Growth of High Quality Al-Doped CsLiB6O10 Crystals Using Cs2O-Li2O-MoO3 Fluxes
Abstract
:1. Introduction
2. Experimental
2.1. Crystal Growth
2.2. Characterization
3. Results and Discussion
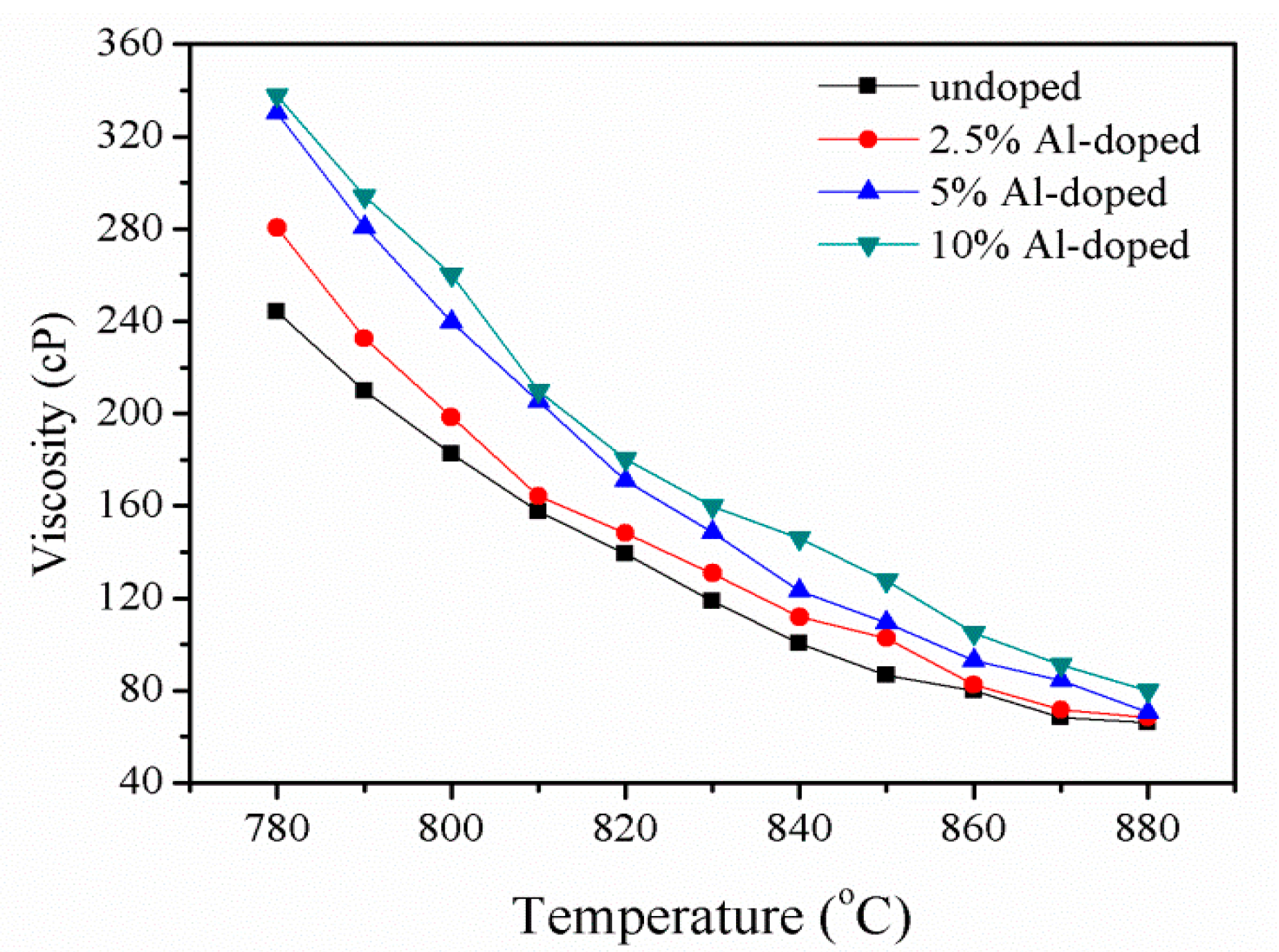
3.1. Crystal Growth and Viscosity Measurement
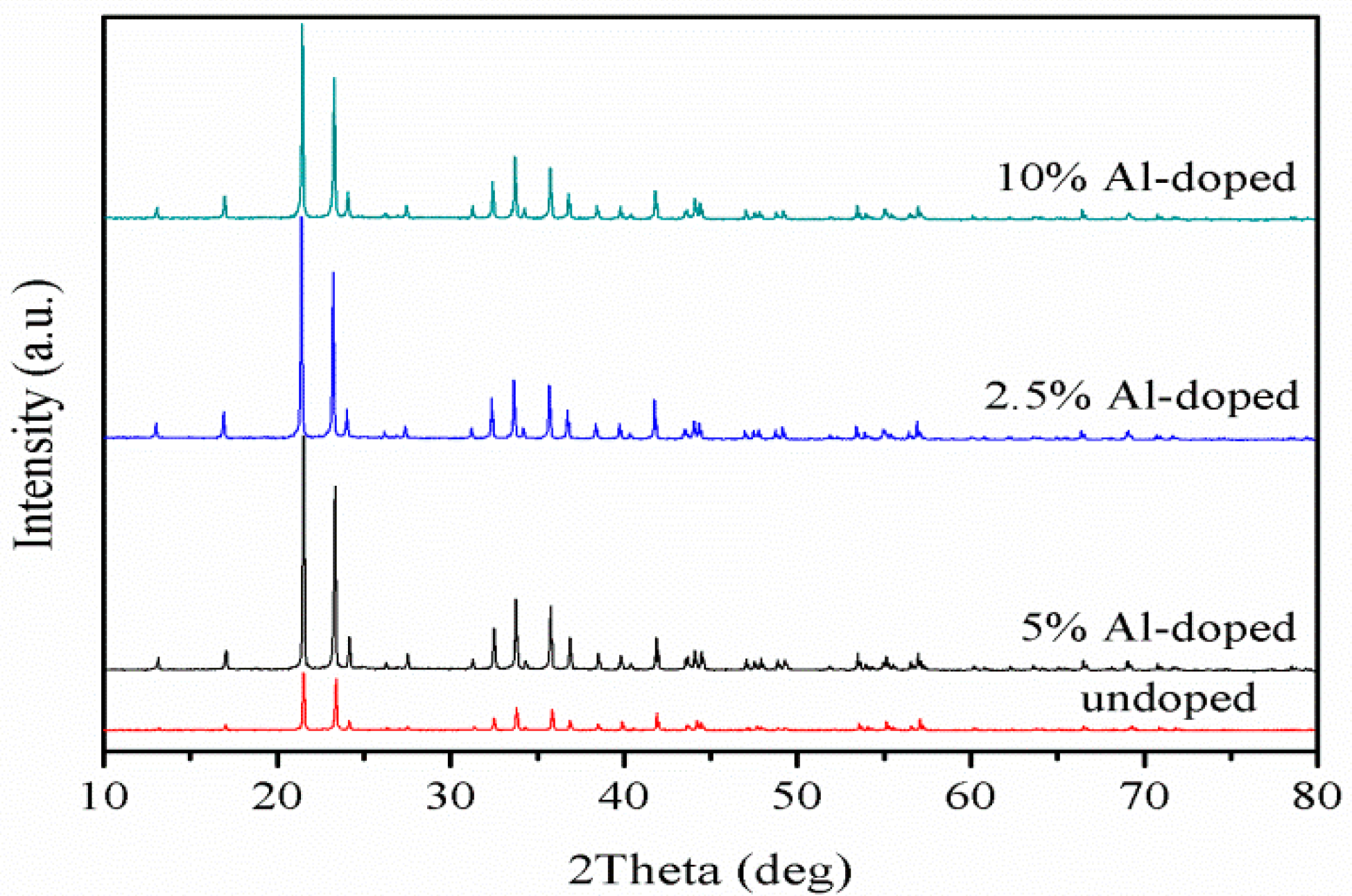
3.2. XRD and ICP Analysis
3.3. Transmission Spectrum
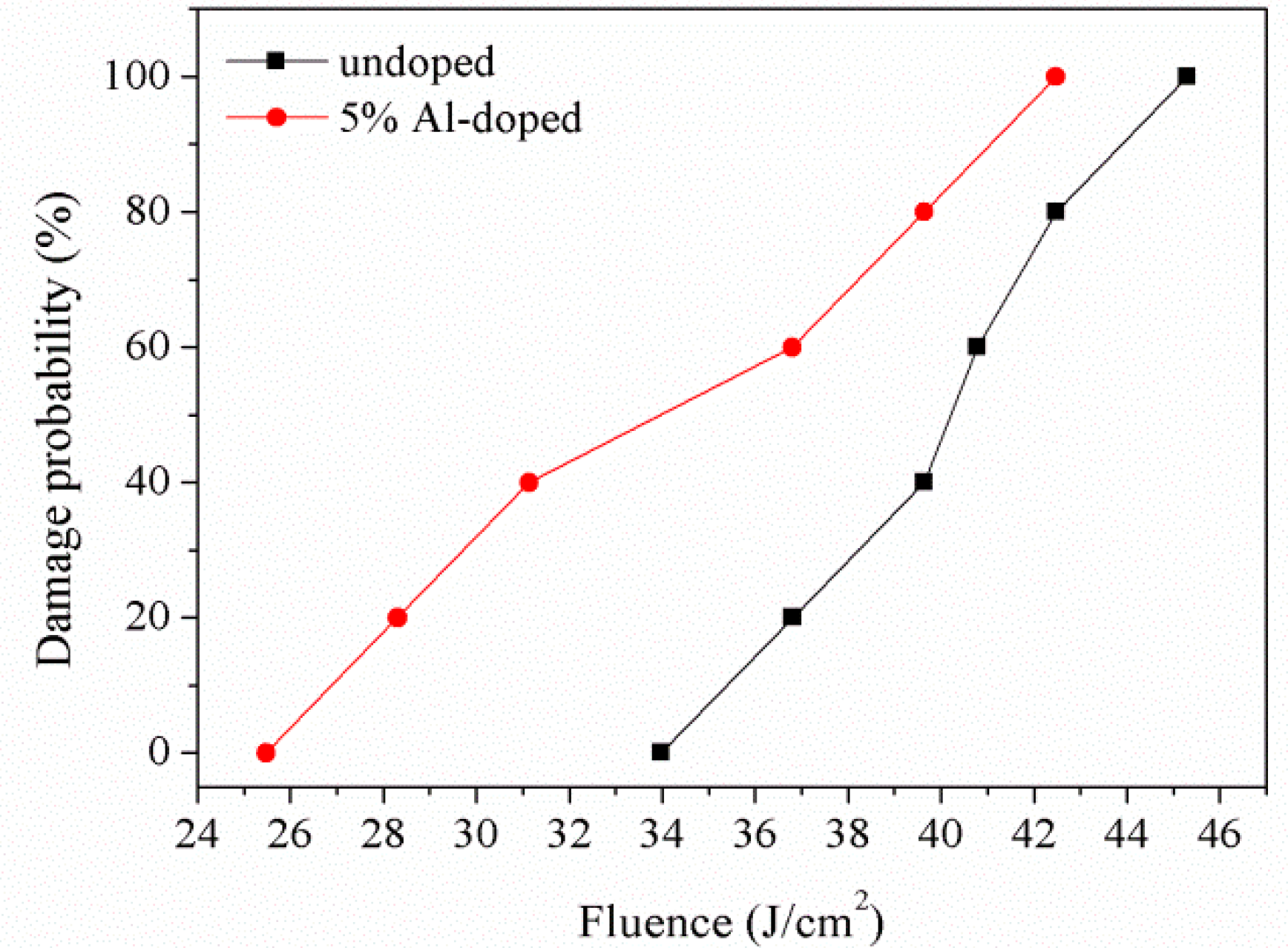
3.4. Laser-Induced Damage Threshold
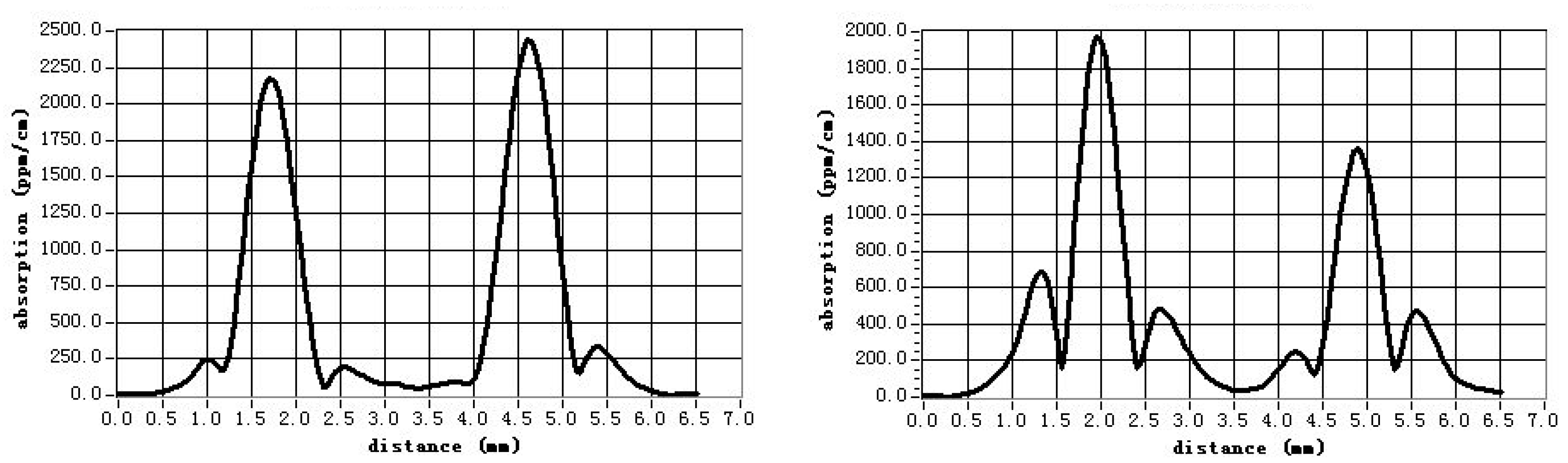
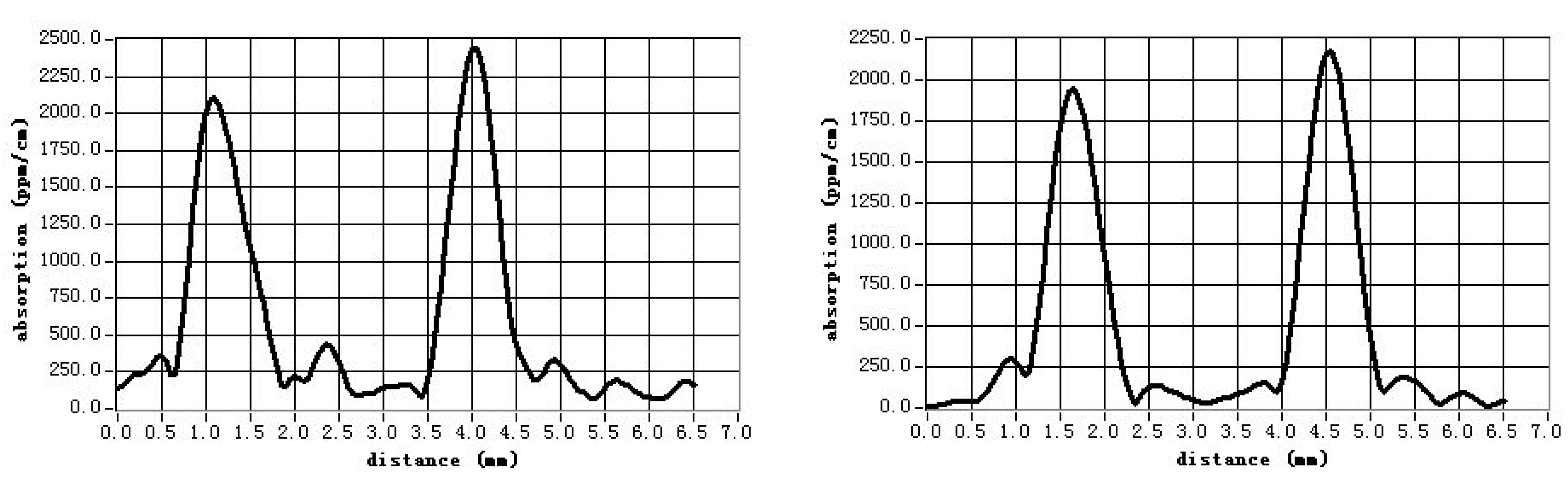
3.5. Optical Weak Absorption
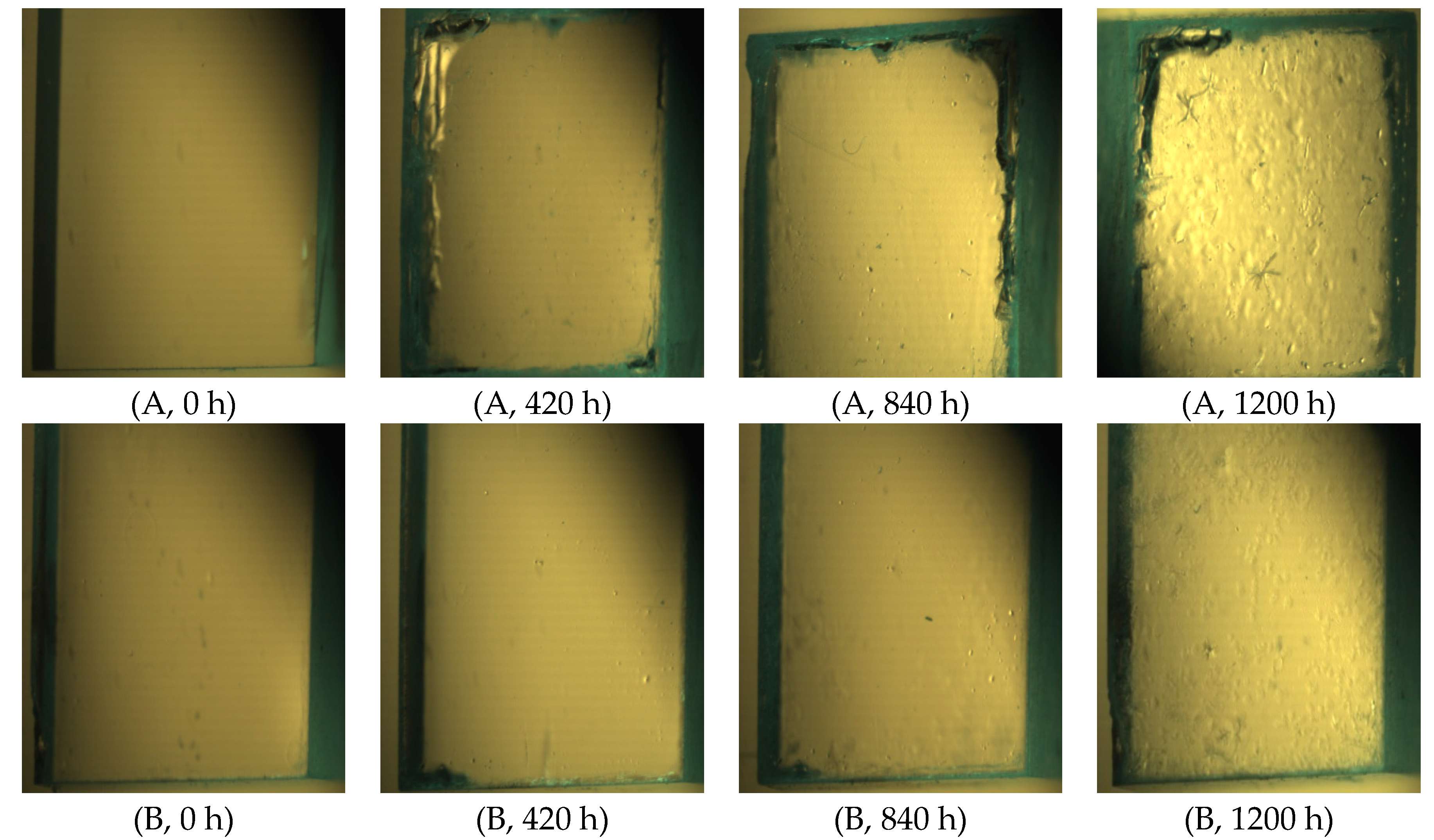
3.6. Humidity Experiment
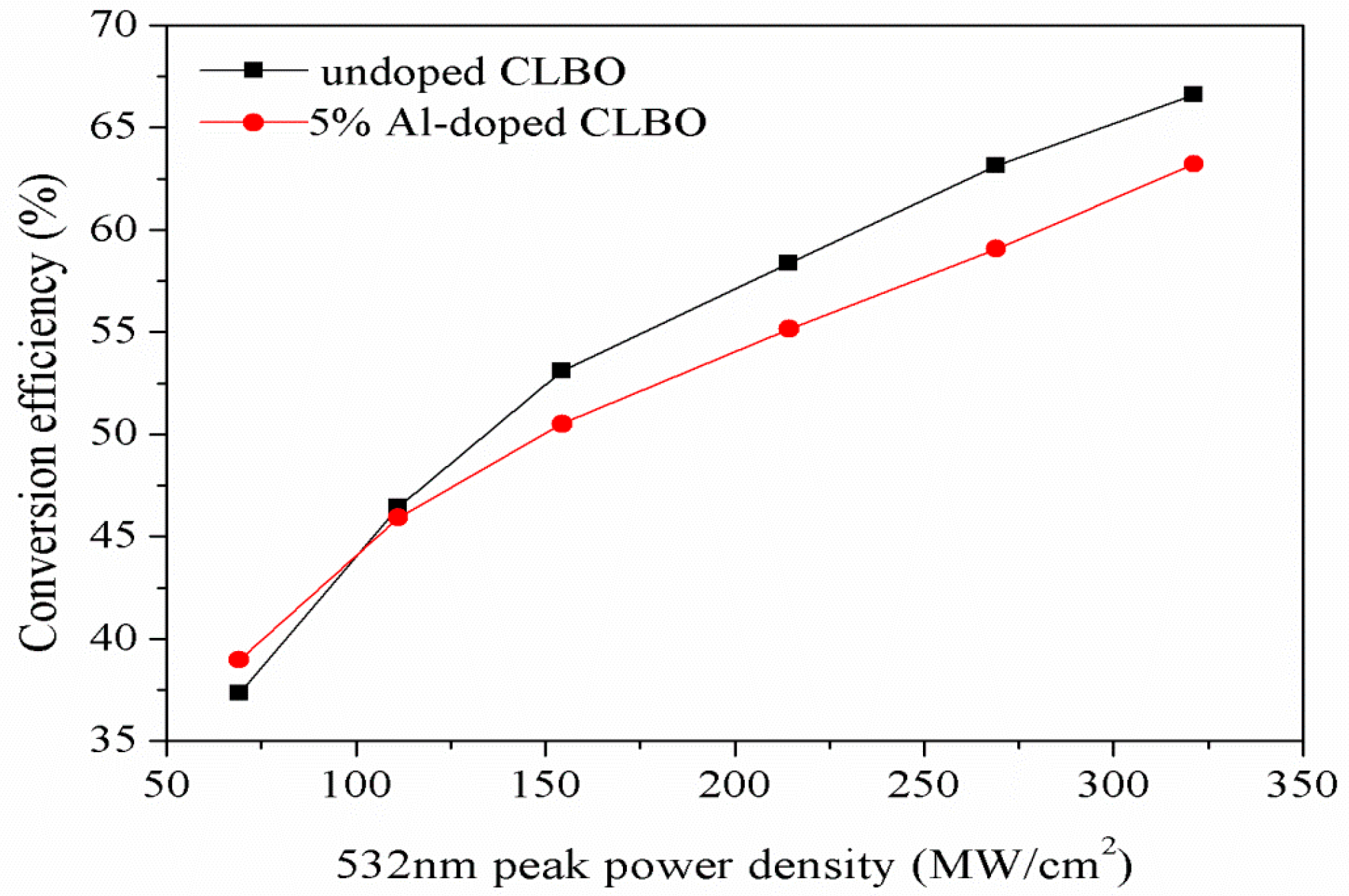
3.7. 266 nm UV Laser Conversion Efficiency
4. Conclusions
Acknowledgment
Author Contributions
Conflicts of Interest
References
- Arizmendi, L. Photonic applications of lithium niobate crystals. Phys. Status Solidi A. 2004, 201, 253–283. [Google Scholar] [CrossRef]
- Wang, G.; Liu, J.; Liu, S.; Liu, M.; Liu, S.; Li, L. Short pulse duration of a 355-nm laser by extracavity sum-frequency mixing with a LiB3O5 (LBO) crystal. Laser Phys. Lett. 2008, 5, 506–509. [Google Scholar] [CrossRef]
- Anisa, M.; Muleya, G.G.; Hakeem, A.; Shirsat, M.D.; Hussaini, S.S. Exploring the influence of carboxylic acids on nonlinear optical (NLO) and dielectric properties of KDP crystal for applications of NLO facilitated photonic devices. Opt. Mater. 2015, 46, 517–521. [Google Scholar] [CrossRef]
- Chen, C.T.; Fan, Y.X.; Eckardt, R.C.; Byeret, R.L. Recent Developments in Barium Borate. Proc. SPIE 1987, 0681, 12–17. [Google Scholar]
- Chen, C.T.; Wu, Y.C.; Jiang, A.D.; Wu, B.C.; You, G.M.; Li, R.K.; Lin, S.J. New nonlinear-optical crystal: LiB3O5. J. Opt. Soc. Am. B. 1989, 6, 616–621. [Google Scholar] [CrossRef]
- Wu, Y.C.; Sasaki, T.; Nakai, S.; Yokotani, A.; Tang, H.G.; Chen, C.T. CsB3O5: A new nonlinear optical crystal. Appl. Phys. Lett. 1993, 62, 2614–2615. [Google Scholar] [CrossRef]
- Sasaki, T.; Mori, Y.; Kuroda, I.; Nakajima, S.; Yamaguchi, K.; Watanabe, S.; Nakai, S. Caesium Lithium Borate: a New Nonlinear Optical Crystal. Acta Crystallogr. Sect. C Struct. Chem. 1995, 51, 2222–2224. [Google Scholar] [CrossRef]
- Mori, Y.; Kuroda, I.; Nakajima, S.; Sasaki, T.; Nakai, S. New nonlinear optical crystal: Cesium lithium borate. Appl. Phys. Lett. 1995, 67, 1818–1820. [Google Scholar] [CrossRef]
- Mei, L.F.; Wang, Y.B.; Chen, C.T.; Wu, B.C. Nonlinear optical materials based on MBe2BO3F2 (M = Na, K). J. Appl. Phys. 1993, 74, 7014–7015. [Google Scholar] [CrossRef]
- Fei, Y.T.; Chai, B.H.T.; Ebbers, C.A.; Liao, Z.M.; Schaffers, K.I; Thelin, P. Large-aperture YCOB crystal growth for frequency conversion in the high average power laser system. J. Cryst. Growth 2006, 290, 301–306. [Google Scholar] [CrossRef]
- Yu, X.S.; Yue, Y.C.; Yao, J.Y.; Hu, Z.G. YAl3(BO3)4: Crystal growth and characterization. J. Cryst. Growth 2010, 312, 3029–3033. [Google Scholar] [CrossRef]
- Wang, G.L.; Geng, A.C.; Bo, Y.; Li, H.Q.; Sun, Z.P.; Bi, Y.; Cui, D.F.; Xu, Z.Y.; Yuan, X.; Wang, X.Q.; et al. 28.4 W 266 nm ultraviolet-beam generation by fourth-harmonic generation of an all-solid-state laser. Opt. Commun. 2006, 259, 820–822. [Google Scholar] [CrossRef]
- Yap, Y.K.; Inagaki, M.; Nakajima, S.; Mori, Y.; Sasaki, T. High-power fourth- and fifth-harmonic generation of a Nd:YAG laser by means of a CsLiB6O10. Opt. Lett. 1996, 21, 1348–1350. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Kuroda, I.; Nakajima, S.; Taguchi, A.; Sasaki, T.; Nakai, S. Growth of a nonlinear optical crystal: Cesium lithium borate. J. Cryst. Growth 1995, 156, 307–309. [Google Scholar] [CrossRef]
- Mori, Y.; Kuroda, I.; Nakajima, S.; Sasaki, T.; Nakai, S. Nonlinear Optical Properties of Cesium Lithium Borate. Jpn. J. Appl. Phys. 1995, 34, 296–298. [Google Scholar] [CrossRef]
- Sasaki, T.; Kuroda, I.; Nakajima, S.; Watanabe, S.; Mori, Y.; Nakai, S. New Nonlinear Optical Crystal Cesium Lithium Borate. Proc. Adv. Solid State Lasers Conf. 1995, 24, 91–95. [Google Scholar]
- Mori, Y.; Yap, Y.K.; Inagaki, M.; Nakajima, S.; Taguchi, A.; Zhou, W.L.; Sasaki, T. High efficiency UV light generation by CLBO. In Proceedings of the Advanced Solid State Lasers, San Francisco, CA, USA, 31 January 1996; pp. 341–345.
- Yuan, X.; Shen, G.Q.; Wang, X.Q.; Shen, D.Z.; Wang, G.L.; Xu, Z.Y. Growth and characterization of large CLBO crystals. J. Cryst. Growth 2006, 293, 97–101. [Google Scholar] [CrossRef]
- Sasaki, T.; Mori, Y.; Yoshimura, M. Progress in the growth of a CsLiB6O10 crystal and its application to ultraviolet light generation. Opt. Mater. 2003, 23, 343–351. [Google Scholar] [CrossRef]
- Ono, R.; Kamimura, T.; Fukumoto, S.; Yap, Y.K.; Yoshimura, M.; Mori, Y.; Sasakia, T.; Yoshida, K. Effect of crystallinity on the bulk laser damage and UV absorption of CLBO crystals. J. Cryst. Growth 2002, 237–239, 645–648. [Google Scholar] [CrossRef]
- Reddy, B.; Elizabeth, S.; Bhat, H.L.; Karnal, A.K. Development of a versatile high temperature top seeded solution growth unit for growing cesium lithium borate crystals. Rev. Sci. Instrum. 2009, 80, 013908. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, E.; Péter, Á; Lengyel, K.; Kovács, L. Effect of melt composition on the electrical conductivity and IR absorption of CsLiB6O10 crystals. Cryst. Res. Technol. 2003, 3–5, 331–335. [Google Scholar] [CrossRef]
- Isaenko, L.; Vasilyeva, I.; Merkulov, A.; Tomilenko, A.; Bogdanova, I.; Malakhov, V.; Drebushchak, V. CsLiB6O10 crystals with Cs deficit: Structure and properties. J. Cryst. Growth 2005, 282, 407–413. [Google Scholar] [CrossRef]
- Kovács, L.; Lengyel, K.; Péter, Á.; Polgár, K.; Beran, A. IR absorption spectroscopy of water in CsLiB6O10 crystals. Opt. Mater. 2003, 24, 457–463. [Google Scholar]
- Yap, Y.K.; Inoue, T.; Sakai, H.; Kagebayashi, Y.; Mori, Y.; Sasaki, T.; Deki, K.; Horiguchi, M. Long-term operation of CsLiB6O10 at elevated crystal temperature. Opt. Lett. 1998, 23, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Wang, X.Q.; Shen, G.Q.; Shen, D.Z. Cracking mechanism in CLBO crystals at room temperature. J. Cryst. Growth 2002, 241, 129–134. [Google Scholar] [CrossRef]
- Ryu, G.; Yoon, C.S.; Han, T.P.J.; Gallagher, H.G. Growth and characterisation of CsLiB6O10 (CLBO) crystals. J. Cryst. Growth 1998, 191, 492–500. [Google Scholar] [CrossRef]
- Taguchi, A.; Miyamoto, A.; Mori, Y.; Haramura, S.; Inoue, T.; Nishijima, K.; Kagebayashi, Y.; Sakai, H.; Yap, Y.K.; Sasaki, T. Effect of the moisture on CLBO. OSA TOPS Adv. Solid State Lasers 1997, 10, 19–23. [Google Scholar]
- Seryotkin, Y.V.; Fomina, E.A.; Isaenko, L.I. Humidity effect on hydration of CsLiB6O10 nonlinear optical crystal: X-ray diffraction study. Opt. Mater. 2013, 35, 1646–1651. [Google Scholar] [CrossRef]
- Takachiho, K.; Yoshimura, M.; Takahashi, Y.; Imade, M.; Sasaki, T.; Mori, Y. Ultraviolet laser-induced degradation of CsLiB6O10 and β-BaB2O4. Opt. Mater. Express 2014, 4, 559–567. [Google Scholar] [CrossRef]
- Kawamura, T.; Yoshimura, M.; Honda, Y.; Nishioka, M.; Shimizu, Y.; Kitaoka, Y.; Mori, Y.; Sasaki, T. Effect of water impurity in CsLiB6O10 crystals on bulk laser-induced damage threshold and transmittance in the ultraviolet region. Appl. Opt. 2009, 48, 1658–1662. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, M.; Kanoh, A.; Yoshimura, M.; Mori, Y.; Sasaki, T.; Katsura, T.; Kojima, T.; Nishimae, J. Improvement in UV Optical Properties of CsLiB6O10 by Reducing Water Molecules in the Crystal. Jpn. J. Appl. Phys. 2005, 44, 699–700. [Google Scholar] [CrossRef]
- Mori, Y.; Sasaki, T. CsLiB6O10 crystal: Growth and properties. Proc. SPIE 1996, 2700, 20–27. [Google Scholar]
- Hu, Z.G.; Zhao, Y.; Yue, Y.C.; Yu, X.S. Large LBO crystal growth at 2 kg-level. J. Cryst. Growth 2011, 335, 133–137. [Google Scholar] [CrossRef]
- Liu, S.S.; Zhang, G.C.; Li, X.M.; Yang, F.; Bo, Y.; Fu, P.Z.; Wu, Y.C. Growth and characterization of CsB3O5 crystals without scattering centers. CrystEngComm 2012, 14, 4738–4744. [Google Scholar] [CrossRef]
- Leonyuk, N.I.; Leonyuk, L.I. Growth and characterization of RM3(BO3)4 crystals. Prog. Cryst. Growth Charact. Mater. 1995, 31, 179–278. [Google Scholar] [CrossRef]
- Sanjay; Kishore, N.; Agarwal, A. Investigation of structural, optical and transport properties of MoO3–PbO–B2O3 glasses. J. Alloys Compd. 2009, 487, 52–57. [Google Scholar] [CrossRef]
- Py1neva, N.A.; Kononova, N.G.; Yurkin, A.M.; Kokh, A.E.; Bazarova, G.G.; Danilov, V.I.; Lisova, I.A.; Tsirkina, N.L. Top-seeded solution growth of CLBO crystals. In Proceedings of the SPIE Conference on Laser Material Crystal Growth and Nonlinear Materials and Devices, San Jose, CA, USA, 27–28 January 1999.
- Yu, X.S.; Hu, Z.G. Growth and characterization of Al-doped CsLiB6O10 crystal. J. Cryst. Growth 2011, 318, 1171–1174. [Google Scholar] [CrossRef]
- Yu, X.S.; Hu, Z.G. Flux growth of CsLiB6O10 crystals. J. Cryst. Growth 2010, 312, 2415–2418. [Google Scholar] [CrossRef]
- Kushwaha, S.K.; Rathee, S.P.; Maurya, K.K.; Bhagavannarayana, G. Enhancement in second harmonic generation efficiency, laser damage threshold and optical transparency of Mn2+ doped L-alanine crystals: A correlation with crystalline perfection. J. Cryst. Growth 2011, 328, 81–88. [Google Scholar] [CrossRef]
- Li, X.M.; Hu, Z.G.; Yue, Y.C.; Yu, X.S.; Lin, Z.S.; Zhang, G.C. Study on optical weak absorption of borate crystals. Opt. Mater. 2013, 35, 2376–2381. [Google Scholar] [CrossRef]
- Bhagavannarayana, G.; Kushwaha, S.K. Enhancement of SHG efficiency by urea doping in ZTS single crystals and its correlation with crystalline perfection as revealed by Kurtz powder and high-resolution X-ray diffraction methods. J. Appl. Cryst. 2010, 43, 154–162. [Google Scholar] [CrossRef]
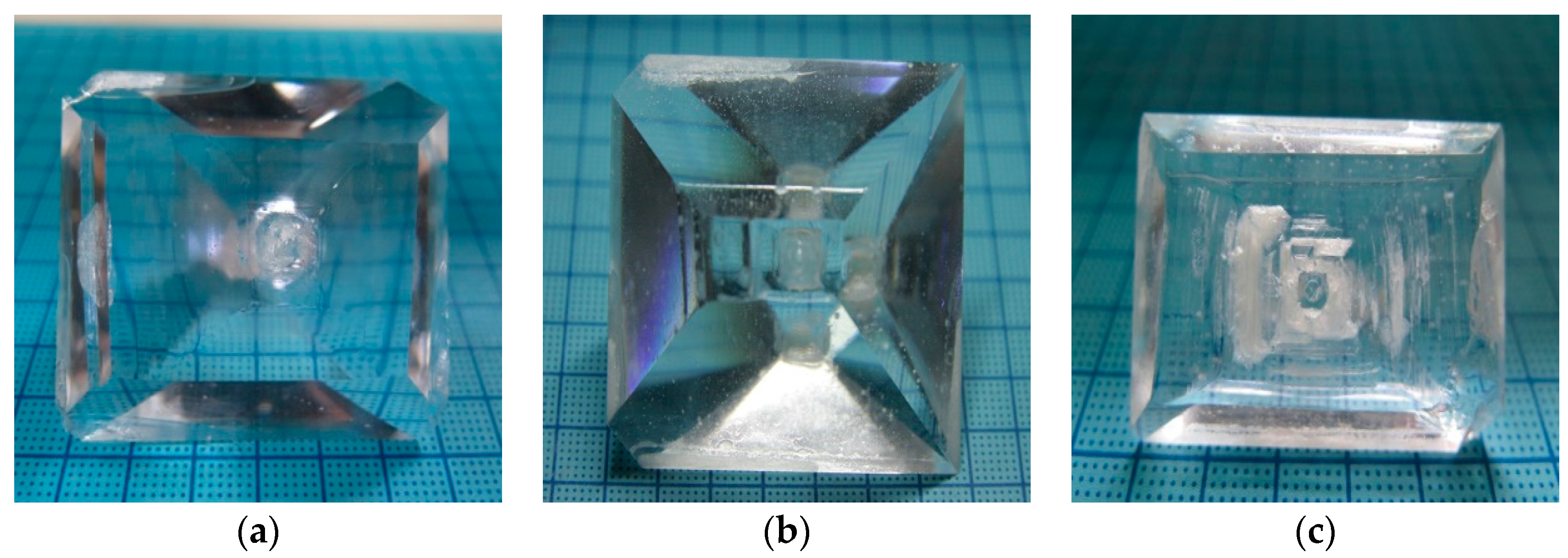

| Al Doping Concentration | Al:Li (Molar Ratio) | Saturation Temperature (°C) | Cooling Range (°C) | Size (mm3), Weight (g) | Inclusions |
|---|---|---|---|---|---|
| undoped | 0 | 800 | 3.4 | 61 × 59 × 35, 125 | free |
| 2.5% Al-doped | 2.5:97.5 | 802 | 4.1 | 68 × 59 × 47, 170 | free |
| 5% Al-doped | 5:95 | 808 | 3.3 | 54 × 35 × 25, 64 | free |
| 10% Al-doped | 10:90 | 811 | 3.2 | 50 × 49 × 26, 61 | almost free |
| CLBO Crystals | Weak Absorption Value at 1064 nm/ppm·cm−1 | |
|---|---|---|
| [100] Direction | [001] Direction | |
| Undoped CLBO | 80 | 50 |
| 5% Al-doped CLBO | 140 | 50 |
| Conventional CLBO [42] | 600 | 150 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Tu, H.; Zhao, Y.; Hu, Z. Growth of High Quality Al-Doped CsLiB6O10 Crystals Using Cs2O-Li2O-MoO3 Fluxes. Crystals 2017, 7, 83. https://doi.org/10.3390/cryst7030083
Zhu X, Tu H, Zhao Y, Hu Z. Growth of High Quality Al-Doped CsLiB6O10 Crystals Using Cs2O-Li2O-MoO3 Fluxes. Crystals. 2017; 7(3):83. https://doi.org/10.3390/cryst7030083
Chicago/Turabian StyleZhu, Xianchao, Heng Tu, Ying Zhao, and Zhanggui Hu. 2017. "Growth of High Quality Al-Doped CsLiB6O10 Crystals Using Cs2O-Li2O-MoO3 Fluxes" Crystals 7, no. 3: 83. https://doi.org/10.3390/cryst7030083





