1. Introduction
By virtue of their high thermal and hydrothermal stability, microporous zeolites are widely used in industries as heterogeneous catalysts, particularly as solid acid catalysts in the fields of oil refining and petrochemistry [
1]. Although the micropores of zeolites have often been described as having excellent potential for chemical functions, their pore size often limits the reaction rate and thus have an adverse effect on practical chemical processes [
2,
3,
4]. In 1992, in an attempt to overcome this problem, Mobil Oil Corporation synthesized a family of mesoporous solids named M41s [
5] which possess an ordered arrangement of larger pores with diameters of 2–10 nm. However, the use of these materials as catalysts is hampered by their relatively low thermal stability and weak acid strength [
6,
7]. Therefore, it would be of great interest to synthesize a new type of material combining the advantages of both mesoporous molecular sieves and zeolites [
8,
9]. Only a few studies have been published to date; for example, Karlsson et al. prepared MFI/MCM-41 composites using two templates (C
6H
13(CH
3)
3NBr and C
14H
29(CH
3)
3NBr), and optimized the template concentrations and reaction temperature [
10]. In previous studies, we have reported the synthesis and characterization of composite molecular sieves M
1-MFI/M
2-MCM-41 (M
1, M
2 = Ni, Co) and their catalytic properties for the hydrocracking of residual oil [
11] and the preparation of micro–mesoporous FeCo-MFI/MCM-41 composites which gave a clear improvement in the hydrocracking of residual oil [
12]. However, the resulting materials are composites of zeolite crystallites embedded in a disordered mesoporous matrix. Kaliaguine et al. described a general method for the production of a new type of material with semi-crystalline zeolite mesopore walls [
13]. Other attempts to crystallize the mesopore walls of mesostructure materials have resulted in the formation of materials with increased thermal stability and catalytic activity. For example, ITQ zeolites having a partially crystalline bimodal pore system with a combination of micropores and mesopores have been synthesized by delaminating the layered zeolite precursors MCM-22 and ferrierite [
14]. However, none of these techniques yields a regular distribution of mesopores, let alone an ideal channel system of mesopores structurally connected with the regular micropores of the zeolite. Jiang et al. reported the synthesis of zeolite ITQ-43, which features a structurally hierarchical system of connected mesopores and micropores. Although high-resolution transmission electron microscopy (HRTEM) did not show the mesopore features, evidence for the presence of mesopores was provided by automated electron diffraction tomography [
15]. W. Chaikittisilp recently demonstrated a new approach for the construction of hierarchically organized zeolites by sequential intergrowth; for the first time, a complex and unusual morphology was observed for MFI zeolites [
16].
In this paper, M-MCM-41 bulk crystal heteroatom molecular sieves with crystalline phase have been synthesized using a direct hydrothermal method. The materials possess a crystalline phase and highly uniform ordered arrangement channels. This is a new type of crystalline mesoporous molecular sieve. Three-dimensional crystal mesoporous MCM-41 was obtained in typical synthesis conditions.
2. Results
The samples were labeled as Cry-Ni and Cry-Co, and the metal heteroatom contents of the samples measured by inductively coupled plasma (ICP) are listed in
Table 1.
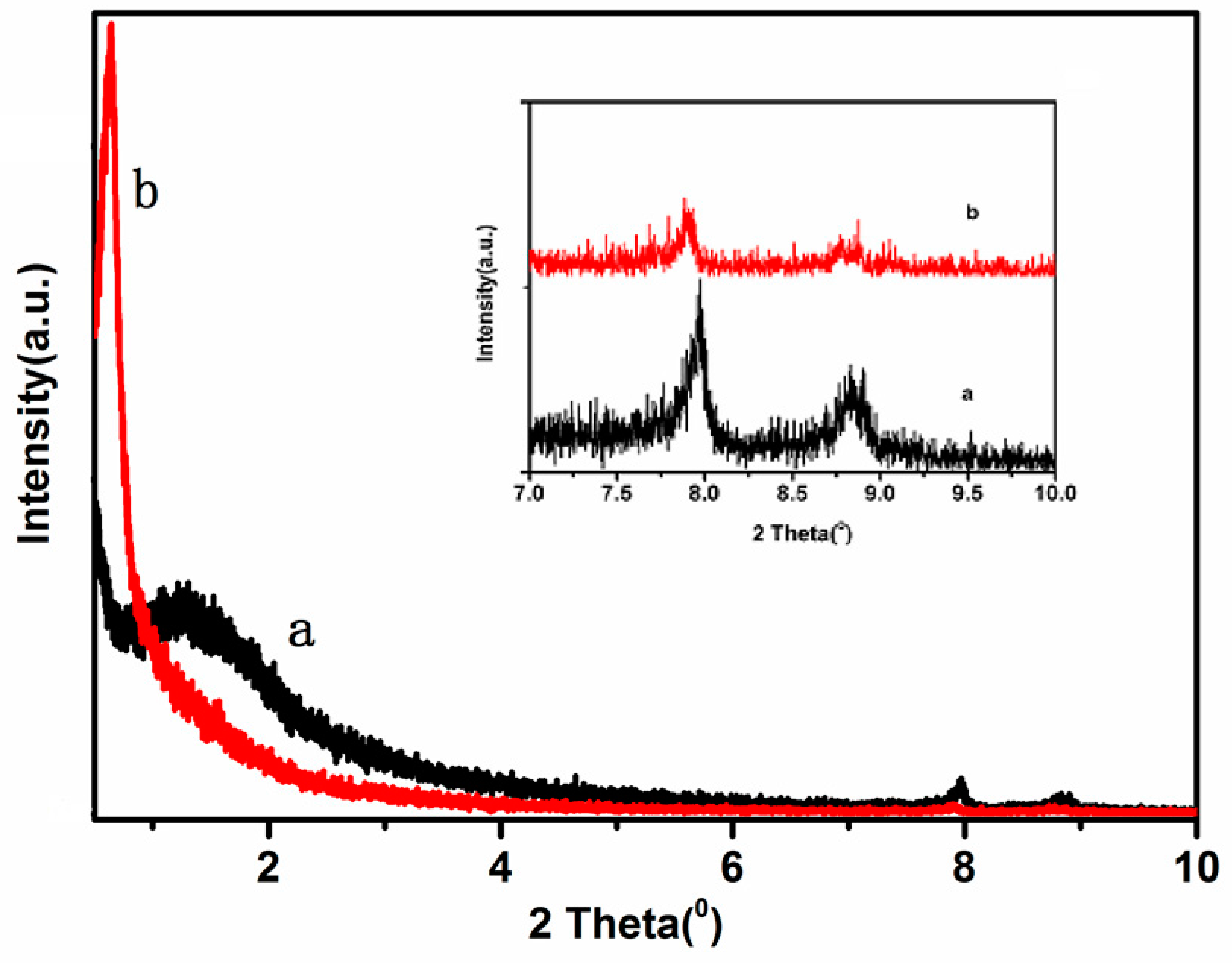
Low angle XRD patterns of the samples are presented in
Figure 1. All samples show an intense broad diffraction peak at very low 2θ angle of 0.7°–1.7°, with an overlapping broader feature at 2°–7°, which can be respectively attributed to the (1 0 0) diffraction peak and the overlapping pair of (1 1 0) and (2 0 0) diffraction peaks, indicating that the samples have good structural order and the obvious mesoporous structure of MCM-41 [
17].
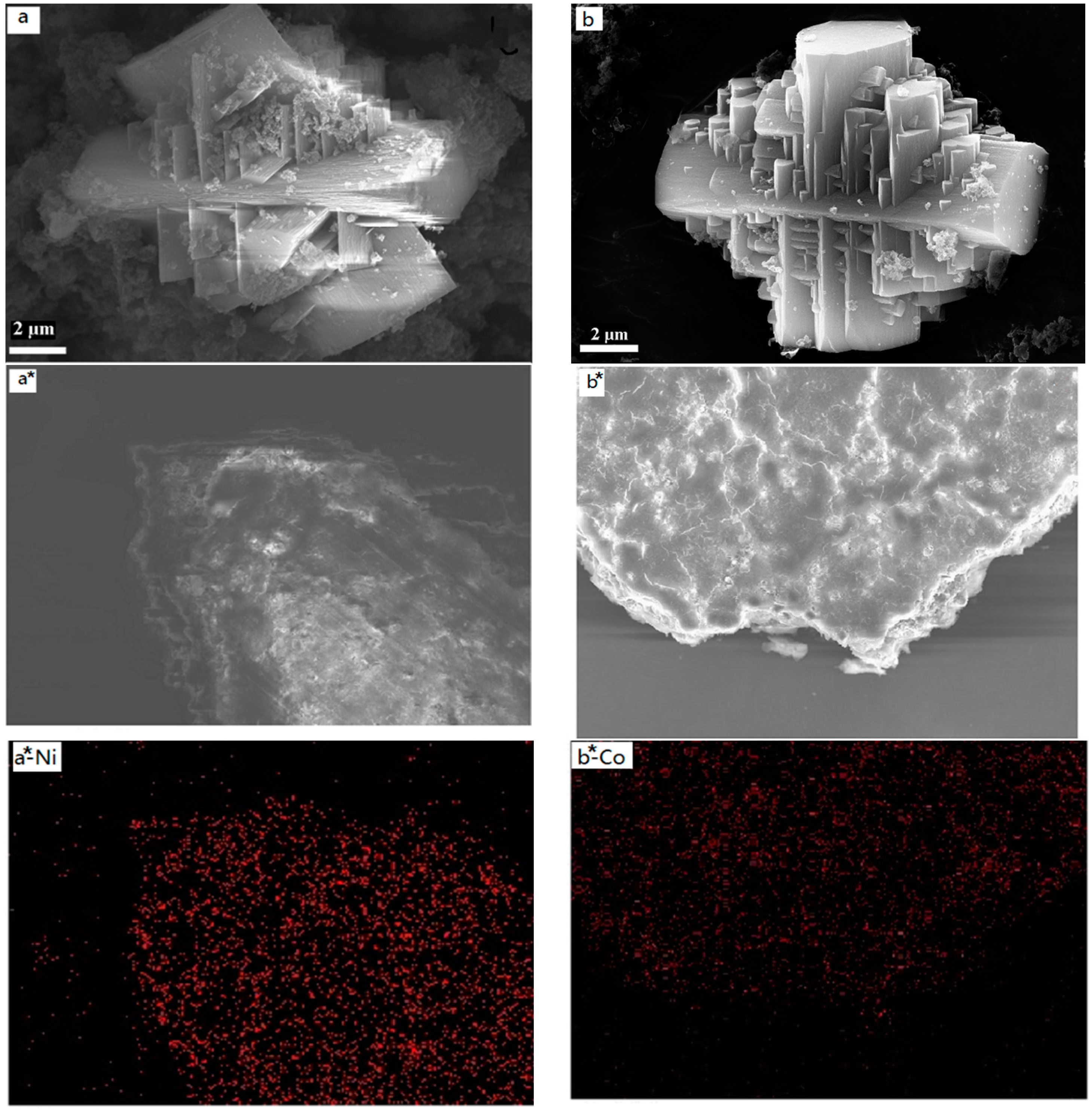
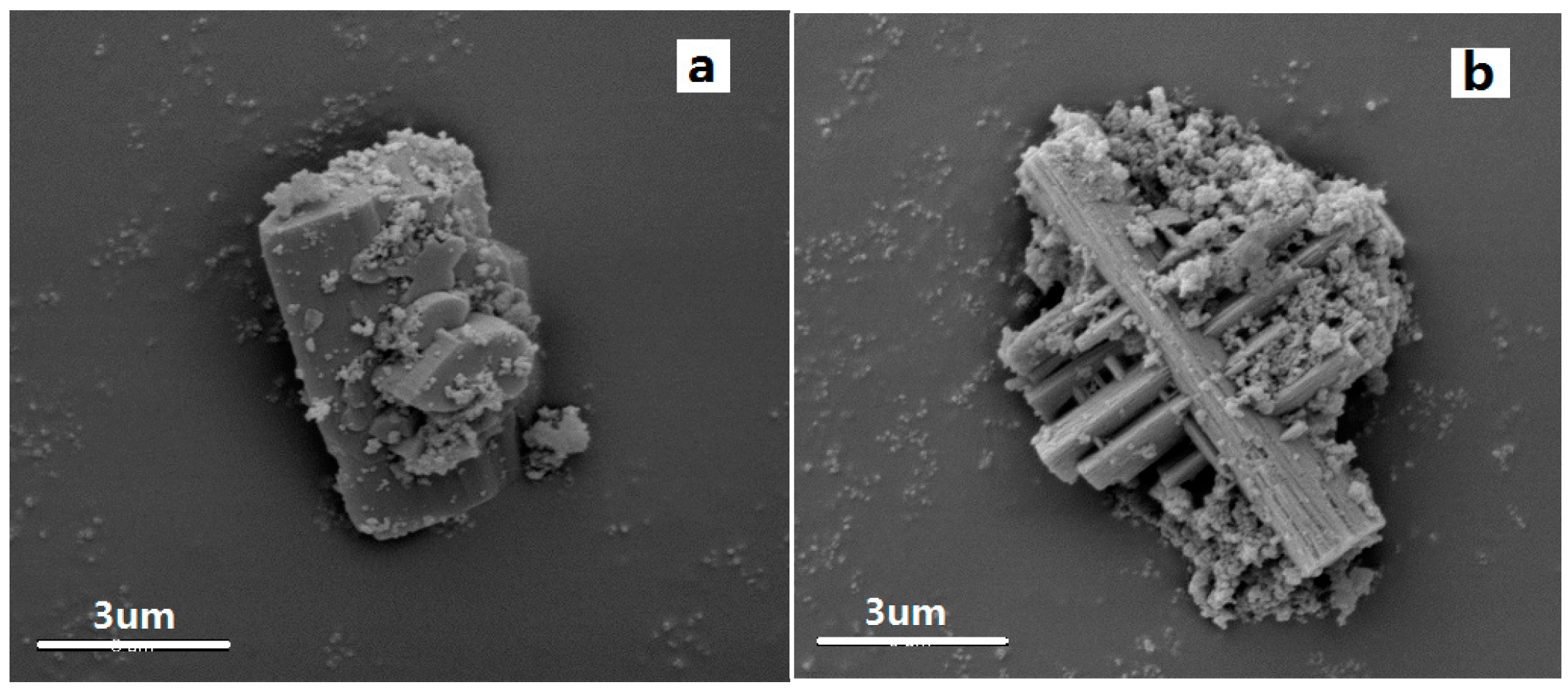
The particle size and shape of the samples were investigated using SEM, and the images are shown in
Figure 2a,b. The results show that the samples have well-defined morphologies with a crystal structure (corresponding to the XRD results), and the crystalline sizes are about 12 µm with a uniform distribution. It can be seen from the images that the two samples have good dispersion. In addition, the materials possess a structure in the vertical cross direction. This is determined presumably by the structure of the metal complex. The SEM-mappings of the samples are also shown in
Figure 2a*,b*, which display the composition and distribution of elements in the samples. It is clear that all samples (Cry-Ni and Cry-Co) have uniform distribution of elements, and the metal atoms (Ni and Co) were highly dispersed in the materials. It can be seen from
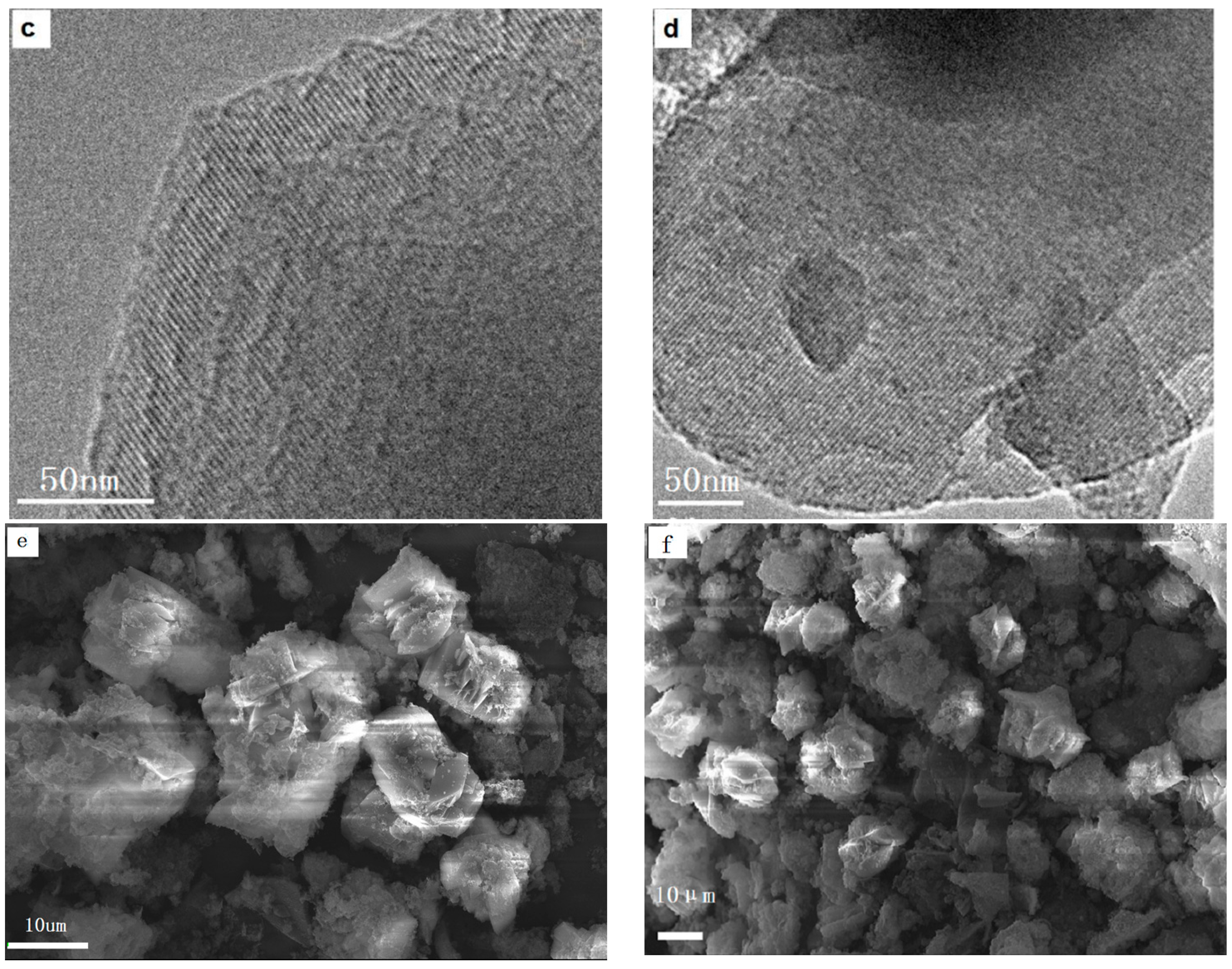
Figure 2e,f that the two samples have good dispersion. After grinding with a ball mill, the samples were tested by HRTEM, and the results are shown in
Figure 2c,d. The images indicate that the materials exhibit a uniform pore size with a highly-ordered pore structure and the characteristic long-range ordered symmetry of a mesophase. The ordered mesopores of the crystals can be easily observed in the HRTEM images.
The handbook of X-ray photoelectron spectroscopy (XPS) showed the binding energy of NiO and CoO. The binding energy peak of NiO was between 854 eV and 855 eV. The peak of CoO and Co
3O
4 centered at 780 eV.
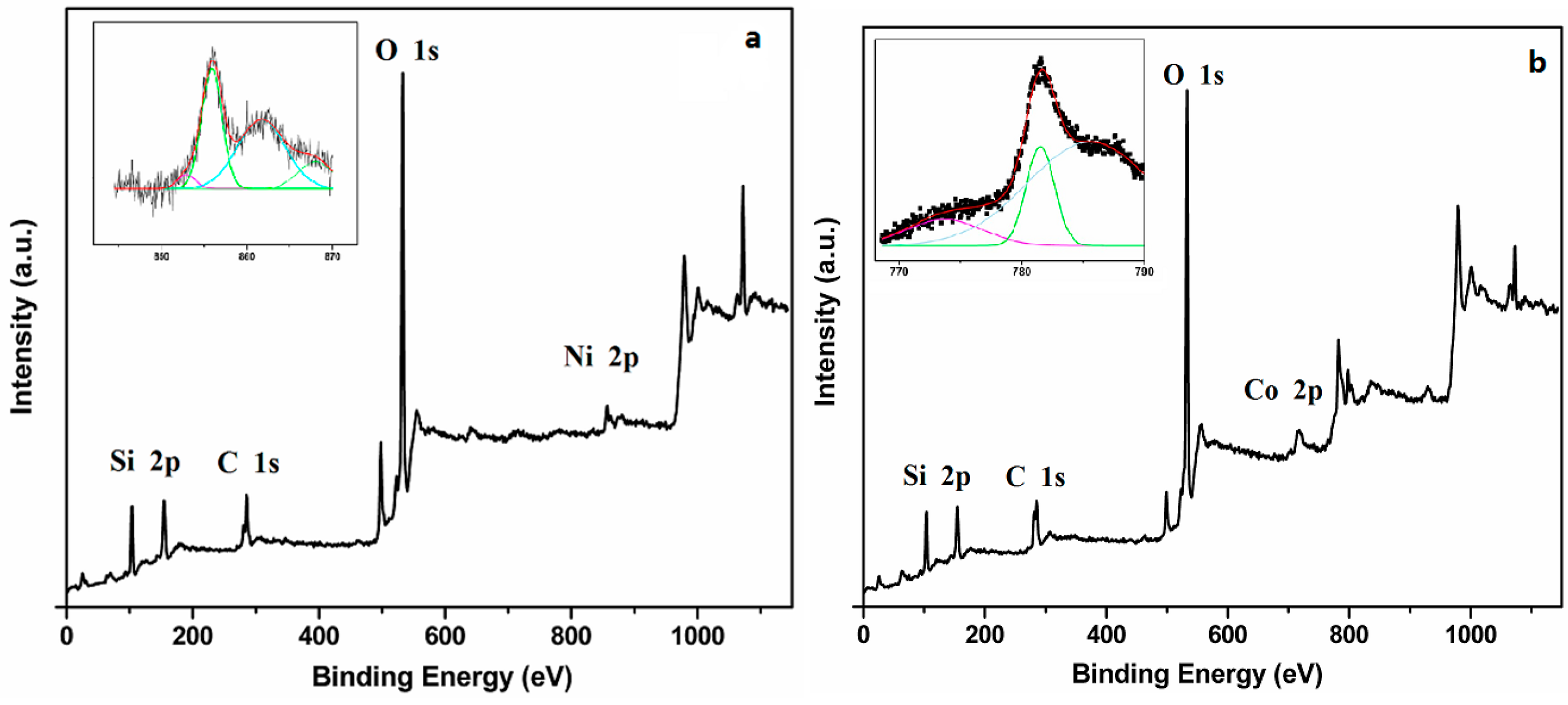
Figure 3 shows the XPS spectra of Cry-Ni and Cry-Co samples, and the insets are the de-convoluted Ni 2p3/2 and Co 2p3/2 spectra. As shown in
Figure 3a, there is a predominant peak centered at 855.95 eV [
18], indicating the strong interaction between nickel atom and the silica base, suggesting the nickel atoms incorporating into the silica framework [
19], which corroborates the previous results; a peak centered at 781.55 eV in the
Figure 3b is the characteristic peak of Co 2p2/3 [
20], indicating that cobalt atoms were also incorporating into the silica framework.
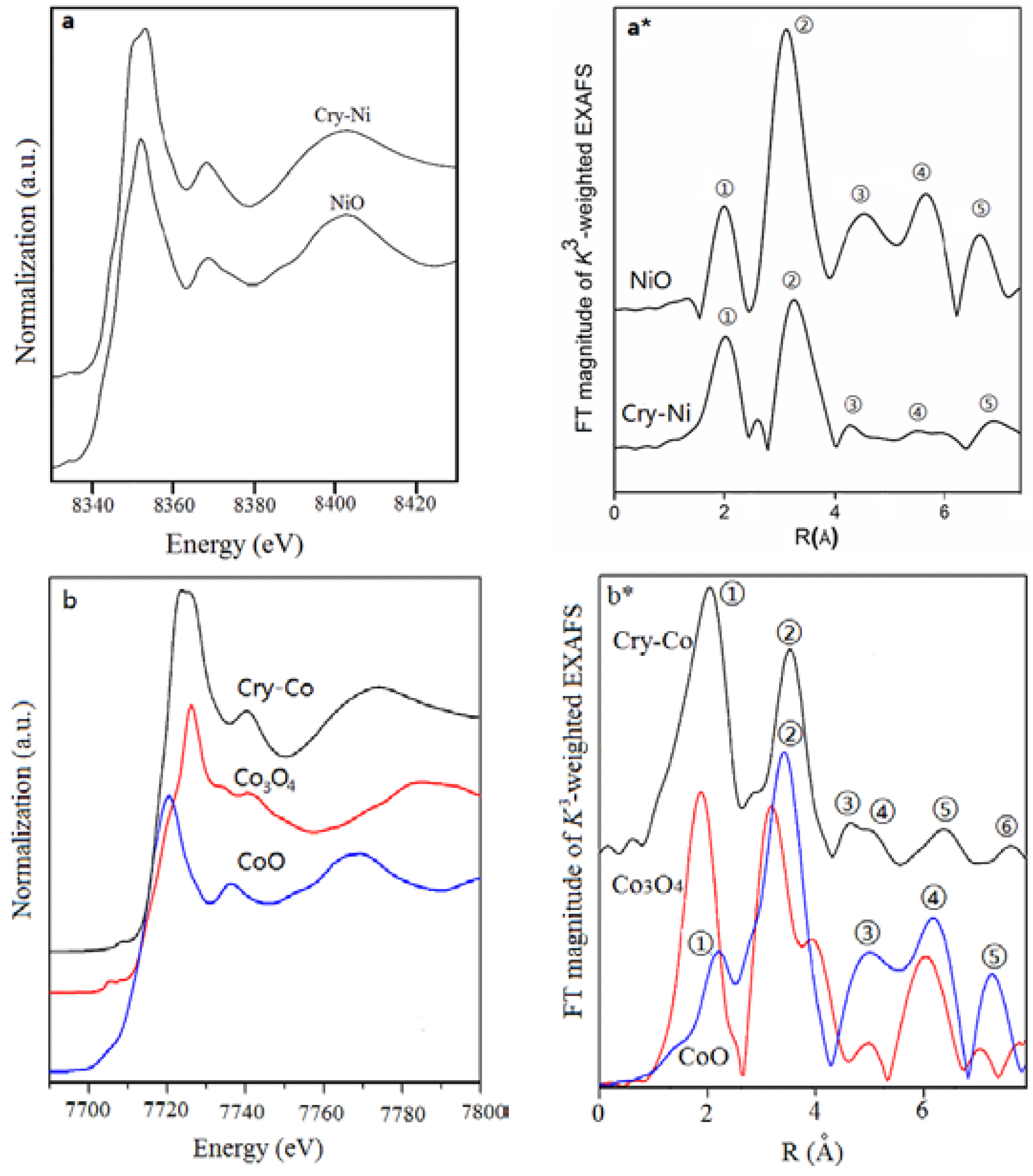
Figure 4 illustrates the X-ray absorption near-edge structure (XANES) spectra of Cry-Ms. The corresponding spectra for metal oxides are given for comparison. The XANES pre-edge peak is attributed to the 1s-3d dipolar forbidden transition [
21]. Principally, this 1s-3d forbidden transition gains additional intensity when the metal center is in a non-centrally symmetric environment through mixing of 3d and 4p orbitals caused by the breakdown of inversion symmetry due to the structure distortion.
Figure 4a illustrates the XANES spectra of NiO and Cry-Ni. The nickel atoms in Cry-Ni have similar pre-edge features to that of NiO, which indicates nickel exists as mainly Ni
2+ in the material.
Figure 4b displays the XANES spectra of CoO, Co
3O
4, and Cry-Co. The cobalt atoms in Cry-Co have similar features to CoO, suggesting that Co
2+ exists in the sample. Note that the peaks are slightly shifted to a longer distance than standard samples because of the scattering phase shifts.
Extended X-ray absorption fine structure (EXAFS) spectroscopy [
22] is one of the most powerful structural characterization methods, especially for interface-confined effects, due to its sensitivity to the three-dimensional short-range order (typically 0–8 Å) and chemical state, including near-neighbor species, distance (R), coordination number (N), and disorder in bond distance (σ
2).
Figure 4a* shows the K
3-weighted spectra of Cry-Ni and NiO, which are the Fourier transformed spectra of Ni K-edge EXAFS spectra in the range of k = 2–6 Å. The peaks correspond to the distance between a Ni atom and its neighboring atoms. In the curve of NiO (a standard reference material), there are five peaks (①–⑤) that can be ascribed to Ni-O, Ni-Ni ②, Ni-O ③, Ni-Ni Ni-Ni-O, Ni-O-Ni-O, Ni-O ④, and Ni-Ni ⑤ scattering paths, respectively. It is clear that the curve of Cry-Ni is different from the curve of NiO, although the two peaks ① and ② of Cry-Ni are similar to the peaks ① and ② of NiO; a new peak between peaks ① and ② of Cry-Ni presents, which is originated in the silicon atoms. The shape and intensity of peaks ③, ④, and ⑤ are also different from that of NiO. The results show that the structure forms of Ni atoms in Cry-Ni are different from in NiO. Similar results were obtained in the Cry-Co sample.
Figure 4b* presents the Fourier transforms of k
3-weighted Co K-edge EXAFS spectra of Cry-Co, CoO, and Co
3O
4 (standard reference materials). The curve of Cry-Co also presents two peaks ① and ②, which are similar with the peaks ① and ② of the curve of CoO, but are much different from the curve of Co
3O
4. The small peak between peaks ① and ② in the curve of Cry-Co is also induced by the silicon atoms. The results are useful for estimation of the crystallinity of the samples, and the analytic results are listed in
Table 2.
The XAFS results indicated that each metal center has a coordination number six with an octahedral coordination with the surrounding atoms. The bonds of M-O-Si present in the Cry-Ms samples, and this suggests that the metal atoms are incorporated in the silica framework rather than existing as a separate oxide phase.
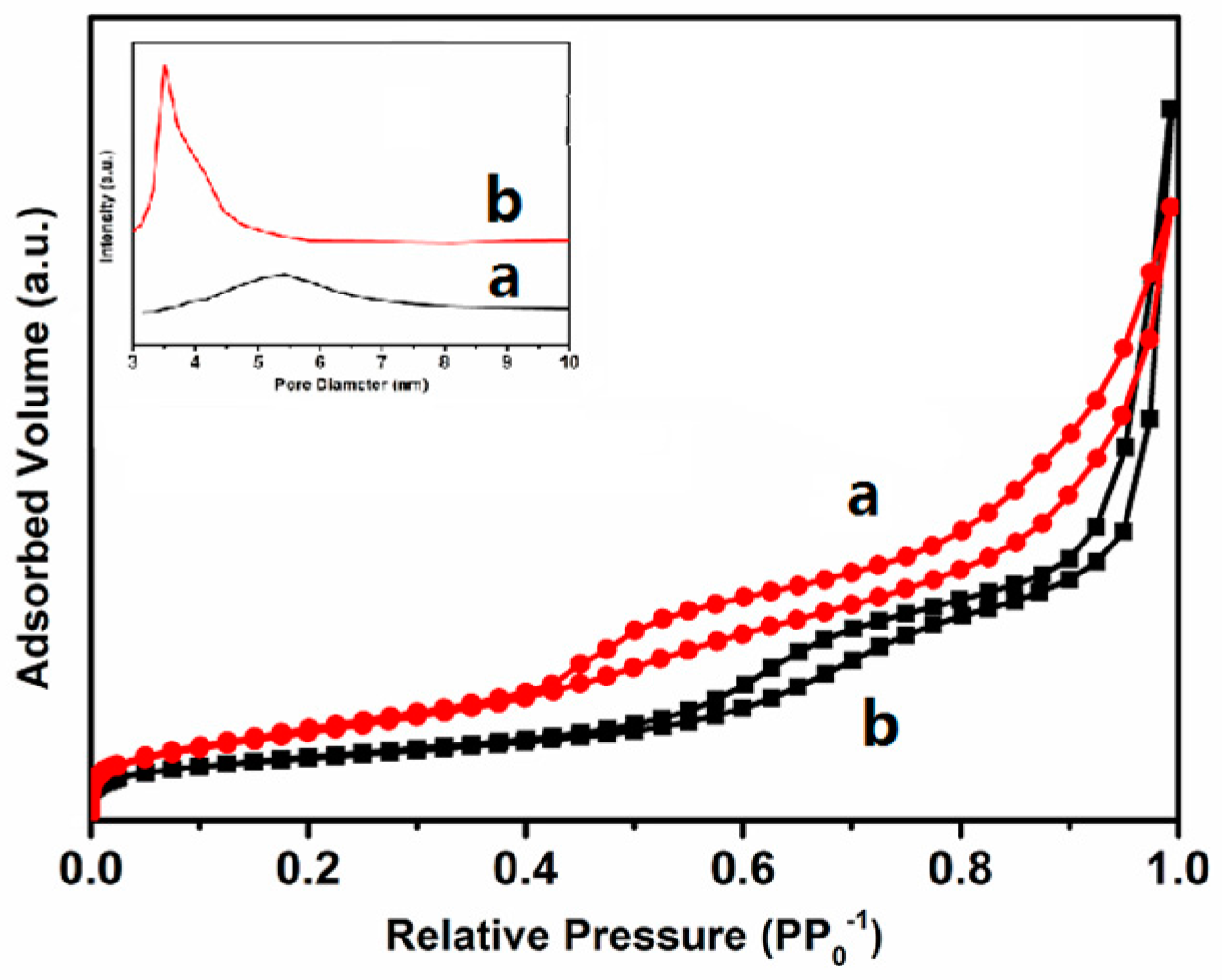
The N
2 adsorption-desorption isotherm method was employed to investigate the pore structure of materials. The isotherms of the samples obtained at −196 °C are illustrated in
Figure 5. It shows that the isotherms of the samples are type IV [
23] and possess type H1 hysteresis loops. It is indicated that the samples are typical mesoporous materials, exhibiting a sharp characteristic of capillary condensation at intermediate partial pressures (0.3 < P/P
0 < 0.5). In the relative pressure range 0.90–0.99, all samples show another sharp with the increase of the nitrogen adsorption volume, resulting from filling the macropores formed by interparticle spaces and untransformed amorphous silica. The textural properties of samples are listed in
Table 1. The pore diameters of the two samples have little difference from each other, due to the different micelle diameter of [(C
4H
9)
4N][M(EDTA)] caused by the difference radius of heteroatoms Ni
2+ and Co
2+, which also influences the specific surface area, pore volume, and the pore diameter.
The 0.5 g samples of Cry-Ni and Cry-Co were placed in 50 mL 1 mol/L NaOH solution, stirring at room temperature for 2 h, then samples were filtered and dried. The morphological changes of the samples were observed under electron microscope, and the morphology of the samples is shown in
Figure 6. The crystal structure of the samples treated by sodium hydroxide solution is shown. The samples covering the surface of the oxide dissolved by the lye treatment, and the crystal exhibits relatively complete form. Mesoporous molecular sieve material by traditional alkali treatment, hole wall collapse phenomenon will appear. In contrast, the crystal structure of metal atoms in the crystalline molecular sieve improves the alkali resistance of the molecular sieve.
According to the above results, a possible mechanism was proposed that is revealed as
Scheme 1. There are two nitrogen atoms and four carboxyl oxygen atoms (marked as A, B, C, D, respectively) in the EDTA molecule which can coordinate with the metal ion to form the complex ion [M(EDAT)]
2− with an octahedral structure. In the chelate ion, the carboxyl oxygen atoms A, B, and two nitrogen atoms are at a flat surface, and the other two carboxyl oxygen atoms C and D are in the vertical direction but not at a flat surface with the two nitrogen atoms. As the complex solution was added into the cetyltrimethyl ammonium bromide (CTAB) solution, the ionic associate [M(EDAT)]
2− (CTA
+)
2 and the special structure micelle would be formed (
Scheme 1). As the micelle suspension was mixed with the solution of SiO
44− formed by SiO
2 and plenty of sodium hydroxide, SiO
44− ions would rapidly hydrolyze and polymerize around the micelle which possessed a structure in the vertical cross direction. Additionally, the crystalline mesoporous zeolite precursors would grow along the vertical cross direction in the following hydrothermal process. Because the structure of the [M(EDAT)]
2− complex is not the same in the vertical cross direction, the micelle structure would not be the same in the two directions; this is the main factor causing the sample to have different structures in the different directions (
Figure 2). A certain amount of the complexes formed by metal ion and EDTA was essential in the formation of the structure. Enough [M(EDTA)]
2− could ensure the formation of single structural micelles with the vertical cross structure, which is the prerequisite of the formation of the crystalline zeolite precursors.
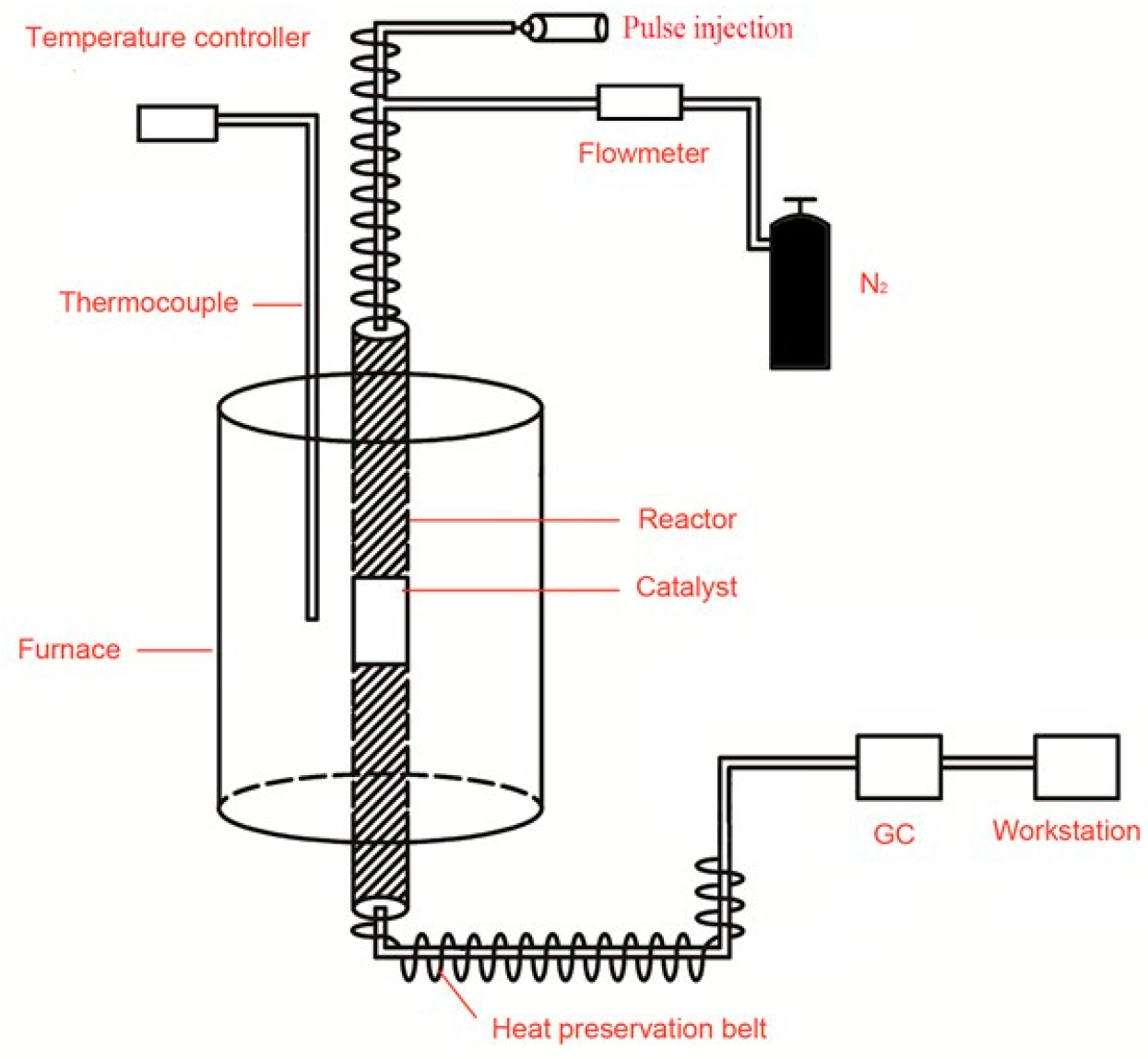
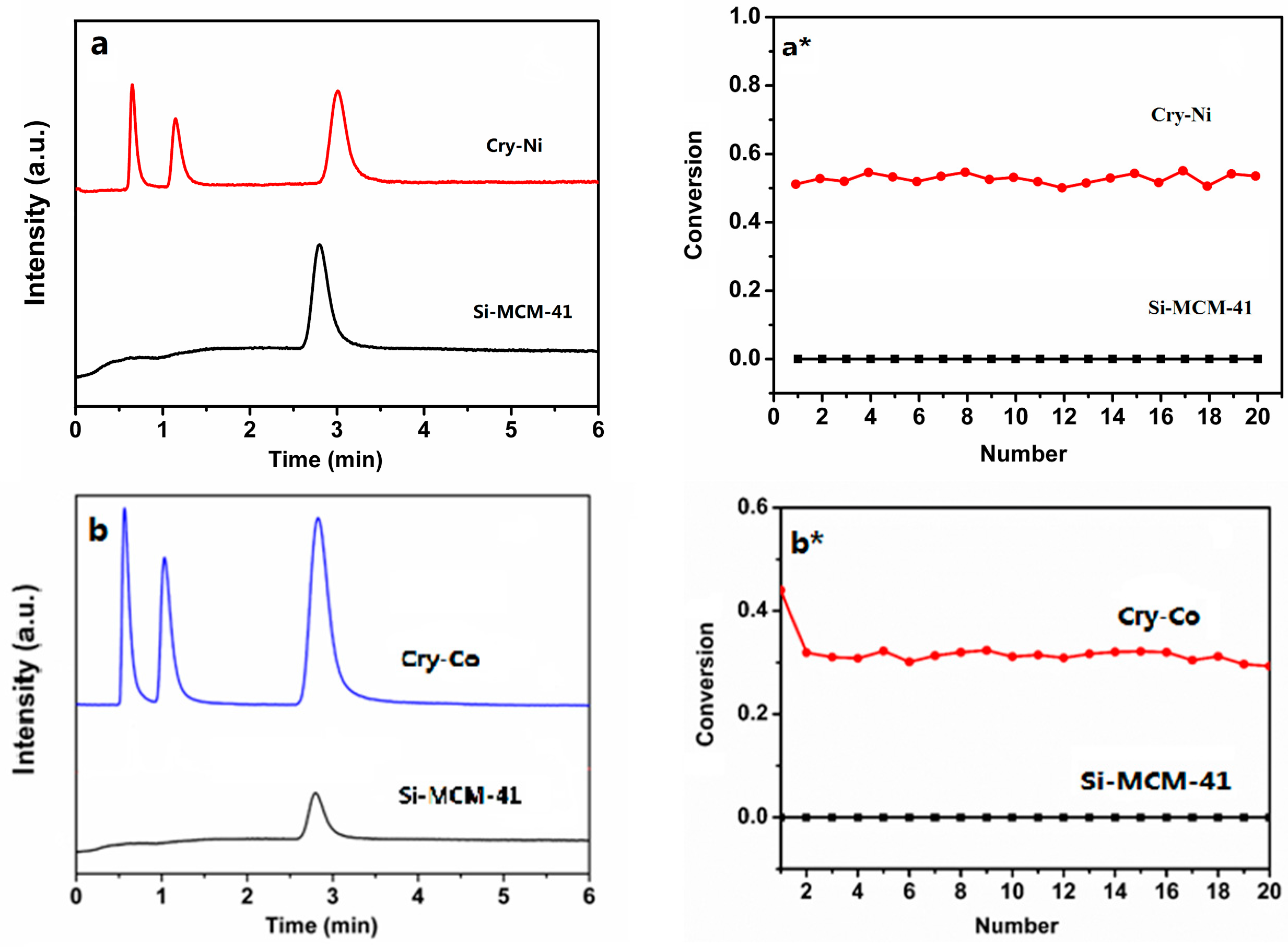
The catalytic performance of the crystalline molecular sieves for cumene cracking was investigated. The device diagram is shown in
Figure 7. Before the reaction, the samples were activated at 300 °C and nitrogen atmosphere for one hour. Reaction conditions were as follows: temperature, 30 °C; nitrogen atmosphere; mass ratio of raw material/catalyst, 100. After a certain reaction time, the results are shown in
Figure 8. The first peak is the cumene peak, the second peak is for small molecule hydrocarbon, the third peak is attributed to benzene homologues. It can be seen from the figure that metal atoms in the crystalline molecular sieves have a catalytic effect on the cracking of cumene.
Figure 8a*,b* for the catalytic conversion curves, the catalytic efficiency of Cry-Ni and Cry-Co samples were not significantly decreased after 20 times, indicating that the samples’ recycling rates are higher, and the highest conversion rate of 54.5% and 44.03% was reached. The cumene cracking reaction is a typical acid catalytic reaction. The pure silicon Si-MCM-41 molecular sieves have almost no acid, so there is no catalytic function of the reaction. Comparing to Si-MCM-41, the samples exhibited better conversion and higher selectivity for cumene cracking. The better catalytic performance is attributed to the samples possessing uniform channels, appropriate pore diameter, and active sites caused by the introduction of Ni and Co atoms into the framework of molecular sieves.
4. Materials and Methods
Nickel nitrate hexahydrate, Ni(NO3)2·6H2O (AR, Beijing Yili Fine Chemical Product Limited Company, Beijing, China), was used as Ni source. Cobalt nitrate hexahydrate, Co(NO3)2·6H2O (AR, Shantou Xilong Chemical Limited Company, Shantou, China), was used as Co source. Cetyltrimethyl ammonium bromide (CTAB, AR, Tianjin Jinke Fine Chemical Institute, Tianjin, China) was used as structure template. Ethylenediaminetetraacetate (EDTA, AR, Beijing Chemical Factory, Beijing, China) was used as structure-directing agent. White carbon black (SiO2, AR, Beijing Chemical Factory, Beijing, China) was used as Si source, and sodium hydroxide (NaOH, AR, Beijing Chemical Factory, Beijing, China) was used to adjust the pH.
The new crystalline sample of Cry-M (M = Ni, Co) was prepared through a direct hydrothermal synthesis method, and the detailed process is as follows: a transparent solution of the ionic complex [M(EDTA)]2− was made by dissolving disodium ethylenediaminetetraacetate solution and a certain amount of M(NO3)2·6H2O in H2O (10 g) and mixing well. CTAB (4.5 g, 12.3 mmol) was dissolved in 34 g of H2O at 70 °C. In another flask, 0.5 g (12.5 mmol) of sodium hydroxide dissolved in 15 g of H2O was mixed with 2.25 g (37.5 mmol) of SiO2 stirred for 0.5 h and then heated to 60 °C. The ionic complex [M(EDTA)]2− and CTAB solutions were then added to the silica solution, and the resulting mixture was stirred for 0.5 h to form a homogeneous emulsion. After adjusting the pH to 11 using sodium hydroxide solution, the mixture was stirred for another 12 h. The mixture was then transferred into a Teflon-lined autoclave and heated at 160 °C under autogenous pressure for 96 h. The resulting solid was filtered and washed with water until the pH of the filtrate reached 7.0. After drying the sample overnight at 100 °C, the template was removed by calcination in air at 550 °C for 5 h.
X-ray powder diffraction (XRD) patterns were recorded on a Philips X′Pert diffractometer equipped with a rotating anode using Cu Kα radiation (λ = 0.1541 nm). The pore morphology of the samples was examined by high-resolution transmission electron microscopy (HRTEM) on a JEM-2100 microscope with an accelerating voltage of 200 kV. The crystal morphology was studied using a Hitachi S-4700 scanning electron microscope (SEM). The chemical compositions of the samples were measured by Optima 8000 inductively coupled plasma (ICP). Nitrogen adsorption–desorption isotherms were obtained using a Quantachrome Autosorb-1 volumetric adsorption analyzer. The volume of the adsorbed N2 was normalized to standard temperature and pressure. Prior to the measurements, the samples were degassed at 300 °C for 16 h. The X-ray photoelectron spectroscopy (XPS) analyses were conducted on an ESCALAB 250 spectrometer equipped with an Al Kα X-ray source. The carbon 1 s peak at 284.6 eV was used as the reference for binding energies. X-ray absorption fine structure (XAFS) spectroscopy of the M (Ni, Co) K-edge of the samples was performed on the U7C beamline at the National Synchrotron Radiation Laboratory (NSRL) of China. The typical energy of the storage ring was 0.8 GeV, with a maximum current of 250 mA. A Si (111) double crystal monochromator was used. The data analysis of all the XAFS spectra was carried out using the IFEFFIT software (NSRL, Beijing, China).