Effect of Diazotated Sulphonated Polystyrene Films on the Calcium Oxalate Crystallization
Abstract
:1. Introduction
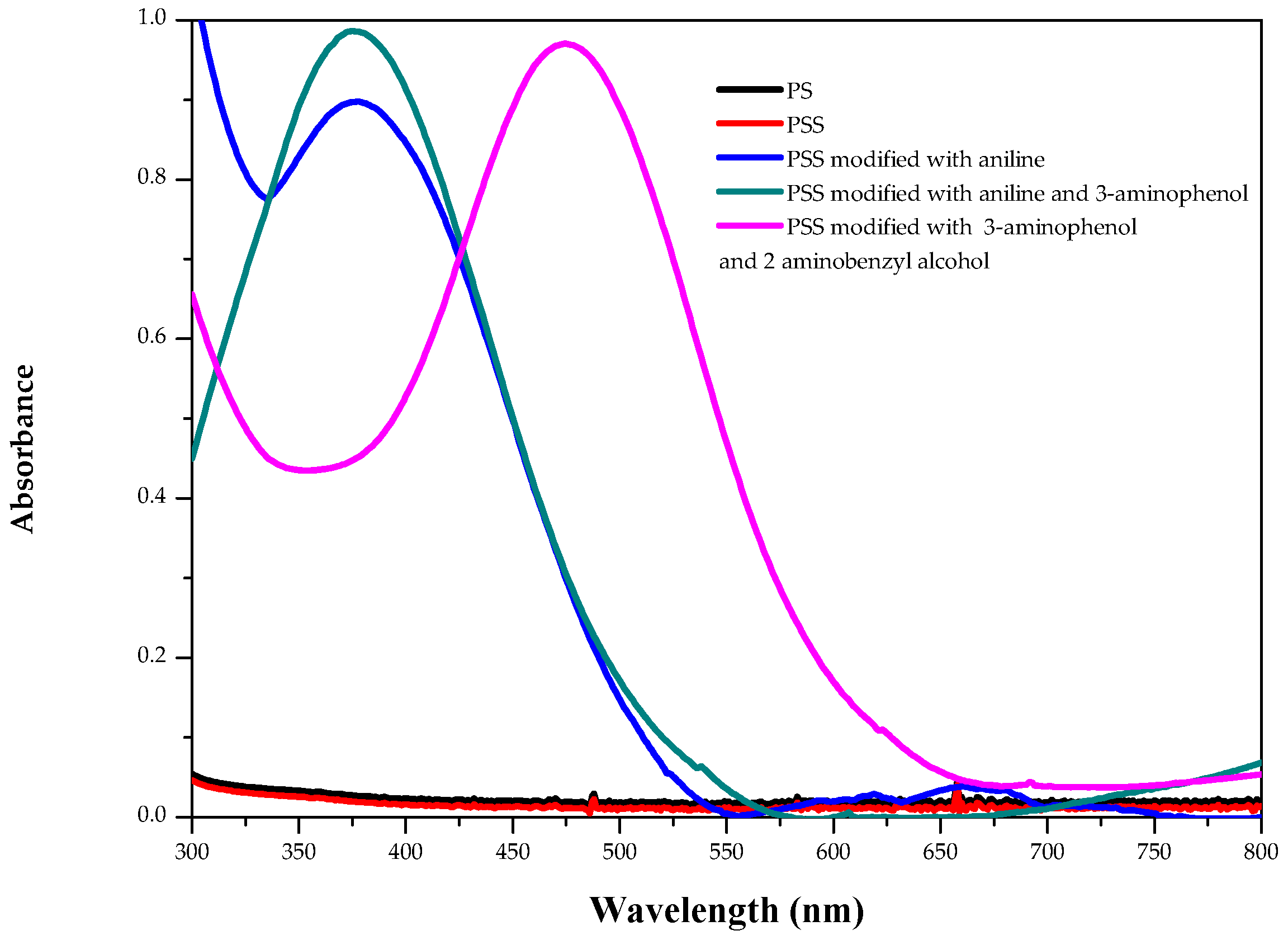
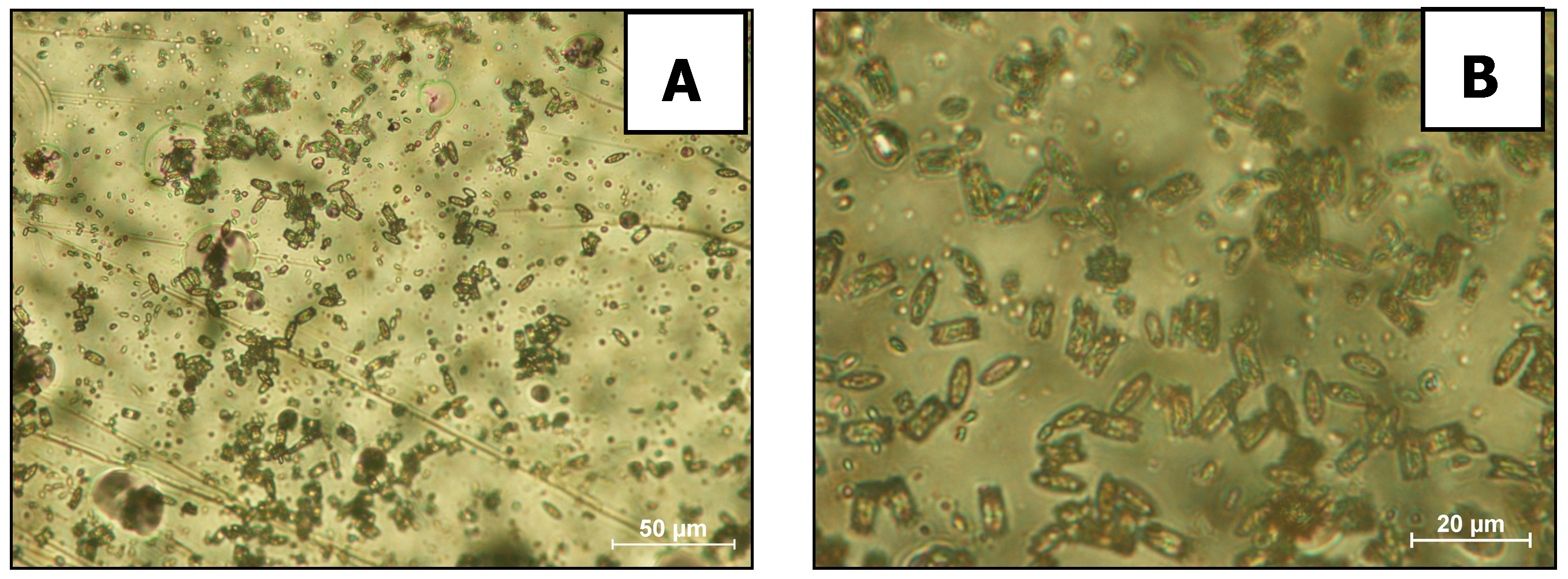
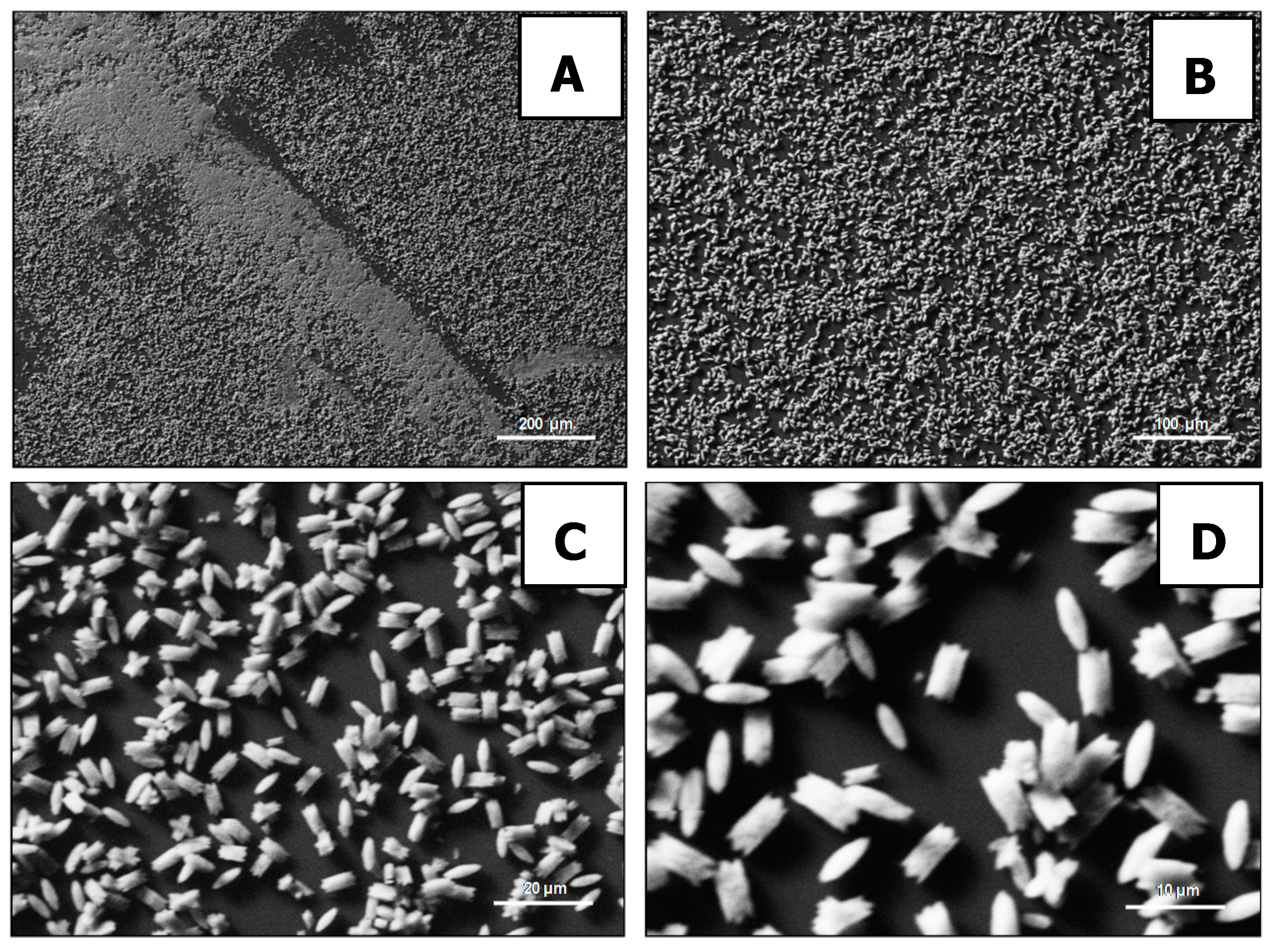
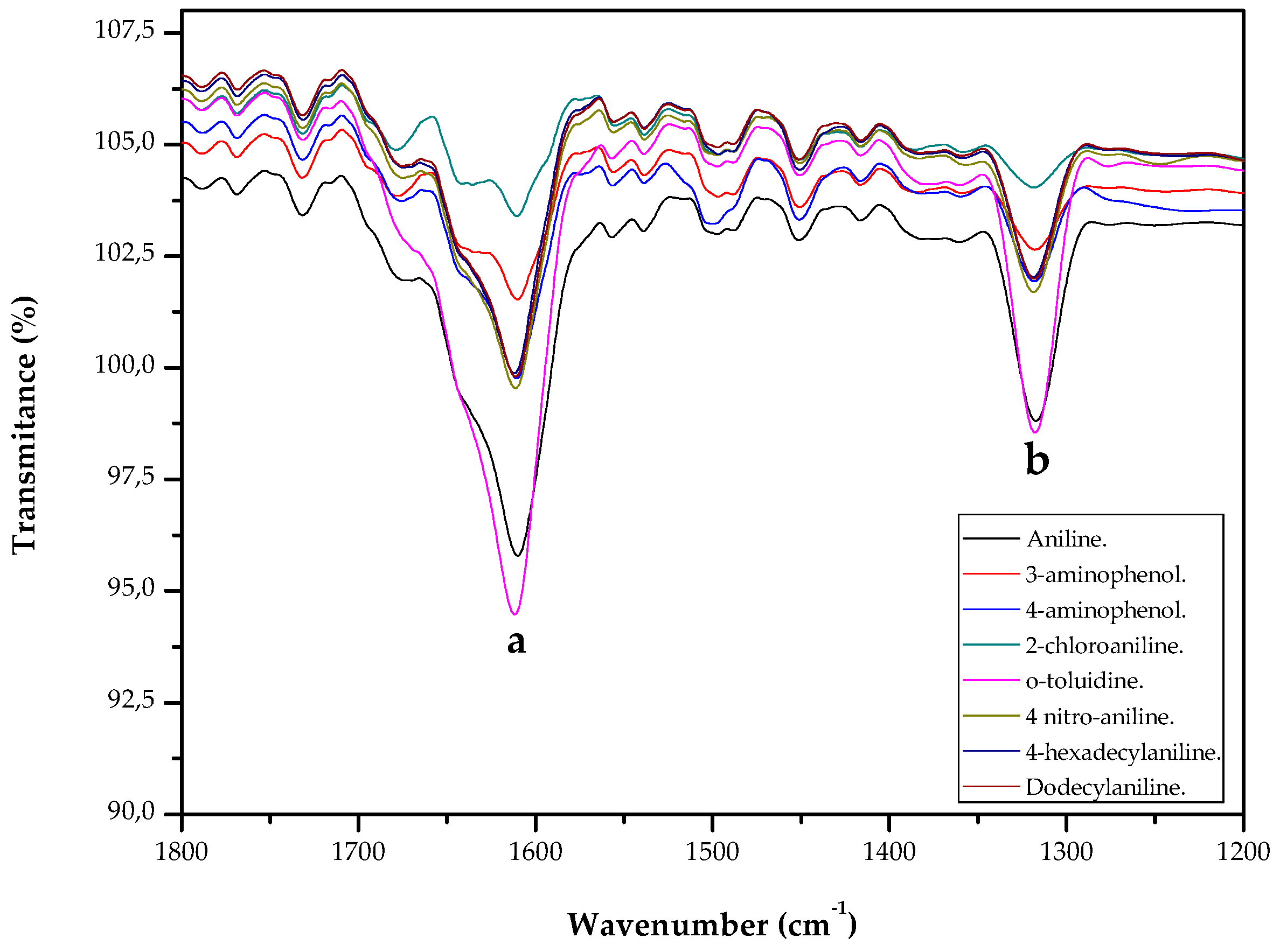
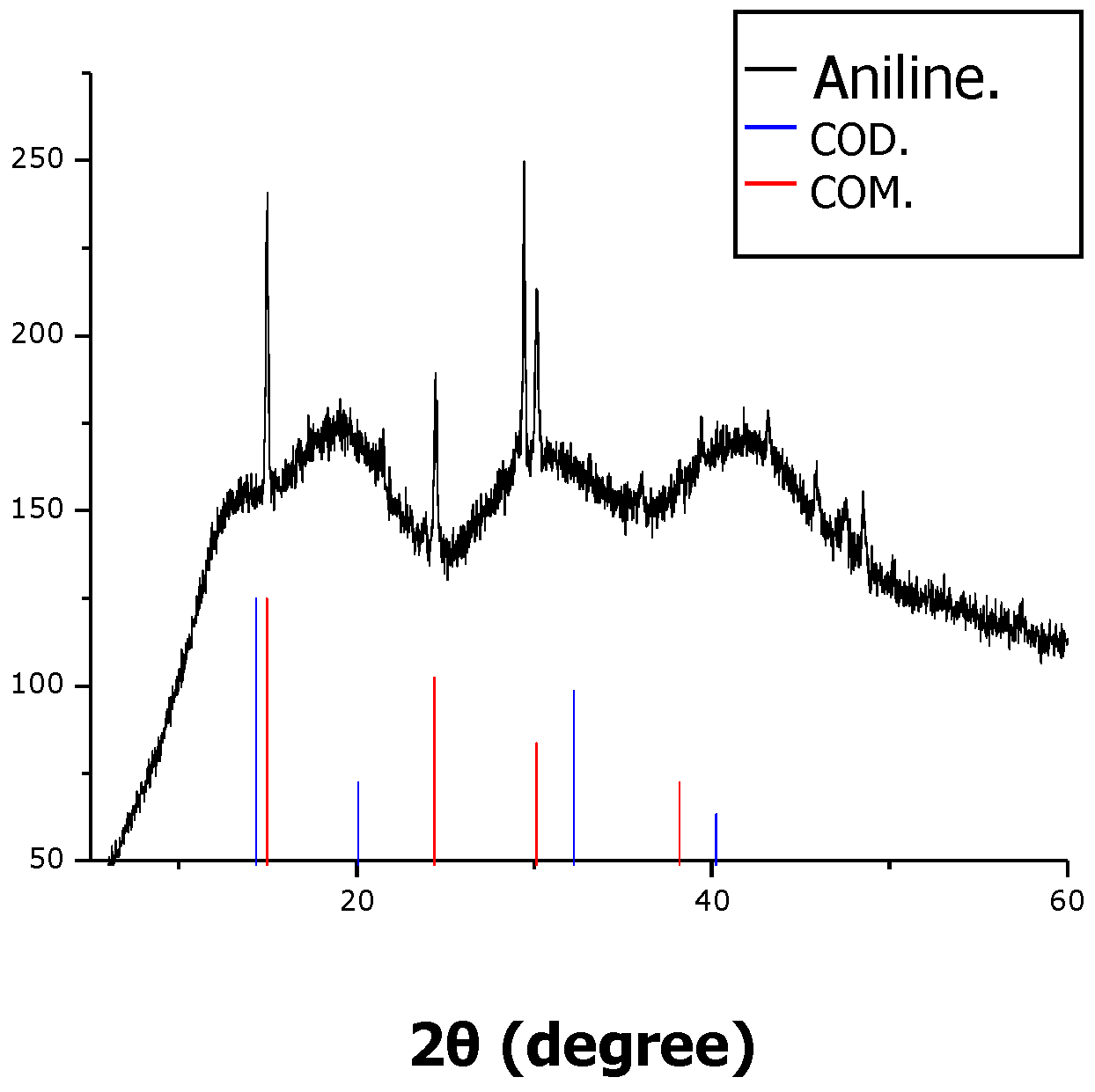
2. Results and Discussion
3. Materials and Methods
3.1. Reactants
3.2. Generation of Polystyrene Films
3.3. Sulphonation of Polystyrene Films
3.4. Diazotation of Sulphonated Polystyrene Films (DSPF)
3.5. Crystallization of CaOx
3.6. Optical, SEM-EDS, FTIR-ATR, UV-VIS and XRD Characterization of DSPF and CaOx Crystals
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aggarwal, K.P.; Narula, S.; Kakkar, M.; Tandon, C. Nephrolithiasis: Molecular mechanism of renal stone formation and the critical role played by modulators. BioMed Res. Int. 2013, 2013, 292953. [Google Scholar] [CrossRef] [PubMed]
- Monje, P.; Baran, E. Characterization of calcium oxalates generated as biominerals in cacti. Plant Physiol. 2002, 128, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Neira-Carrillo, A.; Vásquez-Quitral, P. Formación de calculos renales de oxalato cálcico en mamíferos. Av. Cienc. Vet. 2010, 25. [Google Scholar] [CrossRef]
- Rodgers, A.L.; Webber, D.; Ramsout, R.; Gohel, M.D. Herbal preparations affect the kinetic factors of calcium oxalate crystallization in synthetic urine: Implication for kidney stone therapy. Urolithiasis 2014, 42, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Holmes, R.P.; Assimos, D.G. Glyoxylate synthesis, and its modulation and influence on oxalate synthesis. J. Urol. 1998, 160, 1617–1624. [Google Scholar] [CrossRef]
- Knight, J.; Jiang, J.; Assimos, D.G.; Holmes, R.P. Hydroxyproline ingestion and urinary oxalate and glycolate excretion. Kidney Int. 2006, 70, 1929–1934. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R.; Canales, B.K. Genetic basis of renal celular dysfunction and the formation of kidney stones. Urol. Res. 2009, 37, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Marickar, Y.M.; Salim, A.; Vijay, A. Pattern of family history in stone patients. Urol. Res. 2009, 37, 331. [Google Scholar] [CrossRef] [PubMed]
- Onaran, M.; Yilmaz, A.; Sen, I.; Ergun, M.A.; Camtosun, A.; Küpeli, B.; Menevse, S.; Bozkirli, I. Heparan sulfate gene polymorphism in calcium oxalate nephrolithiasis. Urol. Res. 2009, 37, 47. [Google Scholar] [CrossRef] [PubMed]
- Brikowski, T.H.; Lotan, Y.; Pearle, M.S. Climate-related increase in the prevalence of urolitiasis in the United States. Proc. Natl. Acad. Sci. USA 2008, 105, 9841–9846. [Google Scholar] [CrossRef] [PubMed]
- Basavaraj, D.R.; Biyani, C.S.; Browning, A.J.; Cartledge, J.J. The role of urinary kidney stone inhibitors and promoters in the pathogenesis of calcium containing renal stones. EAU-EBU Update Ser. 2007, 5, 126. [Google Scholar] [CrossRef]
- Beiko, D.; Wollin, T. The Consumer’s Handbook of Urological Health, 1st ed.; Canadian Urological Association: Quebec, QC, Canada, 2013; p. 267. [Google Scholar]
- Wagner, C.A.; Mohebbi, N. Urinary pH and stone formation. J. Nephrol. 2010, 23, S165–S169. [Google Scholar] [PubMed]
- El-Shall, H.; Jeon, J.H.; Abdel-Aal, E.A.; Khan, S.; Gower, L.; Rabinovich, Y. A study of primary nucleation of calcium oxalate monohydrate: II. Effect of urinary species. Cryst. Res. Technol. 2004, 39, 222–229. [Google Scholar] [CrossRef]
- Campieri, C.; Campieri, M.; Bertuzzi, V.; Swennen, E.; Matteuzzi, D.; Stefoni, S.; Pirovano, F.; Centi, C.; Ulisse, S.; Famularo, G.; et al. Reduction of oxaluria after an oral course of lactic acid bacteria at high concentration. Kidney Int. 2001, 60, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Fasano, J.M.; Khan, S.R. Intratubular crystallization of calcium oxalate in the presence of membrane vesicles: An in vitro study. Kidney Int. 2001, 59, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Benitez, I.O.; Talham, D.R. Brewster angle microscopy of calcium oxalate monohydrate precipitation at phospholipid monolayer phase boundaries. Langmuir 2004, 20, 8287–8293. [Google Scholar] [CrossRef] [PubMed]
- Benitez, I.O.; Talham, D.R. Calcium oxalate monohydrate precipitation at membrane lipid rafts. J. Am. Chem. Soc. 2005, 127, 2814–2815. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R.; Maslamani, S.A.; Atmani, F.; Glenton, P.A.; Opalko, F.J.; Thamilselvan, S.; Hammett-Stabler, C. Membranes and their constituents as promoters of calcium oxalate crystal formation in human urine. Calcif. Tissue Int. 2000, 66, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.M.; Novin, F.; Purich, D.L. Calcium oxalate crystal morphology: Influence of phospholipid micelles with compositions based on each leaflet of the erythrocyte membrane. J. Cryst. Growth 1994, 135, 523–532. [Google Scholar] [CrossRef]
- Campbell, A.A.; Fryxell, G.E.; Graff, G.L.; Rieke, P.C.; Tarasevich, B.J. The nucleation and growth of calcium oxalate monohydrate on self-assembled monolayers (SAMS). Scanning Microsc. 1993, 7, 423–429. [Google Scholar] [PubMed]
- Talham, D.R.; Backov, R.; Benítez, I.O.; Sharbaugh, D.M.; Whipps, S.; Khan, S.R. Role of lipids in urinary stones: Studies of calcium oxalate precipitation at phospholipid Langmuir monolayers. Langmuir 2006, 22, 2450–2456. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.P.; Ouyang, J.M. Effects of dipalmitoylphosphatidylcholine monolayers to the crystallization of calcium oxalate monohydrate from the solution containing chondroitin sulfate C. Colloids Surf. A Physicochem. Eng. Asp. 2005, 257–258, 47–50. [Google Scholar] [CrossRef]
- Ouyang, J.-M. Chemical basis in the investigation of calcium oxalate stones. Chem. Bull. 2002, 65, 326. [Google Scholar]
- Shirane, Y.; Kurokawa, Y.; Sumiyoshi, Y.; Kagawa, S. Morphological effects of glycosaminoglycans on calcium oxalate monohydrate crystals. Scanning Microsc. 1995, 9, 1081–1088. [Google Scholar] [PubMed]
- Hess, B.; Meinhardt, U.; Zipperle, L.; Giovanoli, R.; Jaeger, P. Simultaneous measurements of calcium oxalate crystal nucleation and aggregation impact of various modifiers. Urol. Res. 1995, 23, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.-M.; Deng, S.P. Controlled and uncontrolled crystallization of calcium oxalate monohydrate in the presence of citric acid. J. Chem. Dalton Trans. 2003, 63, 2486–2851. [Google Scholar] [CrossRef]
- Tunik, L.; Fueredi-Milhofer, H.; Garti, N. Adsorption of sodium diisooctylsulfosuccinate onto calcium crystals. Langmuir 1998, 14, 3351–3355. [Google Scholar] [CrossRef]
- Khan, S.R.; Whalen, P.O.; Glenton, P.A. Heterogeneous nucleation of calcium oxalate crystals in the presence of membrane vesicles. J. Cryst. Growth 1993, 134, 211–218. [Google Scholar] [CrossRef]
- He, J.-Y.; Ouyang, J.-M. Circular patterns of calcium oxalate monohydrate induced by defective Langmuir-Blodgett film on quartz substrates. Mater. Sci. Eng. C 2009, 29, 288–291. [Google Scholar] [CrossRef]
- Wiessner, J.H.; Hasegawa, A.T.; Hung, L.Y.; Mandel, G.S.; Mandel, N.S. Mechanisms of calcium oxalate crystals attachment to injured renal collecting duct cells. Kidney Int. 2001, 59, 637–644. [Google Scholar] [CrossRef] [PubMed]
- De Koker, R.; McConnell, H.M. Circle to dogbone: Shapes and shape transitions of lipid monolayer domains. J. Phys. Chem. 1993, 97, 13419–13424. [Google Scholar] [CrossRef]
- Kane, S.A.; Compton, M.; Wilder, N. Interactions determining the growth of chiral domains in phospholipid monolayers: Experimental results and comparison with theory. Langmuir 2000, 16, 8447–8455. [Google Scholar] [CrossRef]
- Ouyang, J.-M.; Deng, S.-P. Formation of circular patterns of calcium oxalate crystals at defective sites of Langmuir–Blodgett films. Colloids Surf. A 2008, 317, 155. [Google Scholar] [CrossRef]
- Loste, E.; Diaz-Marti, E.; Zarbakhsh, A.; Meldrum, F.C. Study of calcium carbonate precipitation under a series of fatty acid Langmuir monolayers using Brewster angle microscopy. Langmuir 2003, 19, 2830–2837. [Google Scholar] [CrossRef]
- Cruz, A.; Vazquez, L.; Velez, M.; Perez-Gil, J. Influence of a fluorescent probe on the nanostructure of phospholipid membranes: Dipalmitoylphosphatidyl interfacial monolayers. Langmuir 2005, 21, 5349–5355. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.; Steinem, C.; Galla, H.-J.; Janshoff, A. Visualization of chemical and physical properties of calcium induced domains in DPCC/DPPS Langmuir-Blodgett layers. Langmuir 2001, 17, 2437–2447. [Google Scholar] [CrossRef]
- Kang, Y.S.; Lee, D.K.; Lee, C.S.; Stroeve, P. In situ observation of domain structure in monolayers of arachidonic acid/γ-Fe2O3 nanoparticle complexes at the air/water interface. J. Phys. Chem. B 2002, 106, 9341–9346. [Google Scholar] [CrossRef]
- An, Z.; Lee, S.; Oppenheimer, H.; Wesson, J.; Ward, M. Attachment of calcium oxalate monohydrate crystals on patterned surfaces of proteins and lipid bilayers. J. Am. Chem. Soc. 2010, 132, 13188–13190. [Google Scholar] [CrossRef] [PubMed]
- Hug, S.; Grohe, B.; Jalkanen, J.; Chan, B.; Galarreta, B.; Vincent, K.; Lagugné-Labarthet, F.; Lajoie, G.; Goldberg, H.A.; Karttunena, M.; et al. Mechanism of inhibition of calcium oxalate crystal growth by an osteopontin phosphopeptide. Soft Matter 2012, 8, 1226. [Google Scholar] [CrossRef]
- Sharma, V.; Park, K.; Srinivasarao, M. Colloidal dispersion of gold nanorods: Historical background, optical properties, seed-mediated synthesis, shape separation and self-assembly. Mater. Sci. Eng. R. 2009, 65, 1–38. [Google Scholar] [CrossRef]
- Asselman, M.; Verkoelen, C.F. Crystal-cell interaction in the pathogenesis of kidney stone disease. Curr. Opin. Urol. 2002, 12, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Vargas Fernández, A. Efecto de Nanotubos de Carbono Como Moduladores de la Cristalización In Vitro de Oxalate Cálcico Mediante la Técnica de Electrocristalización. Ph.D. Thesis, Facultad de Ciencias Veterinarias y Pecuarias, Universidad de Chile, Santiago, Chile, 2016. [Google Scholar]
- Fleming, D.E.; van Riessen, A.; Chauvet, M.C.; Grover, P.K.; Hunter, B.; van Bronswijk, W.; Ryall, R.L. Intracrystalline proteins and urolitiasis: A synchotron X-ray diffraction study of calcium oxalate monohydrate. J. Bone Miner. Res. 2003, 18, 1282. [Google Scholar] [CrossRef] [PubMed]
- Kepa, K.; Coleman, R.; Grøndahl, L. In vitro mineralization of functional polymers. Biosurf. Biotribol. 2015, 1, 214–227. [Google Scholar] [CrossRef]
- Leonor, I.B.; Kim, H.-M.; Balas, F.; Kawashita, M.; Reis, R.L.; Kokubo, T.; Nakamura, T. Formation of bone-like apatite on polymeric surfaces modified with –SO3H groups. Mater. Sci. Forum 2006, 514–516, 966–969. [Google Scholar] [CrossRef]
- Leonor, I.B.; Kim, H.-M.; Balas, F.; Kawashita, M.; Reis, R.L.; Kokubo, T.; Nakamura, T. Functionalization of different polymers with sulfonic groups as a way to coat them with biomimetic apatite layer. J. Mater. Sci. Mater. Med. 2007, 18, 1923–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonor, I.B.; Kim, H.-M.; Balas, F.; Kawashita, M.; Reis, R.L.; Kokubo, T.; Nakamura, T. Surface charge of bioactive polyethylene modified with –SO3H groups and tts apatite inducing capability in simulated body fluid. Key Eng. Mater. 2005, 284–286, 453–456. [Google Scholar] [CrossRef]
- Leonor, I.B.; Kim, H.-M.; Balas, F.; Kawashita, M.; Reis, R.L.; Kokubo, T.; Nakamura, T. Surface potential change in bioactive polymer during the process of biomimetic apatite formation in a simulated body fluid. Mater. Chem. 2007, 17, 4057–4063. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, S.; Whittaker, M.R.; Grøndahl, L.; Monteiro, M.J.; Wentrup-Byrne, E. Synthesis of soluble phosphate polymers by RAFT and their in vitro mineralization. Biomacromolecules 2006, 7, 3178–3187. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Rintoul, L.; Monteiro, M. J.; Wentrup-Byrne, E.; Grøndahl, L. In Vitro Mineralisation of Well-Defined Polymers and Surface. Ph.D. Thesis, Queensland University of Technology, Brisbane, Australia, 2007. [Google Scholar]
- Stancu, I.C.; Filmon, R.; Cincu, C.; Marculescu, B.; Zaharia, C.; Tourmen, Y.; Basle, M.F.; Chappard, D. Synthesis of methacryloyloxyethyl phosphate copolymers and in vitro calcification capacity. Biomaterials 2004, 25, 205–213. [Google Scholar] [CrossRef]
- Ethirajan, A.; Ziener, U.; Landfester, K. Surface-functionalized polymeric nanoparticles as templates for biomimetic mineralization of hydroxyapatite. Chem. Mater. 2009, 21, 2218–2225. [Google Scholar] [CrossRef]
- Miyazaki, T.; Ohtsuki, C.; Akioka, Y.; Tanihara, M.; Nakao, J.; Sakaguchi, Y.; Konagaya, S. Apatite deposition on polyamide films containing carboxyl group in a biomimetic solution. Mater. Sci. Mater. Med. 2003, 14, 569–574. [Google Scholar] [CrossRef]
- Kawai, T.; Ohtsuki, C.; Kamitakahara, M.; Miyazaki, T.; Tanihara, M.; Sakaguchi, Y.; Konagaya, S. Coating of an apatite layer on polyamide films containing sulfonic groups by a biomimetic process. Biomaterials 2004, 25, 4529–4534. [Google Scholar] [CrossRef] [PubMed]
- Sıdıra, Y.G.; Sıdıra, İ.; Taşalb, E.; Ermişc, E. Studies on the electronic absorption spectra of some monoazo derivatives. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 78, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Brogli, M.F.; Suarez, S.; Soldera, F.; Mücklich, F.; Barbero, C.A.; Bellingeri, R.; Alustiza, F.; Acevedo, A. Direct laser interference patterning of polystyrene films doped with azo dyes, using 355 nm laser light. Appl. Surf. Sci. 2014, 300, 86–90. [Google Scholar] [CrossRef]
- Deng, H.; Shen, X.C.; Wang, X.M.; Du, C. Calcium carbonate crystallization controlled by functional groups: A mini-review. Front. Mater. Sci. 2013, 7, 62–68. [Google Scholar] [CrossRef]
- Borissova, A.; Goltz, G.; Kavanagh, J.; Wilkins, T. Reverse engineering the kidney: Modelling calcium oxalate monohydrate crystallization in the nephron. Med. Biol. Eng. Comput. 2010, 48, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Whipss, S.; Khan, S.R.; O’Palko, F.J.; Backov, R.; Talham, D.R. Growth of calcium oxalate monohydrate at phospholipid Langmuir monolayers. J. Cryst. Growth 1998, 192, 243–249. [Google Scholar] [CrossRef]
- Letellier, S.R.; Lochhead, M.J.; Campbell, A.A.; Vogel, V. Oriented growth of calcium oxalate monohydrate crystals beneath phospholipid monolayers. Biochim. Biophys. Acta 1998, 1380, 31–45. [Google Scholar] [CrossRef]
- Ouyang, J.M.; Deng, S.P.; Zhong, J.P.; Tieke, B.; Yu, S.Y. Crystallization of calcium oxalate monohydrate at dipalmitophosphatidylcholine monolayers in the presence of chondroitin sulfate A. J. Cryst. Growth 2004, 270, 646–654. [Google Scholar] [CrossRef]
- Deng, S.P.; Ouyang, J.M.; Xie, Y.S.; Xing, F.Y. Circular patterns of calcium oxalate crystals induced by defective Langmuir-Blodgett films. Sci. China Ser. B Chem. 2008, 51, 25–30. [Google Scholar] [CrossRef]
- El-Shall, H.; Jeon, J.-H.; Abdel-Aal, E.A.; Khan, S.; Gower, L.; Rabinovich, Y. A study of primary nucleation of calcium oxalate monohydrate: I. Effect of supersaturation. Cryst. Res. Technol. 2004, 39, 214. [Google Scholar] [CrossRef]
- Didenko, L.; Tolardava, E.; Perpanova, T.; Shevlyagina, T.; Boravaya, Y.; Romanova, Y.; Cazzaniga, M.; Curia, R.; Milani, R.; Savoia, C.; et al. Electron microscopy investigation of urine stones suggests how to prevent post-operation septic complications in nephrolithiasis. J. Appl. Med. Sci. 2014, 3, 2241–2328. [Google Scholar]
- Kumar, N.; Singh, P.; Kumar, S. Physical, X-ray diffraction and SEM studies of uroliths. Indian J. Biochem. Biophys. 2006, 43, 226–332. [Google Scholar] [PubMed]
- Abdel-Aal, E.; Daosukho, S.; El-Shall, H. Effect of supersaturation ratio and Khella extract on nucleation and morphology of kidney stones. J. Cryst. Growth 2009, 311, 2673–2681. [Google Scholar] [CrossRef]
- Neira-Carrillo, A.; Luengo-Ponce, F.; Vásquez-Quitral, P.; Yazdani-Pedram, M.; Fernández, M.S.; Cölfen, H.; Arias, J.L. Sulfonated polymethylsiloxane as an additive for selective calcium oxalate crystallization. Eur. J. Inorg. Chem. 2015, 7, 1167. [Google Scholar] [CrossRef]
- Ghosh, S.; Basu, S.; Chakraborty, S.; Mukherjee, A.K. Structural and microstructural characterization of human kidney stones from eastern India using IR spectroscopy, scanning electron microscopy, thermal study and X-ray Rietveld analysis. J. Appl. Cryst. 2009, 42, 629–635. [Google Scholar] [CrossRef]
- Giannossi, M.L.; Summa, V. New mixed urinary stone: Review of classification scheme. Urologist 2012, 1, 2. [Google Scholar]
- Zhang, D.; Qi, L.; Ma, J.; Cheng, H. Morphological control of calcium oxalate dehydrate by a double-hydrophilic block copolymer. Chem. Mater. 2002, 14, 2450–2457. [Google Scholar] [CrossRef]
- Laffite, G.; Leroy, C.; Bonhomme, C.; Bonhomme-Coury, L.; Letavernier, E.; Daudon, M.; Frochot, V.; Haymann, J.P.; Rouzière, S.; Lucas, I.T.; et al. Calcium oxalate precipitation by diffusion using laminar microfluidics: Toward a biomimetic model of pathological microcalcifications. Lab Chip 2016, 16, 1157. [Google Scholar] [CrossRef] [PubMed]
- Kirboğa, S.; Öner, M. Inhibition of calcium oxalate crystallization by graft copolymers. Cryst. Growth Des. 2009, 9, 2159–2167. [Google Scholar] [CrossRef]
- Neira-Carrillo, A.; Vásquez-Quitral, P.; Sánchez, M.; Vargas-Fernández, A.; Silva, J.F. Control of calcium oxalate morphology through electrocristallization as an electrochemical approach for preventing pathological disease. Ionics 2015, 21, 3141–3149. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vásquez-Quitral, P.; Toledo Arana, J.; Miras, M.C.; Acevedo, D.F.; Barbero, C.A.; Neira-Carrillo, A. Effect of Diazotated Sulphonated Polystyrene Films on the Calcium Oxalate Crystallization. Crystals 2017, 7, 70. https://doi.org/10.3390/cryst7030070
Vásquez-Quitral P, Toledo Arana J, Miras MC, Acevedo DF, Barbero CA, Neira-Carrillo A. Effect of Diazotated Sulphonated Polystyrene Films on the Calcium Oxalate Crystallization. Crystals. 2017; 7(3):70. https://doi.org/10.3390/cryst7030070
Chicago/Turabian StyleVásquez-Quitral, Patricio, Javier Toledo Arana, Maria Cristina Miras, Diego Fernando Acevedo, Cesar Alfredo Barbero, and Andrónico Neira-Carrillo. 2017. "Effect of Diazotated Sulphonated Polystyrene Films on the Calcium Oxalate Crystallization" Crystals 7, no. 3: 70. https://doi.org/10.3390/cryst7030070





