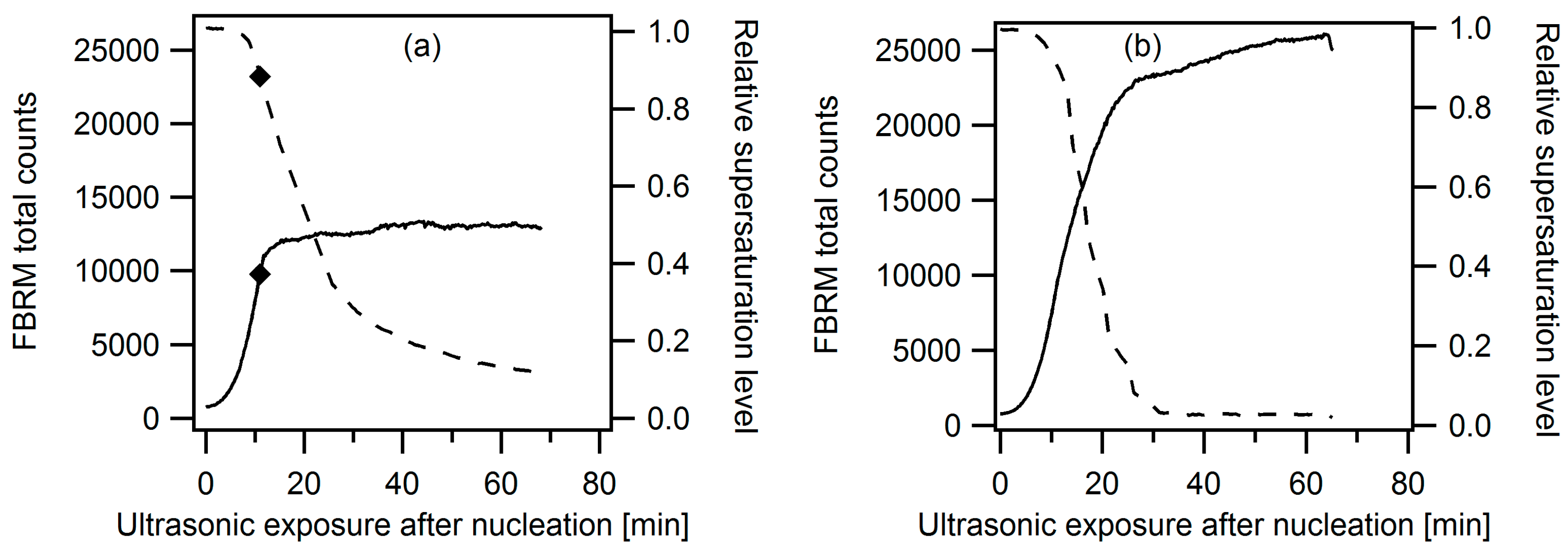
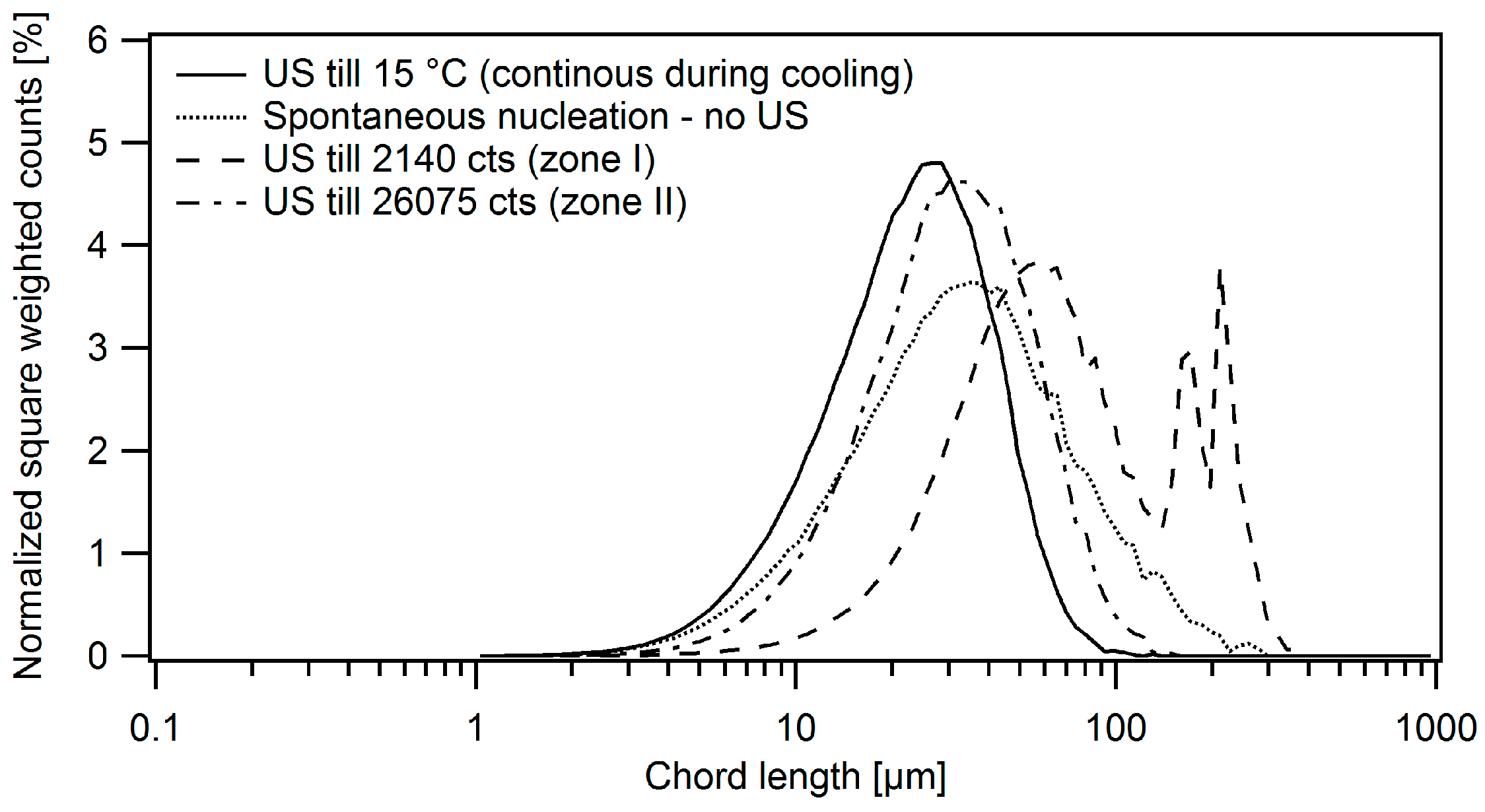
3.2.2. Ultrasonic Treatment during the Entire Cooling Profile
3.2.3. Targeted Ultrasonic Treatment at Seeding Temperature
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Giulietti, M.; Seckler, M.M.; Derenzo, S.; Ré, M.I.; Cekinski, E. Industrial Crystallization and Precipitation from Solutions: State of the Technique. Braz. J. Chem. Eng. 2001, 18, 423–440. [Google Scholar] [CrossRef]
- Zeng, G.; Li, H.; Luo, S.; Wang, X.; Chen, J. Effects of ultrasonic radiation on induction period and nucleation kinetics of sodium sulfate. Korean J. Chem. Eng. 2014, 31, 807–811. [Google Scholar] [CrossRef]
- Bhoi, S.; Sarkar, D. Modelling and experimental validation of ultrasound assisted unseeded batch cooling crystallization of L-asparagine monohydrate. CrystEngComm 2016, 18, 4863–4874. [Google Scholar] [CrossRef]
- Dalvi, S.V.; Dave, R.N. Controlling particle size of a poorly water-soluble drug using ultrasound and stabilizers in antisolvent precipitation. Ind. Eng. Chem. Res. 2009, 48, 7581–7593. [Google Scholar] [CrossRef]
- Castillo-Peinado, L.D.L.S.; Dolores, M.; de Castro, L. The role of ultrasound in pharmaceutical production: Sonocrystallization. J. Pharm. Pharmacol. 2016, 68, 1249–1267. [Google Scholar] [CrossRef] [PubMed]
- Dhumal, R.S.; Biradar, S.V.; Paradkar, A.R.; York, P. Particle engineering using sonocrystallization: Salbutamol sulphate for pulmonary delivery. Int. J. Pharm. 2009, 368, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Ålander, E. Agglomeration of Paracetamol during Crystallization in Pure and Mixed Solvents. Ind. Eng. Chem. Res. 2004, 43, 629–637. [Google Scholar] [CrossRef]
- Brunsteiner, M.; Jones, A.G.; Pratola, F.; Price, S.L.; Simons, S.J.R. Toward a molecular understanding of crystal agglomeration. Cryst. Growth Des. 2005, 5, 3–16. [Google Scholar] [CrossRef]
- Nichols, G.; Byard, S.; Bloxham, M.J.; Botterill, J.; Dawson, N.J.; Dennis, A.; Diart, V.; North, N.C.; Sherwood, J.D. A review of the terms agglomerate and aggregate with a recommendation for nomenclature used in powder and particle characterization. J. Pharm. Sci. 2002, 91, 2103–2109. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, W. Crystallization: Basic Concepts and Industrial Applications; Beckmann, W., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013. [Google Scholar]
- Yu, Z.Q.; Tan, R.B.H.; Chow, P.S. Effects of operating conditions on agglomeration and habit of paracetamol crystals in anti-solvent crystallization. J. Cryst. Growth 2005, 279, 477–488. [Google Scholar] [CrossRef]
- Chang, S.M.; Kim, J.M.; Kim, I.H.; Shin, D.M.; Kim, W.S. Agglomeration control of L-ornithine aspartate crystals by operating variables in drowning-out crystallization. Ind. Eng. Chem. Res. 2006, 45, 1631–1635. [Google Scholar] [CrossRef]
- Kim, J.M.; Chang, S.M.; Kim, K.S.; Chung, M.K.; Kim, W.S. Acoustic influence on aggregation and agglomeration of crystals in reaction crystallization of cerium carbonate. Colloids Surf. A Physicochem. Eng. Asp. 2011, 375, 193–199. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, S.; Lü, X.; Xie, C. Control of the agglomeration of crystals in the reactive crystallization of 5-(difluoromethoxy)-2-mercapto-1H-benzimidazole. Front. Chem. Sci. Eng. 2012, 6, 423–431. [Google Scholar] [CrossRef]
- Schnebelen, M.; Mozet, K.; Jakob, A.; Sy, D.; Plasari, E.; Muhr, H. Agglomeration Mechanisms and Kinetics during the Carbonation of a Suspension of Lime in a Pilot Batch Reactor. Cryst. Struct. Theory Appl. 2015, 4, 35–46. [Google Scholar] [CrossRef]
- Mumtaz, H.S.; Hounslow, M.J.; Seaton, N.A.; Paterson, W.R. Orthokinetic Aggregation During Precipitation. Chem. Eng. Res. Des. 1997, 75, 152–159. [Google Scholar] [CrossRef]
- Hostomsky, J.; Jones, A.G. Calcium carbonate crystallization, agglomeration and form during continuous precipitation from solution. J. Phys. D Appl. Phys. 1991, 24, 165–170. [Google Scholar] [CrossRef]
- Wójcik, J.A.; Jones, A.G. Particle disruption of precipitated CaCO3 crystal agglomerates in turbulently agitated suspensions. Chem. Eng. Sci. 1998, 53, 1097–1101. [Google Scholar] [CrossRef]
- Mullin, J.W. Crystallization; Butterworth-Heinemann: Oxford, UK, 2001. [Google Scholar]
- David, R.; Espitalier, F.; Cameirão, A.; Rouleau, L. Developments in the understanding and modeling of the agglomeration of suspended crystals in crystallization from solutions. KONA Powder Part. J. 2003, 21, 40–53. [Google Scholar] [CrossRef]
- Li, D.; Kaner, R.B. Shape and aggregation control of nanoparticles: Not shaken, not stirred. J. Am. Chem. Soc. 2006, 128, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, H.; Guo, Z.; Liu, Y. The application of power ultrasound to reaction crystallization. Ultrason. Sonochem. 2006, 13, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Sivabalan, R.; Gore, G.M.; Nair, U.R.; Saikia, A.; Venugopalan, S.; Gandhe, B.R. Study on ultrasound assisted precipitation of CL-20 and its effect on morphology and sensitivity. J. Hazard. Mater. 2007, 139, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Bari, A.H.; Chawla, A.; Pandit, A.B. Sono-crystallization kinetics of K2SO4: Estimation of nucleation, growth, breakage and agglomeration kinetics. Ultrason. Sonochem. 2017, 35, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Jones, A.G.; Li, N.; Germana, S. High-speed observation of the effects of ultrasound on liquid mixing and agglomerated crystal breakage processes. Powder Technol. 2007, 171, 146–153. [Google Scholar] [CrossRef]
- Luque de Castro, M.D.; Priego-Capote, F. Ultrasound-assisted crystallization (sonocrystallization). Ultrason. Sonochem. 2007, 14, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Ruecroft, G.; Hipkiss, D.; Ly, T.; Maxted, N.; Cains, P.W. Sonocrystallization: The use of ultrasound for improved industrial crystallization. Org. Process Res. Dev. 2005, 9, 923–932. [Google Scholar] [CrossRef]
- Iyer, S.R.; Gogate, P.R. Ultrasound assisted crystallization of mefenamic acid: Effect of operating parameters and comparison with conventional approach. Ultrason. Sonochem. 2017, 34, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Hem, S.L. The effect of ultrasonic vibrations on crystallization processes. Ultrasonics 1967, 5, 202–207. [Google Scholar] [CrossRef]
- Wohlgemuth, K.; Kordylla, A.; Ruether, F.; Schembecker, G. Experimental study of the effect of bubbles on nucleation during batch cooling crystallization. Chem. Eng. Sci. 2009, 64, 4155–4163. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, M.; Li, H.; Wang, J.; Kougoulos, E. Effect of ultrasound on anti-solvent crystallization process. J. Cryst. Growth 2005, 273, 555–563. [Google Scholar] [CrossRef]
- Wu, T.Y.; Guo, N.; Teh, C.Y.; Hay, J.X.W. Challenges and Recent Developments of Sonochemical Processes. In Advances in Ultrasound Technology for Environmental Remediation; Springer Science & Business Media: New York, NY, USA, 2013; pp. 109–120. [Google Scholar]
- Sutkar, V.S.; Gogate, P.R. Design aspects of sonochemical reactors: Techniques for understanding cavitational activity distribution and effect of operating parameters. Chem. Eng. J. 2009, 155, 26–36. [Google Scholar] [CrossRef]
- Gogate, P.R.; Pandit, A.B. Sonochemical reactors: Scale up aspects. Ultrason. Sonochem. 2004, 11, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Hatkar, U.N.; Gogate, P.R. Ultrasound assisted cooling crystallization of sodium acetate. Ind. Eng. Chem. Res. 2012, 51, 12901–12909. [Google Scholar] [CrossRef]
- Narducci, O.; Jones, A.G. Seeding in Situ the Cooling Crystallization of Adipic Acid using Ultrasound. Cryst. Growth Des. 2012, 12, 1727–1735. [Google Scholar] [CrossRef]
- Abbas, A.; Srour, M.; Tang, P.; Chiou, H.; Chan, H.K.; Romagnoli, J.A. Sonocrystallisation of sodium chloride particles for inhalation. Chem. Eng. Sci. 2007, 62, 2445–2453. [Google Scholar] [CrossRef]
- Kim, S.; Wei, C.; Kiang, S. Crystallization process development of an active pharmaceutical ingredient and particle engineering via the use of ultrasonics and temperature cycling. Org. Process Res. Dev. 2003, 7, 997–1001. [Google Scholar] [CrossRef]
- Addai-Mensah, J.; Prestidge, C.A.; Ralston, J. Interparticle forces, interfacial structure development and agglomeration of gibbsite particles in synthetic Bayer liquors. Miner. Eng. 1999, 12, 655–669. [Google Scholar] [CrossRef]
- Sohnel, O.; Mullin, J.W.; Jones, A.G. Crystallization and agglomeration kinetics in the batch precipitation of strontium molybdate. Ind. Eng. Chem. Res. 1988, 27, 1721–1728. [Google Scholar] [CrossRef]
- Wójcik, J.A.; Jones, A.G. Experimental Investigation into Dynamics and Stability of Continuous MSMPR Agglomerative Precipitation of CaCO3 Crystals. Chem. Eng. Res. Des. 1997, 75, 113–118. [Google Scholar] [CrossRef]
- Majano, G.; Darwiche, A.; Mintova, S.; Valtchev, V. Seed-Induced Crystallization of Nanosized Na-ZSM-5 Crystals. Ind. Eng. Chem. Res. 2009, 48, 7084–7091. [Google Scholar] [CrossRef]
- Zumstein, R.C.; Rousseau, R.W. Agglomeration of copper sulfate pentahydrate crystals within well-mixed crystallizers. Chem. Eng. Sci. 1989, 44, 2149–2155. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Bao, Y.; Guo, Z.; Zhang, M. Rapid sonocrystallization in the salting-out process. J. Cryst. Growth 2003, 247, 192–198. [Google Scholar] [CrossRef]
- Heath, A.R.; Fawell, P.D.; Bahri, P.A.; Swift, J.D. Estimating average particle size by focused beam reflectance measurement (FBRM). Part. Part. Syst. Charact. 2002, 19, 84–95. [Google Scholar] [CrossRef]
- Synowiec, P.; Jones, A.G.; Shamlou, P.A. Crystal break-up in dilute turbulently agitated suspensions. Chem. Eng. Sci. 1993, 48, 3485–3495. [Google Scholar] [CrossRef]
- Bartos, C.; Kukovecz, Á.; Ambrus, R.; Farkas, G.; Radacsi, N.; Szabó-Révész, P. Comparison of static and dynamic sonication as process intensification for particle size reduction using a factorial design. Chem. Eng. Process. Process Intensif. 2015, 87, 26–34. [Google Scholar] [CrossRef]
- Zeiger, B.W.; Suslick, K.S. Sonofragmentation of molecular crystals. J. Am. Chem. Soc. 2011, 133, 14530–14533. [Google Scholar] [CrossRef]
- Sander, J.R.G.; Zeiger, B.W.; Suslick, K.S. Sonocrystallization and sonofragmentation. Ultrason. Sonochem. 2014, 21, 1908–1915. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Sharma, S.K.; Mudhoo, A. Handbook on Applications of Ultrasound: Sonochemistry for Sustainability; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2012. [Google Scholar]
- Wagterveld, R.M.; Boels, L.; Mayer, M.J.; Witkamp, G.J. Visualization of acoustic cavitation effects on suspended calcite crystals. Ultrason. Sonochem. 2011, 18, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Gielen, B.; Kusters, P.; Jordens, J.; Thomassen, L.C.J.; Van Gerven, T.; Braeken, L. Energy efficient crystallization of paracetamol using pulsed ultrasound. Chem. Eng. Process 2017. submitted. [Google Scholar] [CrossRef]
- Louhi-Kultanen, M.; Karjalainen, M.; Rantanen, J.; Huhtanen, M.; Kallas, J. Crystallization of glycine with ultrasound. Int. J. Pharm. 2006, 320, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Jordens, J.; Bamps, B.; Gielen, B.; Braeken, L.; Van Gerven, T. The effect of ultrasound on micromixing. Ultrason. Sonochem. 2015, in press. [Google Scholar] [CrossRef] [PubMed]
- Monnier, H.; Wilhelm, A.M.; Delmas, H. The influence of ultrasound on micromixing in a semi-batch reactor. Chem. Eng. Sci. 1999, 54, 2953–2961. [Google Scholar] [CrossRef]
- Parvizian, F.; Rahimi, M.; Faryadi, M. Macro- and micromixing in a novel sonochemical reactor using high frequency ultrasound. Chem. Eng. Process. Process Intensif. 2011, 50, 732–740. [Google Scholar] [CrossRef]
- Wagterveld, R.M.; Miedema, H.; Witkamp, G. Effect of Ultrasonic Treatment on Early Growth during CaCO3 Precipitation. Cryst. Growth Des. 2012, 12, 4403–4410. [Google Scholar] [CrossRef]
- Chow, R.; Blindt, R.; Chivers, R.; Povey, M. A study on the primary and secondary nucleation of ice by power ultrasound. Ultrasonics 2005, 43, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Chow, R.; Blindt, R.; Chivers, R.; Povey, M. The sonocrystallisation of ice in sucrose solutions: Primary and secondary nucleation. Ultrasonics 2003, 41, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Wohlgemuth, K.; Ruether, F.; Schembecker, G. Sonocrystallization and crystallization with gassing of adipic acid. Chem. Eng. Sci. 2010, 65, 1016–1027. [Google Scholar] [CrossRef]
- Boels, L.; Wagterveld, R.M.; Mayer, M.J.; Witkamp, G.J. Seeded calcite sonocrystallization. J. Cryst. Growth 2010, 312, 961–966. [Google Scholar] [CrossRef]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gielen, B.; Jordens, J.; Thomassen, L.C.J.; Braeken, L.; Van Gerven, T. Agglomeration Control during Ultrasonic Crystallization of an Active Pharmaceutical Ingredient. Crystals 2017, 7, 40. https://doi.org/10.3390/cryst7020040
Gielen B, Jordens J, Thomassen LCJ, Braeken L, Van Gerven T. Agglomeration Control during Ultrasonic Crystallization of an Active Pharmaceutical Ingredient. Crystals. 2017; 7(2):40. https://doi.org/10.3390/cryst7020040
Chicago/Turabian StyleGielen, Bjorn, Jeroen Jordens, Leen C. J. Thomassen, Leen Braeken, and Tom Van Gerven. 2017. "Agglomeration Control during Ultrasonic Crystallization of an Active Pharmaceutical Ingredient" Crystals 7, no. 2: 40. https://doi.org/10.3390/cryst7020040





