The Effects of ACRT on the Growth of ZnTe Crystal by the Temperature Gradient Solution Growth Technique
Abstract
:1. Introduction
2. Modeling Approaches
3. Results and Discussion
3.1. Computational Strategy
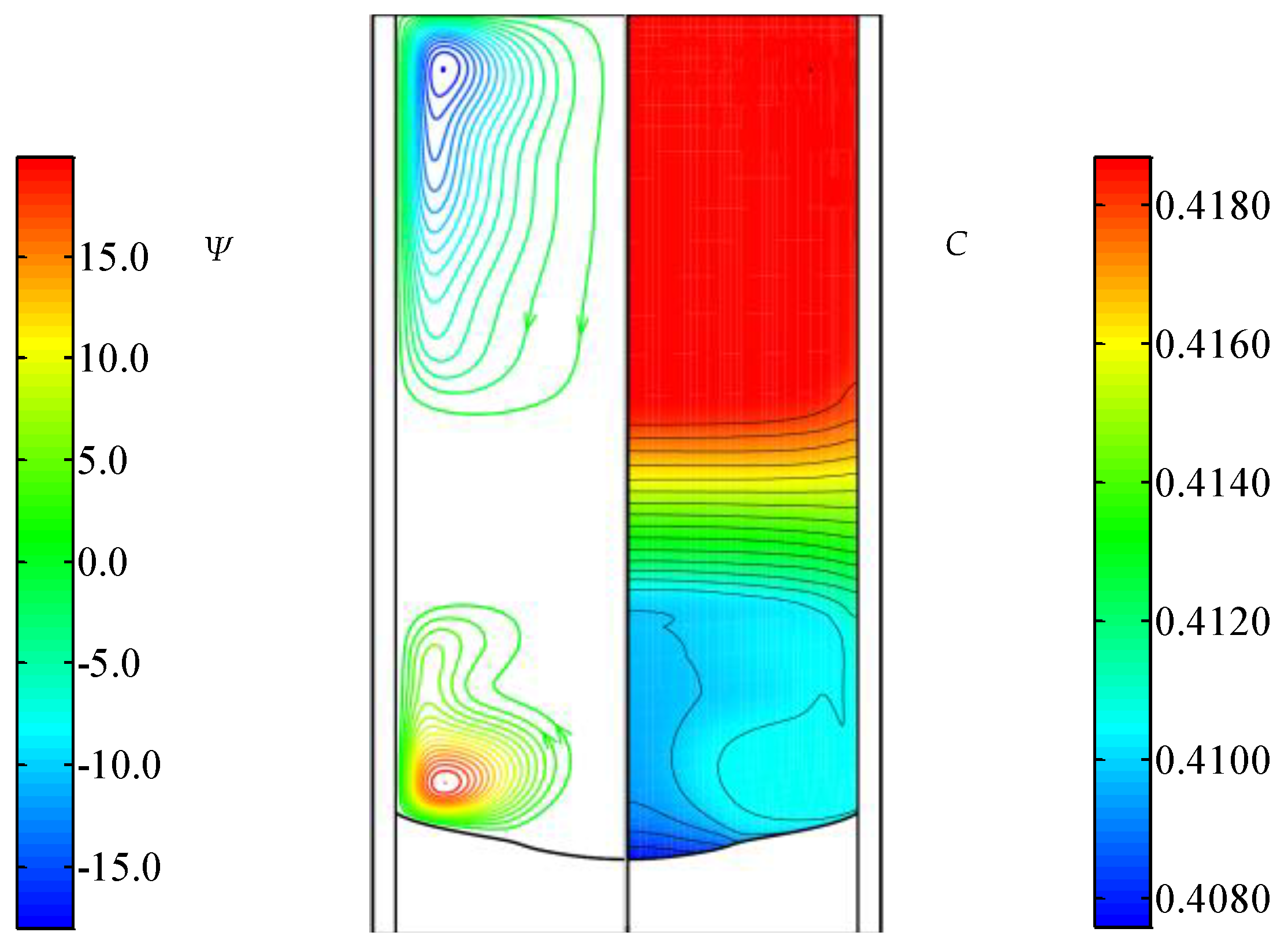
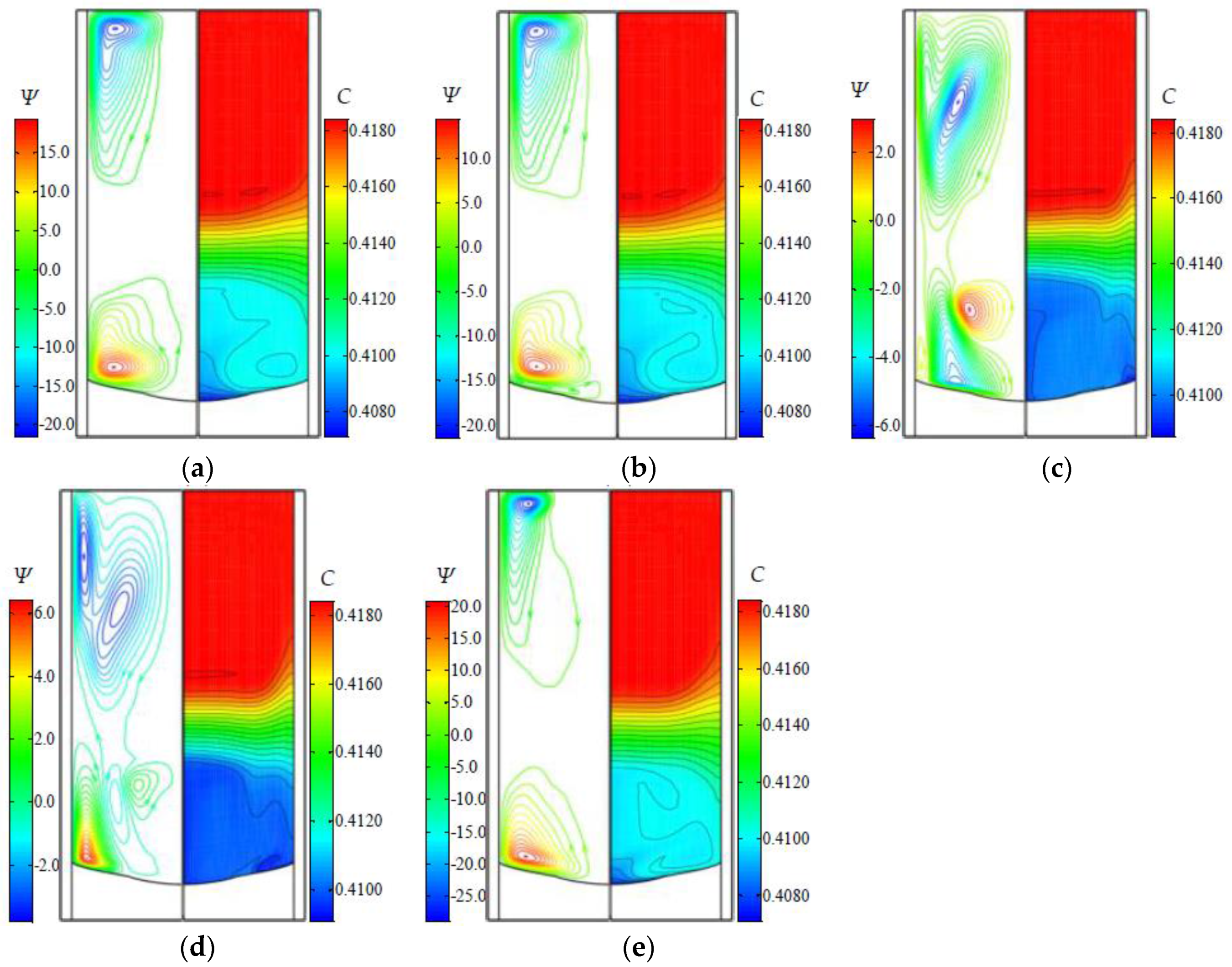
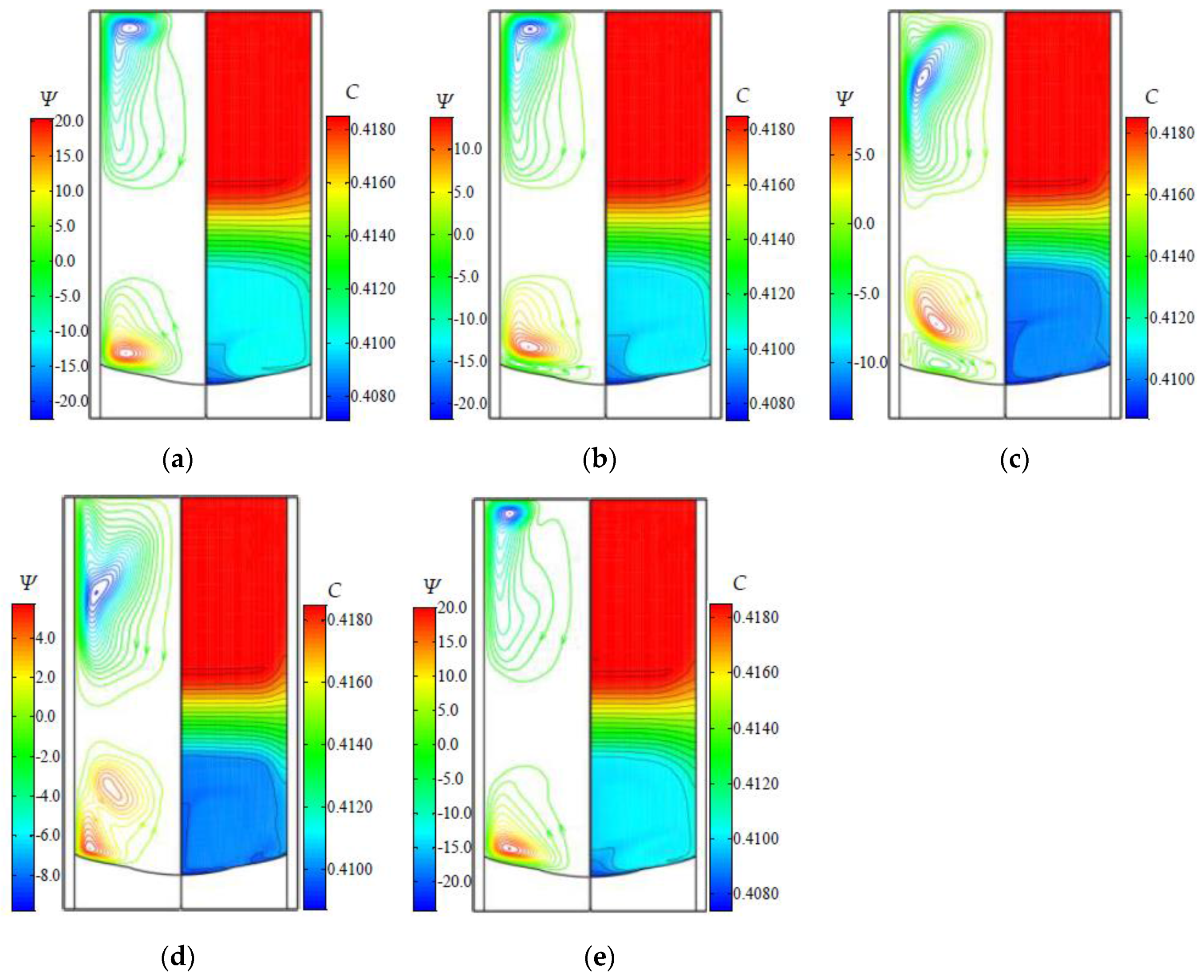
3.2. The Flow and Concentration Fields
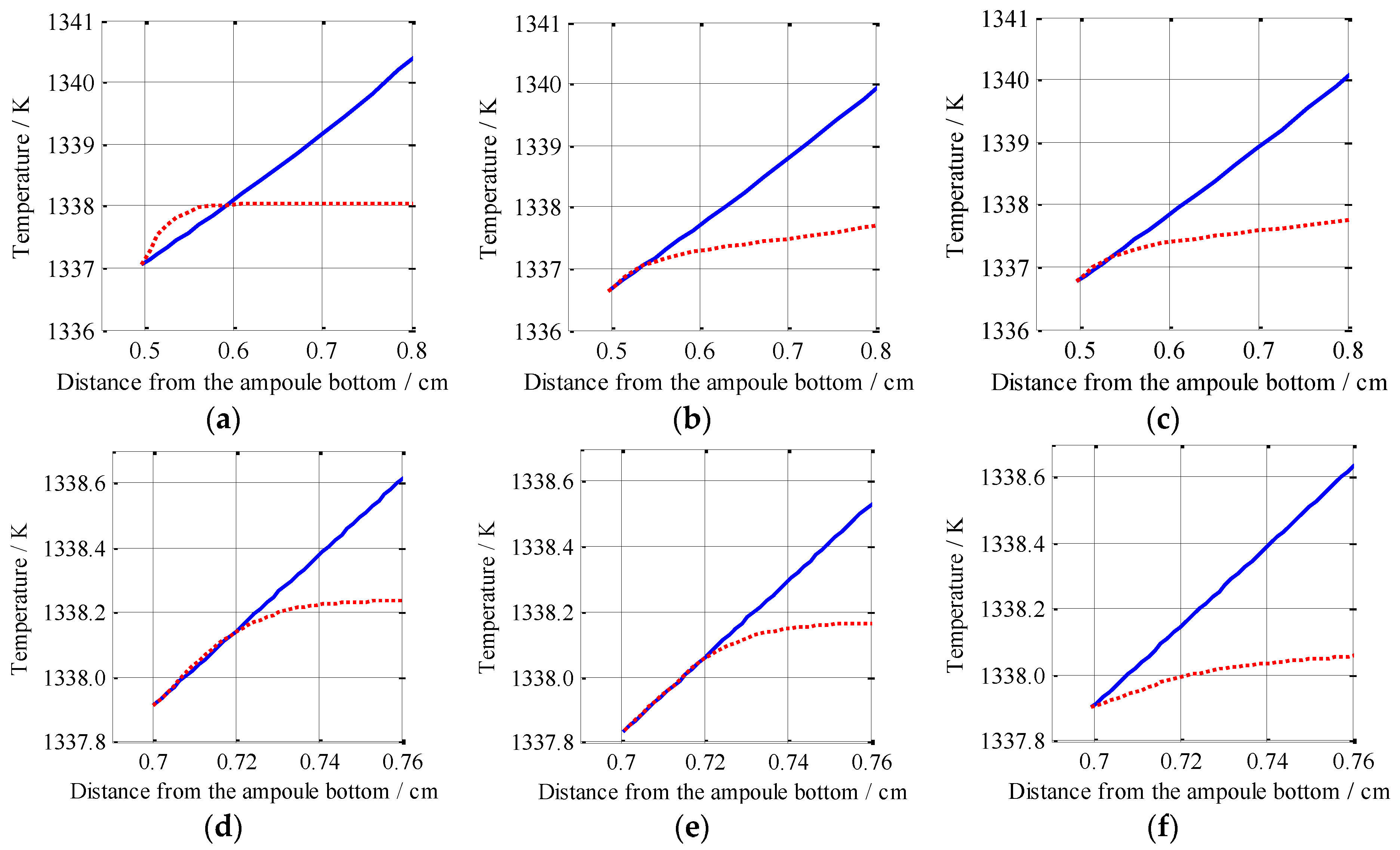
3.3. Analysis on the Constitutional Supercooling
3.4. Effects of ACRT on the Growth Interface Morphology
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yoshino, K.; Yoneta, M.; Ohmori, K.; Saito, H.; Ohishi, M.; Yabe, T. Annealing effects of a high-quality ZnTe substrate. J. Electron. Mater. 2004, 33, 579–582. [Google Scholar] [CrossRef]
- Traversa, M.; Lovergine, N.; Prete, P.; Tapfer, L.; Mancini, A.M. Homoepitaxy of ZnTe on (100) oriented substrates: Technology issues and MOVPE growth aspects. Cryst. Res. Technol. 2005, 40, 1003–1010. [Google Scholar] [CrossRef]
- Kaneta, A.; Adachi, S. Photoreflectance study in the E1 and E1 + Δ1 transition regions of ZnTe. J. Phys. D Appl. Phys. 2000, 33, 901–905. [Google Scholar] [CrossRef]
- Rusu, G.I.; Prepelita, P.; Apetroaei, N.; Popa, G. On the electronic transport and optical properties of ZnTe thin films. J. Optoelectron. Adv. Mater. 2005, 7, 829–883. [Google Scholar]
- Blanchard, F.; Razzari, L.; Bandulet, H.C.; Sharma, G.; Morandotti, R.; Kieffer, J.C.; Ozaki, T.; Reid, M.; Tiedje, H.F.; Haugen, H.K.; et al. Generation of 1.5 μJ single-cycle terahertz pulses by optical rectification from a large aperture ZnTe crystal. Opt. Express 2007, 15, 13212–13220. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.; Zhang, X.C. Materials for terahertz science and technology. Nat. Mater. 2002, 1, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Seki, Y.; Sato, K.; Oda, O. Solution growth of ZnTe single crystals by the vertical Bridgman method using a hetero-seeding technique. J. Cryst. Growth 1997, 171, 32–38. [Google Scholar] [CrossRef]
- Sato, K.; Seki, Y.; Oda, O. Solution growth combined with solvent evaporation: A novel technique in solution growth. Jpn. J. Appl. Phys. 1999, 38, 5772–5774. [Google Scholar] [CrossRef]
- Uen, W.Y.; Chou, S.Y.; Shin, H.Y.; Liao, S.M.; Lan, S.M. Characterizations of ZnTe bulks grown by temperature gradient solution growth. Mater. Sci. Eng. B 2004, 106, 27–32. [Google Scholar] [CrossRef]
- Yang, R.; Jie, W.; Liu, H. Growth of ZnTe single crystals from Te solution by vertical Bridgman method with ACRT. J. Cryst. Growth 2014, 400, 27–33. [Google Scholar] [CrossRef]
- Scheel, H.J.; Schulz-Dubois, E.O. Flux growth of large crystals by accelerated crucible-rotation technique. J. Cryst. Growth 1971, 8, 304–306. [Google Scholar] [CrossRef]
- Schulz-Dubois, E.O. Accelerated crucible rotation: Hydrodynamics and stirring effect. J. Cryst. Growth 1972, 12, 81–87. [Google Scholar] [CrossRef]
- Capper, P.; Gosney, J.J.G.; Jones, C.L. Application of the accelerated crucible rotation technique to the Bridgman growth of CdxHg1−xTe: Simulations and crystal growth. J. Cryst. Growth 1984, 70, 356–364. [Google Scholar] [CrossRef]
- Capper, P.; Gosney, J.J.G.; Jones, C.L.; Pearce, E.J. Fluid flows induced in tall narrow containers by A.C.R.T. J. Electron. Mater. 1986, 15, 361–370. [Google Scholar] [CrossRef]
- Capper, P.; Gosney, J.J.G.; Jones, C.L.; Kenworthy, I. Bridgman growth of CdxHg1−xTe using ACRT. J. Electron. Mater. 1986, 15, 371–376. [Google Scholar] [CrossRef]
- Brice, J.C.; Capper, P.; Jones, C.L.; Gosney, J.J.G. ACRT: A review of models. Prog. Cryst. Growth Charact. 1986, 13, 197–229. [Google Scholar] [CrossRef]
- Capper, P.; Brice, J.C. Interfaces and flow regimes in ACRT grown CdxHg1−xTe crystals. J. Cryst. Growth 1988, 89, 171–176. [Google Scholar] [CrossRef]
- Coates, W.G.; Capper, P.; Jones, C.L.; Gosney, J.J.G.; Ard, C.K.; Kenworthy, I.; Clark, A. Effect of ACRT rotation parameters on Bridgman grown CdxHg1−xTe crystals. J. Cryst. Growth 1989, 94, 959–966. [Google Scholar] [CrossRef]
- Capper, P. The role of accelerated crucible rotation in the growth of Hg1−xCdxTe and CdTe/CdZnTe. Prog. Cryst. Growth Charact. Mater. 1994, 28. [Google Scholar] [CrossRef]
- Yeckel, A.; Derby, J.J. Effect of accelerated crucible rotation on melt composition in high-pressure vertical Bridgman growth of cadmium zinc telluride. J. Cryst. Growth 2000, 209, 734–750. [Google Scholar] [CrossRef]
- Liu, X.; Jie, W.; Zhou, Y. Numerical analysis on Hg1−xCdxTe growth by ACRT-VBM. J. Cryst. Growth 2000, 209, 751–762. [Google Scholar] [CrossRef]
- Liu, X.; Jie, W.; Zhou, Y. Numerical analysis of CdZnTe crystal growth by the vertical Bridgman method using the accelerated crucible rotation technique. J. Cryst. Growth 2000, 219, 22–31. [Google Scholar]
- Lan, C. Flow and segregation control by accelerated rotation for vertical Bridgman growth of cadmium zinc telluride: ACRT versus vibration. J. Cryst. Growth 2005, 274, 379–386. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Yasuda, H.; Okano, Y.; Dost, S. Numerical simulation of crystal growth of CdZnTe by Vertical Gradient Freezing method-Crucible Rotation Effect. Int. J. Transp. Phenom. 2005, 7, 175–187. [Google Scholar]
- Liu, J. ACRT forced convection and its effects on solute segregation and heat and mass transfer during single crystal growth. Cryst. Res. Technol. 2008, 43, 396–408. [Google Scholar]
- Yin, L.; Jie, W.; Wang, T.; Zhou, B.; Yang, F. The transport phenomena during the growth of ZnTe crystal by the temperature gradient solution growth technique. J. Cryst. Growth 2017, 461, 16–24. [Google Scholar] [CrossRef]
- Yeckel, A.; Goodwin, R.T. Cats2D: Crystallization and Transport Simulator, User Manual. 2015. Available online: http://cats2d.com/overview.html (accessed on 9 July 2015).




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, L.; Jie, W.; Wang, T.; Zhou, B.; Yang, F.; Nan, R. The Effects of ACRT on the Growth of ZnTe Crystal by the Temperature Gradient Solution Growth Technique. Crystals 2017, 7, 82. https://doi.org/10.3390/cryst7030082
Yin L, Jie W, Wang T, Zhou B, Yang F, Nan R. The Effects of ACRT on the Growth of ZnTe Crystal by the Temperature Gradient Solution Growth Technique. Crystals. 2017; 7(3):82. https://doi.org/10.3390/cryst7030082
Chicago/Turabian StyleYin, Liying, Wanqi Jie, Tao Wang, Boru Zhou, Fan Yang, and Ruihua Nan. 2017. "The Effects of ACRT on the Growth of ZnTe Crystal by the Temperature Gradient Solution Growth Technique" Crystals 7, no. 3: 82. https://doi.org/10.3390/cryst7030082




