Synthesis, Crystal Structure and Catalytic Activity of a 1D Chained Ca(II) Coordination Polymer with 3,5-Bis(4-pyridylmethoxy)benzoate Ligand
Abstract
:1. Introduction
2. Results and Discussion
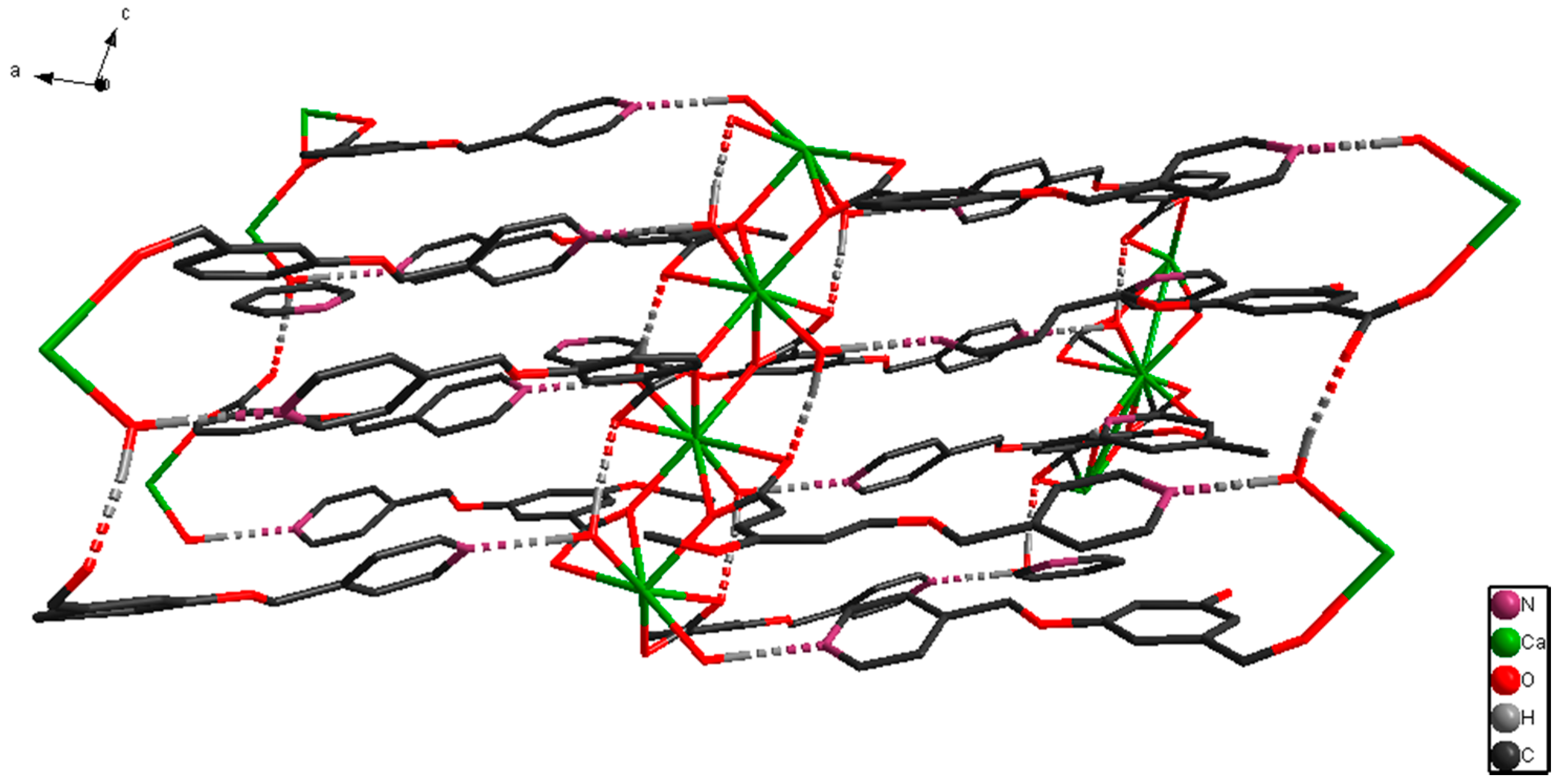
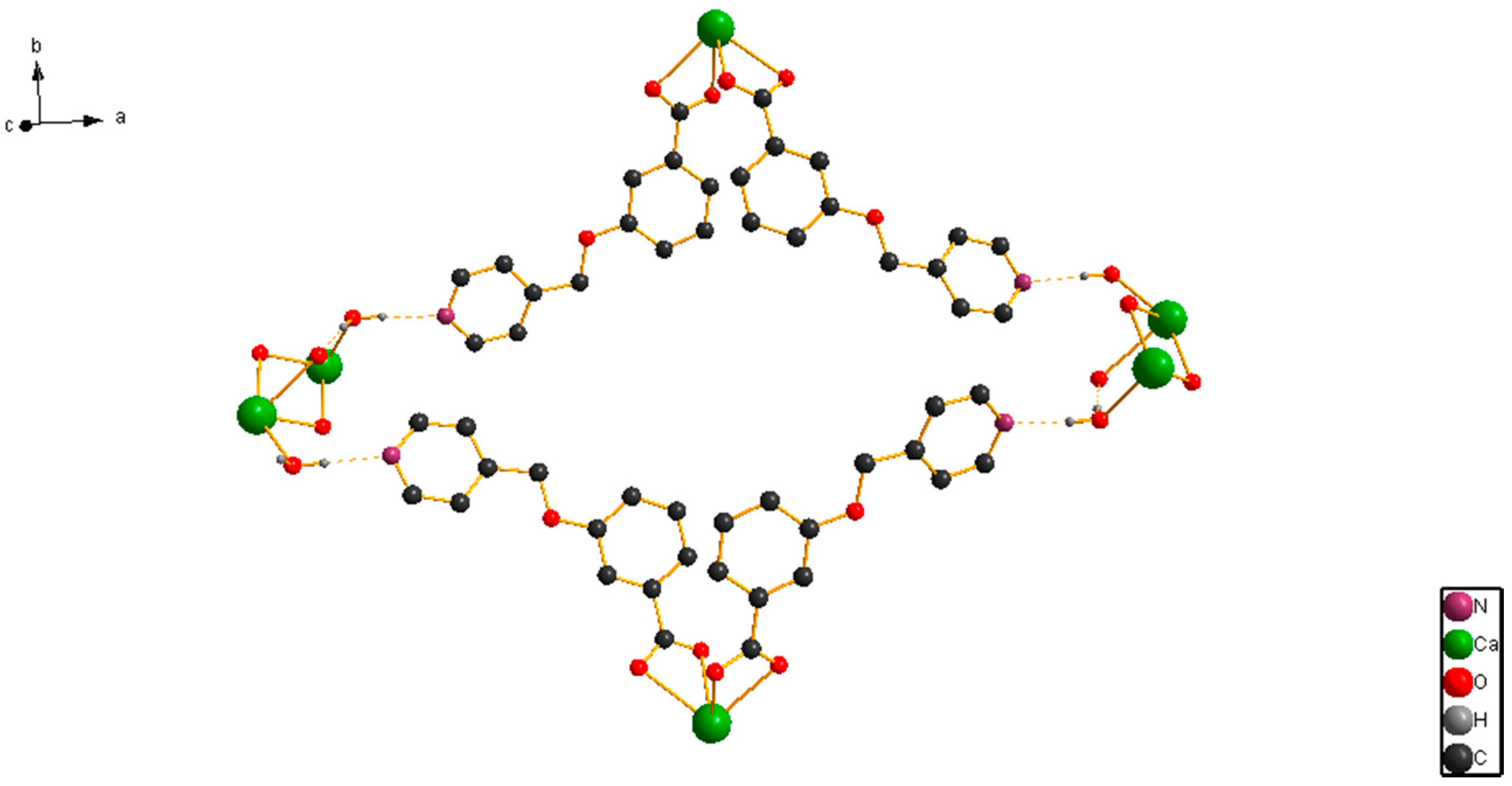
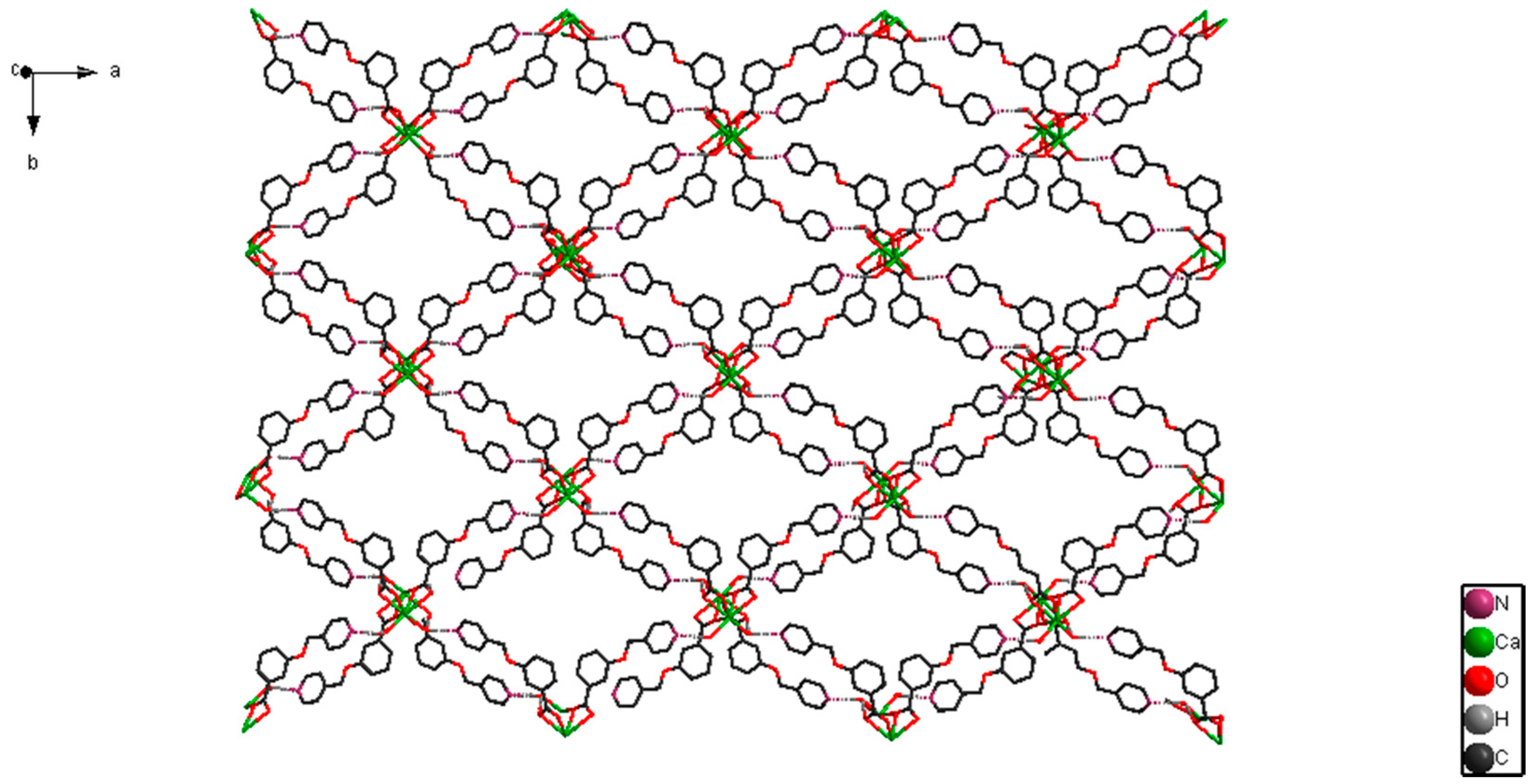
2.1. Structural Description of [CaL2(H2O)2]n
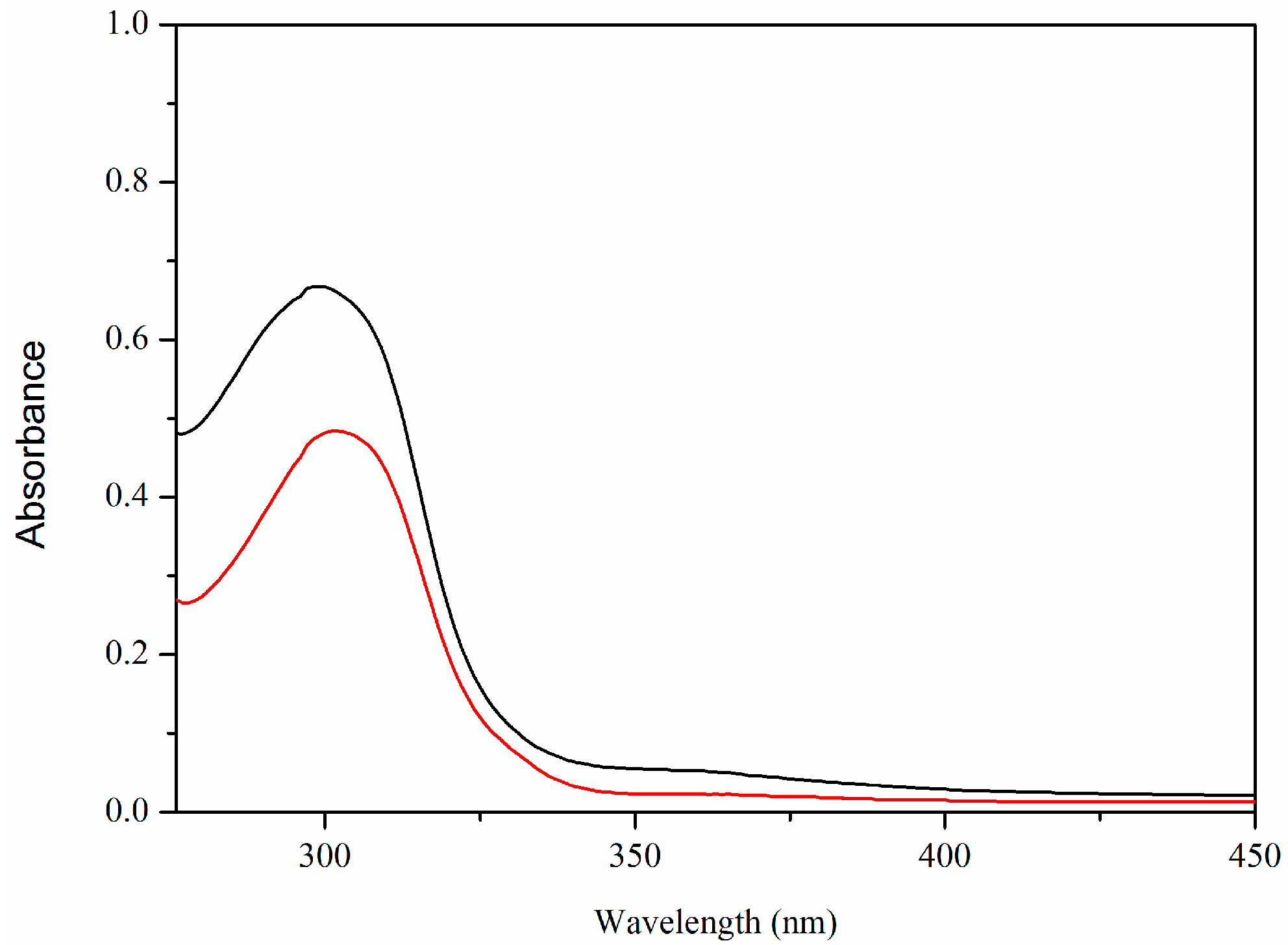
2.2. UV-Vis Spectra
2.3. Catalytic Studies of Three Component Coupling Reaction
3. Experimental Section
3.1. Materials and Instrumentation
3.2. Synthesis of [CaL2(H2O)2]n
3.3. Crystal Structure Determination
3.4. General Procedure for the Three Component Coupling Reaction (A3)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Yaghi, O.M. Reticular chemistry-construction, properties, and precision reactions of frameworks. J. Am. Chem. Soc. 2016, 138, 15507–15509. [Google Scholar] [CrossRef] [PubMed]
- Meundaeng, N.; Rujiwatra, A.; Prior, T.J. Copper coordination polymers constructed from thiazole-5-carboxylic acid: Synthesis, crystal structures, and structural transformation. J. Solid State Chem. 2017, 245, 138–145. [Google Scholar] [CrossRef]
- Chakraborty, P.; Mohanta, S. Syntheses, crystal structures, lone pair functionality and electrospray ionization mass spectral properties of trinuclear, dimer of trinuclear and trinuclear-based one-dimensional systems of copper(II) and lead(II). Inorg. Chim. Acta 2017, 455, 70–80. [Google Scholar] [CrossRef]
- Mukherjee, G.; Biradha, K. Topological Equivalences between Coordination Polymer and Cocrystal: A Tecton Approach in Crystal Engineering. Cryst. Growth Des. 2014, 14, 419–422. [Google Scholar] [CrossRef]
- Wang, X.P.; Han, L.L.; Lin, S.J.; Li, X.Y.; Mei, K.; Sun, D. Synthesis, structure and photoluminescence of three 2D Cd(II) coordination polymers based on varied dicarboxylate ligand. J. Coord. Chem. 2016, 69, 286–294. [Google Scholar] [CrossRef]
- Tai, X.S.; Wang, X. Synthesis and crystal structure of a 1D chained coordination polymers constructed from Ca2+ and 2-[(E)-(2-furoylhydrazono)methyl]benzenesulfonate. Crystals 2015, 5, 458–465. [Google Scholar] [CrossRef]
- Shen, J.J.; Li, M.X.; Wang, Z.X.; Duan, C.Y.; Zhu, S.R.; He, X.S. Unexpected 4-fold [2 + 2] interpenetration and polycatenation behaviors in porous luminescent zinc metal-organic frameworks constructed from flexible 3,5-bis(4-pyridylmethoxy) benzoate ligand. Cryst. Growth Des. 2014, 14, 2818–2830. [Google Scholar] [CrossRef]
- Li, T.; Huang, X.H.; Zhao, Y.F.; Li, H.H.; Wu, S.T.; Huang, C.C. An unusual double T5(2) water tape trapped in silver(I) coordination polymer hosts: Influence of the solvent on the assembly of Ag(I)-4,4′-bipyridine chains with trans-cyclohexanedicarboxylate and their luminescent properties. Dalton Trans. 2012, 41, 12872–12881. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.Y.; Yang, D.D.; Li, N.; Huang, R.D. Ligands effect on the structures of a series of coordination polymers: Syntheses, structures, luminescence and magnetism. Inorg. Chim. Acta 2015, 427, 285–292. [Google Scholar] [CrossRef]
- Bagherzadeh, M.; Ashouri, F.; Đaković, M. Synthesis, structural characterization and application of a 2D coordination polymer of Mn-terephthalate as a heterogeneous catalyst for olefin oxidation. Polyhedron 2014, 69, 167–173. [Google Scholar] [CrossRef]
- Yan, K.K.; Fujita, M. A speedy marriage in supramolecular catalysis. Science 2015, 350, 1165–1166. [Google Scholar] [CrossRef] [PubMed]
- Tai, X.S.; Zhao, W.H. Synthesis, crystal structure and antitumor activity of Ca(II) coordination polymer based on 1,5-naphthalenedisulfonate. J. Inorg. Organomet. Polym. Mater. 2013, 23, 1354–1357. [Google Scholar] [CrossRef]
- Liu, C.B.; Li, Q.; Wang, X.; Che, G.B.; Zhang, X.J. A series of lanthanide(III) coordination polymers derived via in situ hydrothermal decarboxylation of quinoline-2,3-dicarboxylic acid. Inorg. Chem. Commun. 2014, 39, 56–60. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, T.; Zhang, L.; Li, C.C. Structures and properties of coordination polymers based on 5-nitroisophthalic acid and N,N′-bis(4-pyridyl-methyl) piperazine. Z. Anorg. Allg. Chem. 2014, 640, 2503–2507. [Google Scholar] [CrossRef]
- Sharif, S.; Şahin, O.; Khan, B.; Khan, I.U. Hydrothermal synthesis, structural investigation, and magnetic properties of 2-D layered lanthanide (Ln = Pr, Eu, Gd, Tb, and Er) coordination polymers possessing infinite 1-D nanosized cavities. J. Coord. Chem. 2015, 68, 2725–2738. [Google Scholar] [CrossRef]
- Tai, X.S.; Wang, X. Synthesis, characterization and antitumor activity of a Ca(II) coordination polymer based on 3-amino-2-pyrazinecarboxylic acid. Sci. Stud. Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2015, 16, 253–259. [Google Scholar]
- Tai, X.S.; Zhao, W.H. Synthesis, crystal structure, and antibacterial activity of magnesium(II) coordination polymers formed by hydrogen bonding. Res. Chem. Intermed. 2015, 41, 3471–3478. [Google Scholar] [CrossRef]
- Tai, X.S.; Zhao, W.H. Synthesis, structural characterization, and antitumor activity of a Ca(II) coordination polymer based on 1,6-naphthalenedisulfonate and 4,4′-bipyridyl. Materials 2013, 6, 3547–3555. [Google Scholar] [CrossRef]
- Tai, X.S.; Jiang, J.H. Synthesis, crystal structure and luminescent property of Cd(II) complex with N-benzenesulphonyl-l-leucine. Materials 2012, 5, 1626–1634. [Google Scholar] [CrossRef]
- Tai, X.S.; Wang, X.; You, H.Y. Synthesis, crystal structure and antitumor activity of a new Zn(II) complex based on N-acetyl-l-phenylalanine and 1,10-phenanthroline. Chin. J. Struct. Chem. 2016, 35, 586–590. [Google Scholar]
- Ashiry, K.O.; Zhao, Y.H.; Shao, K.Z.; Su, Z.M.; Xu, G.J. Syntheses and characterizations of three coordination polymers based on dipyridylbenzoates and 1,4-bezenedicarboxylate. Polyhedron 2009, 28, 975–979. [Google Scholar] [CrossRef]
- Xu, G.J.; Zhao, Y.H.; Shao, K.Z.; Lan, Y.Q.; Wang, X.L.; Su, Z.M.; Yan, L.K. Secondary ligand-directed assembly of ZnII and CdII coordination architectures: From 1D to 3D compounds based on pyridine carboxylate ligands. J. Mol. Struct. 2010, 983, 93–98. [Google Scholar] [CrossRef]
- Xu, G.J.; Zhao, Y.H.; Shao, K.Z.; Xu, Y.H.; Wang, X.L.; Su, Z.M.; Yan, L.K. Syntheses and characterization of two coordination polymers constructed by the ligand 3,5-bis(pyridin-4-ylmethoxy)benzoic Acid. Z. Anorg. Allg. Chem. 2009, 635, 2671–2675. [Google Scholar]
- Ashiry, K.O.; Zhao, Y.H.; Shao, K.Z.; Su, Z.M.; Fu, Y.M.; Hao, X.R. A metal–organic framework containing meso-helical chains: Synthesis, characterization and luminescent property. Inorg. Chem. Commun. 2008, 11, 1181–1183. [Google Scholar] [CrossRef]
- Xu, G.J.; Zhao, Y.H.; Shao, K.Z.; Yuan, G.; Wang, X.L.; Su, Z.M.; Yan, L.K. A novel luminescent 3D metal–organic framework possessing 4-fold interpenetrating (3,4)-connected net. Inorg. Chem. Commun. 2009, 12, 969–971. [Google Scholar] [CrossRef]
- Tai, X.S.; Liu, L.L.; Yin, J. Synthesis, crystal structure of tetra-nuclear macrocyclic Cu(II) complex material and its application as catalysts for A3 coupling reaction. J. Inorg. Organomet. Polym. 2014, 24, 1014–1020. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]

| Bond | d | Angle | (°) |
|---|---|---|---|
| Ca1–O1W | 2.3811(12) | O1W–Ca1–O1WA | 105.58(6) |
| Ca1–O1WA | 2.3811(12) | O1W–Ca1–O1WA | 76.31(4) |
| Ca1–O1A | 2.3815(11) | O1A–Ca1–O1WA | 87.98(4) |
| Ca1–O1 | 2.3815(11) | O1W–Ca1–O1 | 87.98(4) |
| Ca1–O1B | 2.4935(12) | O1–Ca1–O1WA | 76.31(4) |
| Ca1–O1C | 2.4935(12) | O1–Ca1–O1A | 154.00(5) |
| Ca1–O2B | 2.6350(11) | O1W–Ca1–O1B | 152.63(4) |
| Ca1–O2C | 2.6350(11) | O1B–Ca1–O1WA | 88.93(4) |
| O1–C1 | 1.2592(17) | O1B–Ca1–O1A | 128.26(4) |
| O2–C1 | 1.2512(17) | O1–Ca1–O1B | 72.79(4) |
| O3–C4 | 1.3741(18) | O1W–Ca1–O1C | 88.93 (4) |
| O3–C8 | 1.4302(19) | O1C–Ca1–O1WA | 152.63(4) |
| N1–C12 | 1.324(3) | O1C–Ca1–O1A | 72.79(4) |
| N1–C11 | 1.328(3) | O1–Ca1–O1C | 128.26(4) |
| N2–C17 | 1.323(3) | O1B–Ca1–O1C | 88.08(5) |
| N2–C18 | 1.324(3) | O1W–Ca1–O2B | 152.45(4) |
| O2B–Ca1–O1WA | 82.41(4) | ||
| O2B–Ca1–O1A | 77.71(4) | ||
| O1–Ca1–O2B | 119.57(4) | ||
| O2B–Ca1–O1B | 50.71(4) | ||
| O2B–Ca1–O1C | 74.71(4) | ||
| O2C–Ca1–O1W | 82.41(4) | ||
| O1WA–Ca1–O2C | 152.45(4) | ||
| O2C–Ca1–O1A | 119.57(4) | ||
| O1–Ca1–O2C | 77.71(4) | ||
| O2C–Ca1–O1B | 74.71(4) | ||
| O1C–Ca1–O2C | 50.71(4) | ||
| O2B–Ca1–O2C | 102.80(5) |
| Empirical Formula | C38H34CaN4O10 |
|---|---|
| Formula weight | 746.77 |
| Temperature/K | 293(2) |
| Crystal system | Monoclinic |
| Space group | C2/c |
| a/Å | 25.791(5) |
| b/Å | 18.986(4) |
| c/Å | 7.4359(15) |
| α/° | 90 |
| β/° | 102.17(3) |
| γ/° | 90 |
| Volume/Å3 | 3559.4(12) |
| Z | 4 |
| ρcalc mg/mm3 | 1.394 |
| μ/mm−1 | 0.24 |
| S | 1.06 |
| F(000) | 1560 |
| Index ranges | −30 ≤ h ≤ 30, −22 ≤ k ≤ 22, −8 ≤ l ≤ 8 |
| Reflections collected | 13,423 |
| Independent reflections | 3121 [R(int) = 0.032] |
| Data/restraints/parameters | 3121/2/240 |
| Goodness-of-fit on F2 | 1.06 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0354, wR2 = 0.0903 |
| Final R indexes [all data] | R1 = 0.0403, wR2 = 0.0948 |
| Largest diff. peak/hole/e·Å−3 | 0.321/−0.213 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.-H.; Wang, X.; Tai, X.-S. Synthesis, Crystal Structure and Catalytic Activity of a 1D Chained Ca(II) Coordination Polymer with 3,5-Bis(4-pyridylmethoxy)benzoate Ligand. Crystals 2017, 7, 72. https://doi.org/10.3390/cryst7030072
Wang L-H, Wang X, Tai X-S. Synthesis, Crystal Structure and Catalytic Activity of a 1D Chained Ca(II) Coordination Polymer with 3,5-Bis(4-pyridylmethoxy)benzoate Ligand. Crystals. 2017; 7(3):72. https://doi.org/10.3390/cryst7030072
Chicago/Turabian StyleWang, Li-Hua, Xin Wang, and Xi-Shi Tai. 2017. "Synthesis, Crystal Structure and Catalytic Activity of a 1D Chained Ca(II) Coordination Polymer with 3,5-Bis(4-pyridylmethoxy)benzoate Ligand" Crystals 7, no. 3: 72. https://doi.org/10.3390/cryst7030072






