Two Organic Cation Salts Containing Tetra(isothiocyanate)cobaltate(II): Synthesis, Crystal Structures, Spectroscopic, Optical and Magnetic Properties
Abstract
:1. Introduction
2. Results and Discussion
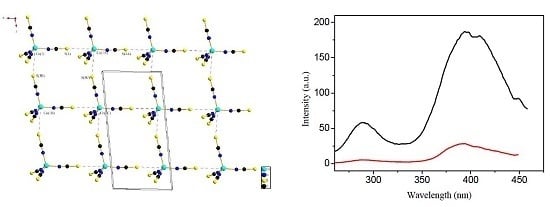
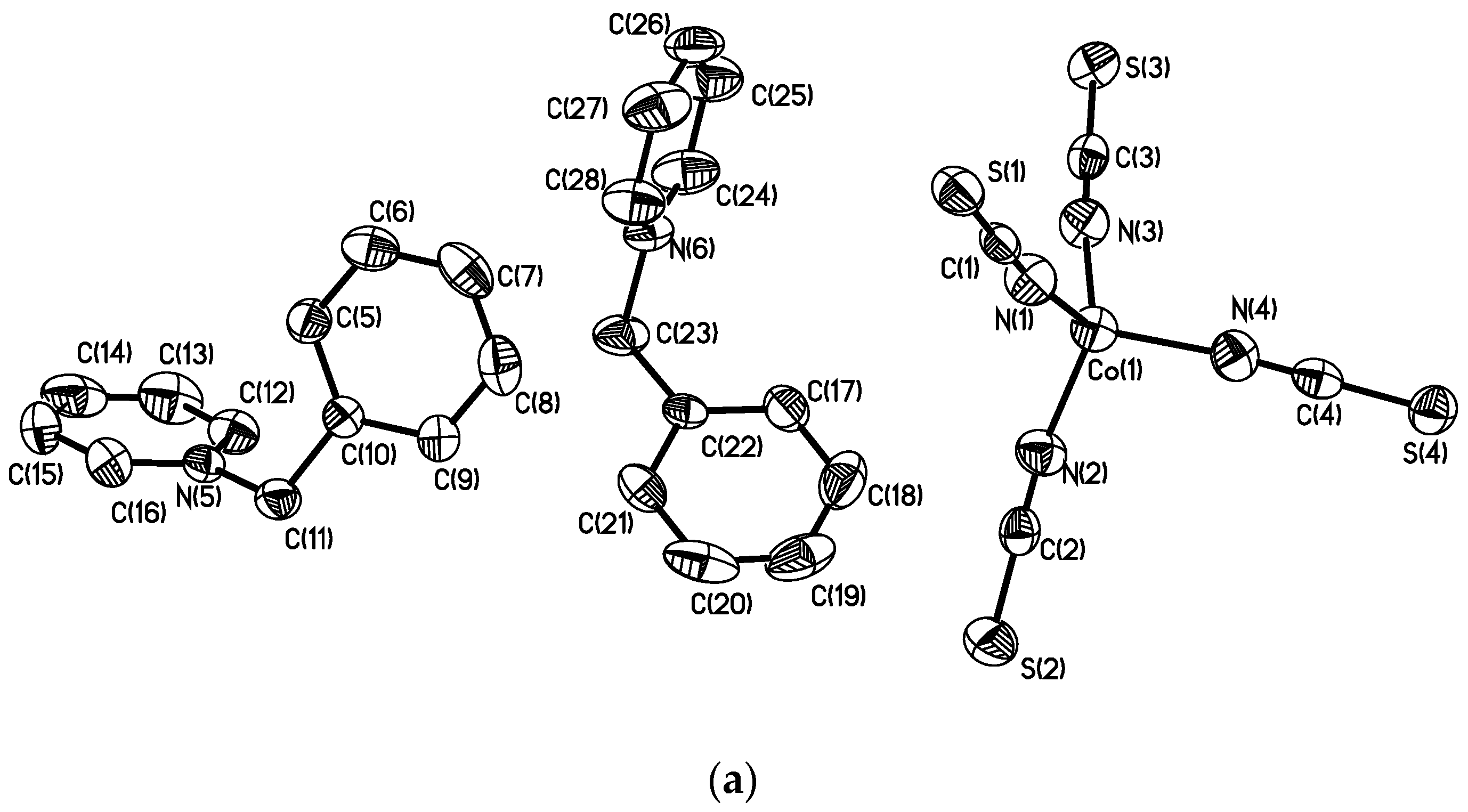
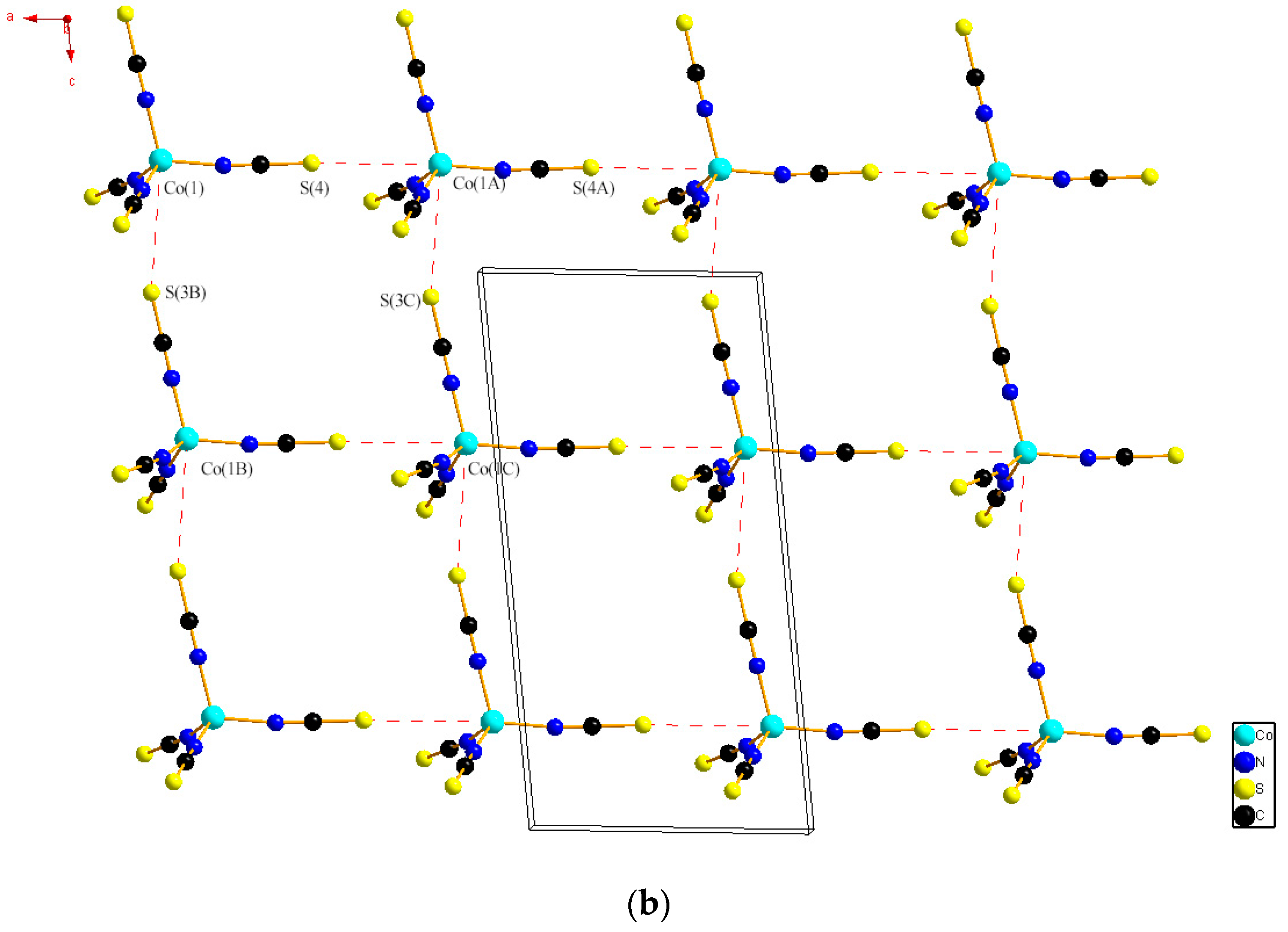
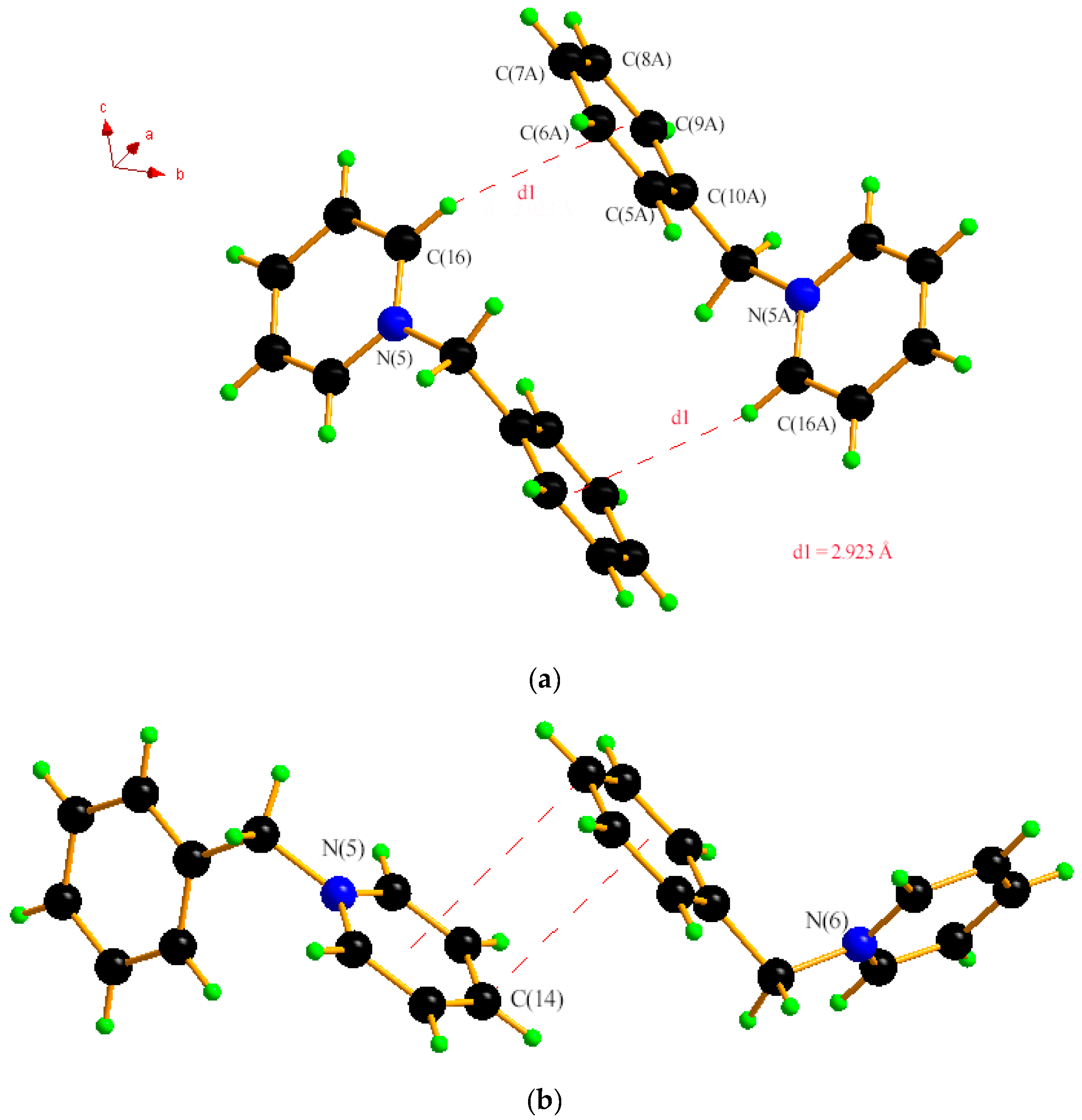
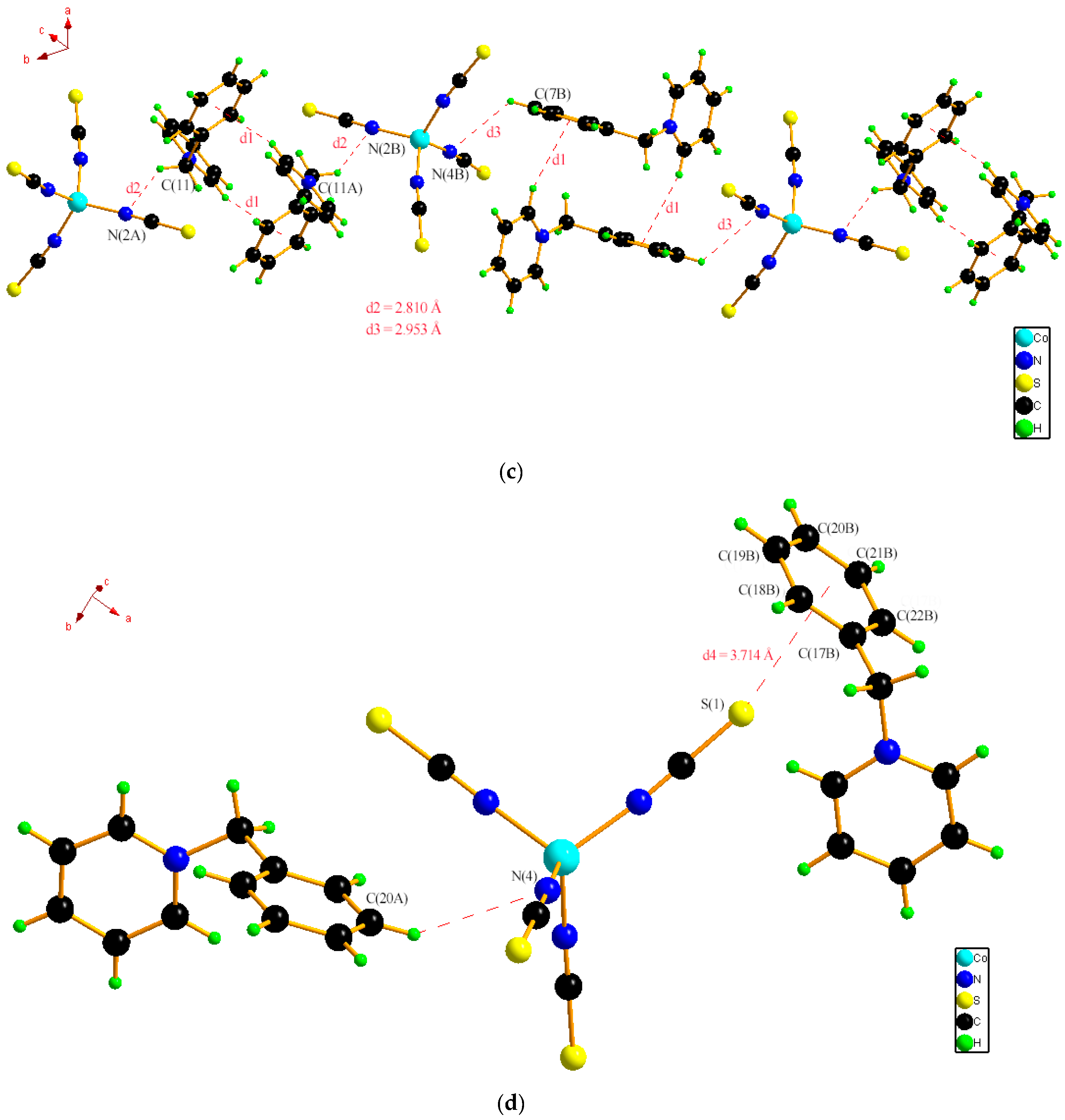
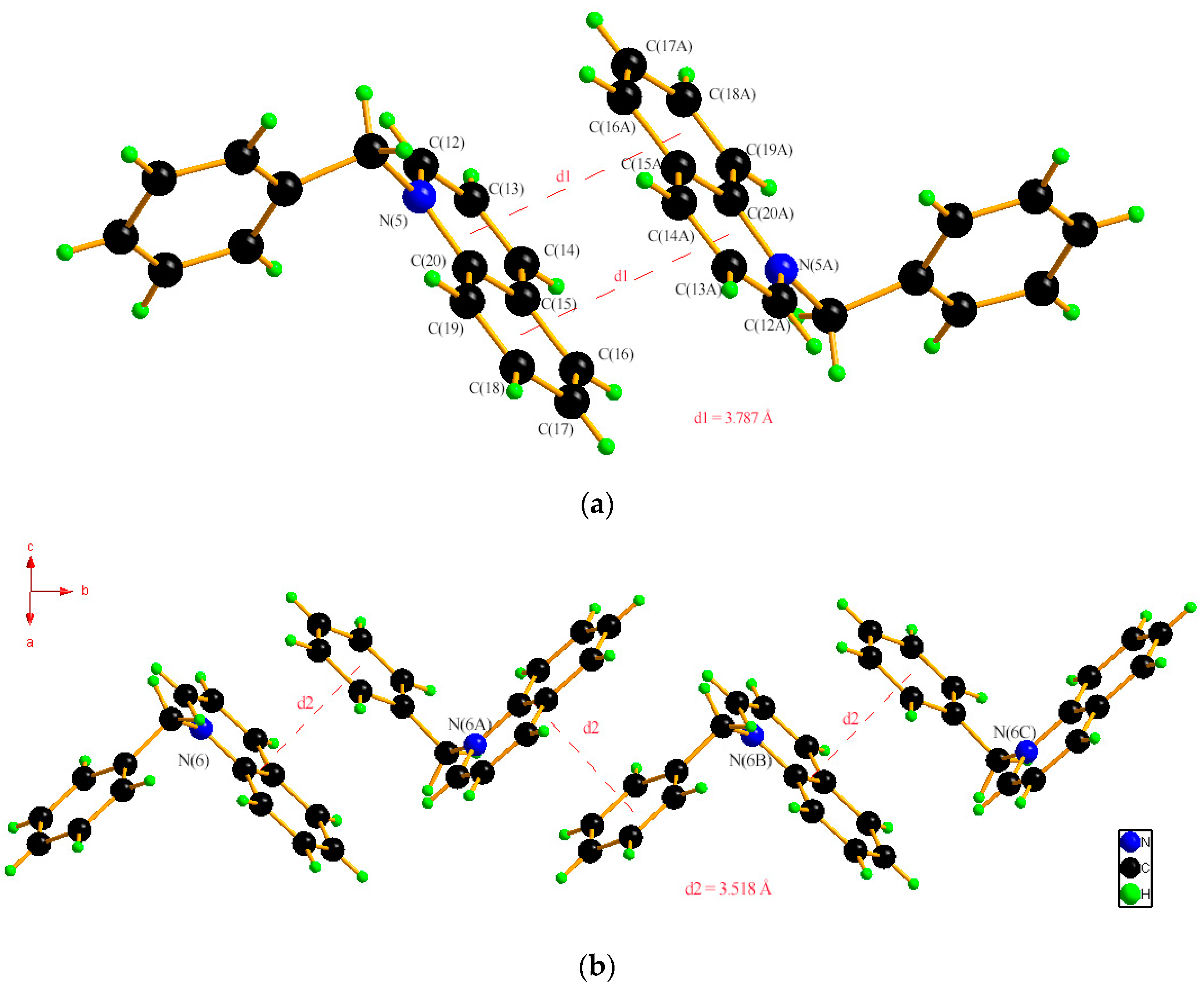
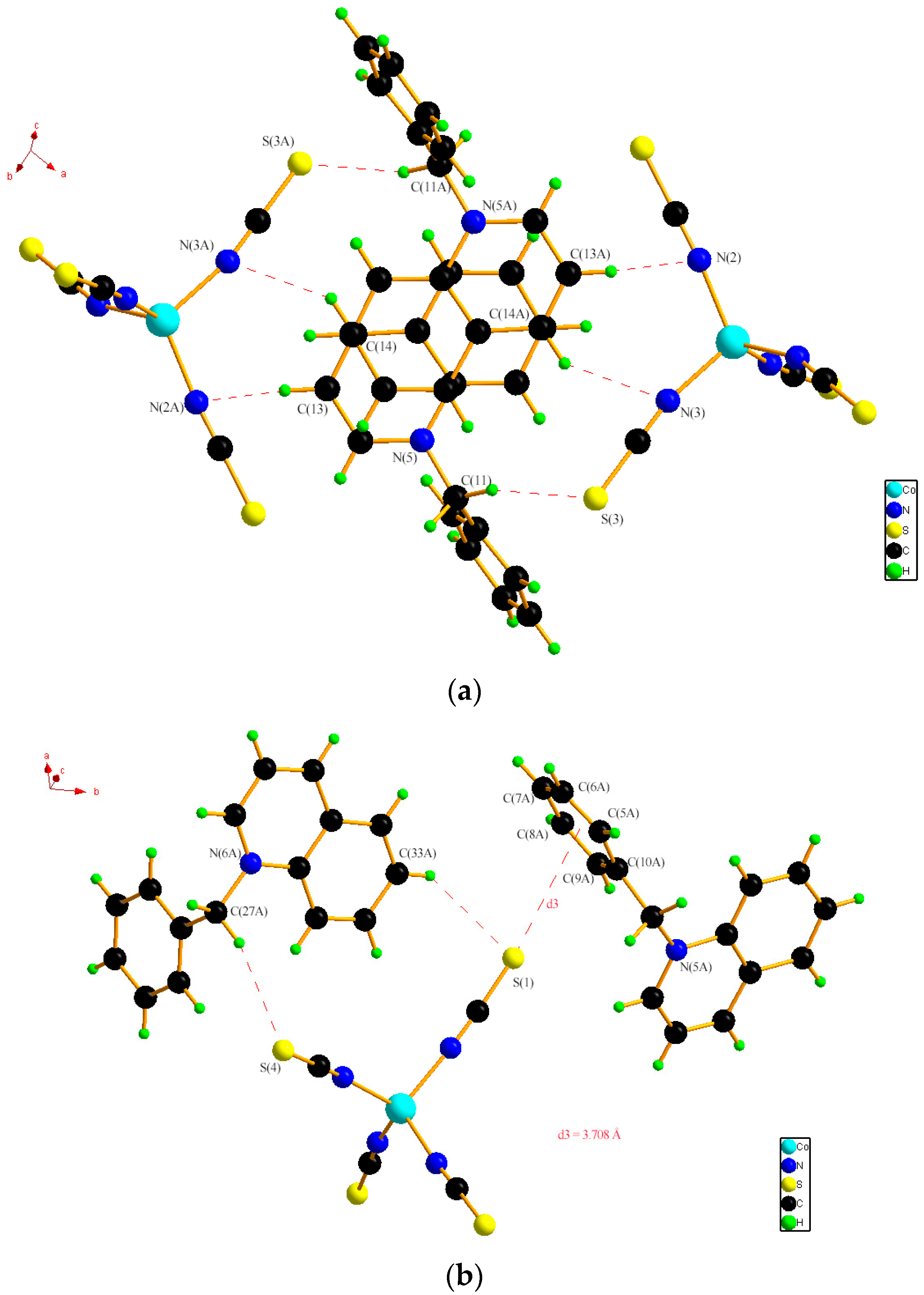
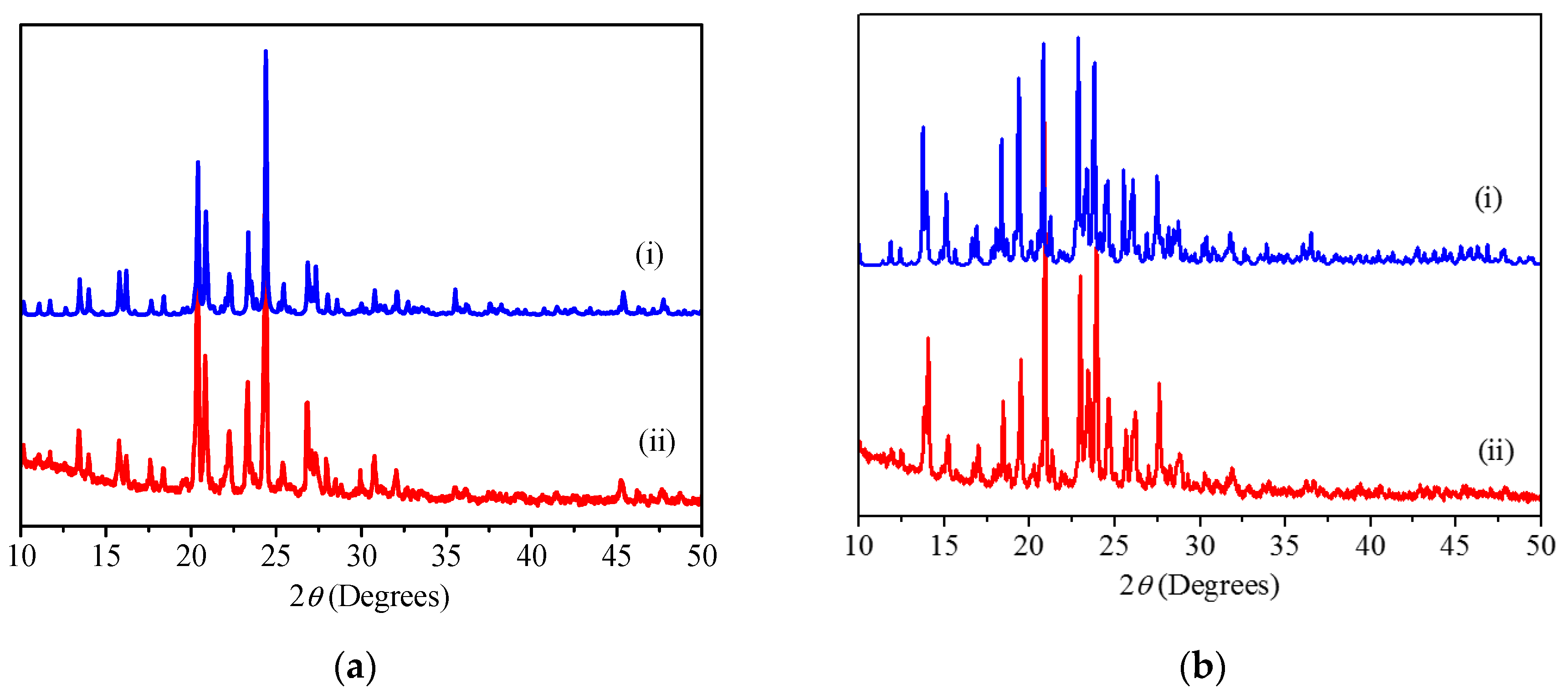
2.1. Single Crystal XRD and Powder XRD
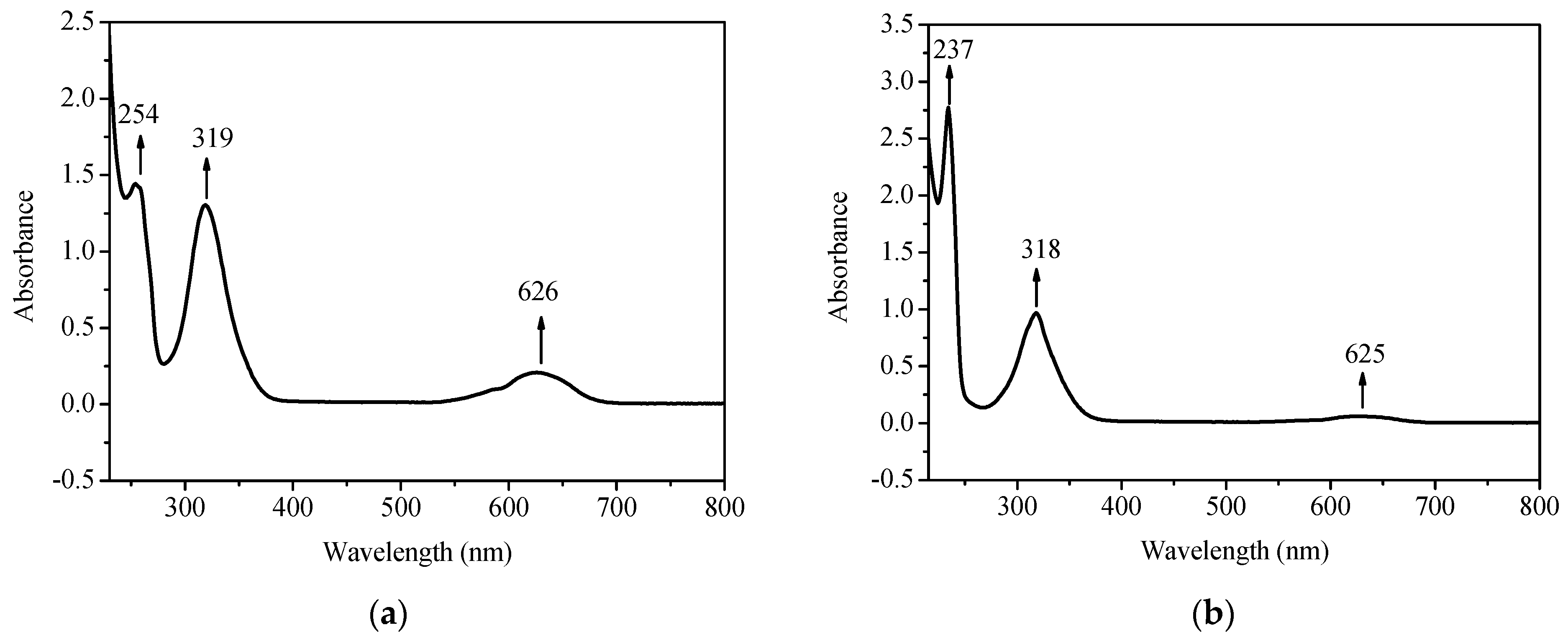
2.2. UV-Vis Spectra
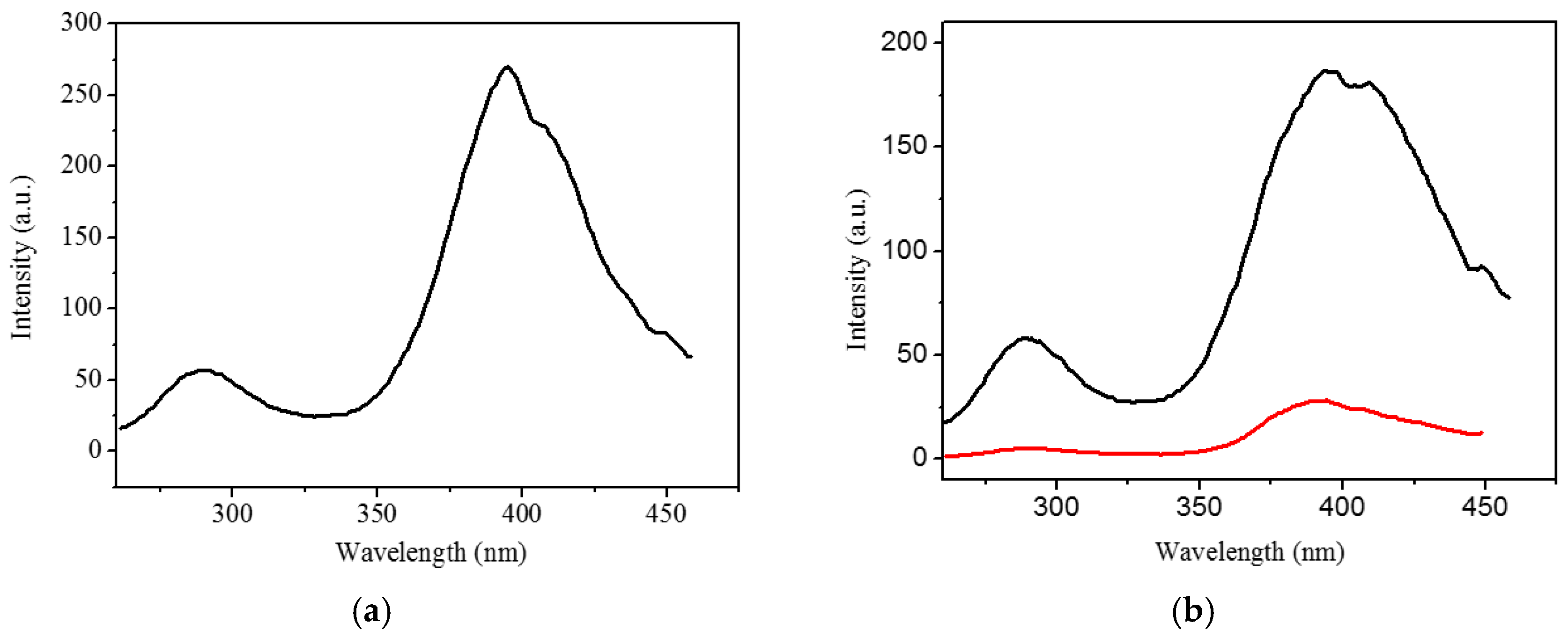
2.3. Luminescent Properties
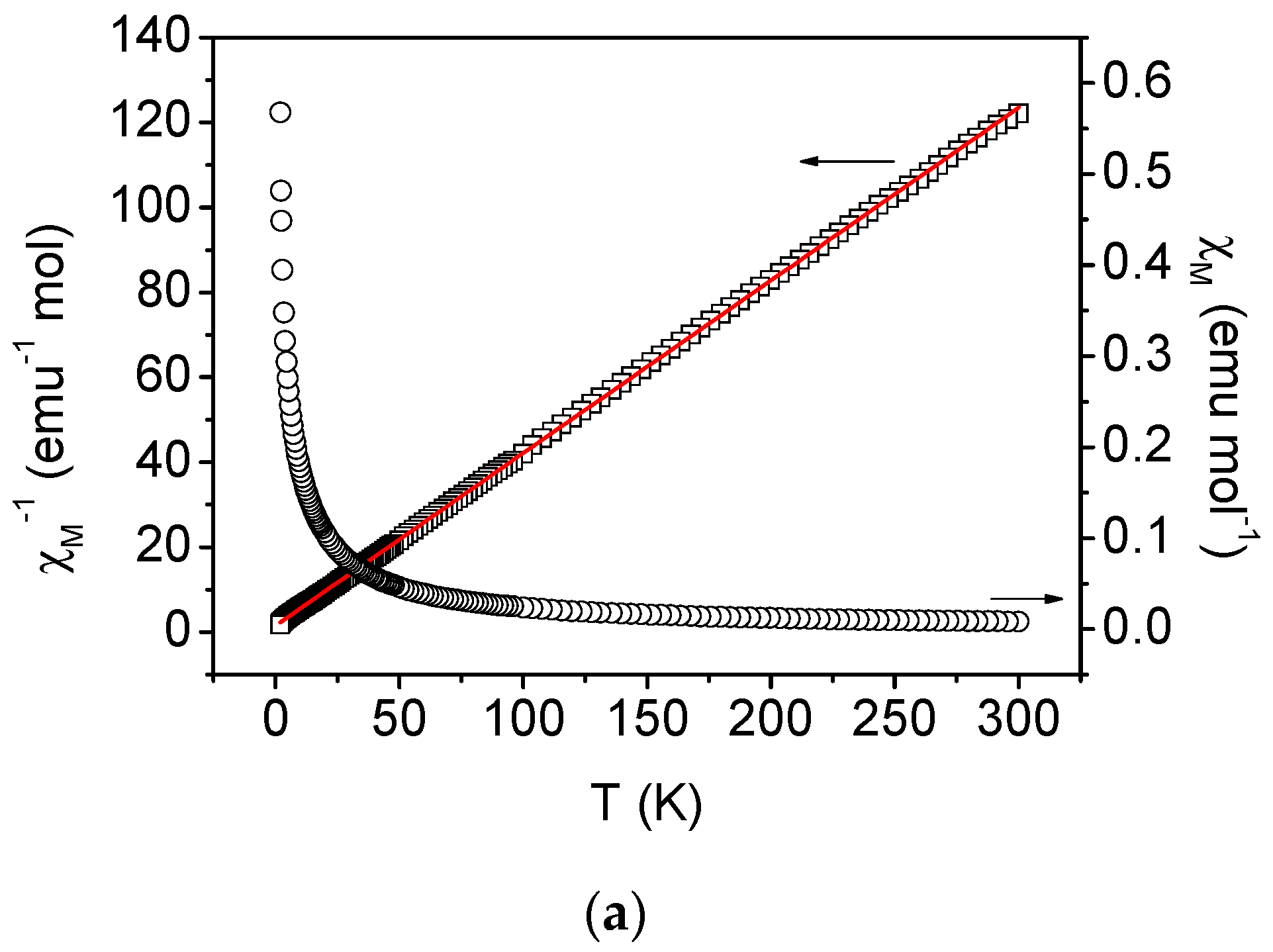
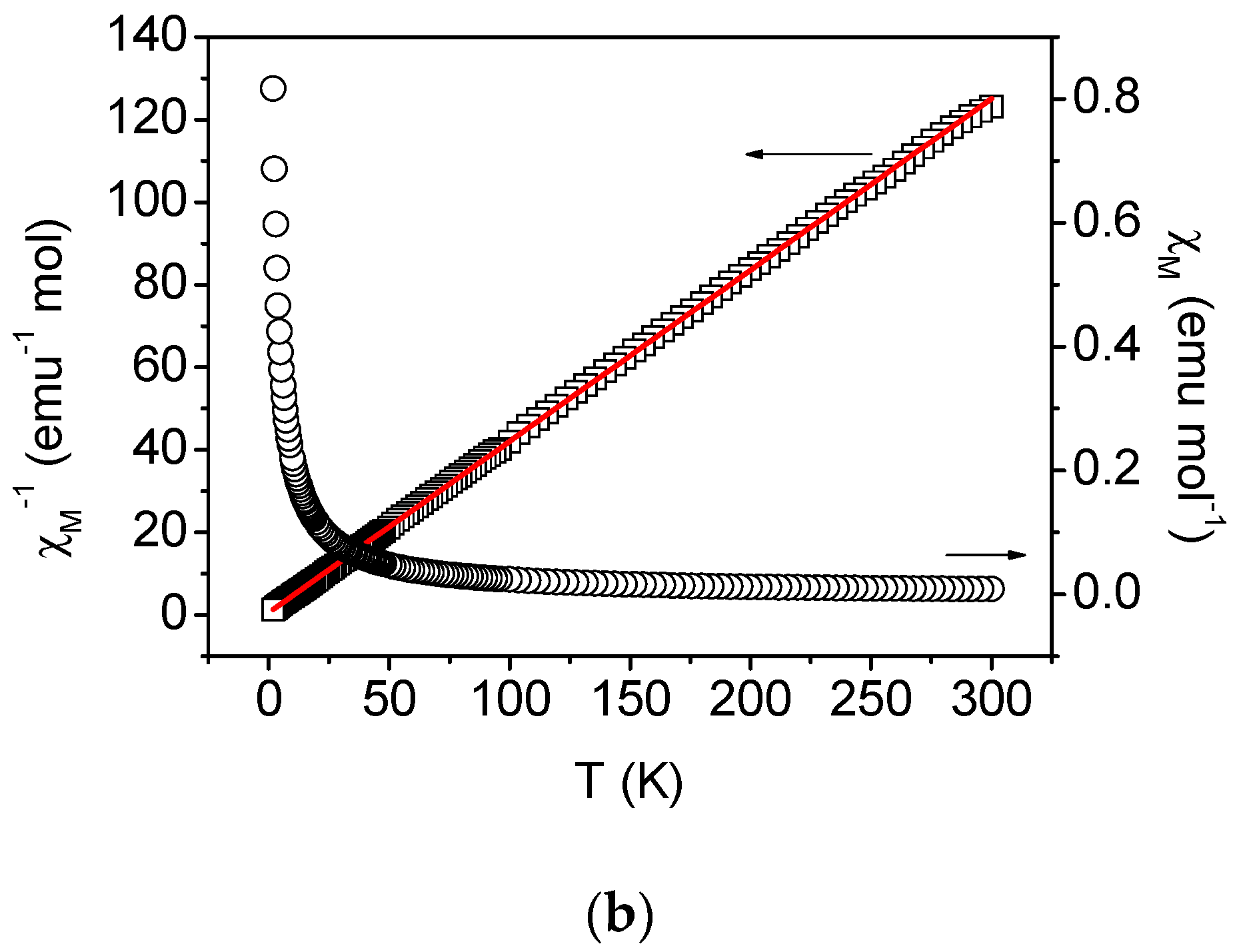
2.4. Magnetic Properties
3. Materials and Methods
3.1. Materials
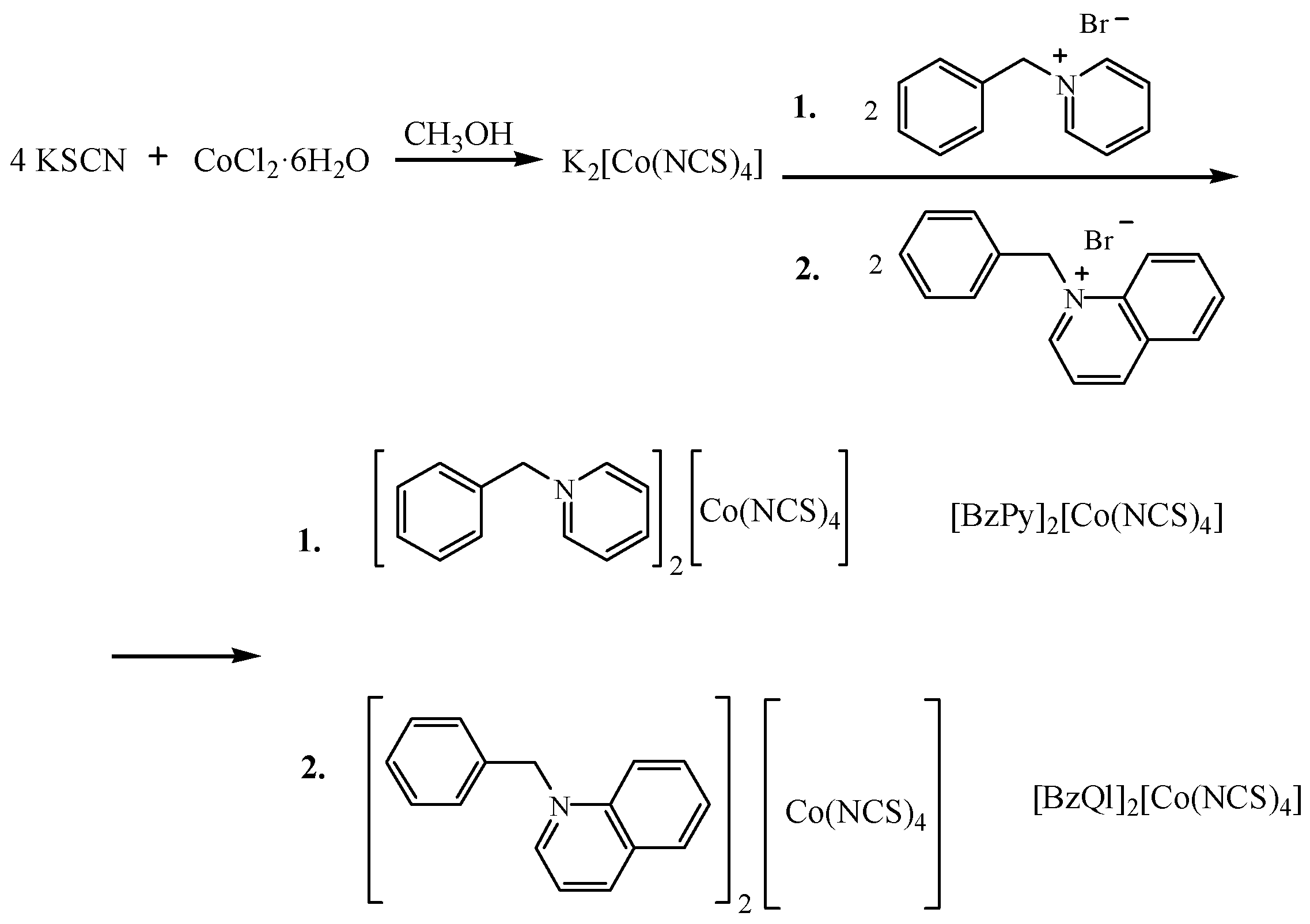
3.2. Syntheses and Crystal Growth Method
3.3. Characterization Technique
3.4. Fluorescence Emission Spectra Measurements
3.5. Magnetic Properties Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflict of Interest
References
- Wang, K.; Stiefel, E.I. Toward separation and purification of olefins using dithiolene complexes: An electrochemical approach. Science 2001, 291, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Tomoyuki, A.; Takayoshi, N. Control of assembly and magnetism of metal-dmit complexes by supramolecular cations. Coord. Chem. Rev. 2001, 226, 3–9. [Google Scholar]
- Robertson, N.; Cronin, L. Metal bis-1,2-dithiolene complexes in conducting or magnetic crystalline assemblies. Coord. Chem. Rev. 2002, 227, 93–127. [Google Scholar] [CrossRef]
- Desiraju, G.R. Crystal engineering: From molecule to crystal. J. Am. Chem. Soc. 2013, 135, 9952–9967. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.; Landee, C.P.; Turnbull, M.M.; Jornet, J.; Deumal, M.; Novoa, J.J.; Robb, M.A.; Lewis, W. Synthesis, structure, and magnetic properties of an antiferromagnetic spin-ladder complex: Bis(2,3-dimethylpyridinium) tetrabromocuprate. J. Am. Chem. Soc. 2007, 129, 952–959. [Google Scholar] [CrossRef]
- Li, L.X.; Turnbull, M.M. Synthesis, structure, and magnetic behavior of bis(2-amino-5-fluoropyridinium) tetrachlorocuprate(II). Inorg. Chem. 2007, 46, 11254–11265. [Google Scholar] [CrossRef] [PubMed]
- Vela, S.; Deumal, M.; Ribas-Arino, J.; Novoa, J.J. Tracing the sources of the different magnetic behavior in the two phases of the bistable (BDTA)2[Co(mnt)2] compound. Inorg. Chem. 2012, 51, 8646–8648. [Google Scholar] [CrossRef] [PubMed]
- Savard, D.; Leznoff, D.B. Synthesis, structure and light scattering properties of tetraalkylammonium metal isothiocyanate salts. Dalton Trans. 2013, 42, 14982–14991. [Google Scholar] [CrossRef] [PubMed]
- Walker, I.M.; McCarthy, P.J. Charge-transfer spectra and photochemistry of the hexakis(isothiocyanato)ferrate(III) ion at cryogenic temperatures in diluent crystals. Inorg. Chem. 1984, 23, 1842–1845. [Google Scholar] [CrossRef]
- Kaye, S.S.; Long, J.R. Hydrogen storage in the dehydrated prussian blue analogues M3[Co(CN)6]2 (M = Mn, Fe, Co, Ni, Cu, Zn). J. Am. Chem. Soc. 2005, 127, 6506–6507. [Google Scholar] [CrossRef] [PubMed]
- Mrozinski, J.; Klak, J.; Kruszynski, R. Crystal structure and magnetic properties of the 1D bimetallic thiocyanate bridged compound: {(CuL1)[Co(NCS)(4)]}(L-1 = N-rac-5,12-Me-2-[14]-4,11-dieneN(4)). Polyhedron 2008, 27, 1401–1407. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Bhar, K.; Das, S.; Chantrapromma, S.; Fun, H.K.; Ghosh, B.K. Syntheses, structures and properties of homo- and heterobimetallic complexes of the type [Zn(tren)NCS](2)[M(NCS)(4)] [tren = tris(2-aminoethyl)amine; M = Zn, Cu]. J. Mol. Struct. 2010, 967, 112–118. [Google Scholar] [CrossRef]
- Quan, Y.P.; Yin, P.; Han, N.N.; Yang, A.H.; Gao, H.L.; Cui, J.Z.; Shib, W.; Cheng, P. Novel hetero-polynuclear metal complexes (CuL)(3)[Mn(NCS)(5)](2) and (NiL)(3)[Mn(NCS)(5)](2) containing trigonal bipyramidal geometric [Mn(NCS)(5)](3-) as bridging ligand. Inorg. Chem. Commun. 2009, 12, 469–472. [Google Scholar] [CrossRef]
- Li, L.L.; Yuan, R.X.; Liu, L.L.; Ren, Z.G.; Zheng, A.X.; Cheng, H.J.; Li, H.X.; Lang, J.P. Formation of [CuSCN](n)-Based topological structures via a family of flexible benzimidazolyl-based linkers with different spacer lengths. Cryst. Growth Des. 2010, 10, 1929–1938. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.M.; Zhang, K.C.; Teo, B.K. Molecular and crystal engineering of a new class of inorganic cadmium-thiocyanate polymers with host–guest complexes as organic spacers, controllers, and templates. Coord. Chem. Rev. 1999, 183, 157–195. [Google Scholar] [CrossRef]
- Ghazzali, M.; Langer, V.; Oehrstroem, L. The role of intermolecular interactions in the assemblies of FeII and CoII tetrakis-isothiocyanatometalates with tris(1,10-phenanthroline)-RuII: Crystal structures of two dual-metal assemblies featuring octahedral cationic and tetrahedral anionic modules. J. Solid State Chem. 2008, 181, 2191–2198. [Google Scholar] [CrossRef]
- Ohba, M.; Okawa, H. Synthesis and magnetism of multi-dimensional cyanide-bridged bimetallic assemblies. Coord. Chem. Rev. 2000, 198, 313–328. [Google Scholar] [CrossRef]
- Chen, H.J.; Zhang, L.Z.; Cai, Z.G.; Yang, G.; Chen, X.M. Organic-inorganic hybrid materials assembled through weak intermolecular interactions: Synthesis, structures and non-linear optical properties of [4,4′-bipyH(2)][M(NCS)(4)] (M = Mn2+, Co2+ or Zn2+; 4,4′-bipy=4,4′-bipyridine). J. Chem. Soc. Dalton Trans. 2000, 14, 2463–2466. [Google Scholar] [CrossRef]
- Chen, X.; Chen, W.Q.; Han, S.; Liu, J.F.; Zhou, J.R.; Yu, L.L.; Yang, L.M.; Ni, C.L.; Hu, X.L. Two hybrid materials self-assembly from tetra(isothiocyanate)cobalt(II) dianion and substituted benzyl triphenylphosphinium. J. Mol. Struct. 2010, 984, 164–169. [Google Scholar] [CrossRef]
- Chen, X.; Dai, S.L.; Cheng, Z.P.; Liang, L.B.; Han, S.; Liu, J.F.; Zhou, J.R.; Yang, L.M.; Ni, C.L. Syntheses, crystal structures, and magnetic properties of two hybrid materials self-assembly from tetra(isothiocyanate)cobalt(II) anion and substituted benzyl triphenylphosphinium. Synth. React. Inorg. Met. Org. Nano Met. Chem. 2012, 42, 987–993. [Google Scholar] [CrossRef]
- Chen, W.Q.; Su, L.J.; Cai, X.Q.; Yang, J.J.; Qian, Y.L.; Liu, X.P.; Yang, L.M.; Zhou, J.R.; Ni, C.L. Syntheses, crystal Structures, and magnetic properties of two meta-substituted benzyl triphenylphosphinium tetra(isothiocyanate)cobaltate(II) complexes. Synth. React. Inorg. Met. Org. Nano Met. Chem. 2014, 44, 980–985. [Google Scholar] [CrossRef]
- Ye, H.Q.; Su, L.J.; Chen, X.X.; Liao, X.; Liu, Q.T.; Wu, X.Y.; Zhou, J.R.; Yang, L.M.; Ni, C.L. Syntheses, crystal structures, weak interaction, magnetic and luminescent properties of two new organic-inorganic molecular solids with substituted chlorobenzyl triphenylphosphinium and tetra (isothiocyanate)cobalt(II) anion. Synth. Met. 2015, 199, 232–240. [Google Scholar] [CrossRef]
- Ye, H.Q.; Xie, J.L.; Yu, J.Y.; Liu, Q.T.; Dai, S.L.; Huang, W.Q.; Zhou, J.R.; Yang, L.M.; Ni, C.L. Syntheses, crystal structures, luminescent properties of two new molecular solids with tetra(isothiocyanate)zinc(II) and substituted benzyl triphenylphosphonium cations. Synth. Met. 2014, 197, 99–104. [Google Scholar] [CrossRef]
- Ye, H.Q.; Li, Y.Y.; Huang, R.K.; Liu, X.-P.; Chen, W.Q.; Zhou, J.R.; Yang, L.M.; Ni, C.L. Unusual layer structure in an ion-paired compound containing tetra(isothiocyanate) cobalt(II) dianion and 4-Nitrobenzylpyridinium: Crystal structure and magnetic properties. J. Struct. Chem. 2014, 55, 691–696. [Google Scholar] [CrossRef]
- Allinger, N.L.; Zhou, X.F.; Bergsma, J. Molecular mechanics parameters. J. Mol. Struct. Theochem. 1994, 31, 269–283. [Google Scholar] [CrossRef]
- Batsanov, S.S. Van der Waals Radii of Elements. Inorg. Mater. 2001, 37, 871–885. [Google Scholar] [CrossRef]
- Ma, L.F.; Wang, L.Y.; Hu, J.L.; Wang, Y.Y.; Yang, G.P. Syntheses, structures, and photoluminescence of a series of d(10) coordination polymers with R-Isophthalate (R = –OH, –CH3, and –C(CH3)(3)). Cryst. Growth Des. 2009, 9, 5334–5342. [Google Scholar] [CrossRef]
- Jiao, C.H.; He, C.H.; Geng, J.C.; Cui, G.H. Syntheses, structures, and photoluminescence of three cadmium(II) coordination polymers with flexible bis(benzimidazole) ligands. J. Coord. Chem. 2012, 65, 2852–2861. [Google Scholar] [CrossRef]
- Ashenhurst, J.; Wu, G.; Wang, S.N. Syntheses, Structures, Solution, and Solid-State 27Al NMR Studies of Blue Luminescent Mononuclear Aluminum Complexes: Al(7-azain)2(7-azain-H)(CH3), Al(7-azain)3(7-azain-H), and Al(7-azain)(7-azain-H)(OCH(CF3)2)2 (7-azain-H) 7-azaindole). J. Am. Chem. Soc. 2000, 122, 2541–2547. [Google Scholar] [CrossRef]
- Li, B.L.; Wang, X.Y.; Zhu, X.; Gao, S.; Zhang, Y. Synthesis, crystal structure and magnetic behavior of two cobalt coordination polymers with 1,2-bis(1,2,4-triazol-1-yl)ethane and dicyanamide. Polyhedron 2007, 26, 5219–5224. [Google Scholar] [CrossRef]
- Wei, Y.Q.; Wu, K.C.; Broer, R.; Zhuang, B.T.; Yu, Y.F. A polymeric cobalt compound [Co(DCNT)(H2O)](n) with novel topology: Synthesis, structure, luminescence, and magnetic property. Inorg. Chem. Commun. 2007, 10, 910–913. [Google Scholar] [CrossRef]
- Holman, K.T.; Hammud, H.H.; Isber, S.; Tabbal, M. One-dimensional coordination polymer [Co(H2O)4(pyz)](NO3)2·2H2O (pyz = pyrazine) with intra- and inter-chain H-bonds: Structure, electronic spectral studies and magnetic properties. Polyhedron 2005, 24, 221–228. [Google Scholar] [CrossRef]
- Bulgarevich, S.B.; Bren, D.V.; Movshovic, D.Y.; Finocchiaro, P.; Failla, S. Conformational investigation of N-aralkylpyridinium ions by cotton-mouten effect method. J. Mol. Struct. 1994, 317, 147–155. [Google Scholar] [CrossRef]
- SMART. Brucker Molecular Analysis Research Tool; Version 6.1; Bruker AXS: Madison, WI, USA, 2000. [Google Scholar]
- SAINTPlus. Data Reduction and Correction Program; Version 6.01; Bruker AXS: Madison, WI, USA, 2000. [Google Scholar]
- SHELXTL. Structure Determination Software Programs; Version 6.10; Shelddrick, G.M., Ed.; Bruker AXS Inc.: Madison, WI, USA, 2000. [Google Scholar]
| Compound | 1 | 2 |
|---|---|---|
| Bond length (Å) | ||
| Co(1)–N(1) | 1.937(3) | 1.958(3) |
| Co(1)–N(2) | 1.945(3) | 1.941(4) |
| Co(1)–N(3) | 1.968(3) | 1.966(3) |
| Co(1)–N(4) | 1.957(3) | 1.987(4) |
| N(1)–C(1) | 1.138(5) | 1.141(5) |
| N(2)–C(2) | 1.146(5) | 1.144(5) |
| N(3)–C(3) | 1.162(4) | 1.111(5) |
| N(4)–C(4) | 1.159(5) | 1.137(5) |
| S(1)–C(1) | 1.609(4) | 1.610(3) |
| S(2)–C(2) | 1.611(4) | 1.614(4) |
| S(3)–C(3) | 1.599(4) | 1.628(4) |
| S(4)–C(4) | 1.610(4) | 1.620(4) |
| Bond angles (°) | ||
| N(1)–Co(1)–N(2) | 115.60(12) | 109.48(14) |
| N(1)–Co(1)–N(3) | 106.98(12) | 110.66(15) |
| N(1)–Co(1)–N(4) | 110.11(12) | 105.50(15) |
| N(2)–Co(1)–N(3) | 110.20(12) | 109.80(14) |
| N(2)–Co(1)–N(4) | 105.08(12) | 117.37(16) |
| N(3)–Co(1)–N(4) | 108.75(12) | 103.81(14) |
| Co(1)–N(1)–C(1) | 173.2(3) | 177.0(3) |
| Co(1)–N(2)–C(2) | 171.5(3) | 172.6(4) |
| Co(1)–N(3)–C(3) | 172.2(3) | 168.8(3) |
| Co(1)–N(4)–C(4) | 173.2(3) | 161.0(3) |
| N(5)–C(11)–C(10) | 112.4(3) | 114.6(3) |
| D−H···A | d (D−H) | d (H···A) | d (D···A) | <(DHA) |
|---|---|---|---|---|
| Compound 1 | ||||
| C(7)–H(7)···N(4)#1 | 0.970 | 2.953 | 3.568(4) | 124.0 |
| C(20)–H(20)···N(4)#2 | 0.930 | 2.871 | 3.668(3) | 144.0 |
| Compound 2 | ||||
| C(9)–H(9)···N(5) | 0.930 | 2.588 | 2.920(5) | 101.0 |
| C(11)–H(11B)···S(3)#3 | 0.970 | 2.753 | 3.700(4) | 166.0 |
| C(13)–H(13)···N(2)#4 | 0.930 | 2.926 | 3.648(4) | 130.0 |
| C(14)–H(14)···N(3)#4 | 0.930 | 2.995 | 3.660(4) | 136.0 |
| Compounds | 1 | 2 |
|---|---|---|
| Empirical formula | C28H24N6CoS4 | C36H28N6CoS4 |
| Formula weight | 631.70 | 731.81 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/c | P21/n |
| a/Å | 8.749(1) | 16.029(3) |
| b/Å | 20.062(3) | 12.731(2) |
| c/Å | 17.527(2) | 19.010(3) |
| β/° | 96.28(1) | 111.29(1) |
| Volume, Å3 | 3058.0(7) | 3614.7(10) |
| Z | 4 | 4 |
| Density (calculated), g/cm3 | 1.372 | 1.345 |
| Absorption coefficient, mm−1 | 0.862 | 0.740 |
| F(000) | 1300 | 1508 |
| Crystal size/mm3 | 0.13 × 0.17 × 0.21 | 0.11 × 0.15 × 0.19 |
| Reflections collected | 26,736 | 25,478 |
| Independent reflections | 5676 (Rint = 0.069) | 6353 (Rint = 0.037) |
| Data/restraints/parameters | 5676/0/352 | 6353/0/424 |
| Goodness of fit on F2 | 1.000 | 1.067 |
| Final R indices [I > 2σ (I)] | R1 = 0.0480, wR2 = 0.0921 | R1 = 0.0462, wR2 = 0.1199 |
| Final R indices (all data) | R1 = 0.1034, wR2 = 0.1023 | R1 = 0.0726, wR2 = 0.1370 |
| Largest Diff Peak and hole (e Å−3) | 0.232 and −0.357 | 0.630 and −0.510 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Xu, J.; Yan, S.; Chen, Y.; Wang, Y.; Chen, Z.; Ni, C. Two Organic Cation Salts Containing Tetra(isothiocyanate)cobaltate(II): Synthesis, Crystal Structures, Spectroscopic, Optical and Magnetic Properties. Crystals 2017, 7, 92. https://doi.org/10.3390/cryst7030092
Zhang Z, Xu J, Yan S, Chen Y, Wang Y, Chen Z, Ni C. Two Organic Cation Salts Containing Tetra(isothiocyanate)cobaltate(II): Synthesis, Crystal Structures, Spectroscopic, Optical and Magnetic Properties. Crystals. 2017; 7(3):92. https://doi.org/10.3390/cryst7030092
Chicago/Turabian StyleZhang, Zheng, Junwei Xu, Sisi Yan, Yangli Chen, Yan Wang, Zhuo Chen, and Chunlin Ni. 2017. "Two Organic Cation Salts Containing Tetra(isothiocyanate)cobaltate(II): Synthesis, Crystal Structures, Spectroscopic, Optical and Magnetic Properties" Crystals 7, no. 3: 92. https://doi.org/10.3390/cryst7030092