Enhanced Framework Rigidity of a Zeolitic Metal-Azolate via Ligand Substitution
Abstract
:1. Introduction
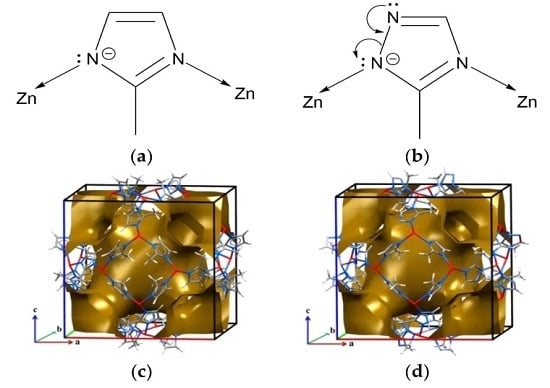
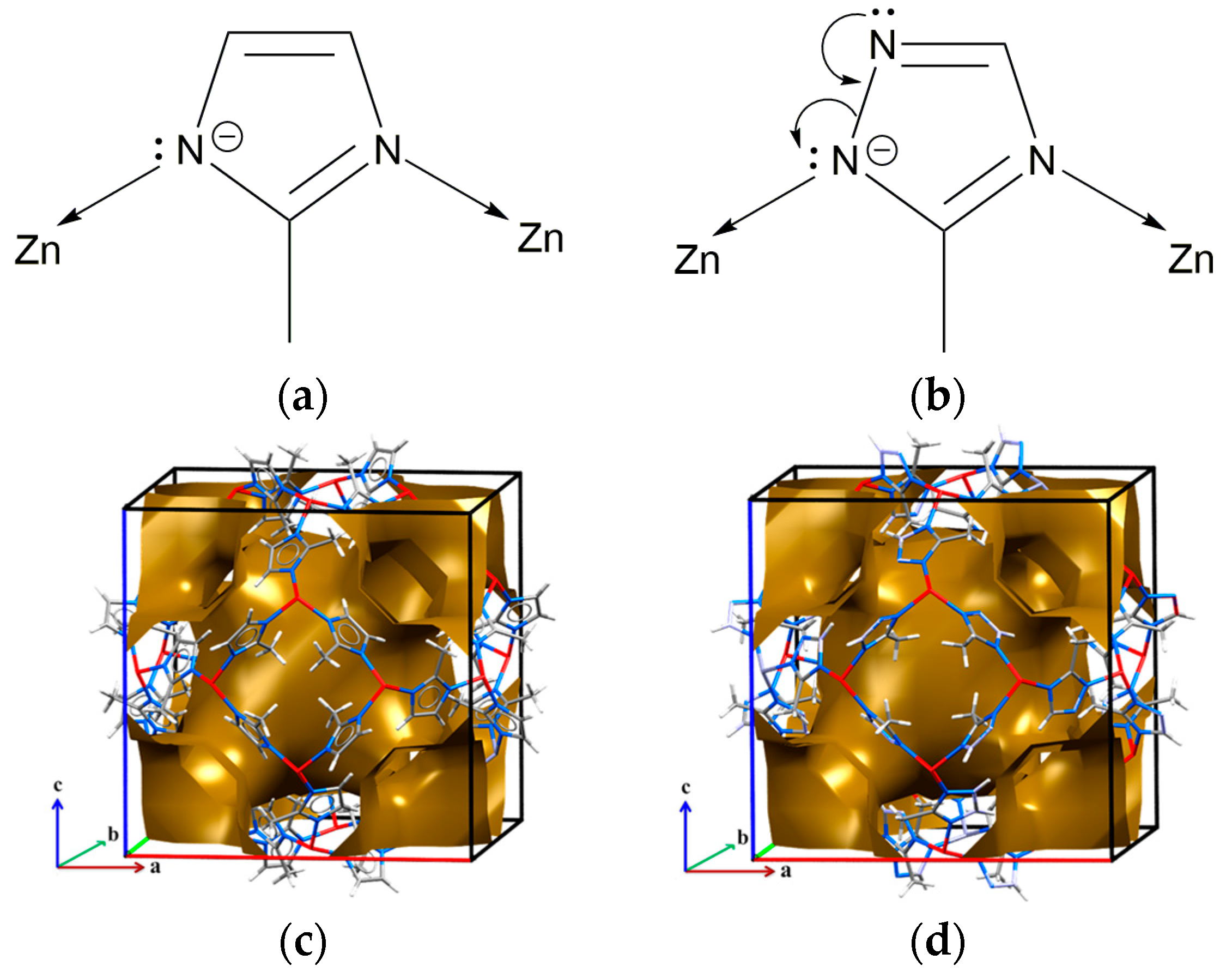
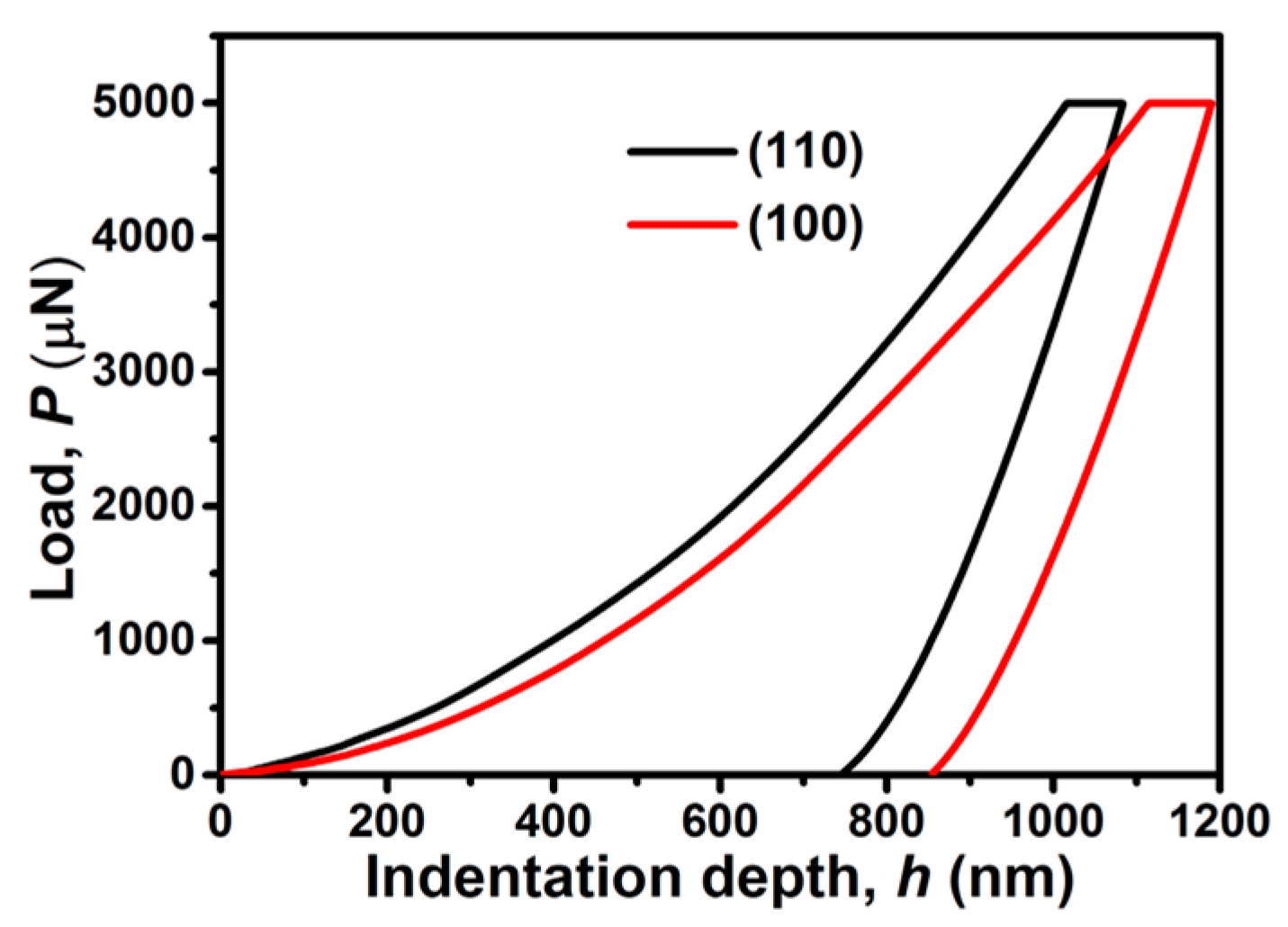
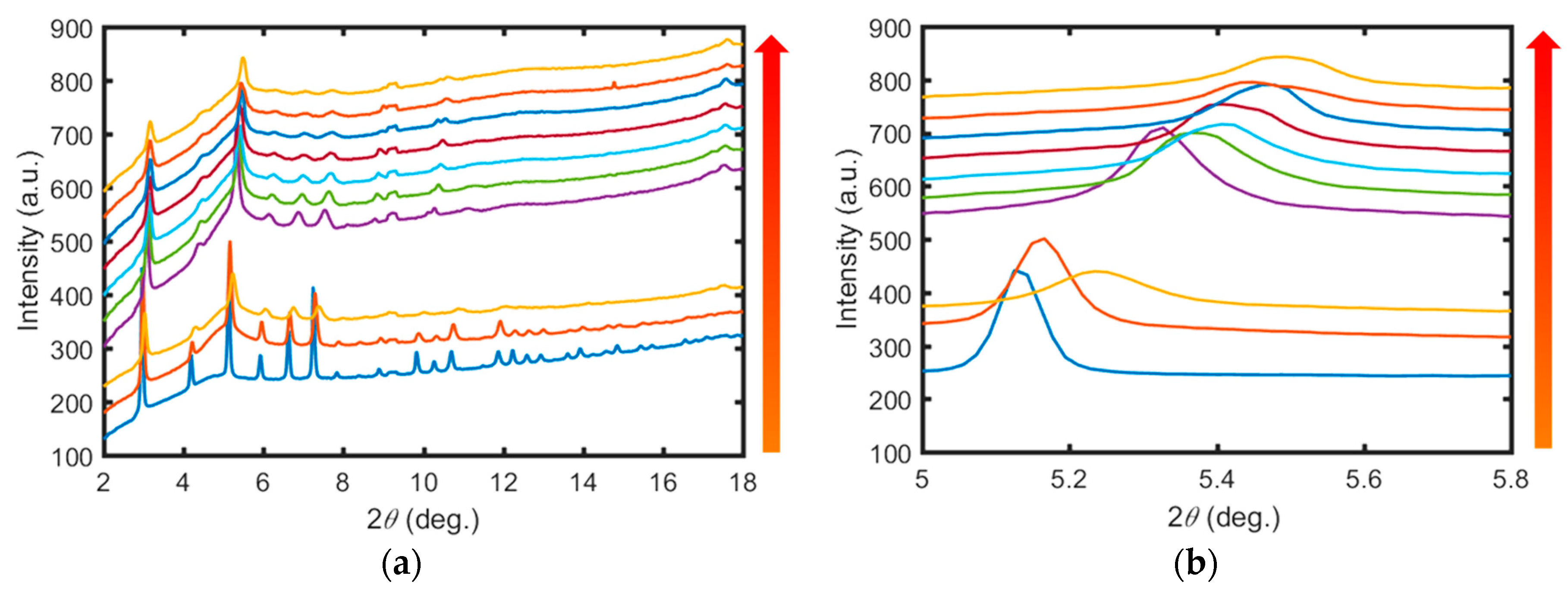
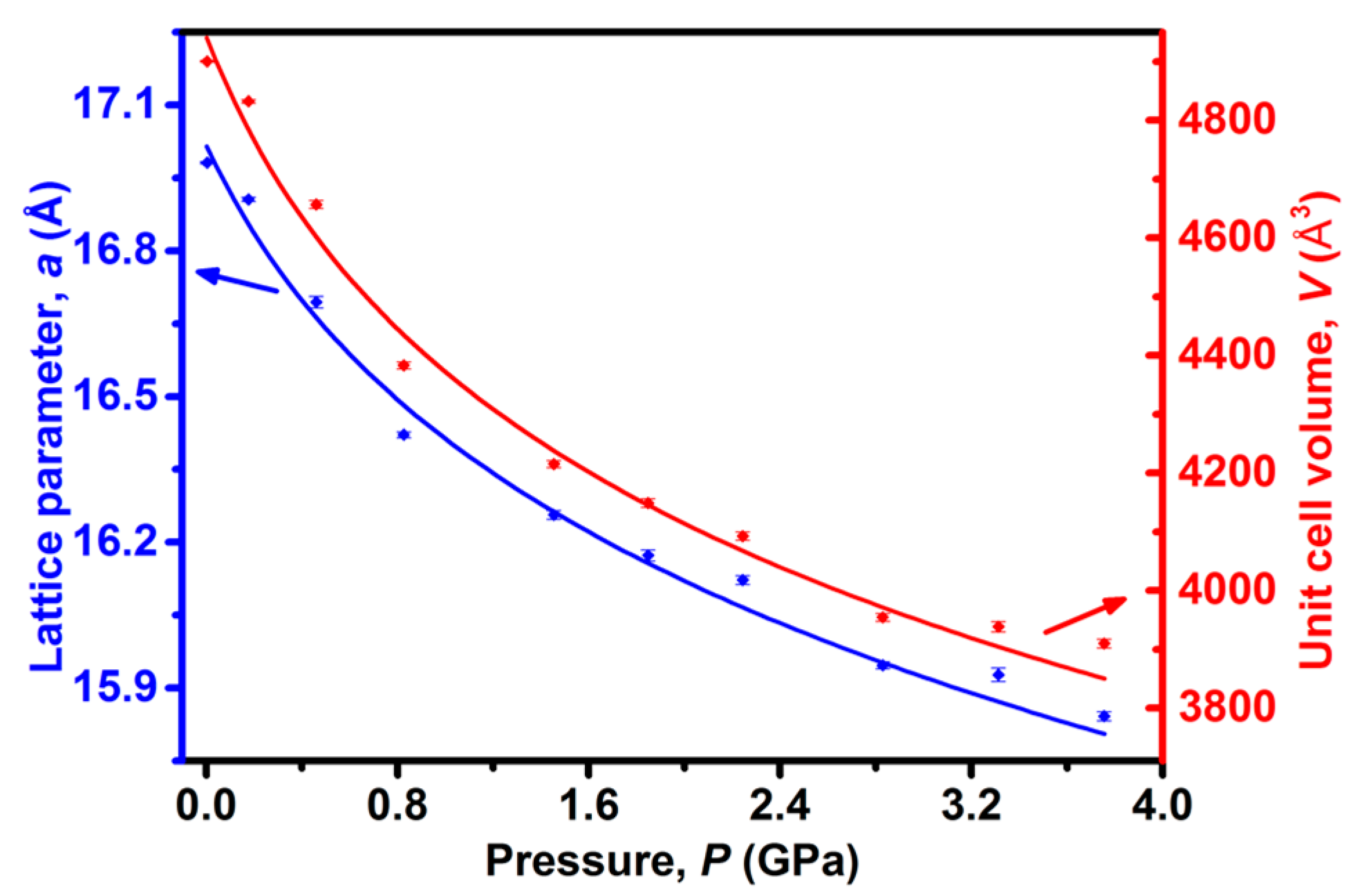
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of MAF-7
3.2. First-Principles Calculations
3.3. High-Pressure Synchrotron X-ray Powder Diffraction
3.4. Nanoindentation Methodology of Single Crystals
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, W.; Wang, Z.; Deschler, F.; Gao, S.; Friend, R.H.; Cheetham, A.K. Chemically diverse and multifunctional hybrid organic–inorganic perovskites. Nat. Rev. Mater. 2017, 16099. [Google Scholar] [CrossRef]
- Deng, H.; Doonan, C.J.; Furukawa, H.; Ferreira, R.B.; Towne, J.; Knobler, C.B.; Wang, B.; Yaghi, O.M. Multiple functional groups of varying ratios in metal-organic frameworks. Science 2010, 327, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Horike, S.; Inubushi, Y.; Nakagawa, K.; Kubota, Y.; Takata, M.; Kitagawa, S. Solid Solutions of Soft Porous Coordination Polymers: Fine-Tuning of Gas Adsorption Properties. Angew. Chem. Int. Ed. 2010, 49, 4820–4824. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, D.; Higuchi, M.; Horike, S.; Matsuda, R.; Kinoshita, Y.; Yanai, N.; Kitagawa, S. Storage and Sorption Properties of Acetylene in Jungle-Gym-Like Open Frameworks. Chem. Asian J. 2008, 3, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Kleist, W.; Jutz, F.; Maciejewski, M.; Baiker, A. Mixed-Linker Metal-Organic Frameworks as Catalysts for the Synthesis of Propylene Carbonate from Propylene Oxide and CO2. Eur. J. Inorg. Chem. 2009, 2009, 3552–3561. [Google Scholar] [CrossRef]
- Cheetham, A.K.; Rao, C.N.R.; Feller, R.K. Structural diversity and chemical trends in hybrid inorganic–organic framework materials. Chem. Commun. 2006, 4780–4795. [Google Scholar] [CrossRef]
- Taylor-Pashow, K.M.; Rocca, J.D.; Xie, Z.; Tran, S.; Lin, W. Postsynthetic modifications of iron-carboxylate nanoscale metal-organic frameworks for imaging and drug delivery. J. Am. Chem. Soc. 2009, 131, 14261–14263. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Kiran, M.S.R.N.; Manson, J.L.; Schlueter, J.A.; Thirumurugan, A.; Ramamurty, U.; Cheetham, A.K. Mechanical properties of a metal–organic framework containing hydrogen-bonded bifluoride linkers. Chem. Commun. 2013, 49, 4471–4473. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Henke, S.; Wharmby, M.T.; Yeung, H.H.M.; Li, W.; Cheetham, A.K. Mechanical Properties of a Calcium Dietary Supplement, Calcium Fumarate Trihydrate. Inorg. Chem. 2015, 54, 11186–11192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jiang, X.; Feng, G.; Lin, Z.; Hu, B.; Li, W. Mechanical properties and negative thermal expansion of a dense rare earth formate framework. J. Solid State Chem. 2016, 233, 289–293. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Cote, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed]
- Moggach, S.A.; Bennett, T.D.; Cheetham, A.K. The Effect of Pressure on ZIF-8: Increasing Pore Size with Pressure and the Formation of a High-Pressure Phase at 1.47 GPa. Angew. Chem. 2009, 121, 7221–7223. [Google Scholar] [CrossRef]
- Chapman, K.W.; Halder, G.J.; Chupas, P.J. Pressure-induced amorphization and porosity modification in a metal-organic framework. J. Am. Chem. Soc. 2009, 131, 17546–17547. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.C.; Bennett, T.D.; Cheetham, A.K. Chemical structure, network topology, and porosity effects on the mechanical properties of Zeolitic Imidazolate Frameworks. Proc. Natl Acad. Sci. USA 2010, 107, 9938–9943. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.C.; Civalleri, B.; Lin, C.C.; Valenzano, L.; Galvelis, R.; Chen, P.F.; Bennett, T.D.; Mellot-Draznieks, C.; Zicovich-Wilson, C.M.; Cheetham, A.K. Exceptionally low shear modulus in a prototypical imidazole-based metal-organic framework. Phys. Rev. Lett. 2012, 108, 095502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.P.; Zhu, A.X.; Lin, R.B.; Qi, X.L.; Chen, X.M. Pore Surface Tailored SOD-Type Metal-Organic Zeolites. Adv. Mater. 2011, 23, 1268–1271. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Lin, R.B.; Qi, X.L.; Liu, Y.; Lin, Y.Y.; Zhang, J.P.; Chen, X.M. Zeolitic metal azolate frameworks (MAFs) from ZnO/Zn(OH)2 and monoalkyl-substituted imidazoles and 1,2,4-triazoles: Efficient syntheses and properties. Microporous Mesoporous Mater. 2012, 157, 42–49. [Google Scholar] [CrossRef]
- Nye, J.F. Physical Properties of Crystals; Clarendon Press: Oxford, UK, 1985; p. 142. [Google Scholar]
- Grimvall, G. Thermophysical Properties of Materials; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Feng, J. Mechanical properties of hybrid organic-inorganic CH3NH3BX3 (B = Sn, Pb; X = Br, I) perovskites for solar cell absorbers. APL Mater. 2014, 2, 081801. [Google Scholar] [CrossRef]
- Marmier, A.; Lethbridge, Z.A.; Walton, R.I.; Smith, C.W.; Parker, S.C.; Evans, K.E. ElAM: A computer program for the analysis and representation of anisotropic elastic properties. Comput. Phys. Commun. 2010, 181, 2102–2115. [Google Scholar] [CrossRef]
- Li, W.; Barton, P.T.; Kiran, M.S.R.N.; Burwood, R.P.; Ramamurty, U.; Cheetham, A.K. Magnetic and Mechanical Anisotropy in a Manganese 2-Methylsuccinate Framework Structure. Chemistry 2011, 17, 12429–12436. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Jiang, X.; Wei, W.; Gong, P.; Kang, L.; Li, Z.; Li, Y.; Li, X.; Wu, X.; Lin, Z.; et al. High pressure behaviour and elastic properties of a dense inorganic–organic framework. Dalton Trans. 2016, 45, 4303–4308. [Google Scholar] [CrossRef] [PubMed]
- Lethbridge, Z.A.; Walton, R.I.; Marmier, A.S.; Smith, C.W.; Evans, K. Elastic anisotropy and extreme Poisson’s ratios in single crystals. Acta Mater. 2010, 58, 6444–6451. [Google Scholar] [CrossRef]
- Cliffe, M.J.; Goodwin, A.L. PASCal: A principal axis strain calculator for thermal expansion and compressibility determination. J. Appl. Cryst. 2012, 45, 1321–1329. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Krist. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Payne, M.C.; Teter, M.P.; Allan, D.C.; Arias, T.A.; Joannopoulos, J.D. Iterative minimization techniques for ab initio total-energy calculations: Molecular dynamics and conjugate gradients. Rev. Mod. Phys. 1992, 64, 1045. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Wang, Y. Pair-distribution function and its coupling-constant average for the spin-polarized electron gas. Phys. Rev. B 1992, 46, 12947. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Portal, D.; Artacho, E.; Soler, J.M. Projection of plane-wave calculations into atomic orbitals. Solid State Commun. 1995, 95, 685–690. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Pfrommer, B.G.; Côté, M.; Louie, S.G.; Cohen, M.L. Relaxation of crystals with the quasi-Newton method. J. Comput. Phys. 1997, 131, 233–240. [Google Scholar] [CrossRef]
- Deyirmenjian, V.B.; Heine, V.; Payne, M.C.; Milman, V.; Lynden-Bell, R.M.; Finnis, M.W. Ab initio atomistic simulation of the strength of defective aluminum and tests of empirical force models. Phys. Rev. B 1995, 52, 15191. [Google Scholar] [CrossRef]
- Mao, H.K.; Xu, J.A.; Bell, P.M. Calibration of the ruby pressure gauge to 800 kbar under quasi-hydrostatic conditions. J. Geophys. Res. 1986, 91, 4673–4676. [Google Scholar] [CrossRef]
- Coelho, A. TOPAS-Academic v5, Coehlo Software: Brisbane, Australia, 2013.
- Gao, H.; Wei, W.; Li, Y.; Wu, R.; Feng, G.; Li, W. Uniaxial Negative Thermal Expansion and Mechanical Properties of a Zinc-Formate Framework. Materials 2017, 10, 151. [Google Scholar] [CrossRef]
- Li, W.; Thirumurugan, A.; Barton, P.T.; Lin, Z.; Henke, S.; Yeung, H.H.M.; Wharmby, M.T.; Bithell, E.G.; Howard, C.J.; Cheetham, A.K.; et al. Mechanical tunability via hydrogen bonding in metal–organic frameworks with the perovskite architecture. J. Am. Chem. Soc. 2014, 136, 7801–7804. [Google Scholar] [CrossRef] [PubMed]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 2004, 19, 3–20. [Google Scholar] [CrossRef]
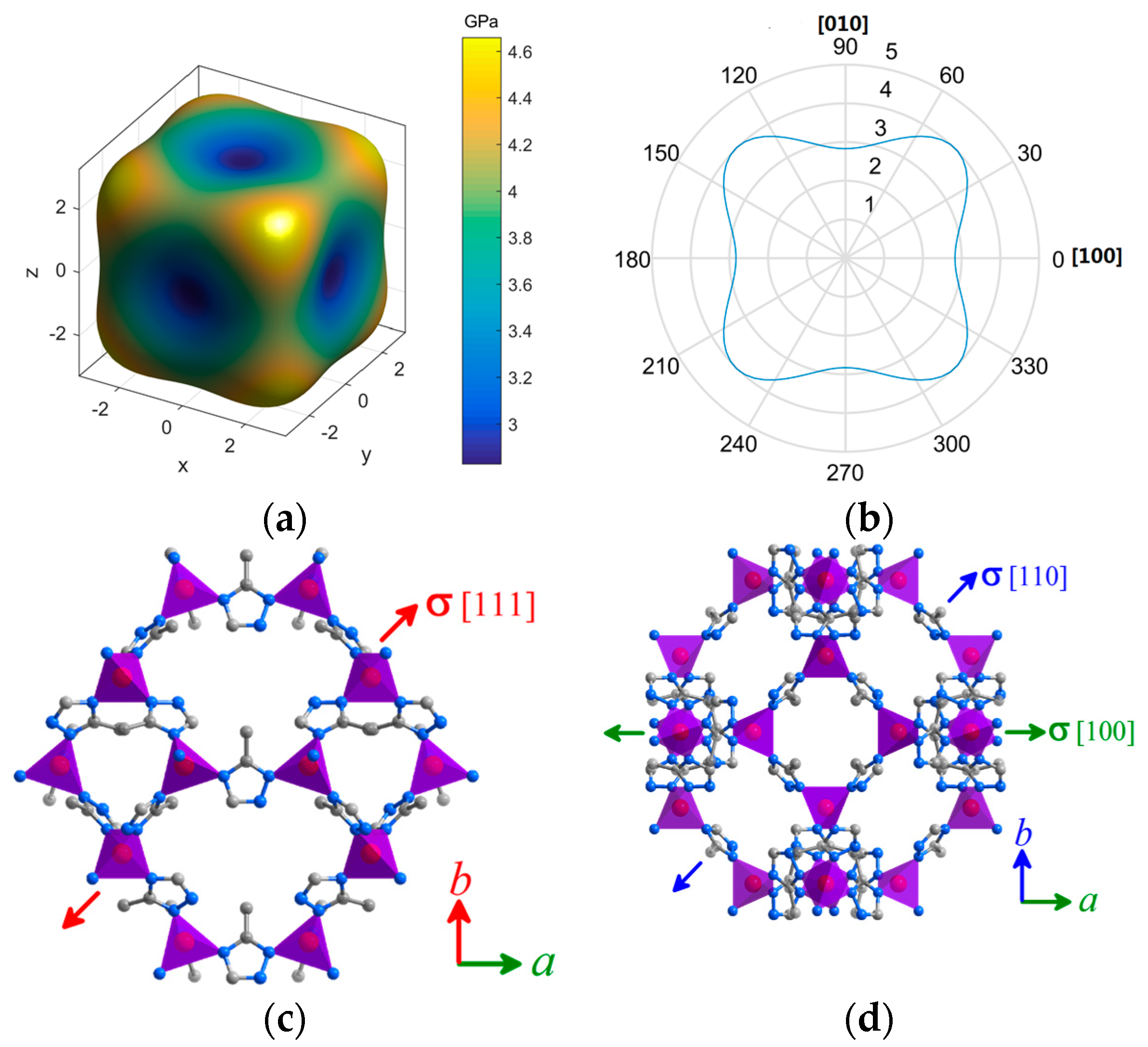

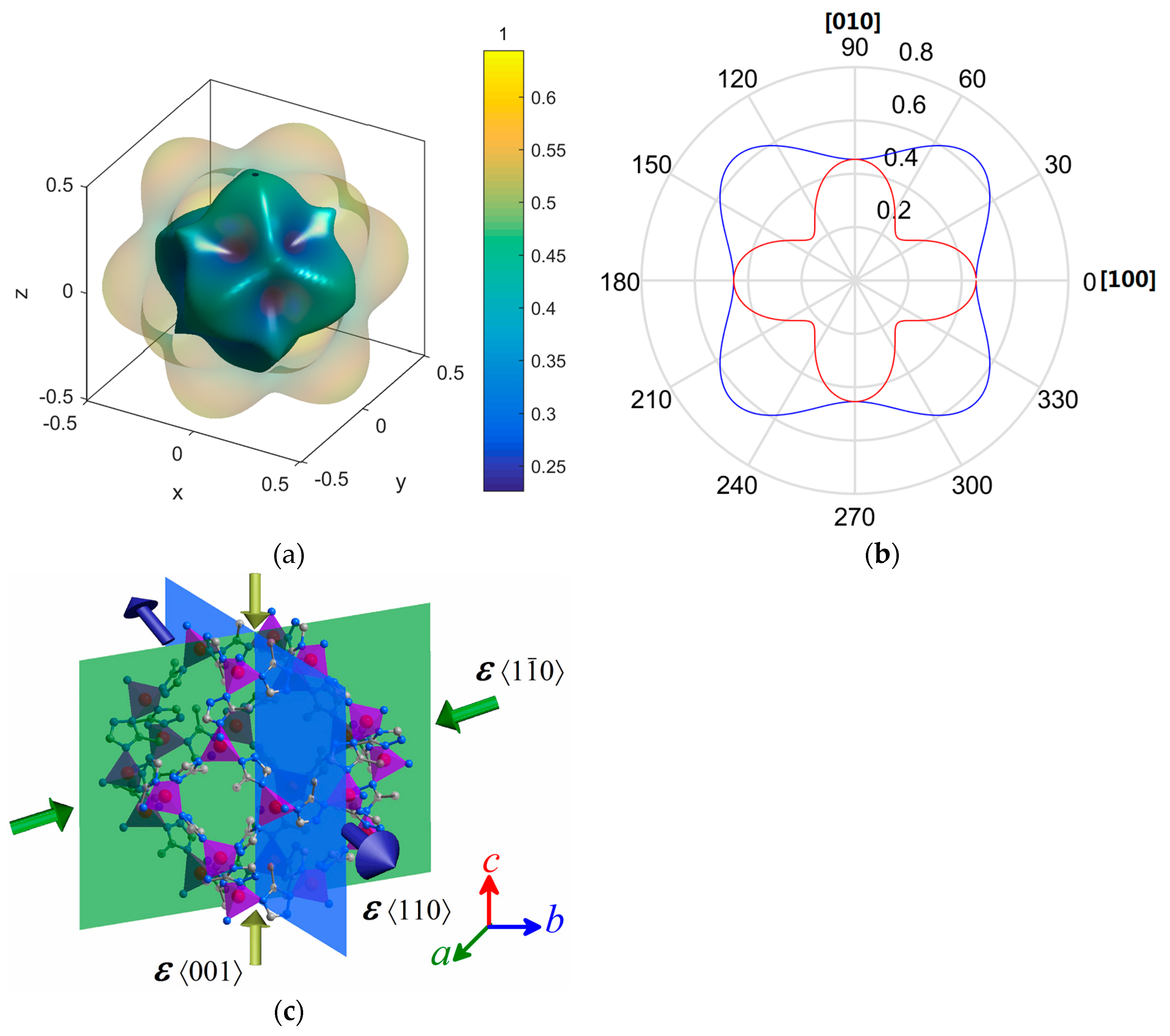
| Elastic Properties | Framework | MAF-7 | ZIF-8 [15] |
|---|---|---|---|
| Stiffness coefficient, Cij (GPa) | C11 | 11.587 | 11.038 |
| C12 | 9.643 | 8.325 | |
| C44 | 1.635 | 0.943 | |
| Young’s modulus, E (GPa) | Emax | E(111) = 4.659 | E(100) = 3.879 |
| E(110) = 4.009 | E(110) = 2.953 | ||
| Emin | E(100) = 2.828 | E(111) = 2.736 | |
| Shear modulus, G (GPa) | Gmax | 1.64 | 1.36 |
| Gmin | 0.97 | 0.94 | |
| Poisson’s ratio, υ | υmax | = 0.64 | = 0.57 |
| υmin | = 0.23 | = 0.33 | |
| Anisotropy measure | Zener, A (=1 if isotropic) | 1.68 | 0.70 |
| Emax/Emin | 1.65 | 1.42 | |
| Bulk modulus K(GPa) | 10.29 | 9.23 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Wei, W.; Dong, L.; Feng, G.; Jiang, X.; Wu, R.; Lin, Z.; Li, W. Enhanced Framework Rigidity of a Zeolitic Metal-Azolate via Ligand Substitution. Crystals 2017, 7, 99. https://doi.org/10.3390/cryst7040099
Gao H, Wei W, Dong L, Feng G, Jiang X, Wu R, Lin Z, Li W. Enhanced Framework Rigidity of a Zeolitic Metal-Azolate via Ligand Substitution. Crystals. 2017; 7(4):99. https://doi.org/10.3390/cryst7040099
Chicago/Turabian StyleGao, Hongqiang, Wenjuan Wei, Liyuan Dong, Guoqiang Feng, Xingxing Jiang, Rong Wu, Zheshuai Lin, and Wei Li. 2017. "Enhanced Framework Rigidity of a Zeolitic Metal-Azolate via Ligand Substitution" Crystals 7, no. 4: 99. https://doi.org/10.3390/cryst7040099