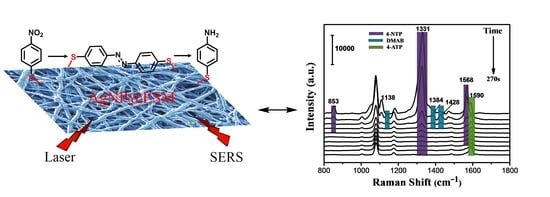
Silver Nanoprism-Loaded Eggshell Membrane: A Facile Platform for In Situ SERS Monitoring of Catalytic Reactions
Abstract
:1. Introduction
2. Results and Discussion
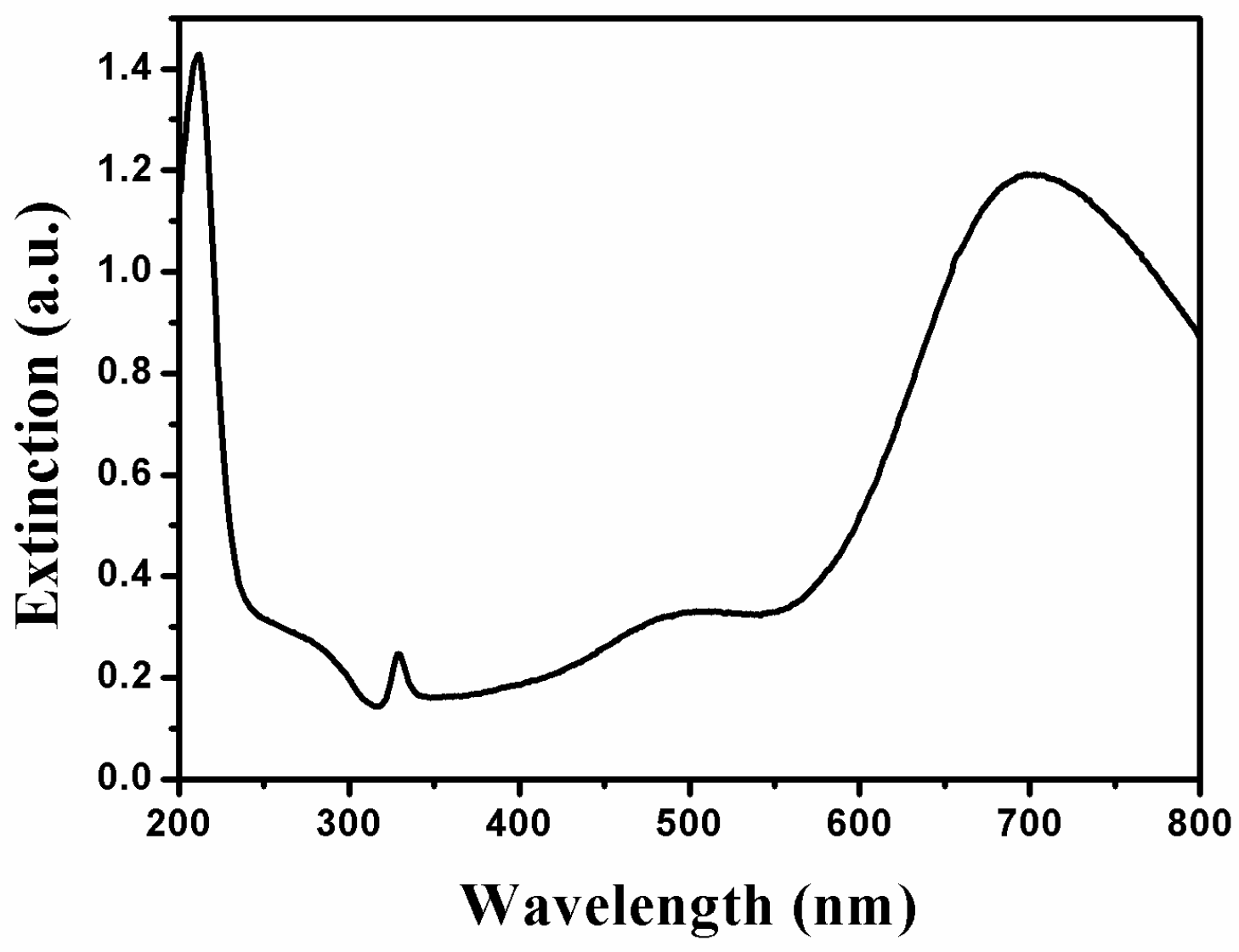
2.1. Optical Properties of AgNPs
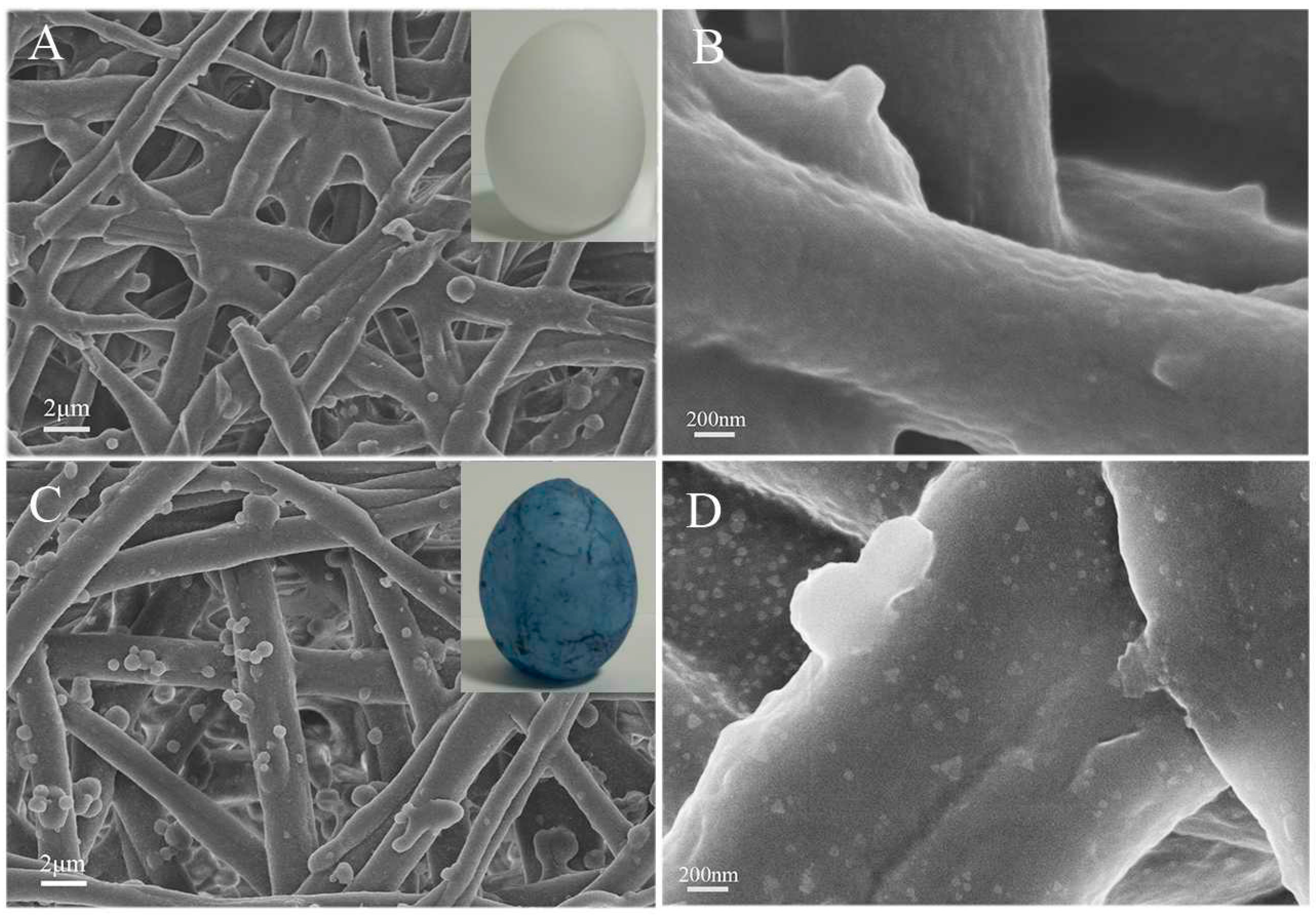
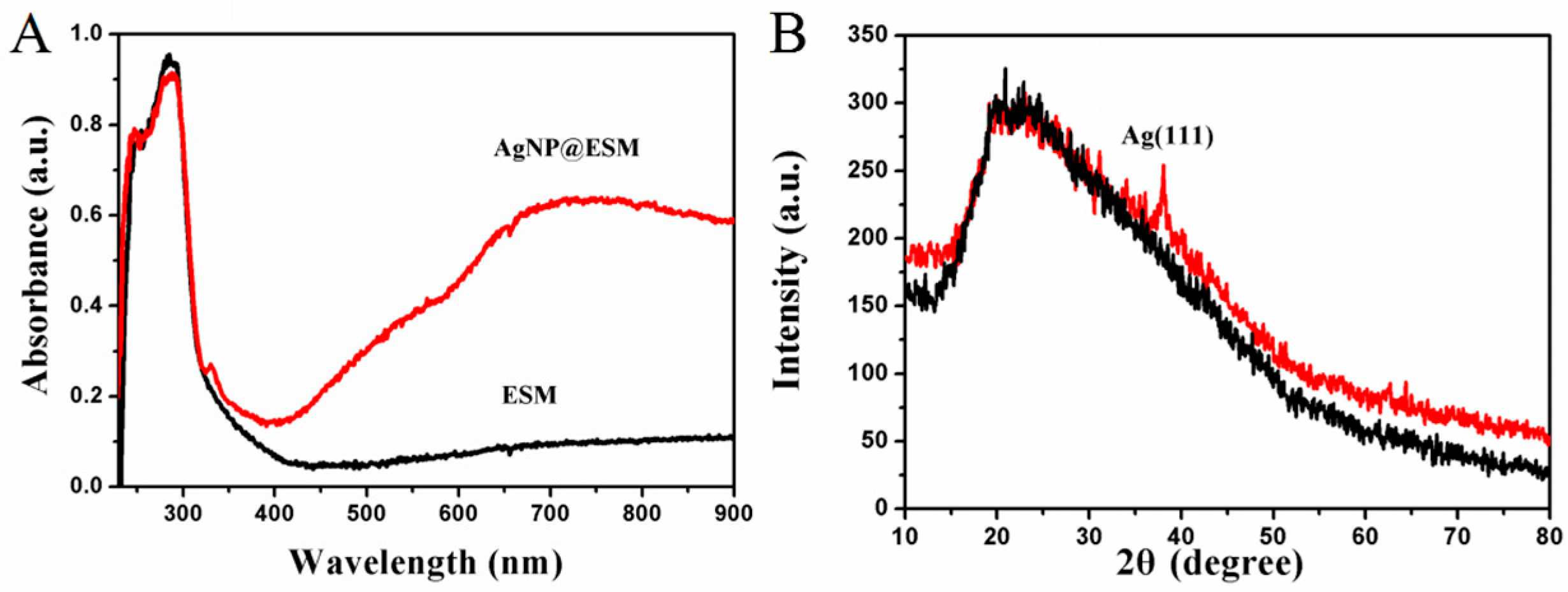
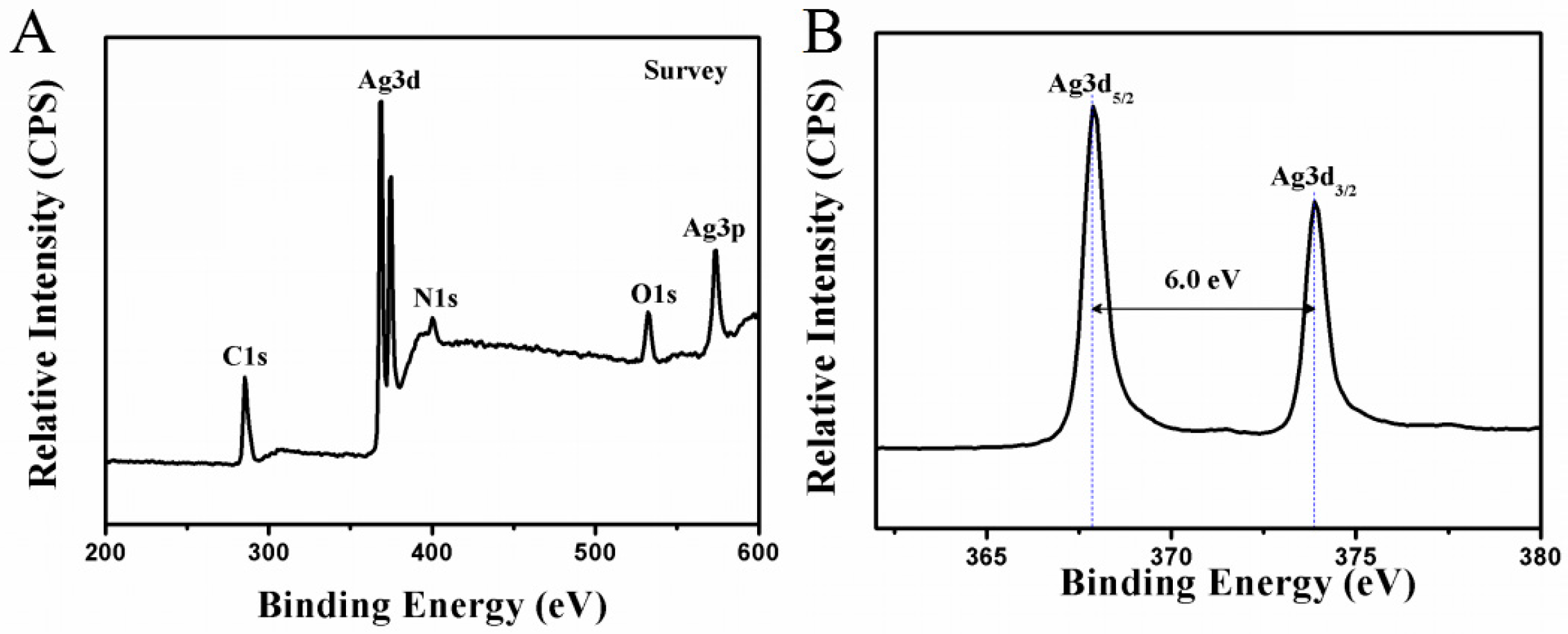
2.2. Characterization of AgNP@ESM Composites
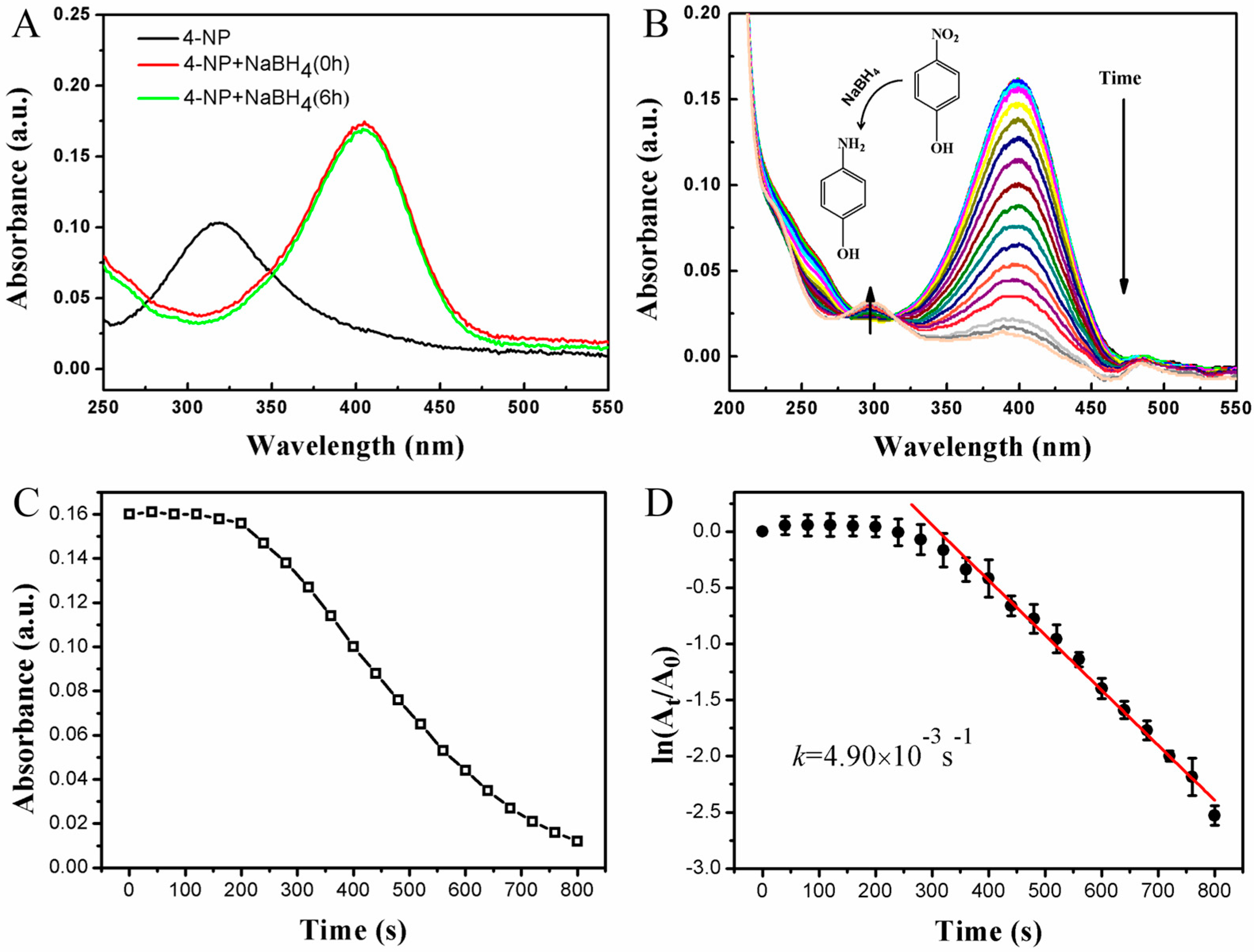
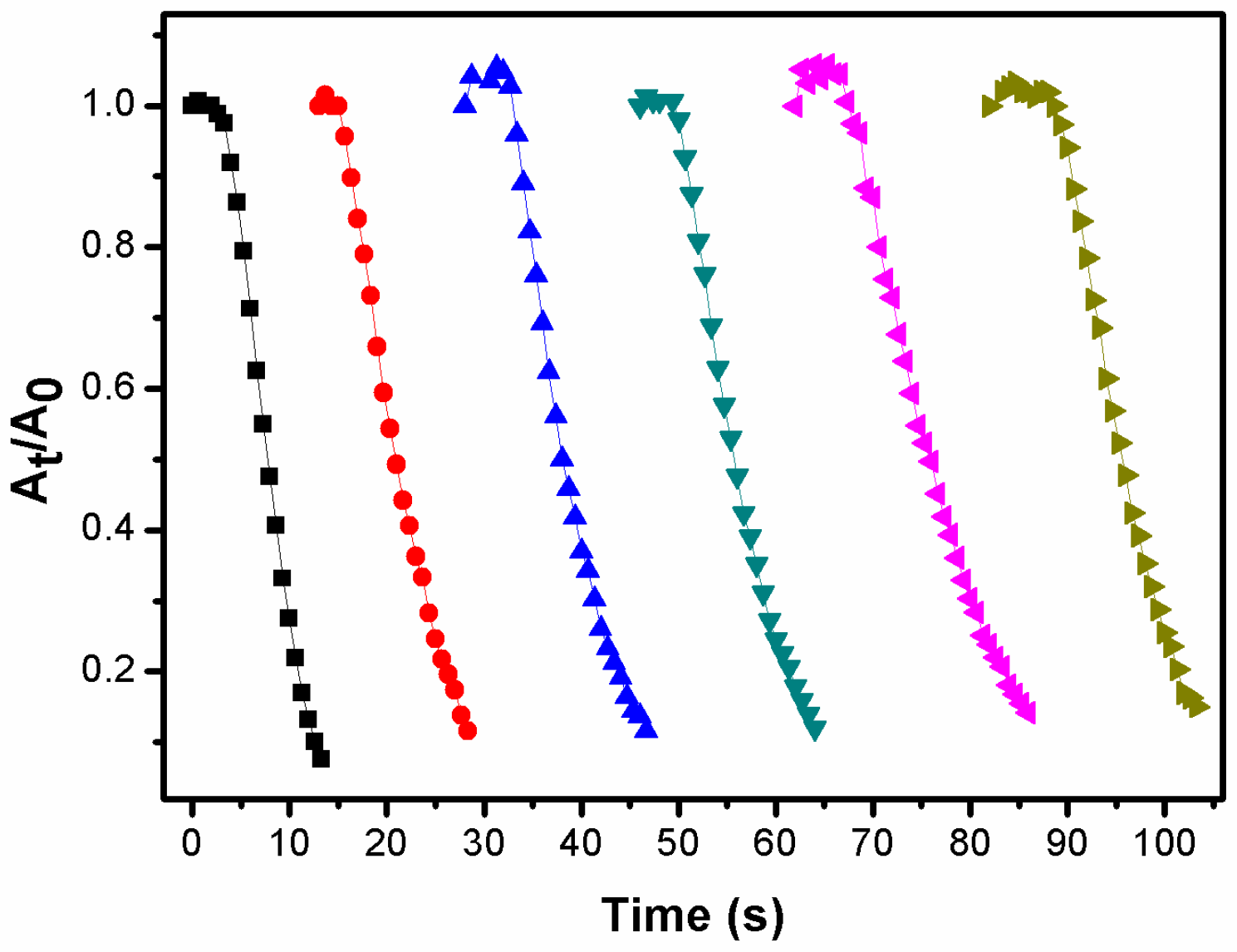
2.3. Monitoring of the Catalytic Reaction with UV-vis
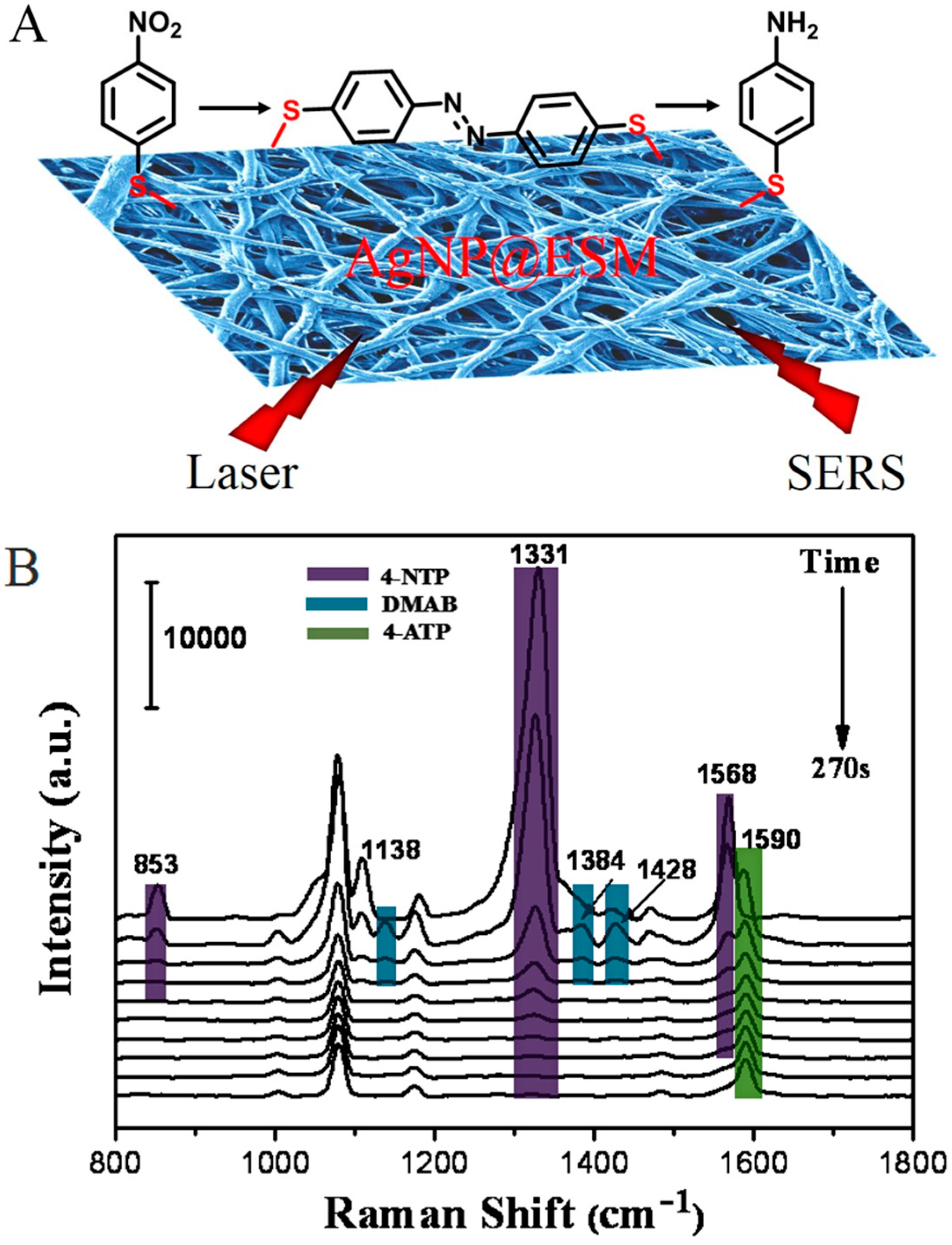
2.4. In Situ Monitoring of Catalytic Reaction with SERS
3. Materials and Methods
3.1. Materials
3.2. Characterization
3.3. Synthesis of Silver Nanoparticles
3.4. Fabrication of AgNP@ESM
3.5. Catalysis and SERS
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zaleski, S.; Wilson, A.J.; Mattei, M.; Chen, X.; Goubert, G.; Cardinal, M.F.; Willets, K.A.; Van Duyne, R.P. Investigating nanoscale electrochemistry with surface and tip-enhanced Raman spectroscopy. Acc. Chem. Res. 2016, 49, 2023–2030. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.V.; Yang, X.; Zhao, K.; Zheng, B.Y.; Nordlander, P.; Halas, N.J. Fan-shaped gold nanoantennas above reflective substrates for surface-enhanced infrared absorption (SEIRA). Nano Lett. 2015, 15, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.Q.; Gao, Z. Plasmonic nanoparticles in biomedicine. Nano Today 2016, 11, 168–188. [Google Scholar] [CrossRef]
- Teng, Z.; Zhang, J.; Li, W.; Zheng, Y.; Su, X.; Tang, Y.; Dang, M.; Tian, Y.; Yuwen, L.; Weng, L.; et al. Facile synthesis of yolk-shell-structured triple-hybridized periodic mesoporous organosilica nanoparticles for biomedicine. Small 2016, 12, 3550–3558. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xin, H.; Liu, X.; Zhang, Y.; Lei, H.; Li, B. Trapping and detection of nanoparticles and cells using a parallel photonic nanojet array. ACS Nano 2016, 10, 5800–5808. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Sahoo, A.; Chattopadhyay, R.; Roy, S.; Bhadra, S.K.; Agrawal, G.P. Implications of a zero-nonlinearity wavelength in photonic crystal fibers doped with silver nanoparticles. Phys. Rev. A 2016, 94, 043835. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.A.; Shimizu, F.M.; Coelho, D.; Piazzeta, M.H.O.; Gobbi, A.L.; Machado, S.A.S.; Oliveira, O.N. A nanostructured bifunctional platform for sensing of glucose biomarker in artificial saliva: Synergy in hybrid Pt/Au surfaces. Biosens. Bioelectron. 2016, 86, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Barsan, M.M.; Brett, C.M.A. Recent advances in layer-by-layer strategies for biosensors incorporating metal nanoparticles. TrAC Trends Anal. Chem. 2016, 79, 286–296. [Google Scholar] [CrossRef]
- Oh, H.S.; Nong, H.N.; Reier, T.; Bergmann, A.; Gliech, M.; de Araujo, J.F.; Willinger, E.; Schlogl, R.; Teschner, D.; Strasser, P. Electrochemical catalyst-support effects and their stabilizing role for irox nanoparticle catalysts during the oxygen evolution reaction. J. Am. Chem. Soc. 2016, 138, 12552–12563. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Shin, H.; Choi, J.Y.; Jung, H.W.; Choi, Y.; Lee, J.S. In-plate and on-plate structural control of ultra-stable gold/silver bimetallic nanoplates as redox catalysts, nanobuilding blocks, and single-nanoparticle surface-enhanced Raman scattering probes. ACS Appl. Mater. Interface 2016, 8, 27140–27150. [Google Scholar] [CrossRef] [PubMed]
- Atarod, M.; Nasrollahzadeh, M.; Sajadi, S.M. Euphorbia heterophylla leaf extract mediated green synthesis of Ag/TiO2 nanocomposite and investigation of its excellent catalytic activity for reduction of variety of dyes in water. J. Colloid Interface Sci. 2016, 462, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Sajadi, S.M.; Hatamifard, A. Waste chicken eggshell as a natural valuable resource and environmentally benign support for biosynthesis of catalytically active Cu/eggshell, Fe3O4/eggshell and Cu/Fe3O4/eggshell nanocomposites. Appl. Catal. B Environ. 2016, 191, 209–227. [Google Scholar] [CrossRef]
- Xie, W.; Walkenfort, B.; Schlucker, S. Label-free SERS monitoring of chemical reactions catalyzed by small gold nanoparticles using 3D plasmonic superstructures. J. Am. Chem. Soc. 2013, 135, 1657–1660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Winget, S.A.; Wu, Y.; Su, D.; Sun, X.; Xie, Z.X.; Qin, D. Ag@Au concave cuboctahedra: A unique probe for monitoring Au-catalyzed reduction and oxidation reactions by surface-enhanced Raman spectroscopy. ACS Nano 2016, 10, 2607–2616. [Google Scholar] [CrossRef] [PubMed]
- Sambur, J.B.; Chen, P. Approaches to single-nanoparticle catalysis. Annu. Rev. Phys. Chem. 2014, 65, 395–422. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.Y.; Lei, D.Y.; Jiang, R.; Liu, X.; Dai, J.; Wang, J.; Chan, H.L.; Tsang, Y.H. Bifunctional Au@Pt core-shell nanostructures for in situ monitoring of catalytic reactions by surface-enhanced Raman scattering spectroscopy. Nanoscale 2014, 6, 9063–9070. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Qiu, L.; Wang, J. A robust site-specific Au@SiO2@AgPt nanorod/nanodots superstructure for in situ SERS monitoring of catalytic reactions. RSC Adv. 2015, 5, 40316–40323. [Google Scholar] [CrossRef]
- Li, P.; Ma, B.; Yang, L.; Liu, J. Hybrid single nanoreactor for in situ SERS monitoring of plasmon-driven and small Aunanoparticles catalyzed reactions. Chem. Commun. 2015, 51, 11394–11397. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Zhang, C.; Gao, W.; Han, Z.; Liu, T.; Li, C.; Wang, Z.; He, E.; Zheng, H. Ag-Au alloy nanoparticles: Synthesis and in situ monitoring SERS of plasmonic catalysis. Sens. Actuator B Chem. 2016, 231, 609–614. [Google Scholar] [CrossRef]
- Ding, Q.; Zhou, H.; Zhang, H.; Zhang, Y.; Wang, G.; Zhao, H. 3D Fe3O4@Au@Ag nanoflowers assembled magnetoplasmonic chains for in situ SERS monitoring of plasmon-assisted catalytic reactions. J. Mater. Chem. A 2016, 4, 8866–8874. [Google Scholar] [CrossRef]
- Cui, Q.; Xia, B.; Mitzscherling, S.; Masic, A.; Li, L.; Bargheer, M.; Möhwald, H. Preparation of gold nanostars and their study in selective catalytic reactions. Colloids Surf. A 2015, 465, 20–25. [Google Scholar] [CrossRef]
- Cui, Q.; Yashchenok, A.; Li, L.; Möhwald, H.; Bargheer, M. Mechanistic study on reduction reaction of nitro compounds catalyzed by gold nanoparticles using in situ SERS monitoring. Colloids Surf. A 2015, 470, 108–113. [Google Scholar] [CrossRef]
- Zheng, G.; Polavarapu, L.; Liz-Marzan, L.M.; Pastoriza-Santos, I.; Perez-Juste, J. Gold nanoparticle-loaded filter paper: A recyclable dip-catalyst for real-time reaction monitoring by surface enhanced Raman scattering. Chem. Commun. 2015, 51, 4572–4575. [Google Scholar] [CrossRef] [PubMed]
- Torkamani, F.; Azizian, S. Green and simple synthesis of Ag nanoparticles loaded onto cellulosic fiber as efficient and low-cost catalyst for reduction of 4-nitrophenol. J. Mol. Liq. 2016, 214, 270–275. [Google Scholar] [CrossRef]
- Zhai, D.; Liu, B.; Shi, Y.; Pan, L.; Wang, Y.; Li, W.; Zhang, R.; Yu, G. Highly sensitive glucose sensor based on Pt nanoparticle/polyaniline hydrogel heterostructures. ACS Nano 2013, 7, 3540–3546. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Su, R.; Qi, W.; Yu, Y.; Wang, L.; He, Z. Synthesis of well-dispersed Ag nanoparticles on eggshell membrane for catalytic reduction of 4-nitrophenol. J. Mater. Chem. 2013, 49, 1639–1647. [Google Scholar] [CrossRef]
- Liang, M.; Su, R.; Huang, R.; Qi, W.; Yu, Y.; Wang, L.; He, Z. Facile in situ synthesis of silver nanoparticles on procyanidin-grafted eggshell membrane and their catalytic properties. ACS Appl. Mater. Interface 2014, 6, 4638–4649. [Google Scholar] [CrossRef] [PubMed]
- Mallampati, R.; Valiyaveettil, S. Eggshell membrane-supported recyclable catalytic noble metal nanoparticles for organic reactions. ACS Sustain. Chem. Eng. 2014, 2, 855–859. [Google Scholar] [CrossRef]
- Sharma, N.C.; Sahi, S.V.; Nath, S.; Parsons, J.G.; Gardea-Torresde, J.L.; Pal, T. Synthesis of plant-mediated gold nanoparticles and catalytic role of biomatrix-embedded nanomaterials. Environ. Sci. Technol. 2007, 41, 5137–5142. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.B.; Park, H.H.; Han, S.S. Synthesis and characterization of biomatrixed-gold nanoparticles by the mushroom flammulina velutipes and its heterogeneous catalytic potential. Chemosphere 2015, 141, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Huang, Y. Polyethyleneimine modified eggshell membrane as a novel biosorbent for adsorption and detoxification of Cr (VI) from water. J. Mater. Chem. 2011, 21, 17413. [Google Scholar] [CrossRef]
- Chung, S.-H.; Manthiram, A. Carbonized eggshell membrane as a natural polysulfide reservoir for highly reversible Li-S batteries. Adv. Mater. 2014, 26, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Wu, H.; Al-Enizi, A.M.; Elzatahry, A.A.; Zheng, G. Freestanding eggshell membrane-based electrodes for high-performance supercapacitors and oxygen evolution reaction. Nanoscale 2015, 7, 14378–14384. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Tang, Q.; Wu, J.; Lin, Y.; Fan, L.; Huang, M.; Lin, J.; Li, Y.; Yu, F. Using eggshell membrane as a separator in supercapacitor. J. Power Sources 2012, 206, 463–468. [Google Scholar] [CrossRef]
- Xue, G.; Yue, Z.; Bing, Z.; Yiwei, T.; Xiuying, L.; Jianrong, L. Highly-sensitive organophosphorus pesticide biosensors based on CdTe quantum dots and bi-enzyme immobilized eggshell membranes. Analyst 2016, 141, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Xu, S.; An, J.; Zhao, B.; Xu, W. Photoinduced shape conversion and reconstruction of silver nanoprisms. J. Phys. Chem. C 2009, 113, 7025–7030. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Kundu, S.; Mandal, M.; Pal, T. Silver and gold nanocluster catalyzed reduction of methylene blue by arsine in a micellar medium. Langmuir 2002, 18, 8756–8760. [Google Scholar] [CrossRef]
- Herves, P.; Perez-Lorenzo, M.; Liz-Marzan, L.M.; Dzubiella, J.; Lu, Y.; Ballauff, M. Catalysis by metallic nanoparticles in aqueous solution: Model reactions. Chem. Soc. Rev. 2012, 41, 5577–5587. [Google Scholar] [CrossRef] [PubMed]
- Muthuchamy, N.; Gopalan, A.; Lee, K.-P. A new facile strategy for higher loading of silver nanoparticles onto silica for efficient catalytic reduction of 4-nitrophenol. RSC Adv. 2015, 5, 76170–76181. [Google Scholar] [CrossRef]
- Cloud, J.E.; Taylor, L.W.; Yang, Y. A simple and effective method for controllable synthesis of silver and silver oxide nanocrystals. RSC Adv. 2014, 4, 24551–24559. [Google Scholar] [CrossRef]
- Tang, B.; Li, J.; Fan, L.; Wang, X. Facile synthesis of silver submicrospheres and their applications. RSC Adv. 2015, 5, 98293–98298. [Google Scholar] [CrossRef]
- Ai, L.; Yue, H.; Jiang, J. Environmentally friendly light-driven synthesis of Ag nanoparticles in situ grown on magnetically separable biohydrogels as highly active and recyclable catalysts for 4-nitrophenol reduction. J. Mater. Chem. 2012, 22, 23447–23453. [Google Scholar] [CrossRef]
- Van Schrojenstein Lantman, E.M.; Deckert-Gaudig, T.; Mank, A.J.G.; Deckert, V.; Weckhuysen, B.M. Catalytic processes monitored at the nanoscale with tip-enhanced Raman spectroscopy. Nat. Nanotechnol. 2012, 7, 583–586. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Ye, Y.; Fan, Y.; Zhou, J.; Jia, L.; Tang, B.; Wang, X. Silver Nanoprism-Loaded Eggshell Membrane: A Facile Platform for In Situ SERS Monitoring of Catalytic Reactions. Crystals 2017, 7, 45. https://doi.org/10.3390/cryst7020045
Li Y, Ye Y, Fan Y, Zhou J, Jia L, Tang B, Wang X. Silver Nanoprism-Loaded Eggshell Membrane: A Facile Platform for In Situ SERS Monitoring of Catalytic Reactions. Crystals. 2017; 7(2):45. https://doi.org/10.3390/cryst7020045
Chicago/Turabian StyleLi, Yaling, Yong Ye, Yunde Fan, Ji Zhou, Li Jia, Bin Tang, and Xungai Wang. 2017. "Silver Nanoprism-Loaded Eggshell Membrane: A Facile Platform for In Situ SERS Monitoring of Catalytic Reactions" Crystals 7, no. 2: 45. https://doi.org/10.3390/cryst7020045