Li2HgMS4 (M = Si, Ge, Sn): New Quaternary Diamond-Like Semiconductors for Infrared Laser Frequency Conversion
Abstract
:1. Introduction
2. Results and Discussion
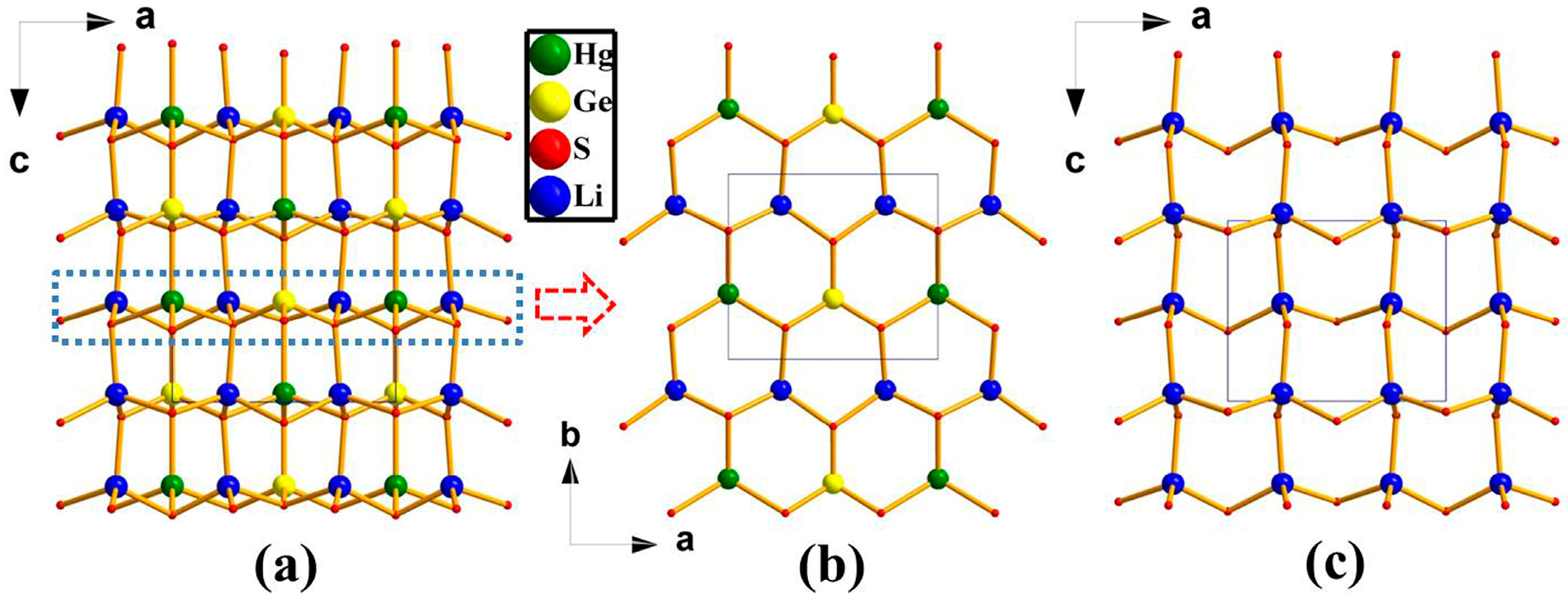
2.1. Crystal Structure
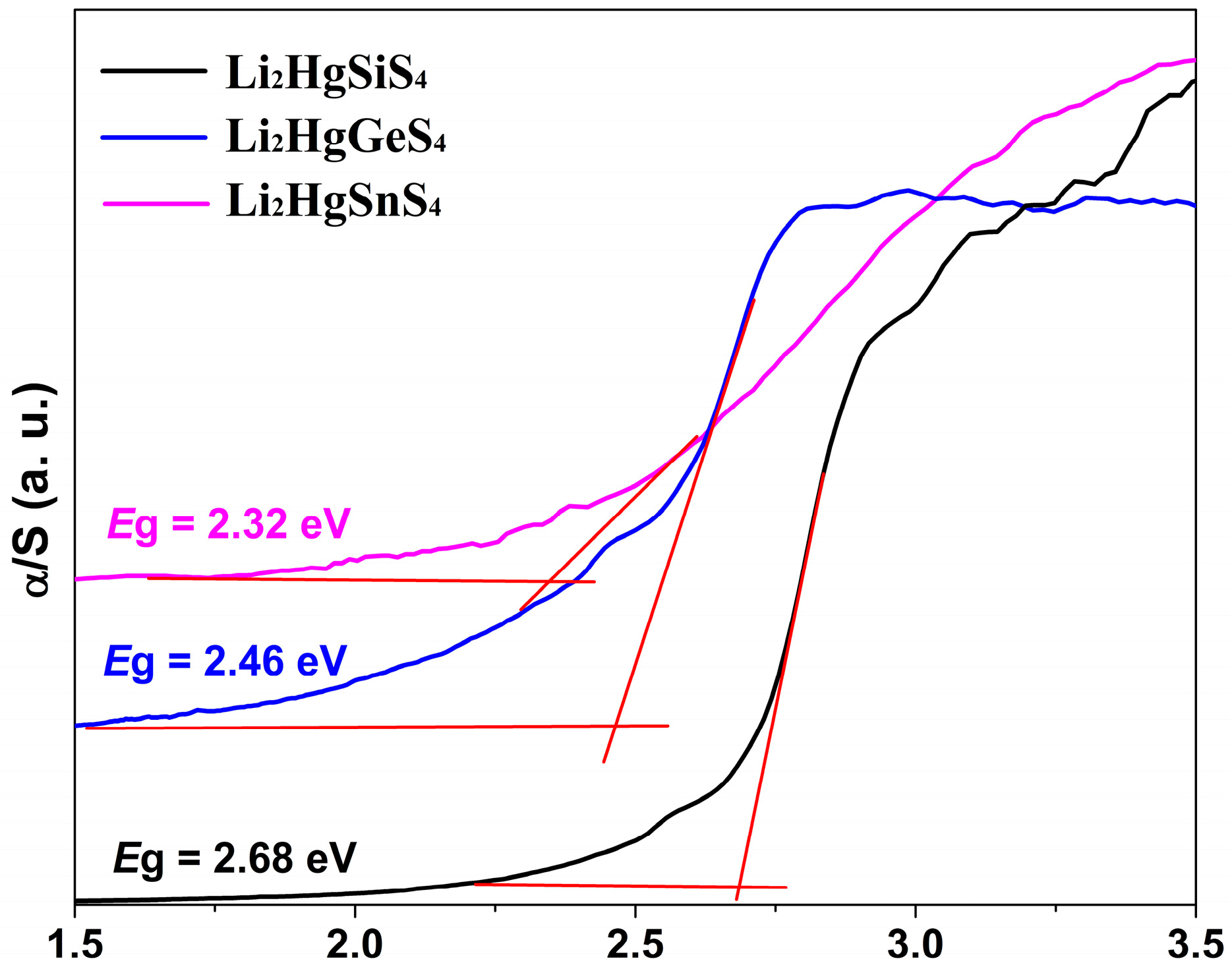
2.2. Optical Properties
3. Materials and Methods
3.1. Synthesis
3.1.1. Li2HgSiS4 and Li2HgSnS4
3.1.2. Li2HgGeS4
3.2. Structure Determination
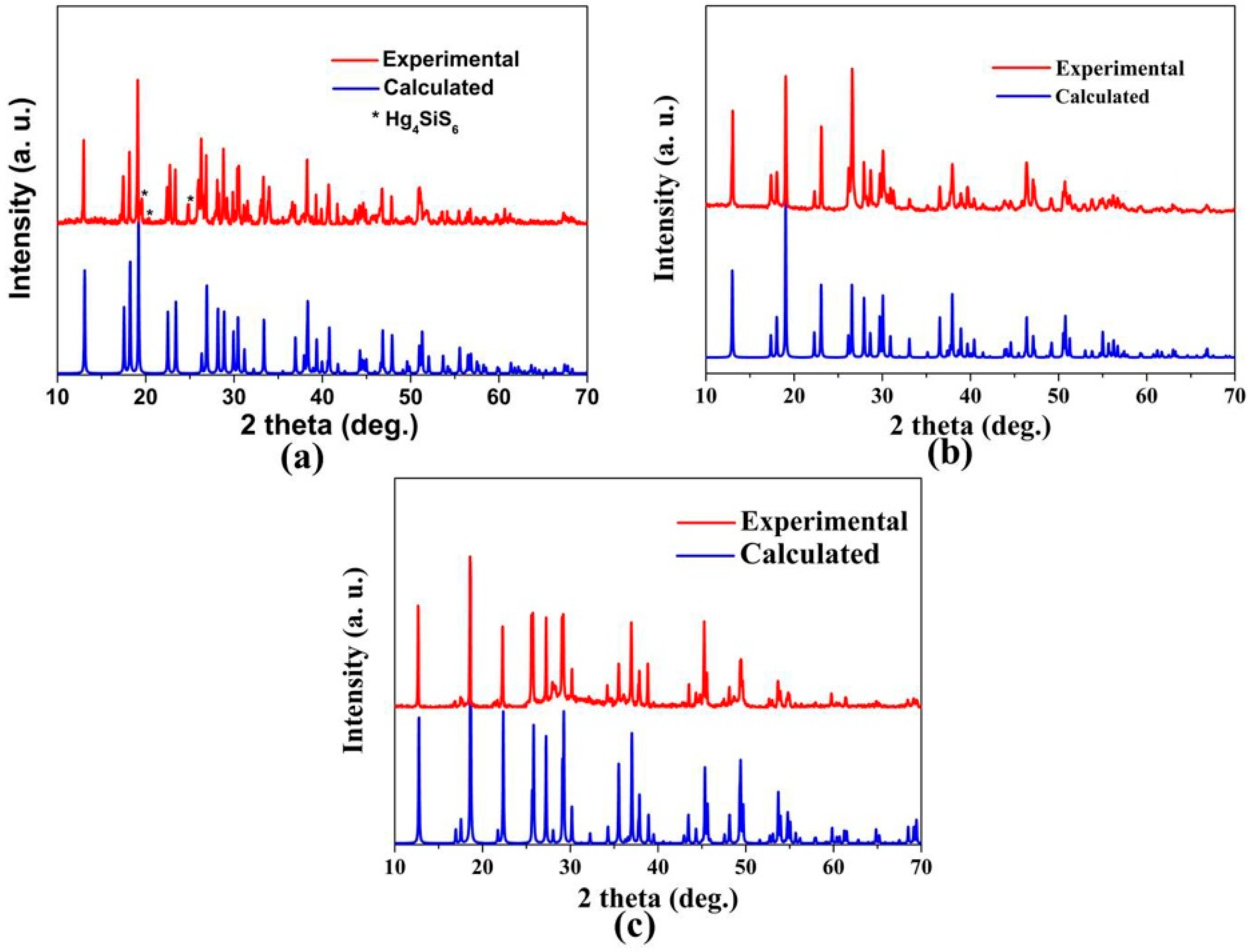
3.3. Powder XRD Measurement
3.4. UV–Vis–NIR Diffuse-Reflectance Spectroscopy
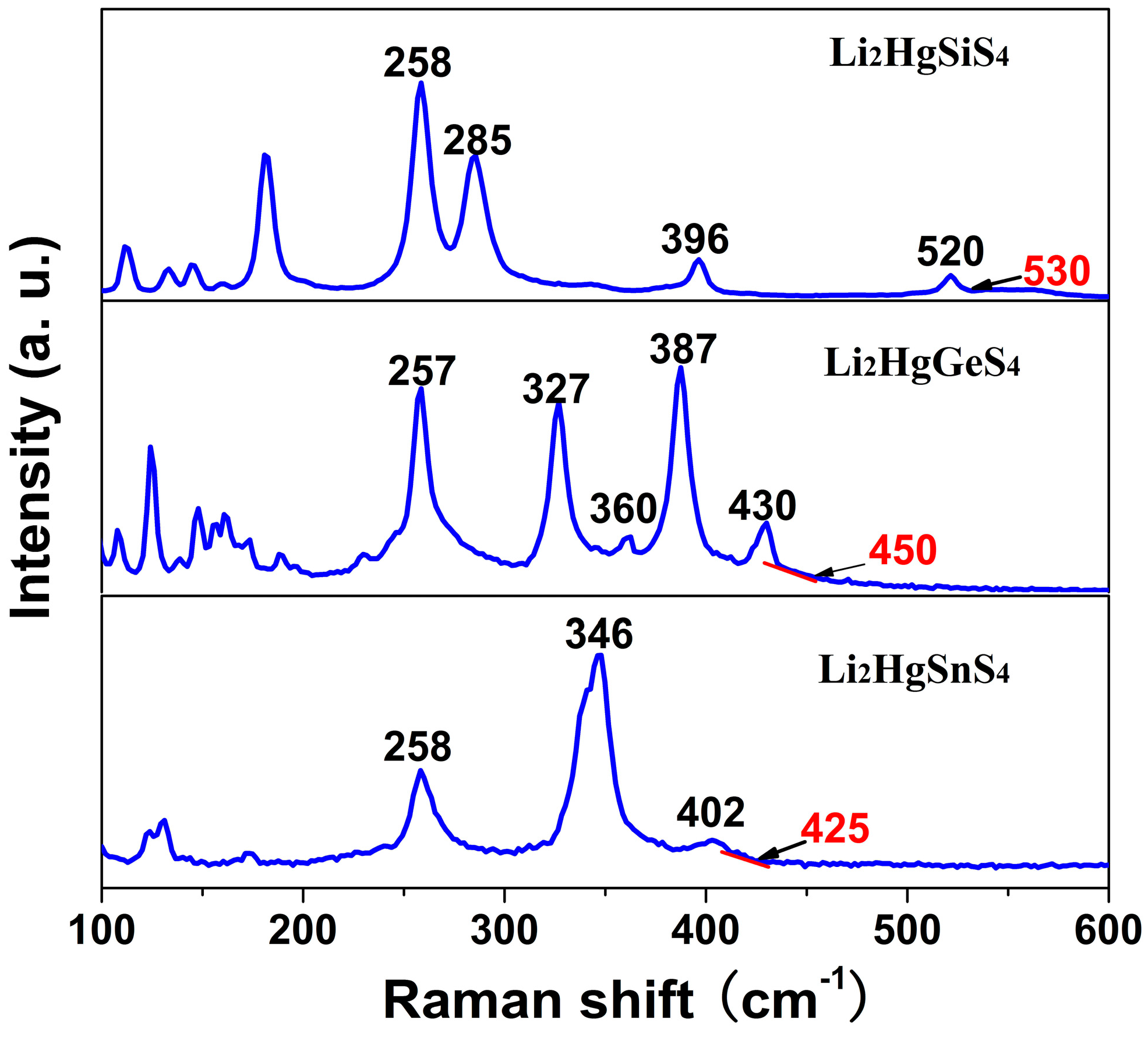
3.5. Raman Spectroscopy
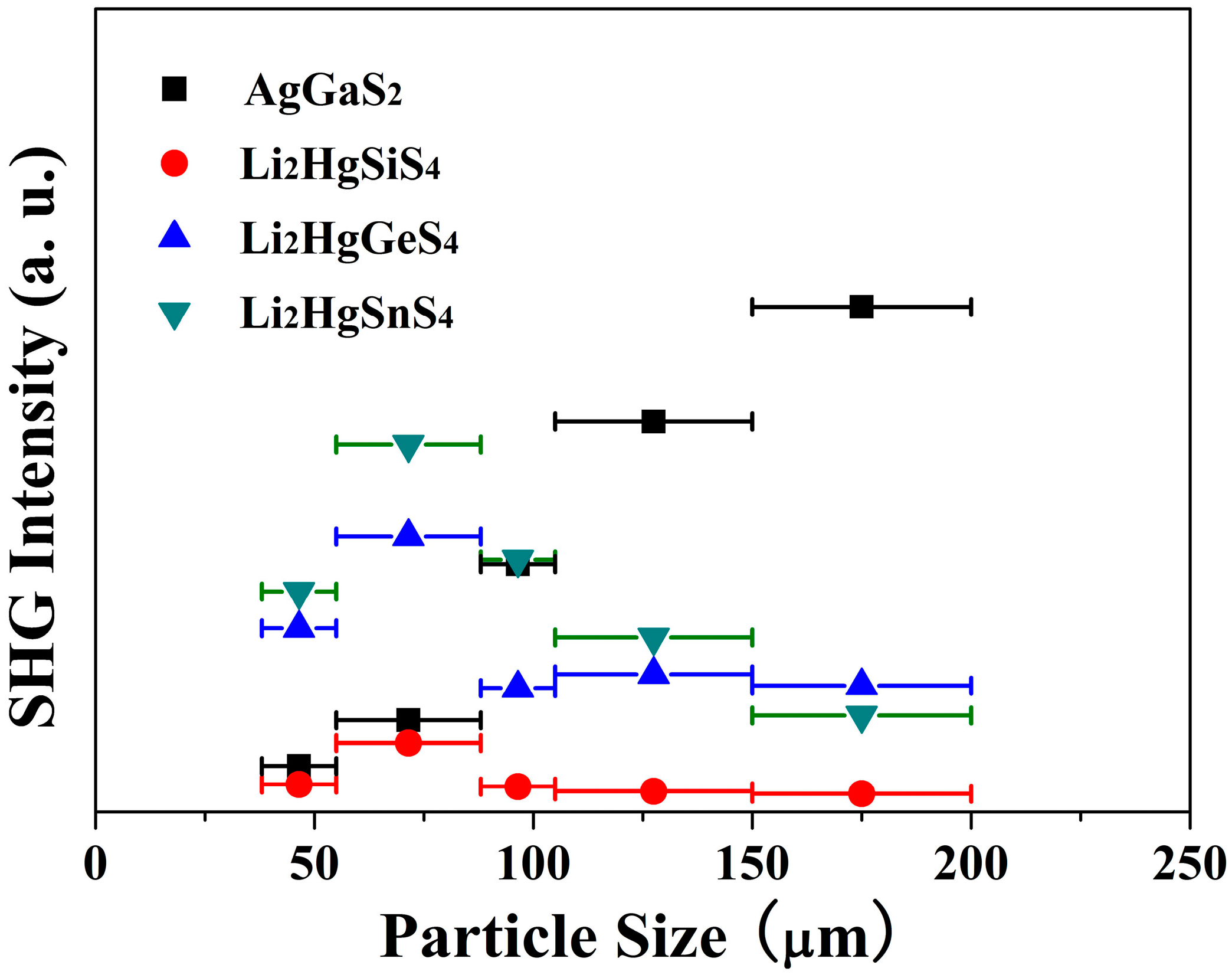
3.6. Second-Harmonic Generation Measurement
3.7. LDT Measurement
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Keller, U. Recent developments in compact ultrafast lasers. Nature 2003, 424, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Byer, R.L. Diode laser-pumped solid-state lasers. Science 1988, 239, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Duarte, F.J. Tunable Laser Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008; Chapters 2, 9 and 12. [Google Scholar]
- Demtröder, W. Laser Spectroscopy, 3rd ed.; Springer: Berlin, Germany, 2009. [Google Scholar]
- Nikogosyan, D.N. Nonlinear Optical Crystals: A Complete Survey, 1st ed.; Springer: New York, NY, USA, 2005. [Google Scholar]
- Chen, C.T.; Wu, Y.C.; Jiang, A.D.; Wu, B.C.; You, G.M.; Li, R.K.; Lin, S.J. New Nonlinear-Optical Crystal: LiB3O5. J. Opt. Soc. Am. B 1989, 6, 616–621. [Google Scholar] [CrossRef]
- Chen, C.T.; Wu, B.C.; Jiang, A.D.; You, G.M. A New-Type Ultraviolet SHG Crystal-β-BaB2O4. Sci. Sin. Ser. B 1985, 28, 235–243. [Google Scholar]
- Mei, L.; Wang, Y.; Chen, C.T.; Wu, B.C. Nonlinear Optical Materials Based on MBe2BO3F2 (M = Na, K). J. Appl. Phys. 1993, 74, 7014–7016. [Google Scholar] [CrossRef]
- Wang, G.L.; Zhang, C.Q.; Chen, C.T.; Yao, A.Y.; Zhang, J.; Xu, Z.Y.; Wang, J.Y. High-Efficiency 266-nm Output of a KBe2BO3F2 Crystal. Appl. Opt. 2003, 42, 4331–4334. [Google Scholar] [CrossRef] [PubMed]
- Becker, P. Borate Materials in Nonlinear Optics. Adv. Mater. 1998, 10, 979–992. [Google Scholar] [CrossRef]
- Sun, C.F.; Hu, C.L.; Xu, X.; Ling, J.B.; Hu, T.; Kong, F.; Long, X.F.; Mao, J.G. BaNbO(IO3)5: A New Polar Material with A Very Large SHG Response. J. Am. Chem. Soc. 2009, 131, 9486–9487. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.L.; Mao, J.G. Recent Advances on Second-Order NLO Materials Based on Metal Iodates. Coord. Chem. Rev. 2015, 288, 1–17. [Google Scholar] [CrossRef]
- Song, J.L.; Hu, C.L.; Xu, X.; Kong, F.; Mao, J.G. A Facile Synthetic Route to a New SHG Material with Two Types of Parallel π-Conjugated Planar Triangular Units. Angew. Chem. Int. Ed. 2015, 54, 3679–3682. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.H.; Ye, N.; Huang, L.; Lin, X.S. Alkaline-Alkaline Earth Fluoride Carbonate Crystals ABCO3F (A = K, Rb, Cs; B = Ca, Sr, Ba) as Nonlinear Optical Materials. J. Am. Chem. Soc. 2011, 133, 20001–20007. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.S.; Peng, G.; Ye, N.; Wang, J.Y.; Luo, M.; Yan, T.; Zhou, Y.Q. Structural Modulation of Anionic Group Architectures by Cations to Optimize SHG Effects: A Facile Route to New NLO Materials in the ATCO3F (A = K, Rb; T = Zn, Cd) Series. Chem. Mater. 2015, 27, 7520–7530. [Google Scholar] [CrossRef]
- Zou, G.H.; Huang, L.; Ye, N.; Lin, C.S.; Cheng, W.D.; Huang, H. CsPbCO3F: A Strong Second-Harmonic Generation Material Derived from Enhancement via p–π Interaction. J. Am. Chem. Soc. 2013, 135, 18560–18566. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.W.; Zhang, W.G.; Young, J.; Rondinelli, J.M.; Halasyamani, P.S. Bidenticity-Enhanced Second Harmonic Generation from Pb Chelation in Pb3Mg3TeP2O14. J. Am. Chem. Soc. 2016, 138, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.W.; Zhang, W.G.; Young, J.; Rondinelli, J.M.; Halasyamani, P.S. Design and Synthesis of the Beryllium-Free Deep-Ultraviolet Nonlinear Optical Material Ba3(ZnB5O10)PO4. Adv. Mater. 2015, 27, 7380–7385. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Tran, T.T.; Choi, W.; You, T.S.; Halasyamani, P.S.; Ok, K.M. Two New Non-centrosymmetric n = 3 Layered Dion–Jacobson Perovskites: Polar RbBi2Ti2NbO10 and Nonpolar CsBi2Ti2TaO10. Chem. Mater. 2016, 28, 2424–2432. [Google Scholar] [CrossRef]
- Zou, G.H.; Nam, G.; Kim, H.G.; Jo, H.; You, T.S.; Ok, K.M. ACdCO3F (A = K and Rb): New Noncentrosymmetric Materials with Remarkably Strong Second-Harmonic Generation (SHG) Responses Enhanced via π-Interaction. RSC Adv. 2015, 5, 84754–84761. [Google Scholar] [CrossRef]
- Cheng, L.; Wei, Q.; Wu, H.Q.; Zhou, L.J.; Yang, G.Y. Ba3M2[B3O6(OH)]2[B4O7(OH)2] (M = Al, Ga): Two Novel UV Nonlinear Optical Metal Borates Containing Two Types of Oxoboron Clusters. Chem. Eur. J. 2013, 19, 17662–17667. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W.; Liu, L.J.; Jin, S.F.; Yao, W.J.; Zhang, Y.H.; Chen, C.T. Deep-Ultraviolet Nonlinear Optical Materials: Na2Be4B4O11 and LiNa5Be12B12O33. J. Am. Chem. Soc. 2013, 135, 18319–18322. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Hou, X.L.; Pan, S.L.; Wang, X.A. Growth, structure, and optical properties of a congruent melting oxyborate, Bi2ZnOB2O6. Chem. Mater. 2009, 21, 2846–2850. [Google Scholar] [CrossRef]
- Wu, H.P.; Pan, S.L.; Poeppelmeier, K.R.; Li, H.Y.; Jia, D.Z.; Chen, Z.H.; Fan, X.Y.; Yang, Y.; Rondinelli, J.M.; Luo, H. K3B6O10Cl: A New Structure Analogous to Perovskite with A Large Second Harmonic Generation Response and Deep UV Absorption Edge. J. Am. Chem. Soc. 2011, 133, 7786–7790. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.W.; Wu, H.P.; Pan, S.L.; Yang, Z.H.; Hou, X.L.; Su, X.; Jing, Q.; Poeppelmeier, K.R.; Rondinelli, J.M. Cs3Zn6B9O21: A Chemically Benign Member of the KBBF Family Exhibiting the Largest Second Harmonic Generation Response. J. Am. Chem. Soc. 2014, 136, 1264–1267. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Y.; Lei, B.H.; Han, S.J.; Yang, Z.H.; Poeppelmeier, K.R.; Pan, S.L. A New Deep-Ultraviolet Transparent Orthophosphate LiCs2PO4 with Large Second Harmonic Generation Response. J. Am. Chem. Soc. 2016, 138, 9101–9104. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pan, S.L. Recent Development of Metal Borate Halides: Crystal Chemistry and Application in Second-order NLO Materials. Coord. Chem. Rev. 2016, 323, 15–35. [Google Scholar] [CrossRef]
- Dong, X.Y.; Jing, Q.; Shi, Y.J.; Yang, Z.H.; Pan, S.L.; Poeppelmeier, K.R.; Young, J.S.; Rondinelli, J.M. Pb2Ba3(BO3)3Cl: A Material with Large SHG Enhancement Activated by Pb-Chelated BO3 Groups. J. Am. Chem. Soc. 2015, 137, 9417–9422. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.P.; Yu, H.W.; Pan, S.L.; Huang, Z.J.; Yang, Z.H.; Su, X.; Poeppelmeier, K.R. Cs2B4SiO9, A Deep-ultraviolet Nonlinear Optical Crystal. Angew. Chem. Int. Ed. 2013, 52, 3406–3410. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.P.; Yu, H.W.; Yang, Z.H.; Hou, X.L.; Su, X.; Pan, S.L.; Poeppelmeier, K.R.; Rondinelli, J.M. Designing a Deep-Ultraviolet Nonlinear Optical Material with a Large Second Harmonic Generation Response. J. Am. Chem. Soc. 2013, 135, 4215–4218. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Gong, P.; Bai, L.; Xu, X.; Zhang, S.; Sun, Z.; Lin, Z.; Hong, M.; Chen, C.; Luo, J. Beryllium-free Li4Sr(BO3)2 for Deep-ultraviolet Nonlinear Optical Applications. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.G.; Gong, P.F.; Luo, S.Y.; Bai, L.; Lin, Z.S.; Tang, Y.Y.; Zhou, Y.L.; Hong, M.C.; Luo, J.H. Tailored Synthesis of a Nonlinear Optical Phosphate with a Short Absorption Edge. Angew. Chem. Int. Ed. 2015, 54, 4217–4221. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.G.; Gong, P.F.; Luo, S.Y.; Liu, S.J.; Li, L.N.; Asghar, M.A.; Khan, T.; Hong, M.C.; Lin, Z.S.; Luo, J.H. Beryllium-Free Rb3Al3B3O10F with Reinforced Inter layer Bonding as a Deep-Ultraviolet Nonlinear Optical Crystal. J. Am. Chem. Soc. 2015, 137, 2207–2210. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.X.; Luo, S.Y.; Kang, L.; Gong, P.F.; Huang, H.W.; Wang, S.C.; Lin, Z.S.; Chen, C.T. First-Principles Evaluation of the Alkali and/or Alkaline Earth Beryllium Borates in Deep Ultraviolet Nonlinear Optical Applications. ACS Photonics 2015, 2, 1183–1191. [Google Scholar] [CrossRef]
- Okorogu, A.O.; Mirov, S.B.; Lee, W.; Crouthamel, D.I.; Jenkins, N.; Dergachev, A.Y.; Vodopyanov, K.L.; Badikov, V.V. Tunable Middle Infrared Downconversion in GaSe and AgGaS2. Opt. Commun. 1998, 155, 307–312. [Google Scholar] [CrossRef]
- Boyd, G.D.; Storz, F.G.; McFee, J.H.; Kasper, H.M. Linear and Nonlinear Optical Properties of Some Ternary Selenides. IEEE J. Quantum Electron. 1972, 8, 900–908. [Google Scholar] [CrossRef]
- Boyd, G.D.; Buehler, E.; Storz, F.G. Linear and Nonlinear Optical Properties of ZnGeP2 and CdSe. Appl. Phys. Lett. 1971, 18, 301–304. [Google Scholar] [CrossRef]
- Chung, I.; Kanatzidis, M.G. Metal chalcogenides: A rich source of nonlinear optical materials. Chem. Mater. 2014, 26, 849–869. [Google Scholar] [CrossRef]
- Jiang, X.M.; Guo, S.P.; Zeng, H.Y.; Zhang, M.J.; Guo, G.C. Large Crystal Growth and New Crystal Exploration of Mid-Infrared Second-Order Nonlinear Optical Materials. Struct. Bond. 2012, 145, 1–43. [Google Scholar]
- Liang, F.; Kang, L.; Lin, Z.S.; Wu, Y.C.; Chen, C.T. Analysis and Prediction of Mid-IR Nonlinear Optical Metal Sulfides with Diamond-like Structures. Coord. Chem. Rev. 2017, 333, 57–70. [Google Scholar] [CrossRef]
- Guo, S.P.; Chi, Y.; Guo, G.C. Recent Achievements on Middle and Far-infrared Second-order Nonlinear Optical Materials. Coord. Chem. Rev. 2017, 335, 44–57. [Google Scholar] [CrossRef]
- Bera, T.K.; Jang, J.I.; Ketterson, J.B.; Kanatzidis, M.G. Strong Second Harmonic Generation from the Tantalum Thioarsenates A3Ta2AsS11 (A = K and Rb). J. Am. Chem. Soc. 2009, 131, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, F.; Kim, S.H.; Halasyamani, P.S. Top-Seeded Solution Crystal Growth and Functional Properties of a Polar Material Na2TeW2O9. Cryst. Growth Des. 2010, 10, 4091–4095. [Google Scholar] [CrossRef]
- Lin, H.; Zhou, L.J.; Chen, L. Sulfides with Strong Nonlinear Optical Activity and Thermochromism: ACd4Ga5S12 (A = K, Rb, Cs). Chem. Mater. 2012, 24, 3406–3414. [Google Scholar] [CrossRef]
- Chen, M.C.; Wu, L.M.; Lin, H.; Zhou, L.J.; Chen, L. Disconnection Enhances the Second Harmonic Generation Response: Synthesis and Characterization of Ba23Ga8Sb2S38. J. Am. Chem. Soc. 2012, 134, 6058–6060. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Zhou, L.J.; Chen, L. Noncentrosymmetric Inorganic Open-Framework Chalcohalides with Strong Middle IR SHG and Red emission: Ba3AGa5Se10Cl2 (A = Cs, Rb, K). J. Am. Chem. Soc. 2012, 134, 2227–2235. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Li, L.H.; Chen, Y.B.; Chen, L. In-Phase Alignments of Asymmetric Building Units in Ln4GaSbS9 (Ln = Pr, Nd, Sm, Gd–Ho) and Their Strong Nonlinear Optical Responses in Middle IR. J. Am. Chem. Soc. 2011, 133, 4617–4624. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.K.; Chen, M.C.; Zhou, L.J.; Chen, L.; Wu, L.M. Syntheses, Structures, and Nonlinear Optical Properties of Quaternary Chalcogenides: Pb4Ga4GeQ12 (Q = S, Se). Inorg. Chem. 2013, 52, 8334–8341. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.Y.; Mei, D.J.; Bai, L.; Lin, Z.S.; Yin, W.L.; Fu, P.Z.; Wu, Y.C. BaGa4Se7: A New Congruent-Melting IR Nonlinear Optical Material. Inorg. Chem. 2010, 49, 9212–9216. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.S.; Zhang, G.; Ye, N. Growth and Characterization of BaGa4S7: A New Crystal for Mid-IR Nonlinear Optics. Cryst. Growth Des. 2009, 9, 1186–1189. [Google Scholar] [CrossRef]
- Luo, Z.Z.; Lin, C.S.; Cui, H.H.; Zhang, W.L.; Zhang, H.; Chen, H.; He, Z.Z.; Cheng, W.D. PbGa2MSe6 (M = Si, Ge): Two Exceptional Infrared Nonlinear Optical Crystals. Chem. Mater. 2015, 27, 914–922. [Google Scholar] [CrossRef]
- Luo, Z.Z.; Lin, C.S.; Cui, H.H.; Zhang, W.L.; Zhang, H.; He, Z.Z.; Cheng, W.D. SHG Materials SnGa4Q7 (Q = S, Se) Appearing with Large Conversion Efficiencies, High Damage Thresholds, and Wide Transparencies in the Mid-Infrared Region. Chem. Mater. 2014, 26, 2743–2749. [Google Scholar] [CrossRef]
- Geng, L.; Cheng, W.D.; Lin, C.S.; Zhang, W.L.; Zhang, H.; He, Z.Z. Syntheses and Characterization of New Mid-Infrared Transparency Compounds: Centric Ba2BiGaS5 and Acentric Ba2BiInS5. Inorg. Chem. 2011, 50, 5679–5686. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.Z.; Lin, C.S.; Zhang, W.L.; Zhang, H.; He, Z.Z.; Cheng, W.D. Ba8Sn4S15: A Strong Second Harmonic Generation Sulfide with Zero-Dimensional Crystal Structure. Chem. Mater. 2013, 26, 1093–1099. [Google Scholar] [CrossRef]
- Liu, B.W.; Zeng, H.Y.; Zhang, M.J.; Fan, Y.H.; Guo, G.C.; Huang, J.S.; Dong, Z.C. Syntheses, Structures, and Nonlinear-optical Properties of Metal Sulfides Ba2Ga8MS16 (M = Si, Ge). Inorg. Chem. 2014, 54, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Li, S.F.; Liu, B.W.; Zhang, M.J.; Fan, Y.H.; Zeng, H.Y.; Guo, G.C. Syntheses, Structures, and Nonlinear Optical Properties of Two Sulfides Na2In2MS6 (M = Si, Ge). Inorg. Chem. 2016, 55, 1480–1485. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Meng, X.G.; Zhong, C.; Chen, X.G.; Qin, J.G. Rb2CdBr2I2: A New IR Nonlinear Optical Material with a Large Laser Damage Threshold. J. Am. Chem. Soc. 2014, 136, 5683–5686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, Y.J.; Jiang, K.; Zeng, H.Y.; Liu, T.; Chen, X.G.; Qin, J.G.; Lin, Z.S.; Fu, P.Z.; Wu, Y.C.; et al. A New Mixed Halide, Cs2HgI2Cl2: Molecular Engineering for a New Nonlinear Optical Material in the Infrared Region. J. Am. Chem. Soc. 2012, 134, 14818–14822. [Google Scholar] [CrossRef] [PubMed]
- Haynes, A.S.; Saouma, F.O.; Otieno, C.O.; Clark, D.J.; Shoemaker, D.P.; Jang, J.I.; Kanatzidis, M.G. Phase-Change Behavior and Nonlinear Optical Second and Third Harmonic Generation of The One-Dimensional K(1–x)CsxPSe6 and Metastable β-CsPSe6. Chem. Mater. 2015, 27, 1837–1846. [Google Scholar] [CrossRef]
- Wu, K.; Yang, Z.H.; Pan, S.L. Na4MgM2Se6 (M = Si, Ge): The First Noncentrosymmetric Compounds with Special Ethane-Like [M2Se6]6− Units Exhibiting Large Laser-Damage Thresholds. Inorg. Chem. 2015, 54, 10108–10110. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Yang, Z.H.; Pan, S.L. Na2Hg3M2S8 (M = Si, Ge, and Sn): New Infrared Nonlinear Optical Materials with Strong Second Harmonic Generation Effects and High Laser-Damage Thresholds. Chem. Mater. 2016, 28, 2795–2801. [Google Scholar] [CrossRef]
- Wu, K.; Yang, Z.H.; Pan, S.L. Na2BaMQ4 (M =Ge, Sn; Q=S, Se): Infrared Nonlinear Optical Materials with Excellent Performances and that Undergo Structural Transformations. Angew. Chem. Int. Ed. 2016, 128, 6825–6827. [Google Scholar] [CrossRef]
- Zhen, N.; Nian, L.Y.; Li, G.M.; Wu, K.; Pan, S.L. A High Laser Damage Threshold and a Good Second-Harmonic Generation Response in a New Infrared NLO Material: LiSm3SiS7. Crystals 2016, 6, 121. [Google Scholar] [CrossRef]
- Li, G.M.; Wu, K.; Liu, Q.; Yang, Z.H.; Pan, S.L. Na2ZnGe2S6: A New Infrared Nonlinear Optical Material with Good Balance between Large Second-Harmonic Generation Response and High Laser Damage Threshold. J. Am. Chem. Soc. 2016, 138, 7422–7428. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.Y.; Ma, Z.J.; Liu, X.C.; Xia, S.Q.; Tao, X.T.; Wu, K.C. Ba4AgGa5Pn8 (Pn = P, As): New Pnictide-Based Compounds with Nonlinear Optical Potential. J. Mater. Chem. C 2015, 3, 9695–9700. [Google Scholar] [CrossRef]
- Kuo, S.M.; Chang, Y.M.; Chung, I.; Jang, J.I.; Her, B.H.; Yang, S.H.; Ketterson, J.B.; Kanatzidis, M.G.; Hsu, K.F. New Metal Chalcogenides Ba4CuGa5Q12 (Q = S, Se) Displaying Strong Infrared Nonlinear Optical Response. Chem. Mater. 2013, 25, 2427–2433. [Google Scholar] [CrossRef]
- Bera, T.K.; Jang, J.I.; Song, J.H.; Malliakas, C.D.; Freeman, A.J.; Ketterson, J.B.; Kanatzidis, M.G. Soluble Semiconductors AAsSe2 (A = Li, Na) with a Direct-Band-Gap and Strong Second Harmonic Generation: A Combined Experimental and Theoretical Study. J. Am. Chem. Soc. 2010, 132, 3484–3495. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.H.; Marking, G.M.; Hsu, K.F.; Matsushita, Y.; Ewbank, M.D.; Borwick, R.; Cunningham, P.; Rosker, M.J.; Kanatzidis, M.G. α- and β-A2Hg3M2S8 (A = K, Rb; M= Ge, Sn): Polar Quaternary Chalcogenides with Strong Nonlinear Optical Response. J. Am. Chem. Soc. 2003, 125, 9484–9493. [Google Scholar] [CrossRef] [PubMed]
- Parthé, E. Crystal Chemistry of Tetrahedral Structures; Gordon and Breach Science: New York, NY, USA, 1964. [Google Scholar]
- Aitken, J.A.; Larson, P.; Mahanti, S.D.; Kanatzidis, M.G. Li2PbGeS4 and Li2EuGeS4: Polar Chalcopyrites with a Severe Tetragonal Compression. Chem. Mater. 2001, 13, 4714–4721. [Google Scholar] [CrossRef]
- Bai, L.; Lin, Z.S.; Wang, Z.Z.; Chen, C.T. Mechanism of Linear and Nonlinear Optical Effects of Chalcopyrites LiGaX2 (X = S, Se and Te) Crystals. J. Appl. Phys. 2008, 103, 083111. [Google Scholar] [CrossRef]
- Kim, Y.; Seo, I.S.; Martin, S.W.; Baek, J.; Shiv Halasyamani, P.; Arumugam, N.; Steinfink, H. Characterization of New Infrared Nonlinear Optical Material with High Laser Damage Threshold, Li2Ga2GeS6. Chem. Mater. 2008, 20, 6048–6052. [Google Scholar] [CrossRef]
- Shi, Y.F.; Chen, Y.K.; Chen, M.C.; Wu, L.M.; Lin, H.; Zhou, L.J.; Chen, L. Strongest Second Harmonic Generation in the Polar R3MTQ7 Family: Atomic Distribution Induced Nonlinear Optical Cooperation. Chem. Mater. 2015, 27, 1876–1884. [Google Scholar] [CrossRef]
- Brant, J.A.; Clark, D.J.; Kim, Y.S.; Jang, J.I.; Zhang, J.H.; Aitken, J.A. Li2CdGeS4, A Diamond-Like Semiconductor with Strong Second-Order Optical Nonlinearity in the Infrared and Exceptional Laser Damage Threshold. Chem. Mater. 2014, 26, 3045–3048. [Google Scholar] [CrossRef]
- Brant, J.A.; Clark, D.J.; Kim, Y.S.; Jang, J.I.; Weiland, A.; Aitken, J.A. Outstanding Laser Damage Threshold in Li2MnGeS4 and Tunable Optical Nonlinearity in Diamond-Like Semiconductors. Inorg. Chem. 2015, 4, 2809–2819. [Google Scholar] [CrossRef] [PubMed]
- Rosmus, K.A.; Brant, J.A.; Wisneski, S.D.; Clark, D.J.; Kim, Y.S.; Jang, J.I.; Brunetta, C.D.; Zhang, J.H.; Srnec, M.N.; Aitken, J.A. Optical Nonlinearity in Cu2CdSnS4 and α/β-Cu2ZnSiS4: Diamond-like Semiconductors with High Laser-Damage Thresholds. Inorg. Chem. 2014, 53, 7809–7811. [Google Scholar] [CrossRef] [PubMed]
- Brant, J.A.; Cruz, C.D.; Yao, J.L.; Douvalis, A.P.; Bakas, T.; Sorescu, M.; Aitken, J.A. Field-Induced Spin-Flop in Antiferromagnetic Semiconductors with Commensurate and Incommensurate Magnetic Structures: Li2FeGeS4 (LIGS) and Li2FeSnS4 (LITS). Inorg. Chem. 2014, 53, 12265–12274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Clark, D.J.; Brant, J.A.; Sinagra, C.W.; Kim, Y.S.; Jang, J.I.; Aitken, J.A. Infrared Nonlinear Optical Properties of Lithium-Containing Diamond-Like Semiconductors Li2ZnGeSe4 and Li2ZnSnSe4. Dalton Trans. 2015, 44, 11212–11222. [Google Scholar] [CrossRef] [PubMed]
- Devlin, K.P.; Glaid, A.J.; Brant, J.A.; Zhang, J.H.; Srnec, M.N.; Clark, D.J.; Kim, Y.S.; Jang, J.I.; Daley, K.R.; Moreau, M.A.; et al. Polymorphism and Second Harmonic Generation in a Novel Diamond-Like Semiconductor: Li2MnSnS4. J. Solid State Chem. 2015, 231, 256–266. [Google Scholar] [CrossRef]
- Lekse, J.W.; Leverett, B.M.; Lake, C.H.; Aitken, J.A. Synthesis, physicochemical characterization and crystallographic twinning of Li2ZnSnS4. J. Solid State Chem. 2008, 181, 3217–3222. [Google Scholar] [CrossRef]
- Rotermund, F.; Petrov, V.; Noack, F. Difference-Frequency Generation of Intense Femtosecond Pulses in the Mid-IR (4–12 μm) Using HgGa2S4 and AgGaS2. Opt. Commun. 2000, 185, 177–183. [Google Scholar] [CrossRef]
- Wu, K.; Su, X.; Pan, S.L.; Yang, Z.H. Synthesis and Characterization of Mid-Infrared Transparency Compounds: Acentric BaHgS2 and Centric Ba8Hg4S5Se7. Inorg. Chem. 2015, 54, 2772–2779. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yin, W.L.; Gong, P.F.; Li, X.X.; Zhou, M.L.; Mar, A.; Lin, Z.S.; Yao, J.Y.; Wu, Y.C.; Chen, C.T. Trigonal Planar [HgSe3]4− Unit: A New Kind of Basic Functional Group in IR Nonlinear Optical Materials with Large Susceptibility and Physicochemical Stability. J. Am. Chem. Soc. 2016, 138, 6135–6138. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Yang, Z.H.; Pan, S.L. The First Quaternary Diamond-like Semiconductor with 10-membered LiS4 Rings Exhibiting Excellent Nonlinear Optical Performances. Chem. Commun. 2017, 53, 3010–3013. [Google Scholar] [CrossRef] [PubMed]
- I Brese, N.E.; O’Keeffe, M. Bond-Valence Parameters for Solid. Acta Crystallogr. B 1991, 47, 192–197. [Google Scholar] [CrossRef]
- Brown, I.D.; Altermatt, D. Bond-Valence Parameters Obtained from a Systematic Analysis of the Inorganic Crystal Structure Database. Acta Crystallogr. B 1985, 41, 244–247. [Google Scholar] [CrossRef]
- Brown, I.D. The Chemical Bond in Inorganic Chemistry: The Bond Valence Model, 1st ed.; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Preiser, C.; Losel, J.; Brown, I.D.; Kunz, M.; Skowron, A. Long Range Coulomb Forces and Localized Bonds. Acta Crystallogr. B 1999, 55, 69–711. [Google Scholar] [CrossRef]
- Salinas-Sanchez, A.; Garcia-Munoz, J.L.; Rodriguez-Carvajal, J.; Saez-Puche, R.; Martinez, J.L. Structural Characterization of R2BaCuO5 (R = Y, Lu, Yb, Tm, Er, Ho, Dy, Gd, Eu and Sm) Oxides by X-ray and Neutron Diffraction. J. Solid State Chem. 1992, 100, 201–211. [Google Scholar] [CrossRef]
- Chen, S.; Walsh, A.; Luo, Y.; Yang, J.H.; Gong, X.G.; Wei, S.H. Wurtzite-Derived Polytypes of Kesterite and Stannite Quaternary Chalcogenide Semiconductors. Phys. Rev. B 2010, 82, 195203. [Google Scholar] [CrossRef]
- Bhar, G.C.; Smith, R.C. Optical Properties of II–IV–V2 and I–III–VI2 crystals with Particular Reference to Transmission Limits. Phys. Status Solidi A 1972, 13, 157–168. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXTL, version 6.14; Bruker Analytical X-ray Instruments, Inc.: Madison, WI, USA, 2008. [Google Scholar]
- Spek, A.L. Single-Crystal Structure Validation with the Program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
| Compounds | Li+ | Hg2+ | Si/Ge/Sn4+ | S2− | GII |
|---|---|---|---|---|---|
| Li2HgSiS4 | 1.125 | 2.092 | 4.030 | 2.069–2.149 | 0.10 |
| Li2HgGeS4 | 1.118 | 2.090 | 4.152 | 2.055–2.229 | 0.14 |
| Li2HgSnS4 | 1.085 | 2.133 | 4.138 | 2.061–2.163 | 0.13 |
| Compounds | Damage Energy (mJ) | Spot Diameter (mm) | LDT (MW/cm2) |
|---|---|---|---|
| AgGaS2 | 0.33 | 0.375 | 29.6 |
| Li2HgSiS4 | 1.02 | 0.375 | 91.6 |
| Li2HgGeS4 | 0.78 | 0.375 | 70.2 |
| Li2HgSnS4 | 0.34 | 0.375 | 30.5 |
| Empirical Formula | Li2HgSiS4 | Li2HgGeS4 | Li2HgSnS4 |
|---|---|---|---|
| fw | 370.80 | 415.30 | 461.40 |
| crystal system | orthorhombic | orthorhombic | orthorhombic |
| space group | Pmn21 | Pmn21 | Pmn21 |
| a (Å) | 7.592 (2) | 7.709 (9) | 7.9400 (17) |
| b (Å) | 6.7625 (19) | 6.812 (8) | 6.9310 (15) |
| c (Å) | 6.3295 (18) | 6.384 (7) | 6.5122 (14) |
| Z, V (Å3) | 2, 324.96 (16) | 2, 335.3 (7) | 2, 358.38 (13) |
| Dc (g/cm3) | 3.790 | 4.114 | 4.276 |
| μ (mm−1) | 25.014 | 28.463 | 25.918 |
| GOF on F2 | 1.022 | 1.161 | 0.985 |
| R1, wR2 (I > 2σ(I)) a | 0.0217, 0.0443 | 0.0422, 0.0994 | 0.0318, 0.0633 |
| R1, wR2 (all data) | 0.0229, 0.0445 | 0.0438, 0.0999 | 0.0423, 0.0682 |
| absolute structure parameter | 0.003 (11) | 0.04 (3) | −0.019 (19) |
| largest diff. peak and hole (e Å−3) | 1.318, −1.170 | 5.723, −1.070 | 0.959, −1.797 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.; Pan, S. Li2HgMS4 (M = Si, Ge, Sn): New Quaternary Diamond-Like Semiconductors for Infrared Laser Frequency Conversion. Crystals 2017, 7, 107. https://doi.org/10.3390/cryst7040107
Wu K, Pan S. Li2HgMS4 (M = Si, Ge, Sn): New Quaternary Diamond-Like Semiconductors for Infrared Laser Frequency Conversion. Crystals. 2017; 7(4):107. https://doi.org/10.3390/cryst7040107
Chicago/Turabian StyleWu, Kui, and Shilie Pan. 2017. "Li2HgMS4 (M = Si, Ge, Sn): New Quaternary Diamond-Like Semiconductors for Infrared Laser Frequency Conversion" Crystals 7, no. 4: 107. https://doi.org/10.3390/cryst7040107





