Crystallographic Characteristics of Hydroxylapatite in Hard Tissues of Cololabis saira
Abstract
:1. Introduction
2. Results
2.1. Phase Analysis
2.2. Lattice Parameters
2.3. Crystallinity
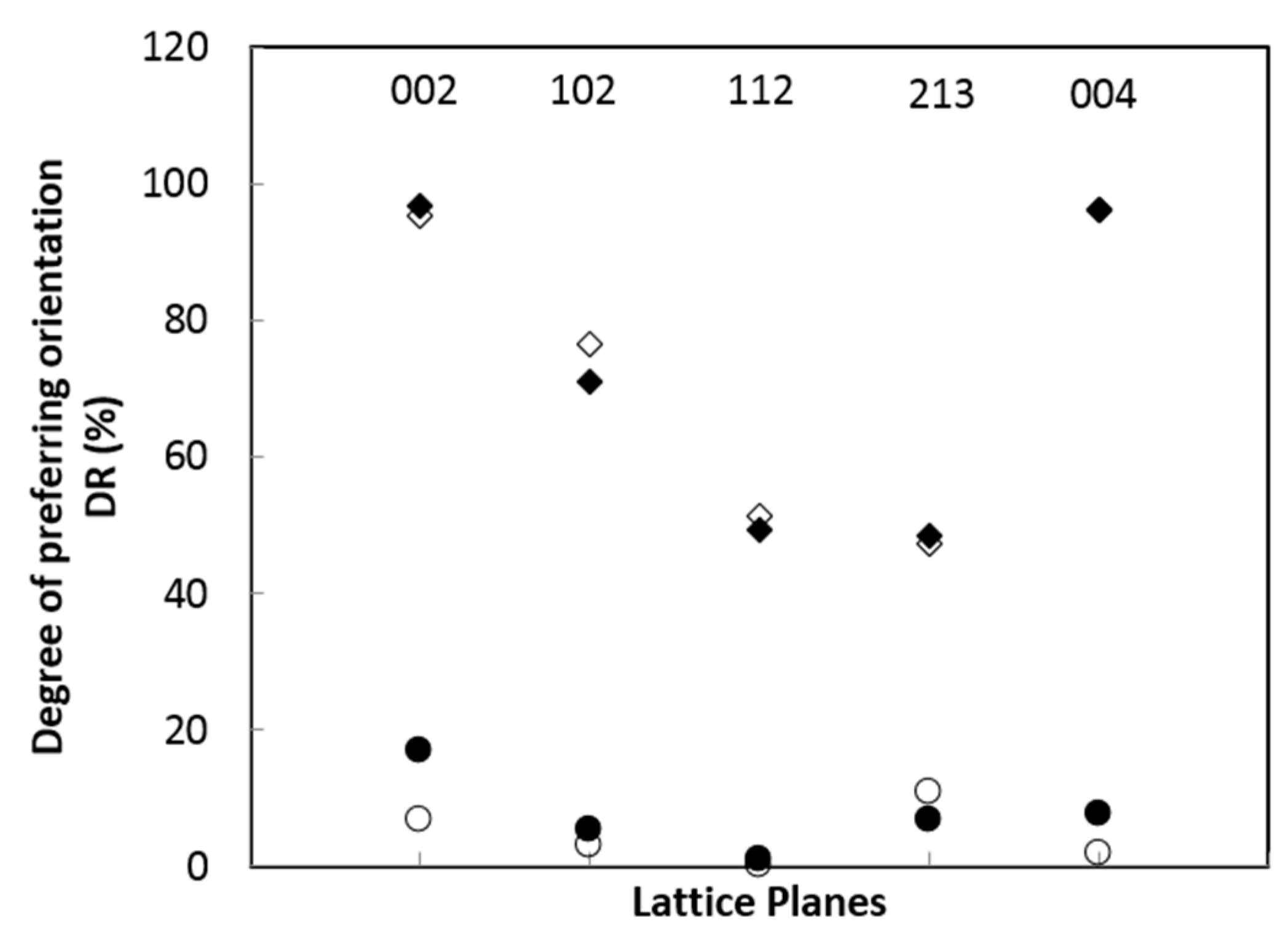
2.4. Crystal Orientation
3. Discussions
3.1. Substitution in HAP
3.2. Particle Size and Crystallinity
3.3. Orientation of HAP
4. Materials and Methods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rountrey, A.N.; Coulson, P.J.; Meeuwig, J.J.; Meekan, M. Water temperature and fish growh: Otoliths predict growth patterns of marine fish in a changing climate. Glob. Chang. Biol. 2014, 20, 2450–2458. [Google Scholar] [CrossRef] [PubMed]
- Phillis, C.C.; Ostrach, D.J.; Ingram, B.L.; Weber, P.K. Evaluating otolith Sr/Ca as a tool for reconstructing estuarine habitat use. Can. J. Fish. Aquat. Sci. 2011, 68, 360–373. [Google Scholar]
- Milton, D.A.; Chenery, S.R. Sources and uptake of trace metals in otoliths of juvenile barramundi (Lates calcarifer). J. Exp. Mar. Biol. Ecol. 2001, 264, 47–65. [Google Scholar] [CrossRef]
- Devereux, I. Temperature measurements from oxygen isotope ratios of fish otoliths. Science 1967, 155, 1684–1685. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.Y.; Li, Z.H.; Zhu, M.W. Study on scale surface structure of colossomabrachypomum by scanning electron microscopy. Chin. J. Zool. 1999, 34, 8–12. [Google Scholar]
- Gu, Y.J.; Li, J.; Li, F.W.; Bao, J.Q. The research progress in chemical composition of fish scales. J. Shanxi Agric. Sci. 2011, 39, 1227–1231. [Google Scholar]
- Ikoma, T.; Kobayashi, H.; Tanaka, J.; Walsh, D.; Mann, S. Microstructure, mechanical, and biomimetic properties of fish scales from Pagrus major. J. Struct. Biol. 2003, 142, 327–333. [Google Scholar] [CrossRef]
- Panda, N.N.; Pramanik, K.; Sukla, L.B. Extraction and characterization of biocompatible hydroxyapatite from fresh water fish scales for tissue engineering scaffold. Bioprocess Biosyst. Eng. 2014, 37, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Cui, F.Z.; Wang, X.M. Variation of nanomechanical properties in the gene mutated Zebra fish bone. Mater. Rev. 2004, 18, 65–68. [Google Scholar]
- Mann, S. Biomineralization: Principals and Concepts in Bioinorganic Materials Chemistry; Oxford University Press: Oxford, UK, 2001; pp. 24–37. [Google Scholar]
- Xue, J.; Zavgorodniy, A.V.; Kennedy, B.J.; Swain, M.V.; Li, W. X-ray microdiffraction, TEM characterization and texture analysis of human dentin and enamel. J. Microsc. 2013, 251, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.F.; Qiao, L.; Feng, Q.L. Research progress on biomineralization mechanism of freshwater pearl. J. Inorg. Mater. 2013, 28, 109–116. [Google Scholar] [CrossRef]
- Wei, W.; Mao, J.; Peng, Z.; Xiao, Z.J.; Liu, Y.; Gong, S.Q.; Wang, D.; Zhou, B. High-resolution X-ray microdiffraction analysis of NaOH-treated dentin. J. Appl. Crystallogr. 2009, 42, 616–620. [Google Scholar] [CrossRef]
- Hughes, J.M.; Cameron, M.; Crowley, K.D. Structural variations in natural Fo OH, and Cl apatites. Am. Mineral. 1989, 74, 870–876. [Google Scholar]
- Hughes, J.M.; Cameron, M.; Crowley, K.D. Crystal structures of natural ternary apatites: Solid solution in the Ca5(PO4)3X (X = F, OH, Cl) system. Am. Mineral. 1990, 75, 295–304. [Google Scholar]
- Wilson, R.M.; Elliott, J.C.; Dower, S.E.P. Rietveld refinement of the crystallographic structure of human dental enamel apatites. Am. Mineral. 1999, 84, 1406–1414. [Google Scholar] [CrossRef]
- International Center for Diffraction Database (ICDD). Powder Diffraction File (PDF-2), version 2005; ICDD: Newtown Square, PA, USA, 2005. [Google Scholar]
- Sasaki, T.; Sakai, Y.; Iizuka, A.; Nakae, T.; Kato, S.; Kojima, T.; Yamasaki, A. Evaluation of the Capacity of Hydroxyapaptite Prepared from Concrete Sludge to Remove Lead from Water. Ind. Eng. Chem. Res. 2011, 50, 9564–9568. [Google Scholar] [CrossRef]
- Liao, J.G.; Li, Y.Q.; Duan, X.Z.; Liu, Q. Synthesis and Characterization of CO32- Doping Nano-Hydroxyapatite. Spectrosc. Spectr. Anal. 2014, 34, 3011–3014. [Google Scholar]
- Okada, M.; Fujiwara, K.; Uehira, M.; Matsumoto, N.; Takeda, S. Expansion of nanosized pores in low-crystallinity nanoparticle-assembled plates via a thermally induced increase in solid-state density. J. Colloid Interface Sci. 2013, 405, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Karamian, E.; Khandan, A.; Eslami, M.; Gheisari, H.; Rafiaei, N. Investigation of HA vanocrystallite size crystallographic characterizations in NHA, BHA and HA pure powders and their influence on biodegradation of HA. Adv. Mater. Res. 2014, 829, 314–318. [Google Scholar] [CrossRef]
- Locardi, B.; Pazzaglia, U.E.; Gabbi, C.; Profilo, B. Thermal behaviour of hydroxyapatite intended for medical applications. Biomaterials 1993, 14, 437–441. [Google Scholar] [CrossRef]
- Sillen, A.; Sealy, J.C. Diagenesis if strontium in fossil bone: A reconsideration of Nelson et al. (1986). J. Arch. Sci. 1995, 22, 313–320. [Google Scholar] [CrossRef]
- Wilson, A.J.C. X-ray Optics: The Diffraction of X-rays by Finite and Imperfect Crystals; Methuen: London, UK, 1962; pp. 37–54. [Google Scholar]
- Klug, H.P.; Alexander, L.E. X-ray Diffraction Procedures for Polycrystalline and Amorphous Materials; Wily: New York, NY, USA, 1974; pp. 618–708. [Google Scholar]
- Low, I.M. Depth-profiling of crystal structure, texture, and microhardness in a functionally graded tooth enamel. J. Am. Ceram. Soc. 2004, 87, 2125–2131. [Google Scholar] [CrossRef]
- Kalita, S.J.; Bhardwaj, A.; Bhatt, H.A. Nanocrystalline calcium phosphate ceramics in biomedical engineering. Mater. Sci. Eng. C 2007, 27, 441–449. [Google Scholar] [CrossRef]
- Cui, F.Z. Biomineralization; Tsinghua University Press: Beijing, China, 2007; pp. 17–21. [Google Scholar]
- Zhou, L.D.; Liu, Y.K.; Zhou, G.F. A study on modern biological apatite and fossil apatite. Act. Miner. Sin. 1999, 19, 41–52. [Google Scholar]
- Hench, L.L.; Wilson, J. An Introduction to Bioceramics; World Scientific Publishing Co.: Singapore, 1993; pp. 140–141. [Google Scholar]
- Yuan, L.; Wang, H.J.; Zhou, Z.; An, J.L. Crystallographic Characteristics of Hydroxylapatite in Hard Tissues of Freshwater LateolabraxJaponicus. Act. Miner. Sin. 2017, in press. [Google Scholar]
- Cai, Y.R.; Tang, R.K. Calcium phosphate nanoparticles in biomineralization and biomaterials. J. Mater. Chem. 2008, 18, 3775–3787. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Gonzalez-Calbet, J.M. Calcium phosphates as substitution of bone tissues. Prog. Solid State Chem. 2004, 32, 1–31. [Google Scholar] [CrossRef]
- Gao, H.J.; Ji, B.H.; Jager, I.L.; Arzt, E.; Fratzl, P. Materials become insensitive to flaws at nanoscales: Lessons from nature. Proc. Natl. Acad. Sci. USA 2003, 100, 5597–5600. [Google Scholar] [CrossRef] [PubMed]
- Cuy, J.L.; Mann, A.B.; Livi, K.J.; Teaford, M.F.; Weihs, T.P. Nanoindentation mapping of the mechanical properties of human molar tooth enamel. Arch. Oral Biol. 2002, 47, 281–291. [Google Scholar] [CrossRef]
- Wang, K.L.; Zhang, Y.X.; Naab, F.U. Calibration for IR measurements of OH in apatite. Am. Mineral. 2011, 96, 1392–1397. [Google Scholar] [CrossRef]
- Clark, K.; Zhang, Y.; Naab, F.U. Quantification of CO2 concentration in apatite. Am. Mineral. 2016, 101, 2443–2451. [Google Scholar] [CrossRef]
- Dumont, M.; Borbely, A.; Kaysser-Pyzalla, A.; Sande, P.M. Long bone cortices in a growth series of Apatosaurus sp. (Dinosauria: Diplodocidae): Geometry, body mass, and crystallite orientation of giant animals. Biol. J. Linn. Soc. 2014, 112, 782–798. [Google Scholar] [CrossRef]
- Landis, W.J.; Paine, M.C.; Glimcher, M.J. Electron-microscopic observations of bone tissue prepared anhydrously in organic-solvents. J. Ultrastruct. Res. 1977, 59, 1–30. [Google Scholar] [CrossRef]
- Akazawa, H.; Ueno, Y. Control of composition and crystallinity in hydroxyapatite films deposited by electron cyclotron resonance plasma sputtering. J. Phys. Chem. Solids 2014, 75, 94–99. [Google Scholar] [CrossRef]
- Naleway, S.E.; Taylor, J.R.A.; Porter, M.M.; Meyers, M.A.; McKittrick, J. Structure and mechanical properties of selected protective systems in marine organisms. Mater. Sci. Eng. C 2016, 59, 1143–1167. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.H.; Gao, H.J. Mechanical principles of biological nanocomposites. Annu. Rev. Mater. Res. 2010, 40, 77–100. [Google Scholar] [CrossRef]
- Traub, W.; Arad, T.; Weiner, S. 3-dimensional ordered distribution of crystals in turkey tendon collagen-fibers. Proc. Natl. Acad. Sci. USA 1989, 86, 9822–9826. [Google Scholar] [CrossRef] [PubMed]

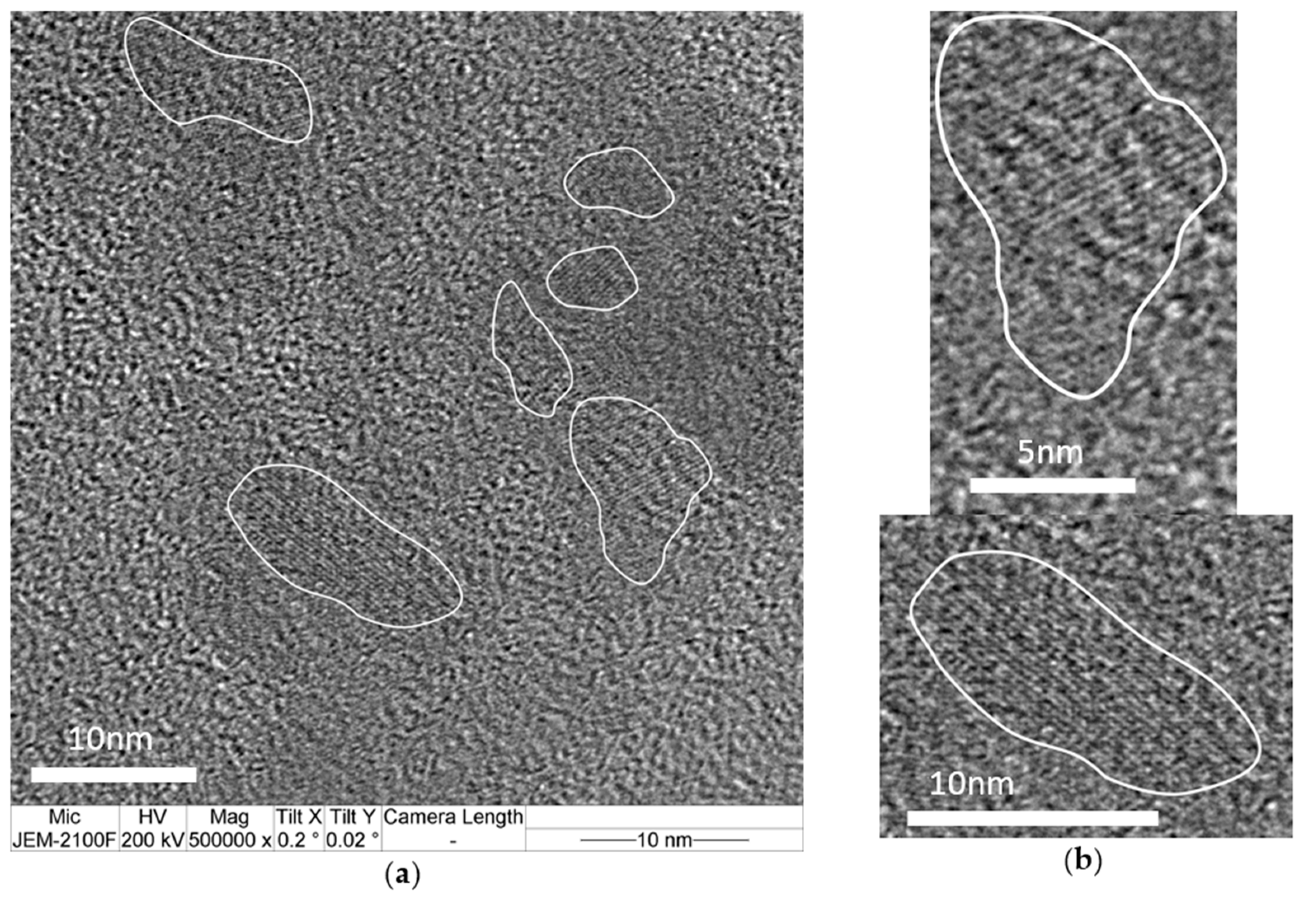

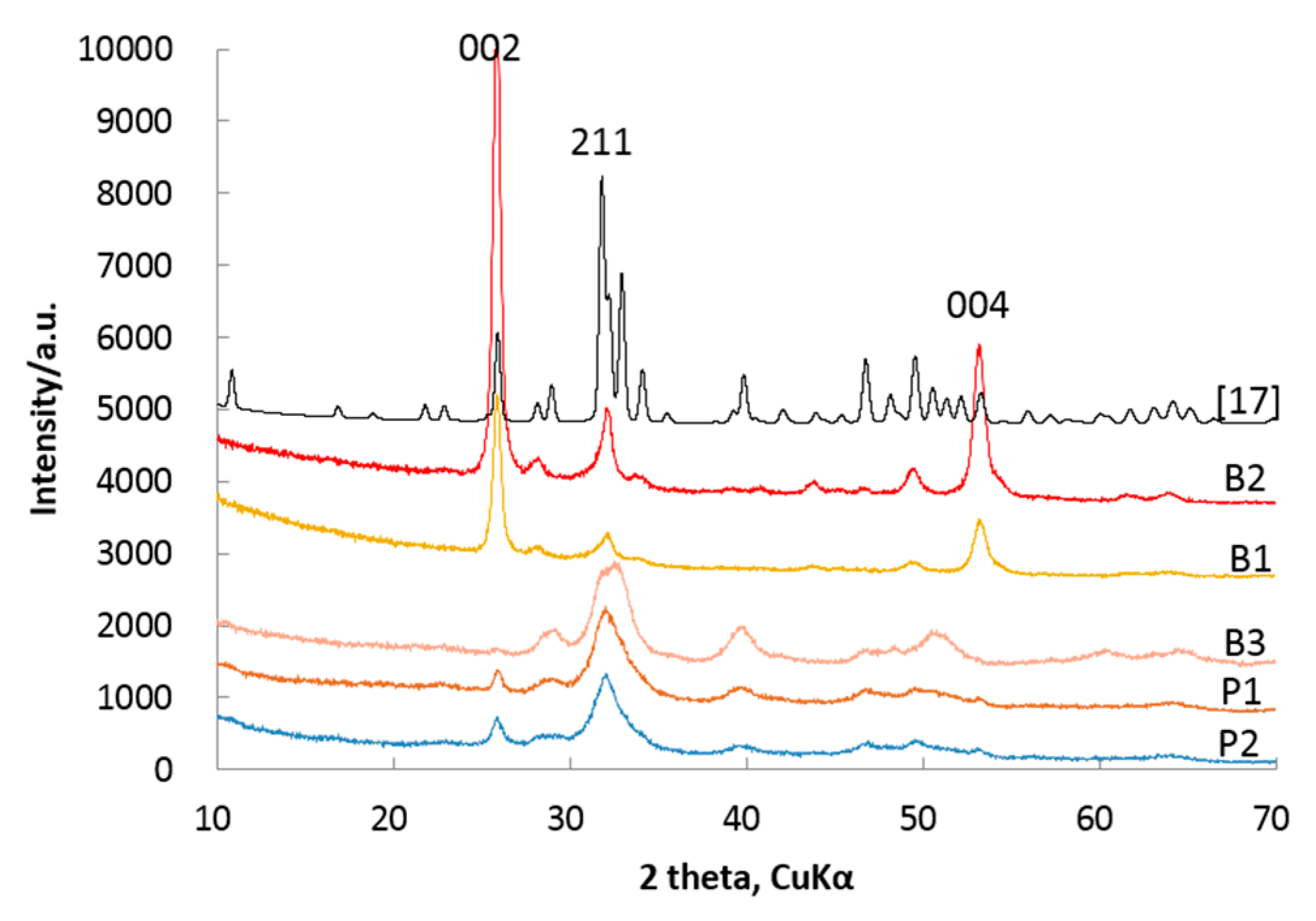
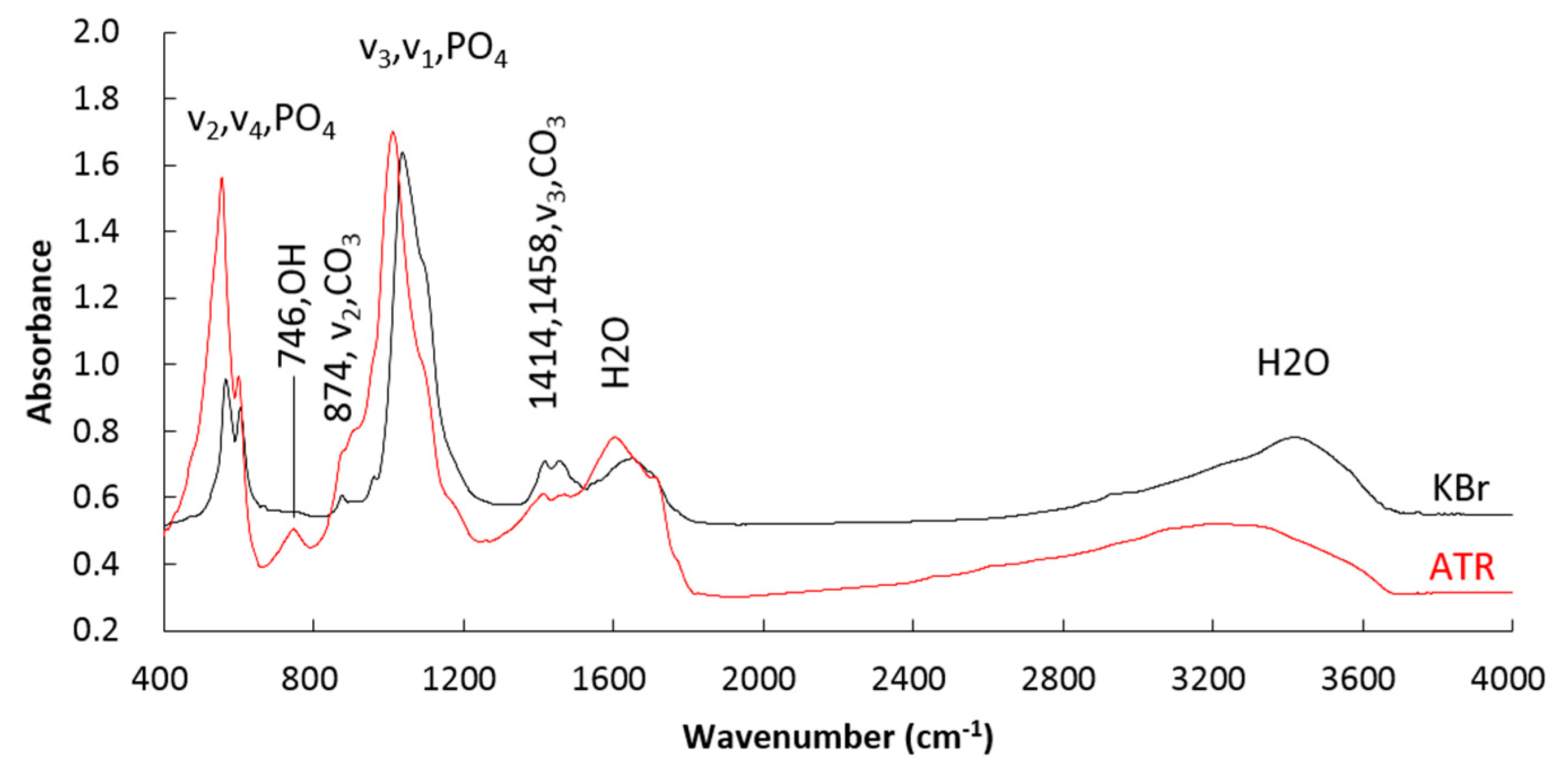
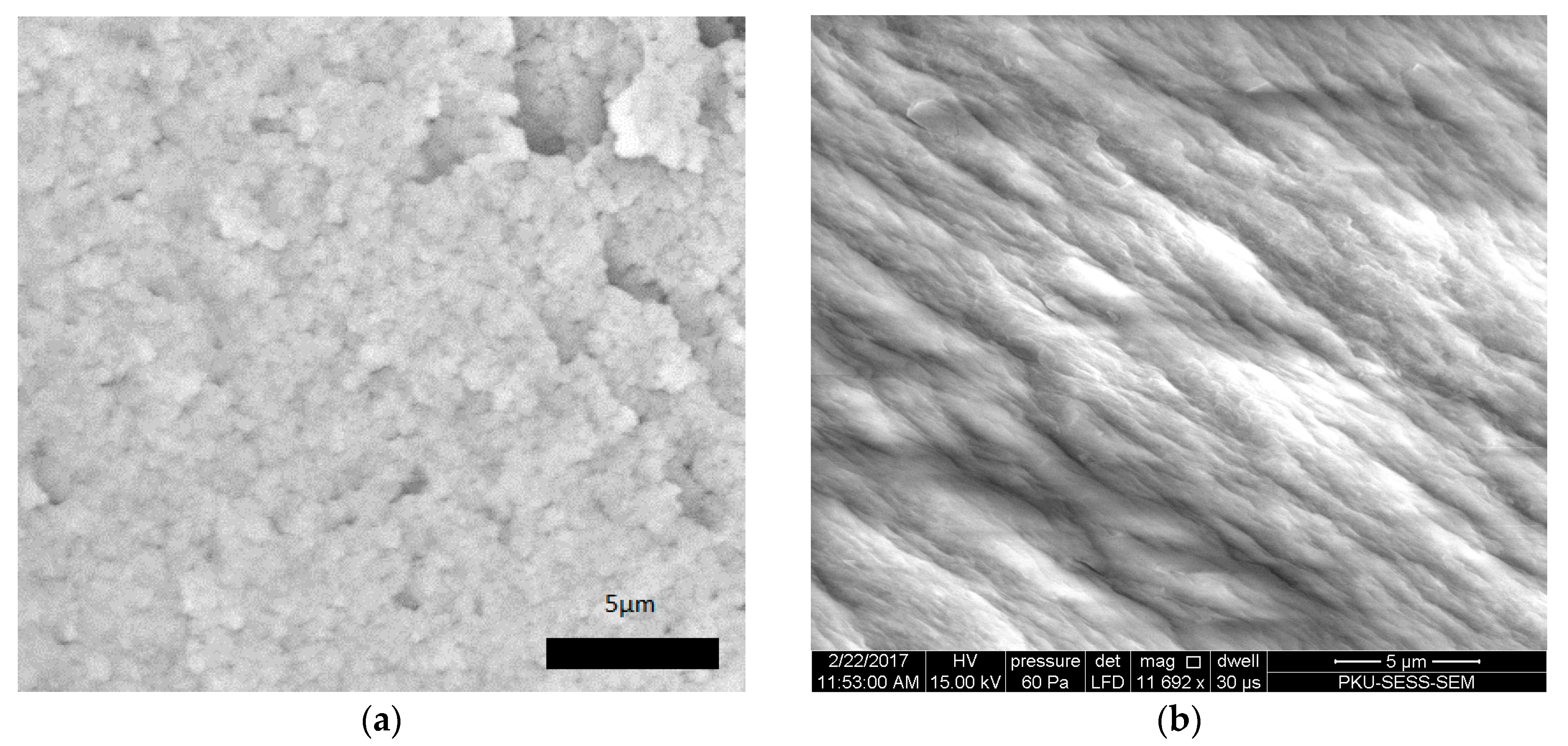
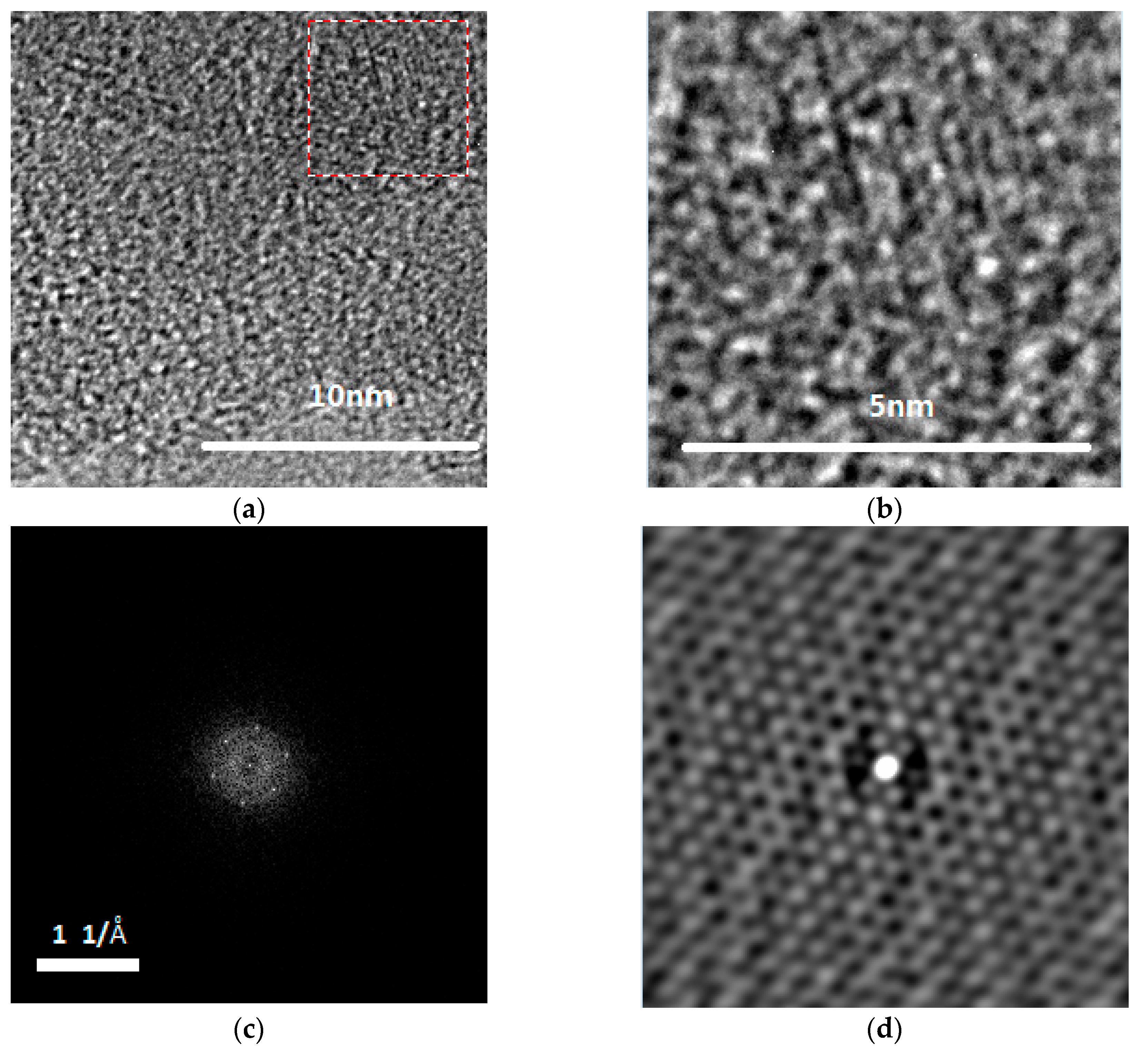
| hkl | 01-086-0740 a | B1 | B2 | P1 | P2 |
|---|---|---|---|---|---|
| 100 | 0.80991 | 0.80803 | 0.80965 | ||
| 101 | 0.52444 | 0.52713 | 0.52779 | ||
| 111 | 0.38677 | 0.39296 | 0.39089 | ||
| 002 | 0.34410 | 0.34312 | 0.34432 | 0.34335 | 0.34432 |
| 102 | 0.31670 | 0.31664 | 0.31679 | 0.31563 | 0.31631 |
| 210 | 0.30612 | 0.30867 | 0.30485 | ||
| 211 | 0.27970 | 0.27851 | 0.27902 | ||
| 112 | 0.27715 | 0.27852 | 0.27770 | 0.27748 | |
| 202 | 0.26222 | 0.26374 | 0.26212 | ||
| 212 | 0.22871 | 0.22997 | 0.22792 | 0.22801 | |
| 130 | 0.22463 | 0.22495 | |||
| 103 | 0.22072 | 0.22102 | 0.22077 | ||
| 113 | 0.20595 | 0.20677 | 0.20655 | 0.20601 | 0.20666 |
| 203 | 0.19960 | 0.20058 | 0.19801 | 0.19996 | |
| 222 | 0.19339 | 0.19427 | 0.19431 | ||
| 213 | 0.18357 | 0.18433 | 0.18274 | 0.18342 | |
| 004 | 0.17205 | 0.17220 | 0.17225 | 0.17147 | 0.17187 |
| 322 | 0.16349 | 0.16469 | 0.16349 | ||
| 124 | 0.14998 | 0.15046 | 0.15057 | 0.150541 | |
| 502 | 0.14656 | 0.14599 | 0.14562 | ||
| 304 | 0.14509 | 0.14519 | 0.14557 | ||
| 523 | 0.12896 | 0.12968 | |||
| 234 | 0.12624 | 0.12611 | |||
| 144 | 0.12328 | 0.12374 | 0.13209 |
| Element | QDY-45-1 | QDY-45-2 | QDY-26 |
|---|---|---|---|
| O | 68.00 | 63.05 | 57.17 |
| Na | - | - | 0.45 |
| Mg | 1.28 | 1.25 | 1.52 |
| Fe | 0.20 | - | 0.16 |
| Ca | 18.72 | 22.29 | 25.87 |
| Al | - | 0.49 | - |
| Si | 1.21 | 0.3 | - |
| P | 10.59 | 12.58 | 14.84 |
| Σ | 100 | 100 | 100 |
| VI/IV | 1.71 | 1.76 | 1.89 |
| Ca/P | 1.76 | 1.77 | 1.74 |
| Parameters | B1 | B2 | P1 | P2 |
|---|---|---|---|---|
| a/nm | 0.93787 | 0.93740 | 0.93636 | 0.93622 |
| c/nm | 0.68892 | 0.69011 | 0.68549 | 0.68823 |
| V/nm3 | 0.52479 | 0.52517 | 0.52050 | 0.52242 |
| Planes | B1 | B2 | P1 | P2 |
|---|---|---|---|---|
| FWHM100 | - | - | 1.513 | 0.965 |
| Size100 | - | - | 6.0 | 9.6 |
| FWHM002 | 0.511 | 0.482 | 0.524 | 0.675 |
| Size002 | 19.4 | 20.7 | 18.9 | 14.4 |
| Plane | B1 | B2 | P1 | P2 |
|---|---|---|---|---|
| 002 | 0.05 | 0.03 | 1.08 | 0.83 |
| 102 | 0.23 | 0.29 | 1.03 | 0.95 |
| 112 | 0.49 | 0.51 | 1.00 | 1.01 |
| 213 | 0.53 | 0.52 | 1.11 | 1.07 |
| 004 | 0.04 | 0.04 | 1.02 | 0.92 |
| Plane | B1 | B2 | P1 | P2 |
|---|---|---|---|---|
| 002 | 95.33 | 96.93 | 6.83 | 16.89 |
| 102 | 76.55 | 70.88 | 2.89 | 5.33 |
| 112 | 51.20 | 49.28 | 0.06 | 1.03 |
| 213 | 47.22 | 48.37 | 10.80 | 6.55 |
| 004 | 96.11 | 96.30 | 1.63 | 7.55 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Yuan, L.; An, J. Crystallographic Characteristics of Hydroxylapatite in Hard Tissues of Cololabis saira. Crystals 2017, 7, 103. https://doi.org/10.3390/cryst7040103
Wang H, Yuan L, An J. Crystallographic Characteristics of Hydroxylapatite in Hard Tissues of Cololabis saira. Crystals. 2017; 7(4):103. https://doi.org/10.3390/cryst7040103
Chicago/Turabian StyleWang, Hejing, Lei Yuan, and Jiali An. 2017. "Crystallographic Characteristics of Hydroxylapatite in Hard Tissues of Cololabis saira" Crystals 7, no. 4: 103. https://doi.org/10.3390/cryst7040103






