Crystal and Magnetic Structures in Layered, Transition Metal Dihalides and Trihalides
Abstract
:1. Introduction
2. Crystal Structures of Layered, Binary, Transition Metal Halides
2.1. MX Compounds
2.2. MX Compounds
3. Magnetic Structures of Layered, Binary, Transition Metal Halides
3.1. MX Compounds
3.1.1. TiX and ZrX
3.1.2. VX
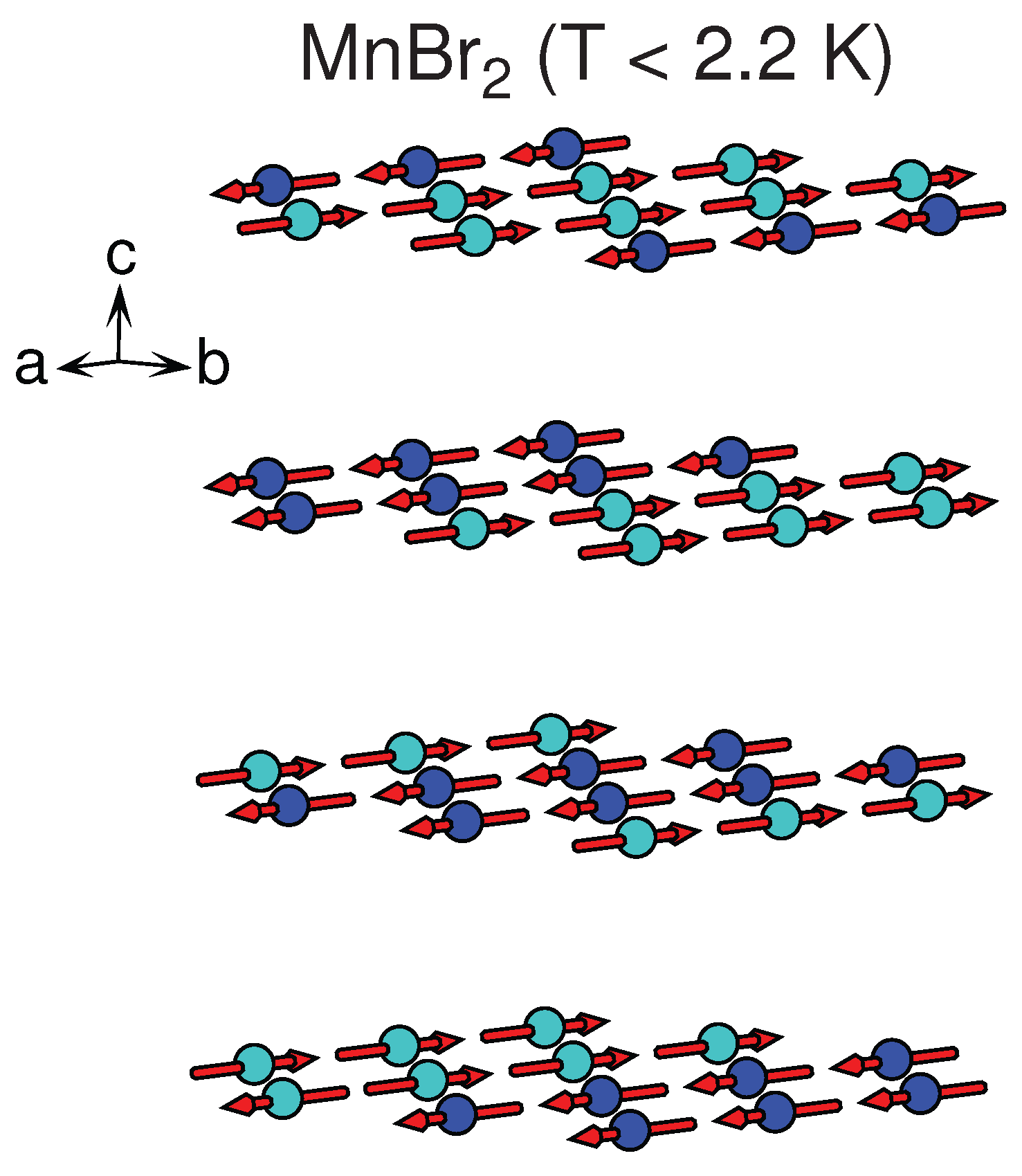
3.1.3. MnX
3.1.4. FeX
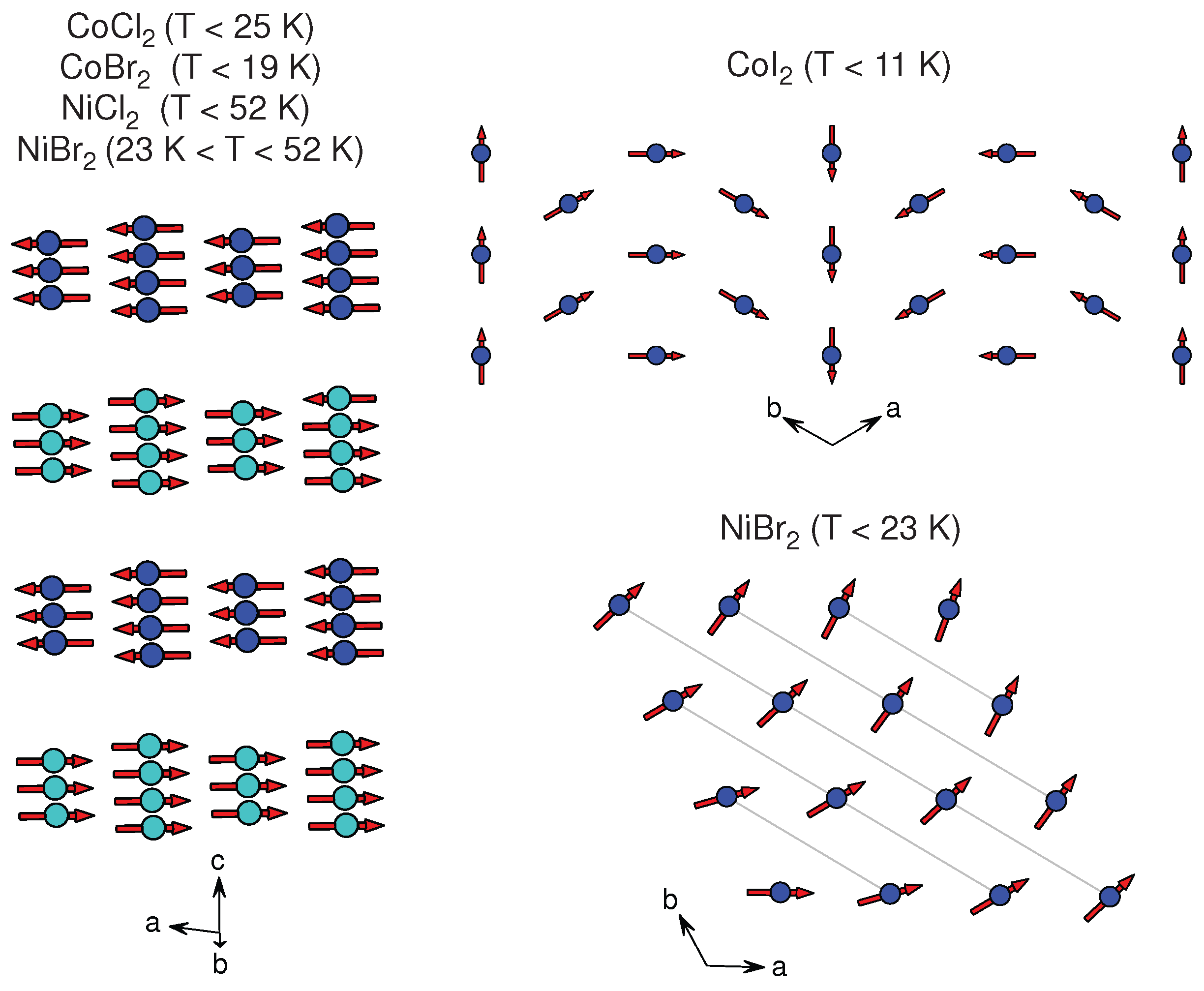
3.1.5. CoX
3.1.6. NiX
3.2. MX Compounds
3.2.1. VX
3.2.2. CrX
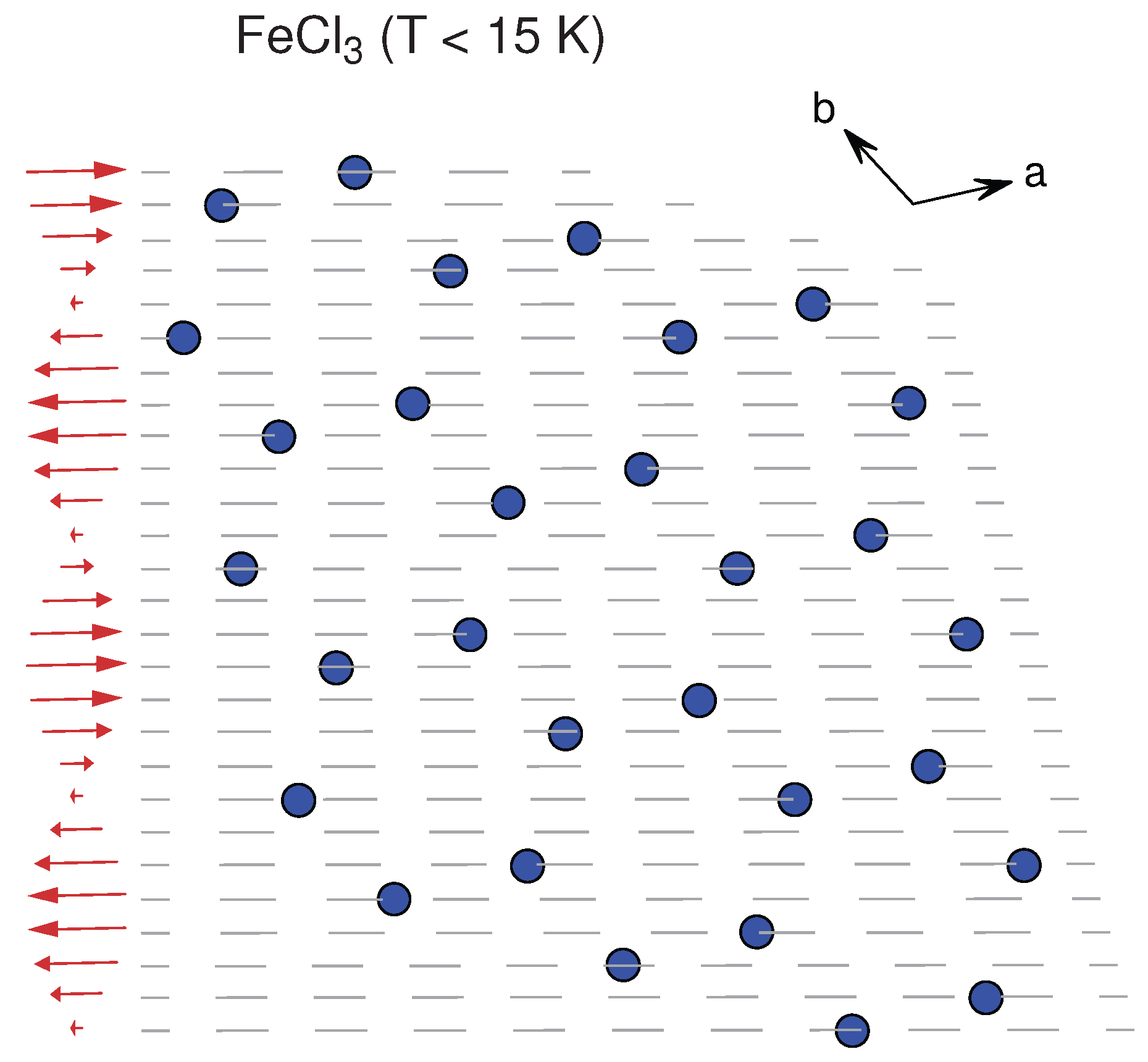
3.2.3. FeX
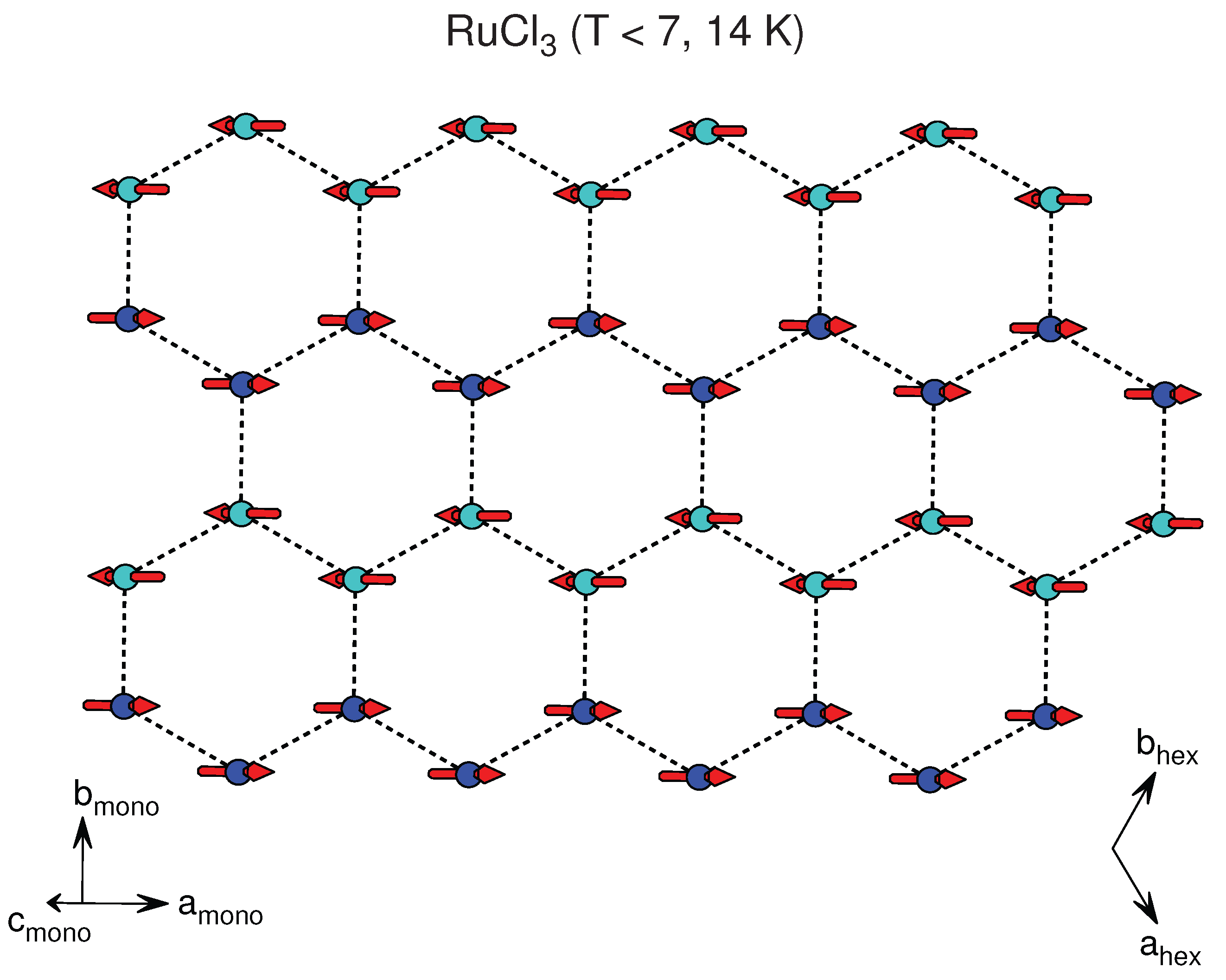
3.2.4. RuX
4. Summary and Conclusions
Acknowledgments
Conflicts of Interest
References
- De Jongh, L.J. Magnetic Properties of Layered Transition Metal Compounds; Kluwer Academic Press: Dordrecht, The Netherlands, 1990. [Google Scholar]
- Kadowaki, H.; Ubukoshi, K.; Hirakawa, K.; Martinez, J.L.; Shirane, G. Experimental Study of New Type Phase Transition in Triangular Lattice Antiferromagnet VCl2. J. Phys. Soc. Jpn. 1987, 56, 4027–4039. [Google Scholar] [CrossRef]
- Ramirez, A.P. Strongly Geometrically Frustrated Magnets. Annu. Rev. Mater. Sci. 1994, 24, 453–480. [Google Scholar] [CrossRef]
- Collins, M.F.; Petrenko, O.A. Triangular antiferromagnets. Can. J. Phys. 1997, 75, 605–655. [Google Scholar] [CrossRef]
- Tokunaga, Y.; Okuyama, D.; Kurumaji, T.; Arima, T.; Nakao, H.; Murakami, Y.; Taguchi, Y.; Tokura, Y. Multiferroicity in NiBr2 with long-wavelength cycloidal spin structure on a triangular lattice. Phys. Rev. B 2011, 84, 060406. [Google Scholar] [CrossRef]
- Kurumaji, T.; Seki, S.; Ishiwata, S.; Murakawa, H.; Tokunaga, Y.; Kaneko, Y.; Tokura, Y. Magnetic-Field Induced Competition of Two Multiferroic Orders in a Triangular-Lattice Helimagnet MnI2. Phys. Rev. Lett. 2011, 106, 167206. [Google Scholar] [CrossRef]
- Wu, X.; Cai, Y.; Xie, Q.; Weng, H.; Fan, H.; Hu, J. Magnetic ordering and multiferroicity in MnI2. Phys. Rev. B 2012, 86, 134413. [Google Scholar] [CrossRef]
- Kurumaji, T.; Seki, S.; Ishiwata, S.; Murakawa, H.; Kaneko, Y.; Tokura, Y. Magnetoelectric responses induced by domain rearrangement and spin structural change in triangular-lattice helimagnets NiI2 and CoI2. Phys. Rev. B 2013, 87, 014429. [Google Scholar] [CrossRef]
- Plumb, K.W.; Clancy, J.P.; Sandilands, L.J.; Shankar, V.V.; Hu, Y.F.; Burch, K.S.; Kee, H.Y.; Kim, Y.J. α-RuCl3: A spin-orbit assisted Mott insulator on a honeycomb lattice. Phys. Rev. B 2014, 90, 041112. [Google Scholar] [CrossRef]
- Kim, H.; Shankar, V.; Catuneanu, A.; Kee, H. Kitaev magnetism in honeycomb RuCl3 with intermediate spin-orbit coupling. Phys. Rev. B 2015, 91, 241110. [Google Scholar] [CrossRef]
- Sears, J.A.; Songvilay, M.; Plumb, K.W.; Clancy, J.P.; Qiu, Y.; Zhao, Y.; Parshall, D.; Kim, Y.J. Magnetic order in α-RuCl3: A honeycomb-lattice quantum magnet with strong spin-orbit coupling. Phys. Rev. B 2015, 91, 144420. [Google Scholar] [CrossRef]
- Johnson, R.D.; Williams, S.C.; Haghighirad, A.A.; Singleton, J.; Zapf, V.; Manuel, P.; Mazin, I.I.; Li, Y.; Jeschke, H.O.; Valenti, R.; et al. Monoclinic crystal structure of α-RuCl3 and the zigzag antiferromagnetic ground state. Phys. Rev. B 2015, 92, 235119. [Google Scholar] [CrossRef]
- Banerjee, A.; Bridges, C.A.; Yan, J.Q.; Aczel, A.A.; Li, L.; Stone, M.B.; Granroth, G.E.; Lumsden, M.D.; Yiu, Y.; Knolle, J.; et al. Proximate Kitaev quantum spin liquid behaviour in a honeycomb magnet. Nat. Mater. 2016, 15, 733. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Geigorieva, I.V. Van der Waals heterostructures. Nature 2013, 499, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Eyert, V.; Schwingenschlögl, U. Electronic structure and magnetic ordering of the semiconducting chromium trihalides CrCl3, CrBr3, and CrI3. J. Phys. Condens. Matter 2011, 23, 116003. [Google Scholar] [CrossRef] [PubMed]
- McGuire, M.A.; Dixit, H.; Cooper, V.R.; Sales, B.C. Coupling of Crystal Structure and Magnetism in the Layered, Ferromagnetic Insulator CrI3. Chem. Mater. 2015, 27, 612–620. [Google Scholar] [CrossRef]
- Zhang, W.B.; Qu, Q.; Zhu, P.; Lam, C.H. Robust intrinsic ferromagnetism and half semiconductivity in stable two-dimensional single-layer chromium trihalides. J. Mater. Chem. C 2015, 3, 12457–12468. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Q.; Kawazoe, Y.; Jena, P. Exfoliating biocompatible ferromagnetic Cr-trihalide monolayers. Phys. Chem. Chem. Phys. 2016, 18, 8777–8784. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fan, F.; Zhu, S.; Wu, H. Doping enhanced ferromagnetism and induced half-metallicity in CrI3 monolayer. EPL 2016, 114, 47001. [Google Scholar] [CrossRef]
- Zhong, D.; Seyler, K.L.; Linpeng, X.; Cheng, R.; Sivadas, N.; Schmidgall, B.H.E.; Taniguchi, T.; Watanabe, K.; McGuire, M.A.; Yao, W.; et al. Van der Waals Engineering of Ferromagnetic Semiconductor Heterostructures for Spin and Valleytronics. arXiv, 2017; arXiv:1704.00841. [Google Scholar]
- Huang, B.; Clark, G.; Navarro-Moratalla, E.; Klein, D.R.; Cheng, R.; Seyler, K.L.; Zhong, D.; Schmidgall, E.; McGuire, M.A.; Cobden, D.H.; et al. Layer-dependent Ferromagnetism in a van der Waals Crystal down to the Monolayer Limit. arXiv, 2017; arXiv:1703.05892. [Google Scholar]
- Lebègue, S.; Björkman, T.; Klintenberg, M.; Nieminen, R.M.; Eriksson, O. Two-Dimensional Materials from Data Filtering and Ab Initio Calculations. Phys. Rev. X 2013, 3, 031002. [Google Scholar]
- Ajayan, P.; Kim, P.; Banerjee, K. Two-dimensional van der Waals materials. Phys. Today 2016, 69, 38. [Google Scholar] [CrossRef]
- Park, J. Opportunities and challenges of 2D magnetic van der Waals materials: Magnetic graphene? J. Phys. Condens. Matter 2016, 28, 301001. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.H.; Tsoi, M. Antiferromagnetic metal spintronics. Philos. Trans. R. Soc. A 2011, 369, 3098–3114. [Google Scholar] [CrossRef]
- Gomonay, E.V.; Loktev, V.M. Spintronics of antiferromagnetic systems. Low Temp. Phys. 2014, 40, 17. [Google Scholar] [CrossRef]
- Jungwirth, T.; Marti, X.; Wadley, P.; Wunderlich, J. Antiferromagnetic spintronics. Nat. Nanotechnol. 2016, 11, 231. [Google Scholar] [CrossRef] [PubMed]
- Klemm, W.; Krose, E. Die Kristallstrukturen von ScCl3, TiCl3 und VCl3. Z. Anorg. Chem. 1947, 253, 218–225. [Google Scholar] [CrossRef]
- Natta, G.; Corradini, P.; Allegra, G. The different crystalline modifications of TiCl3, a catalyst component for the polymerization of α-olefins. I: α-, β-, γ-TiCl3. II: δ-TiCl3. J. Polym. Sci. 1961, 51, 399–410. [Google Scholar] [CrossRef]
- Von Schnering, H.G.; Wöhrle, H.; Schäfer, H. Die Kristallstruktur der Verbindung Nb3Cl8. Naturwissenschaften 1961, 48, 159. [Google Scholar] [CrossRef]
- Sheckelton, J.P.; Plumb, K.W.; Trump, B.A.; Broholm, C.L.; McQueen, T.M. Rearrangement of van der Waals stacking and formation of a singlet state at T = 90 K in a cluster magnet. Inorg. Chem. Front. 2017, 4, 481–490. [Google Scholar] [CrossRef]
- Jiang, J.; Liang, Q.; Meng, R.; Yang, Q.; Tan, C.; Sun, X.; Chen, X. Exploration of new ferromagnetic, semiconducting and biocompatible Nb3X8 (X = Cl, Br or I) monolayers with considerable visible and infrared light absorption. Nanoscale 2017, 9, 2992–3001. [Google Scholar] [CrossRef] [PubMed]
- Brehzer, B. Röntgenographische Untersuchungen an ZnCl2. Naturwissenschaften 1959, 46, 106b. [Google Scholar] [CrossRef]
- Yamaguchi, S. Determining the crystal structure of hygroscopic substances by electron diffraction: ZnBr2. Sci. Pap. Inst. Phys. Chem. Res. (Jpn.) 1942, 39, 291. [Google Scholar]
- Yamaguchi, S. Determining the crystal structure of hygroscopic substances by electron diffraction (continued): ZnI2. Sci. Pap. Inst. Phys. Chem. Res. (Jpn.) 1942, 39, 357. [Google Scholar]
- Pauling, L. On the crystal structure of the chlorides of certain bivalent elements. Proc. Natl. Acad. Sci. USA 1929, 15, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.S. Single crystal x-ray study of structural polytypism in cadmium bromide. Z. Kristallogr. 1962, 117, 309. [Google Scholar] [CrossRef]
- Bozorth, R.M. The crystal structure of cadmium iodide. J. Am. Chem. Soc. 1922, 44, 2232–2236. [Google Scholar] [CrossRef]
- Bijvoet, J.M.; Claassen, A.; Karssen, A. The Crystal Structure of Red Mercuric Iodide. Proc. K. Ned. Akad. Wet. 1926, 29, 529–546. [Google Scholar]
- Schneider, M.; Kuske, P.; Lutz, H.D. Novel High-Temperature Polymorphs of MgBr2 and MnBr2—Limits of Powder Diffraction for Structure Determination. Acta Crystallogr. B 1992, 48, 761–763. [Google Scholar] [CrossRef]
- Kuindersma, S.R.; Sanchez, J.P.; Haas, C. Magnetic and Structural Investigations on NiI2 and CoI2. Phys. B Condens. Matter 1981, 111, 231–248. [Google Scholar] [CrossRef]
- Narath, A.; Schirber, J.E. Effect of Hydrostatic Pressure on the Metamagnetic Transitions in FeCl2·2H2O, CoCl2·2H2O, FeCl2, and FeBr2. J. Appl. Phys. 1966, 37, 1124. [Google Scholar] [CrossRef]
- Rozenberg, G.K.; Pasternak, M.P.; Gorodetsky, P.; Xu, W.M.; Dubrovinsky, L.S.; LeBihan, T.; Taylor, R.D. Pressure-induced structural, electronic, and magnetic phase transitions in FeCl2 studied by x-ray diffraction and resistivity measurements. Phys. Rev. B 2009, 79, 214105. [Google Scholar] [CrossRef]
- Baenziger, N.C.; Rundle, R.E. The structure of TiCl2. Acta Crystallogr. 1948, 1, 274. [Google Scholar] [CrossRef]
- Ehrlich, P.; Gutsche, W.; Seifert, H.J. Darstellung und Kristallstruktur von Titandibromid. Z. Anorg. Allg. Chem. 1961, 312, 80–86. [Google Scholar] [CrossRef]
- Klemm, W.; Grimm, L. Zur Kenntnis der Dihalogenide des Titans und Vanadins. Z. Anorg. Allg. Chem. 1942, 249, 198–208. [Google Scholar] [CrossRef]
- Villadsen, J. Note on the Crystal Structure of Vanadium Dichloride. Acta Chem. Scand. 1959, 13, 2146. [Google Scholar] [CrossRef]
- Kuindersma, S.R.; Hass, C.; Sanchez, J.P.; Al, R. Magnetic structures and properties of VI2. Solid State Commun. 1979, 30, 403. [Google Scholar] [CrossRef]
- Tornero, J.D.; Fayos, J. Single crystal structure refinement of MnCl2. Z. Kristallogr. 1990, 192, 147. [Google Scholar] [CrossRef]
- Wollan, E.O.; Koehler, W.C.; Wilkinson, M.K. Neutron Diffraction Study of the Magnetic Properties of MnBr2. Phys. Rev. 1958, 110, 638. [Google Scholar] [CrossRef]
- Ferrari, A.; Giorgi, F. La struttura cristallina degli ioduri anidri dei metalli bivalenti. I. Ioduri di cobalto, di ferro e di manganese. Atti Accad. Naz. Lincei Cl. Sci. Fis. Mat. Nat. Rend. 1929, 10, 522. [Google Scholar]
- Vettier, C.; Yellon, W.B. The structure of FeCl2 at high pressures. J. Phys. Chem. Solids 1975, 36, 401–405. [Google Scholar] [CrossRef]
- Haberecht, J.; Borrmann, H.; Kniep, R. Refinement of the crystal structure of iron dibromide, FeBr2. Z. Kristallogr. New Cryst. Struct. 2001, 216, 510. [Google Scholar] [CrossRef]
- Gelard, J.; Fert, A.R.; Mériel, P.; Allain, Y. Magnetic structure of FeI2 by neutron diffraction experiments. Solid State Commun. 1974, 14, 187–189. [Google Scholar] [CrossRef]
- Grimme, H.; Santos, J.A. The Structure and Colour of Anhydrous Cobalt Chloride, CoCl2, at Room and very Low Temperatures. Z. Kristallogr. 1934, 88, 136–141. [Google Scholar] [CrossRef]
- Ferrari, A.; Giorgi, F. La struttura cristallina dei bromuri di metalli bivalenti. Atti Accad. Naz. Lincei Cl. Sci. Fis. Mat. Nat. Rend. 1929, 9, 1134. [Google Scholar]
- Ferrari, A.; Braibanti, A.; Bigliardi, G. Refinement of the crystal structure of NiCl2 and of unit-cell parameters of some anhydrous chlorides of divalent metals. Acta Crystallogr. 1963, 16, 846–847. [Google Scholar] [CrossRef]
- Nasser, J.A.; Kiat, J.M.; Gabilly, R. X-ray investigation of magnetostriction in NiBr2. Solid State Commun. 1992, 82, 49–54. [Google Scholar] [CrossRef]
- Ketalaar, J.A.A. Die Kristallstruktur des Nickelbromids und -jodids. Z. Kristallogr. 1934, 88, 26. [Google Scholar] [CrossRef]
- Cisar, A.; Corbett, J.D.; Daake, R.L. The zirconium dichloride phase region. Synthesis, structure, and photoelectron spectral studies of 3R-ZrCl2, 6T-Zr1.05Cl2, and related phases. Inorg. Chem. 1979, 18, 836–843. [Google Scholar] [CrossRef]
- Guthrie, D.H.; Corbett, J.D. Synthesis and Structure of an Infinite-Chain Form of ZrI2 (α). J. Solid State Chem. 1981, 37, 256–263. [Google Scholar] [CrossRef]
- Corbett, J.D.; Guthrie, D.H. A second infinite-chain form of zirconium diiodide (β) and its coherent intergrowth with α-zirconium diiodide. Inorg. Chem. 1982, 21, 1747. [Google Scholar] [CrossRef]
- Imoto, H.; Corbett, J.D.; Cisar, A. Synthesis by hydrogen-driven disproportionation reactions. Synthesis and structure of the hexazirconium dodecahalide clusters Zr6Cl12 and Zr6Br12 and the double salt Zr6Cl12·M2ZrCl6 (M= Na, K, Cs). Inorg. Chem. 1981, 20, 145. [Google Scholar] [CrossRef]
- Men’kov, A.A.; Komissarova, L.N. An X-ray diffraction study of scandium bromide. Russ. J. Inorg. Chem. 1964, 9, 952. [Google Scholar]
- Men’kov, A.A.; Komissarova, L.N. X-ray diffraction study of scandium iodide. Russ. J. Inorg. Chem. 1964, 9, 425. [Google Scholar]
- Templeton, D.H.; Darter, G.F. The crystal structure of Yttrium Trichloride and similar compounds. J. Phys. Chem. 1954, 58, 940–944. [Google Scholar] [CrossRef]
- Brown, D.; Fletcher, S.; Holah, D.G. The Preparation and Crystallographic Properties of Certain Lanthanide and Actinide Tribromides and Tribromide Hexahydrates. J. Chem. Soc. A 1968, 1889–1894. [Google Scholar] [CrossRef]
- Jongen, L.; Meyer, G. Yttrium triiodide, YI3. Acta Crystallogr. E 2005, 61, i151–i152. [Google Scholar] [CrossRef]
- Poineau, F.; Johnstone, E.V.; Czerwinski, K.R.; Sattelberger, A.P. Recent Advances in Technetium Halide Chemistry. Acc. Chem. Res. 2013, 47, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Troyanov, S.I.; Snigireva, E.M.; Rybakov, V.B. An X-ray structural investigation of the phase transition in α-TiCl3. Russ. J. Inorg. Chem. 1991, 36, 634. [Google Scholar]
- Troyanov, S.I.; Rybakov, V.B.; Ionov, V.M. The synthesis and crystal structures of TiBr4, TiBr3, and Ti(AlBr4)2. Russ. J. Inorg. Chem. 1990, 35, 494. [Google Scholar]
- McCarley, R.E.; Roddy, J.W.; Berry, K.O. Transport reactions of some vanadium(III) halides. Mixed halide formation. Inorg. Chem. 1964, 3, 50. [Google Scholar] [CrossRef]
- Morosin, B.; Narath, A. X-Ray Diffraction and Nuclear Quadrupole Resonance Studies of Chromium Trichloride. J. Chem. Phys. 1964, 40, 1958–1967. [Google Scholar] [CrossRef]
- Handy, L.L.; Gregory, N.W. Structural Properties of Chromium(III) Iodide and Some Chromiurn(III) Mixed Halides. J. Am. Chem. Soc. 1952, 74, 891–893. [Google Scholar] [CrossRef]
- Hashimoto, S.; Forster, K.; Moss, S.C. Structure refinement of an FeCl3 crystal using a thin plate sample. J. Appl. Crystallogr. 1989, 22, 173–180. [Google Scholar] [CrossRef]
- Troyanov, S. Crystal Structure of FeCl3 Polytypes. Russ. J. Inorg. Chem. 1993, 38, 1821. [Google Scholar]
- Armbrüster, M.; Ludwig, T.; Rotter, H.W.; Thiele, G.; Oppermann, H. About Irontribromide: Equilibrium Studies, Crystal Structure, and Spectroscopic Characterization. Z. Anorg. Allg. Chem. 2000, 626, 187. [Google Scholar]
- Schäfer, H.; von Schnering, H.G.; Tillack, J.V.; Kuhnen, F.; Wörle, H.; Baumann, H. Neue Untersuchungen über die Chloride des Molybdäns. Z. Anorg. Allg. Chem. 1967, 353, 281–310. [Google Scholar] [CrossRef]
- Poineau, F.; Johnstone, E.V.; Weck, P.F.; Forster, P.M.; Kim, E.; Czerwinski, K.R.; Sattelberger, A.P. β-technetium trichloride: Formation, structure, and first-principles calculations. Inorg. Chem. 2012, 51, 4915. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.B.; Banerjee, A.; Yan, J.; Bridges, C.A.; Lumsden, M.D.; Mandrus, D.G.; Tennant, D.A.; Chakoumakos, B.C.; Nagler, S.E. Low-temperature crystal and magnetic structure of α-RuCl3. Phys. Rev. B 2016, 93, 134423. [Google Scholar] [CrossRef]
- Fletcher, J.M.; Gardner, W.E.; Fox, A.C.; Topping, G. X-Ray, Infrared, and Magnetic Studies of α- and β-Ruthenium Trichloride. J. Chem. Soc. A 1967, 1038–1045. [Google Scholar] [CrossRef]
- Stroganov, E.V.; Ovchinnikov, K.V. Crystal structure of ruthenium trichloride. Vestn. Leningr. Univ. Ser. 4 1957, 22, 152. [Google Scholar]
- Bärnighausen, H.; Handa, B.K. Die Kristallstruktur von Rhodium(III)-Chlorid. J. Less-Common Met. 1964, 6, 226–231. [Google Scholar] [CrossRef]
- Brodersen, K.; Thiele, G.; Recke, I. Strukturuntersuchungen an Rhodiumhalogeniden. J. Less-Common Met. 1968, 14, 151–152. [Google Scholar] [CrossRef]
- Brodersen, K.; Moers, F.; von Schnering, H.G. Zur Struktur des Iridium(III)- und des Ruthenium(III)-chlorids. Naturwissenschaften 1965, 52, 205–206. [Google Scholar] [CrossRef]
- Brodersen, K.; Thiele, G.; Ohnsorge, H.; Recke, I.; Moers, F. Die Struktur des IrBr3 und über die Ursachen der Fehlordnungserscheinungen bei den in Schichtenstrukturen kristallisierenden Edelmetalltrihalogeniden. J. Less-Common Met. 1968, 15, 347–354. [Google Scholar] [CrossRef]
- Ogawa, S. Magnetic Transition in TiCl3. J. Phys. Soc. Jpn. 1960, 15, 1901. [Google Scholar] [CrossRef]
- Troyanov, S.I.; Snigireva, E.M.; Pirarevskii, A.P.; Yanovskii, A.I.; Struchkov, Y.T. Crystal Structure of α-TiBr3 Low-Temperature Modification. Russ. J. Inorg. Chem. 1994, 39, 360. [Google Scholar]
- Troyanov, S.I.; Snigireva, E.M. Crystal Structures of Transition-Metal Halides TiCl4, α-TiCl3, WCl4, and TiI2. Russ. J. Inorg. Chem. 2000, 45, 580. [Google Scholar]
- Park, S.Y.; Do, S.H.; Choi, K.Y.; Jang, D.; Jang, T.H.; Schefer, J.; Wu, C.M.; Gardner, J.S.; Park, J.M.S.; Park, J.H.; Ji, S. Emergence of the Isotropic Kitaev Honeycomb Lattice with Two-dimensional Ising Universality in α-RuCl3. arXiv, 2016; arXiv:1609.05690. [Google Scholar]
- Babel, D.; Deigner, P. Die Kristallstruktur von β-Iridium(III)-Chlorid. Z. Anorg. Allg. Chem. 1965, 339, 57–66. [Google Scholar]
- Kim, H.S.; Kee, H.Y. Crystal structure and magnetism in α-RuCl3: An ab initio study. Phys. Rev. B 2016, 93, 155143. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, H.; Zu, X.; Gao, F. Evidencing the existence of exciting half-metallicity in two-dimensional TiCl3 and VCl3 sheets. Sci. Rep. 2016, 6, 19407. [Google Scholar] [CrossRef] [PubMed]
- Khomskii, D.I. Transition Metal Compounds; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Niel, M.; Cros, C.; Le Flem, G.; Pouchard, M.; Hagenmuller, P. Magnetic behaviour of vanadium +II in one- and two-dimensional systems. Phys. B Condens. Matter 1977, 702, 86–88. [Google Scholar] [CrossRef]
- Lewis, J.; Machin, D.J.; Newnham, I.E.; Nyholm, R.S. The Magnetic Properties of Some Halides of Titanium and Zirconium. J. Chem. Soc. 1962, 40, 2036–2041. [Google Scholar] [CrossRef]
- Starr, C.; Bitter, R.; Kaufmann, A.R. The Magnetic Properties of the Iron Group Anhydrous Chlorides at Low Temperatures. Phys. Rev. 1940, 58, 977. [Google Scholar] [CrossRef]
- Hirakawa, K.; Kadowaki, H.; Ubukoshi, K. Study of Frustration Effects in Two-Dimensional Triangular Lattice Antiferromagnets-Neutron Powder Diffraction Study of VX2, X ≡ Cl, Br and I. J. Phys. Soc. Jpn. 1983, 52, 1814–1824. [Google Scholar] [CrossRef]
- Kadowaki, H.; Ubukoshi, K.; Hirakawa, K. Neutron Scattering Study of the Triangular-Lattice Antiferromagnet VBr2. J. Phys. Soc. Jpn. 1985, 54, 363–373. [Google Scholar] [CrossRef]
- Abdul Wasey, A.H.M.; Karmakar, D.; Das, G.P. Manifestation of long-range ordered state in layered VX2 (X = Cl, Br, I) systems. J. Phys. Condens. Matter 2013, 25, 476001. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.B. Specific Heat of Single-Crystal MnCl2 in Applied Magnetic Fields. Phys. Rev. 1962, 128, 1570. [Google Scholar] [CrossRef]
- Wilkinson, M.K.; Cable, J.W.; Wollan, E.O.; Koehler, W.C. Oak Ridge National Laboratory Report; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1958; Volume ORNL-2430, p. 65. [Google Scholar]
- Wiesler, D.G.; Suzuki, M.; Suzuki, I.S.; Rosov, N. Determination of Anomalous Superexchange in MnCl2 and Its Graphite Intercalation Compound. Phys. Rev. Lett. 1995, 75, 942. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Kadowaki, H.; Iio, K. Successive phase transitions in the hexagonal-layered Heisenberg antiferromagnets MnX2 (X = Br, I). Phys. B Condens. Matter 1995, 224, 213–214. [Google Scholar] [CrossRef]
- Cable, J.W.; Wilkinson, M.K.; Wollan, E.O.; Koehler, W.C. Neutron Diffraction Investigation of the Magnetic Order in MnI2. Phys. Rev. 1962, 125, 1860–1864. [Google Scholar] [CrossRef]
- Katsumata, K.; Aruga Katori, H.; Kimura, S.; Narumi, Y.; Hagiwara, M.; Kindo, K. Phase transition of a triangular lattice Ising antiferromagnet FeI2. Phys. Rev. B 2010, 82, 104402. [Google Scholar] [CrossRef]
- Bertrand, Y.; Fert, A.R.; Gélard, J. Susceptibilité magnétique des halogénures ferreux FeCl2, FeBr2, Fel2. J. Phys. 1974, 35, 385–391. [Google Scholar] [CrossRef]
- Wilkinson, M.K.; Cable, J.W.; Wollan, E.O.; Koehler, W.C. Neutron Diffraction Investigations of the Magnetic Ordering in FeBr2, CoBr2, FeCl2, and CoCl2. Phys. Rev. 1959, 113, 497. [Google Scholar] [CrossRef]
- Fert, A.R.; Carrara, P.; Lanusse, M.C.; Mischler, G.; Redoules, J.P. Transition de phase metamagnetique du bromure ferreux. J. Phys. Chem. Solids 1973, 34, 223–230. [Google Scholar] [CrossRef]
- Xu, W.M.; Pasternak, M.P. Magnetism in FeCl2 at High Pressures. Hyperfine Interact. 2002, 175, 144–145. [Google Scholar]
- Pasternak, M.P.; Xu, W.M.; Rozenberg, G.K.; Taylor, R.D.; Hearne, G.R.; Sterer, E. Pressure-induced magnetic and electronic transitions in the layered Mott insulator FeI2. Phys. Rev. B 2001, 65, 035106. [Google Scholar] [CrossRef]
- Jacobs, I.S.; Lawrence, P.E. Metarnagnetic Phase Transitions and Hysteresis in FeCl2. Phys. Rev. 1967, 164, 866. [Google Scholar] [CrossRef]
- Fert, A.R.; Gelard, J.; Carrara, P. Phase Transitions of FeI2 in High Magnetic Field Parallel to the Spin Direction, Static Field up to 150 kOe, Pulsed Field up to 250 kOe. Solid State Commun. 1973, 13, 1219–1223. [Google Scholar] [CrossRef]
- Lines, M.E. Magnetic Properties of CoCl2 and NiCl2. Phys. Rev. 1963, 131, 546. [Google Scholar] [CrossRef]
- Binek, C.; Kleemann, W. Domainlike antiferromagnetic correlations of paramagnetic FeCl2: A field-induced Griffiths phase? Phys. Rev. Lett. 1994, 72, 1287. [Google Scholar] [CrossRef] [PubMed]
- Binek, C.; Bertrand, D.; Regnault, L.P.; Kleemann, W. Magnetic neutron-scattering investigation of the field-induced Griffiths phase in FeCl2. Phys. Rev. B 1996, 54, 9015. [Google Scholar] [CrossRef]
- Katsumata, K.; Aruga Katori, H.; Shapiro, S.M.; Shirane, G. Neutron-scattering studies of a phase transition in the metamagnet FeBr2 under external magnetic fields. Phys. Rev. B 1997, 55, 11466. [Google Scholar] [CrossRef]
- Chisholm, R.C.; Stout, J.W. Heat capacity and entropy of CoCl2 and MnCl2 from 11 degrees to 300 degrees K-thermal anomaly associated with antiferromagnetic ordering in CoCl2. J. Chem. Phys. 1962, 36, 972–979. [Google Scholar] [CrossRef]
- Yoshizawa, H.; Ubukoshi, K.; Hirakawa, K. Neutron Scattering Investigation of the Magnetic Excitations in CoBr2. J. Phys. Soc. Jpn. 1980, 48, 42–49. [Google Scholar] [CrossRef]
- Mekata, M.; Kuriyama, H.; Ajiro, Y.; Mitsuda, S.; Yoshizawa, H. First-order magnetic transition in CoI2. J. Magn. Magn. Mater. 1992, 859, 104–107. [Google Scholar] [CrossRef]
- Friedt, J.M.; Sanchez, J.P.; Shenoy, G.K. Electronic and magnetic properties of metal diiodides MI2 (M = V, Cr, Mn, Fe, Co, Ni, Cd) from I-129 Mössbauer spectroscopy. J. Chem. Phys. 1976, 65, 5093–5102. [Google Scholar] [CrossRef]
- Busey, R.H.; Giauque, W.F. The Heat Capacity of Anhydrous NiCl2 from 15 to 300 K. The Antiferromagnetic Anomaly near 52 K. Entropy and Free Energy. J. Am. Chem. Soc. 1952, 74, 4443–4446. [Google Scholar] [CrossRef]
- Adam, A.; Billery, D.; Terrier, C.; Mainard, R.; Regnault, L.P.; Rossat-Mignod, J.; Mériel, P. Neutron diffraction study of the commensurate and incommensurate magnetic structures of NiBr2. Solid State Commun. 1980, 35, 1–5. [Google Scholar] [CrossRef]
- De Gunzbourg, J.; Papassimacopoulos, S.; Miedan-Gros, A.; Allain, Y. Étude Expérimentale du Chlorure de Nickel par Rotation Faraday et Mesures Magnétiques en Champ Statique et Pulsé. J. Phys. Colloq. 1971, 32, C1–C125. [Google Scholar]
- Pollard, R.J.; McCann, V.H.; Ward, J.B. Electronic and magnetic properties of 57Fe in NiCl2, NiBr2, NiI2 and CoI2 from Mössbauer spectroscopy. J. Phys. C Solid State Phys. 1982, 15, 6807. [Google Scholar] [CrossRef]
- Day, P.; Dinsdale, A.; Krausz, E.R.; Robbins, D.J. Optical and neutron diffraction study of the magnetic phase diagram of NiBr2. J. Phys. C Solid State Phys. 1976, 9, 2481. [Google Scholar] [CrossRef]
- Day, P.; Ziebeck, K.R.A. Incommensurate spin structure in the low-temperature magnetic phase of NiBr2. J. Phys. C Solid State Phys. 1980, 13, L523. [Google Scholar] [CrossRef]
- Billery, D.; Terrier, C.; Ciret, N.; Kleinclauss, J. Neutron diffraction study and specific heat of antiferromagnetic NiI2. Phys. Lett. A 1977, 61, 138. [Google Scholar] [CrossRef]
- He, J.; Ma, S.; Lyu, P.; Nachtigall, P. Unusual Dirac half-metallicity with intrinsic ferromagnetism in vanadium trihalide monolayers. J. Mater. Chem. C 2016, 4, 2518–2526. [Google Scholar] [CrossRef]
- Tsubokawa, I. On the Magnetic Properties of a CrBr3 Single Crystal. J. Phys. Soc. Jpn. 1960, 15, 1664–1668. [Google Scholar] [CrossRef]
- Hansen, W.N. Some Magnetic Properties of the Chromium (III) Halides at 4.2 K. J. Appl. Phys. 1959, 30, S304–S305. [Google Scholar] [CrossRef]
- Dillon, J.F., Jr.; Olson, C.E. Magnetization, Resonance, and Optical Properties of the Ferromagnet CrI3. J. Appl. Phys. 1965, 36, 1259–1260. [Google Scholar] [CrossRef]
- Cable, J.W.; Wilkinson, M.K.; Wollan, E.O. Neutron Diffraction Investigation of Antiferromagnetism in CrCl3. J. Phys. Chem. Solids 1961, 19, 29–34. [Google Scholar] [CrossRef]
- Kuhlow, B. Magnetic Ordering in CrCl3 at the Phase Transition. Phys. Stat. Sol. A 1982, 72, 161–168. [Google Scholar] [CrossRef]
- Cable, J.W.; Wilkinson, M.K.; Wollan, E.O.; Koehler, W.C. Neutron-Diffraction Study of Antiferromagnetic FeCl3. Phys. Rev. 1962, 127, 714. [Google Scholar] [CrossRef]
- Jones, E.R., Jr.; Morton, O.B.; Cathey, L.; Auel, T.; Amma, E.L. Low-Temperature Magnetic Susceptibility of FeCl3. J. Chem. Phys. 1969, 50, 4755–4757. [Google Scholar] [CrossRef]
- Johnson, P.B.; Friedberg, S.A.; Rayne, J.A. Field-induced magnetic phase transitions in FeCl3. J. Appl. Phys. 1981, 52, 1932–1934. [Google Scholar] [CrossRef]
- Stampfel, J.P.; Oosterhuis, W.T.; Window, B.; Barros, F. Mössbauer-Effect Measurements in Antiferromagnetic FeCl3. Phys. Rev. B 1973, 8, 4371. [Google Scholar] [CrossRef]
- Oosterhuis, W.T.; Window, B.; Spartalian, K. Sublattice magnetization in FeBr3 below the critical region. Phys. Rev. B 1975, 10, 4616. [Google Scholar] [CrossRef]
- Kim, B.J.; Jin, H.; Moon, S.J.; Kim, J.Y.; Park, B.G.; Leem, C.S.; Yu, J.; Noh, T.W.; Kim, C.; Oh, S.J.; et al. Novel Jeff = 1/2 Mott State Induced by Relativistic Spin-Orbit Coupling in Sr2IrO4. Phys. Rev. Lett. 2008, 101, 076402. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.M.; Gardner, W.E.; Hooper, E.W.; Hyde, K.R.; Moore, F.H.; Woodhead, J.L. Anhydrous Ruthenium Chlorides. Nature 1963, 199, 1089–1090. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Okada, T.; Asai, K.; Katada, M.; Sano, H.; Ambe, F. Mössbauer Spectroscopy and Magnetization Studies of α- and β-RuCl3. Inorg. Chem. 1992, 31, 4570–4574. [Google Scholar] [CrossRef]
- Majumder, M.; Schmidt, M.; Rosner, H.; Tsirlin, A.A.; Yasuoka, H.; Baenitz, M. Anisotropic Ru3+ 4d5 magnetism in the α-RuCl3 honeycomb system: Susceptibility, specific heat, and zero-field NMR. Phys. Rev. B 2015, 91, 180401. [Google Scholar] [CrossRef]
- Kitaev, A. Anyons in an exactly solved model and beyond. Ann. Phys. 2006, 321, 2–111. [Google Scholar] [CrossRef]
- Baskaran, G.; Mandal, S.; Shankar, R. Exact Results for Spin Dynamics and Fractionalization in the Kitaev Model. Phys. Rev. Lett. 2007, 98, 247201. [Google Scholar] [CrossRef] [PubMed]
- Knolle, J.; Kovrizhin, D.L.; Chalker, J.T.; Moessner, R. Dynamics of a Two-Dimensional Quantum Spin Liquid: Signatures of Emergent Majorana Fermions and Fluxes. Phys. Rev. Lett. 2014, 112, 207203. [Google Scholar] [CrossRef]


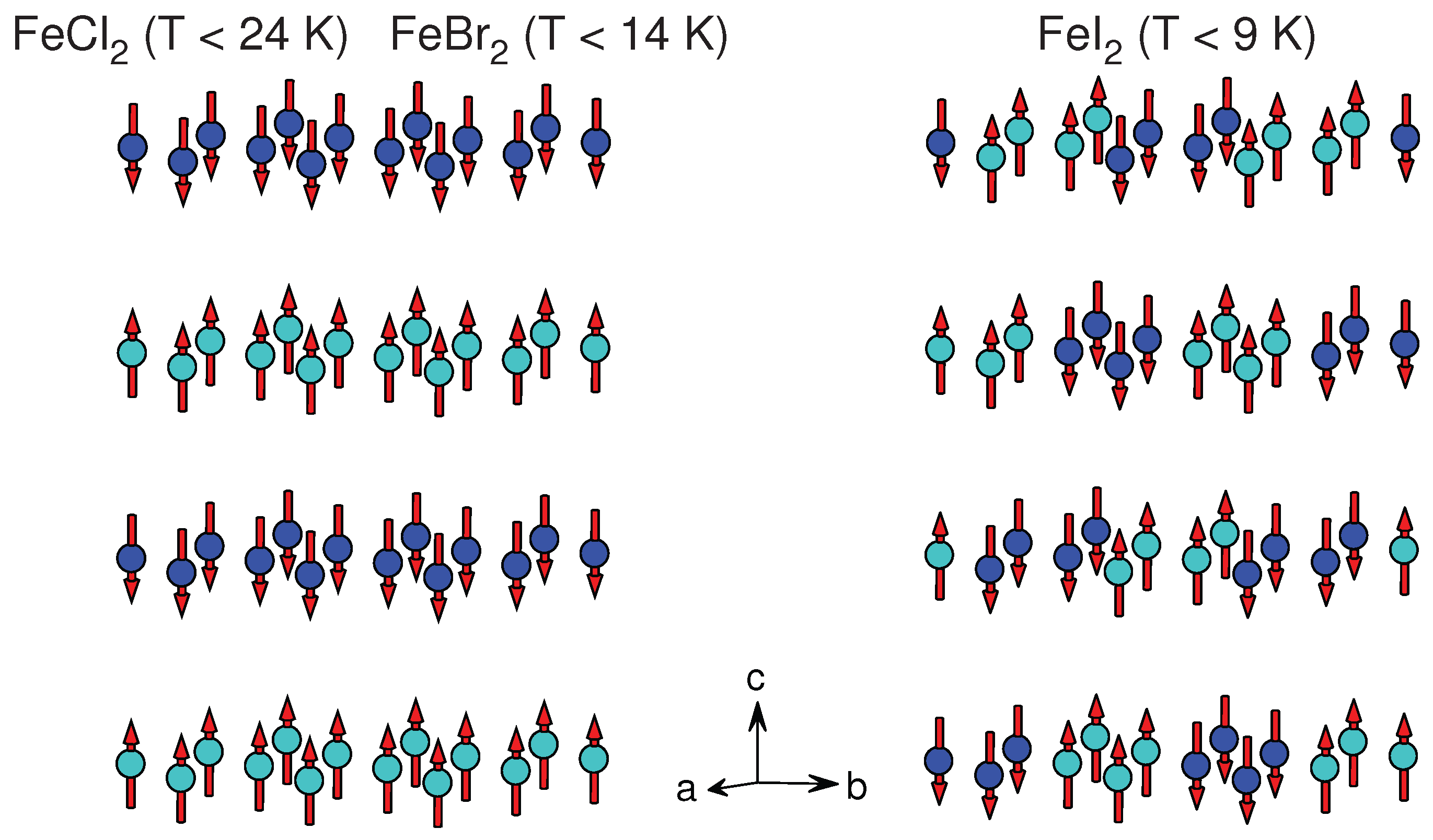
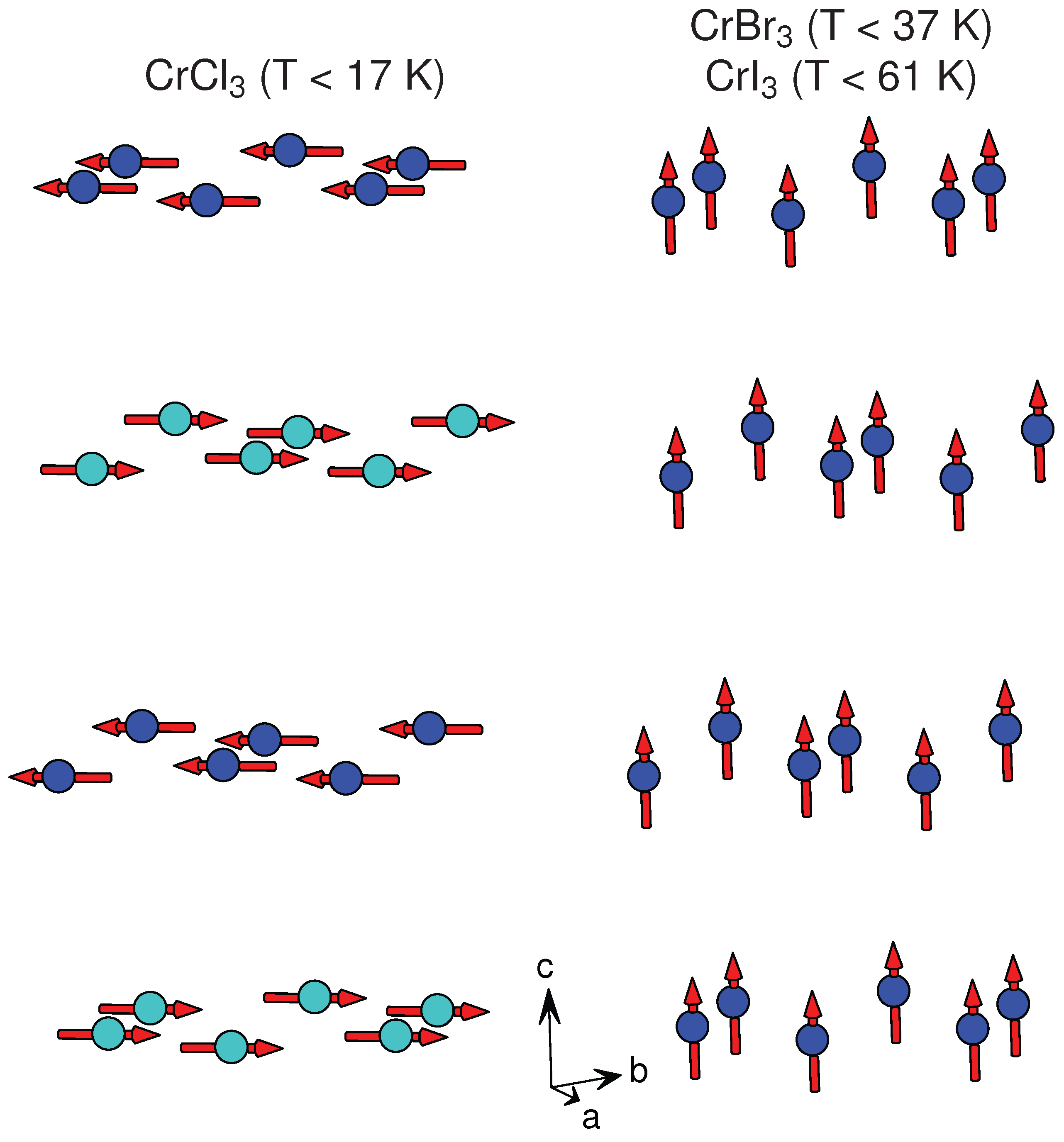
| Compound | Structure Type | Reference | in Plane | Layer | Magnetic Order | Moments | , (K) | , (K) |
|---|---|---|---|---|---|---|---|---|
| Distance (Å) | Spacing (Å) | in Layer | ||||||
| TiCl | CdI () | [44] | 3.56 | 5.88 | AFM | – | 85 | −702 |
| TiBr | CdI () | [45] | 3.63 | 6.49 | – | – | – | – |
| TiI | CdI () | [46] | 4.11 | 6.82 | – | – | – | – |
| VCl | CdI () | [47] | 3.6 | 5.83 | AFM | 120 | 36 | −565, −437 |
| VBr | CdI () | [46] | 3.77 | 6.18 | AFM | 120 | 30 | −335 |
| VI | CdI () | [48] | 4.06 | 6.76 | AFM | – | 16.3, 15 | −143 |
| MnCl | CdCl () | [49] | 3.71 | 5.86 | AFM or HM | stripe or HM | 2.0, 1.8 | −3.3 |
| MnBr * | CdI () | [50] | 3.89 | 6.27 | AFM | stripe | 2.3, 2.16 | – |
| MnI | CdI () | [51] | 4.16 | 6.82 | HM | HM | 3.95, 3.8, 3.45 | – |
| FeCl | CdCl () | [52] | 3.6 | 5.83 | AFM | FM ⊥ | 24 | 9 (), 21 (⊥) |
| FeBr | CdI () | [53] | 3.78 | 6.23 | AFM | FM ⊥ | 14 | −3.0 (), 3.5 (⊥) |
| FeI | CdI () | [54] | 4.03 | 6.75 | AFM | stripe ⊥ | 9 | 24 (), 21.5 (⊥) |
| CoCl | CdCl () | [55] | 3.54 | 5.81 | AFM | FM | 25 | 38 |
| CoBr | CdI () | [56] | 3.69 | 6.12 | AFM | FM | 19 | – |
| CoI | CdI () | [51] | 3.96 | 6.65 | HM | HM | 11 | – |
| NiCl | CdCl () | [57] | 3.48 | 5.8 | AFM | FM | 52 | 68 |
| NiBr | CdCl () | [58] | 3.7 | 6.09 | AFM, HM | FM , HM | 52, 23 | – |
| NiI * | CdCl () | [59] | 3.9 | 6.54 | HM | HM | 75 | – |
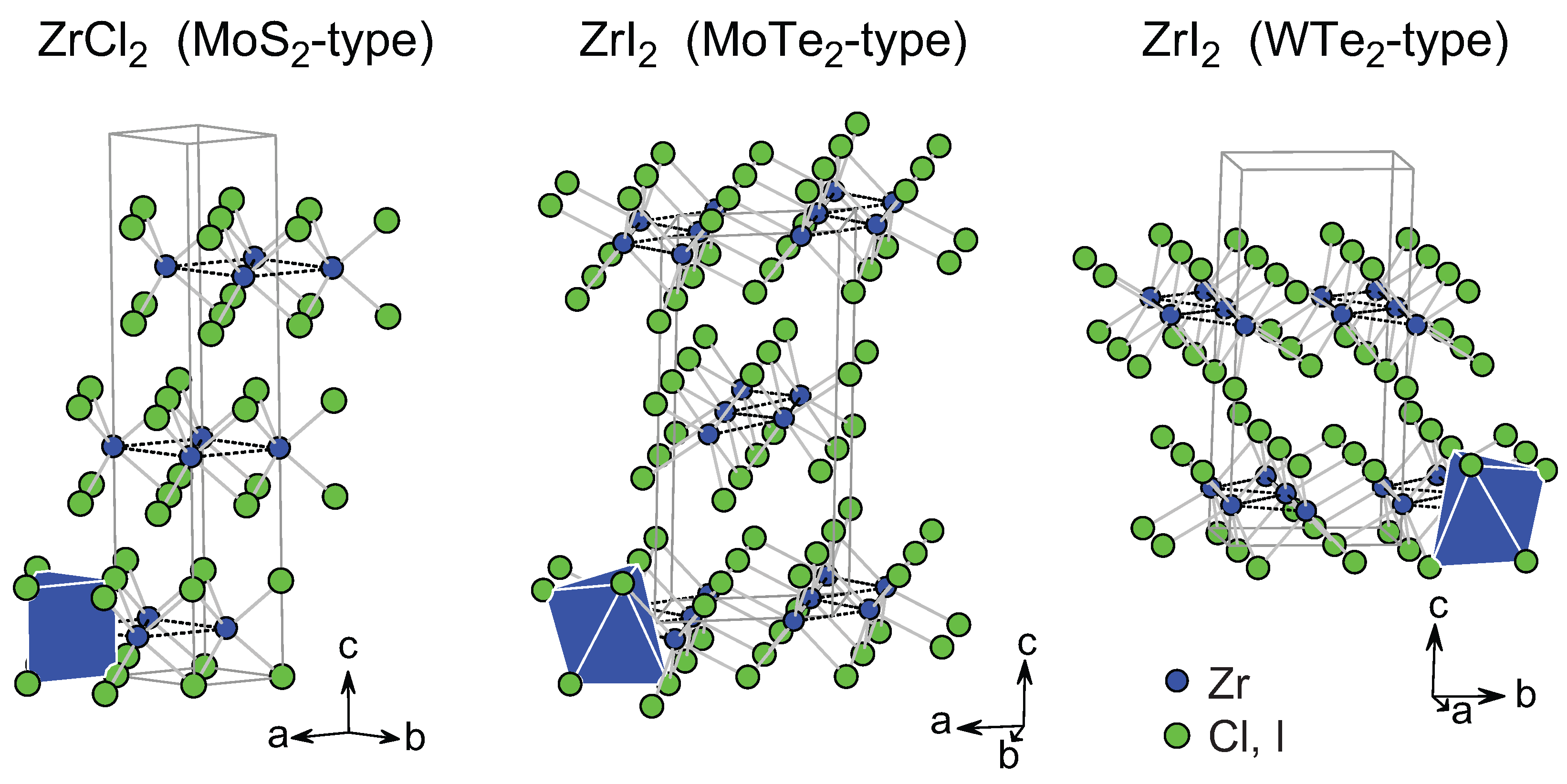
| ZrCl | MoS () | [60] | 3.38 | 6.45 | – | – | – | – |
| ZrI | MoTe () | [61] | 3.18, 3.74, 4.65 | 7.43 | – | – | – | – |
| ZrI | WTe2 () | [62] | 3.19, 3.74, 4.65 | 7.44 |
| Compound | Structure Type | Reference | in Plane | Layer | Magnetic Order | Moments | or , (K) | , (K) |
|---|---|---|---|---|---|---|---|---|
| Distance (Å) | Spacing (Å) | in Layer | ||||||
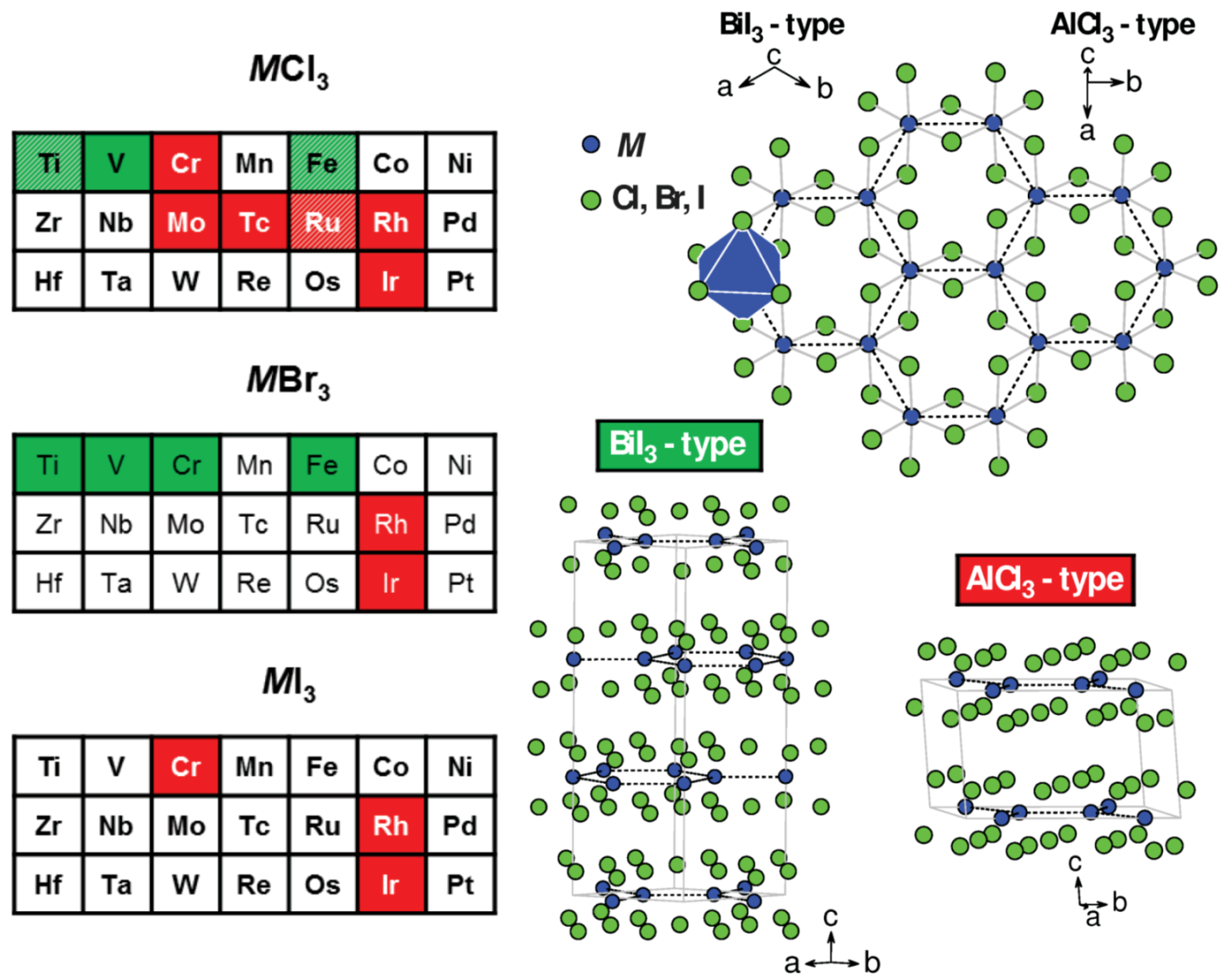
| TiCl * | BiI () | [28] | 3.53 | 5.83 | – | – | – | – |
| TiCl * | TiO () | [70] | 3.55 | 5.86 | ||||
| TiBr * | BiI () | [71] | 3.74 | 6.21 | – | – | – | – |
| VCl | BiI () | [28] | 3.47 | 5.78 | AFM | – | ∼20 | −30 |
| VBr | BiI () | [72] | 3.7 | 6.21 | – | – | – | – |
| CrCl * | AlCl () | [73] | 3.44, 3.44 | 5.80 | AFM | FM | 15.5, 16.8 | 27 |
| CrBr * | BiI () | [74] | 3.64 | 6.11 | FM | FM ⊥ | 37 | 47 |
| CrI * | AlCl () | [16] | 3.96, 3.97 | 6.62 | FM | FM ⊥ | 61 | 70 |
| FeCl | BiI () | [75] | 3.50 | 5.80 | HM | HM | 9–10 | −11.5 |
| FeCl | TiO () | [76] | 3.50 | 5.80 | ||||
| FeCl | FeCl () | [76] | 3.50 | 5.81 | ||||
| FeBr | BiI () | [77] | 3.69 | 6.13 | AFM | – | 15.7 | – |
| MoCl | AlCl () | [78] | 2.76, 3.71 | 5.99 | – | – | – | – |
| TcCl | AlCl () | [79] | 2.86, 3.60 | 5.86 | – | – | – | – |
| RuCl * | AlCl () | [80] | 3.45, 3.45 | 5.69 | AFM | zig-zag canted | 7–8, 13–14 | 37 (), −150(⊥) |
| RuCl * | TiO () | [81] | 3.45 | 5.72 | ||||
| RuCl * | CrCl () | [82] | 3.44, 3.45 | 5.73 | ||||
| RhCl | AlCl () | [83] | 3.44, 3.43 | 5.70 | – | – | – | – |
| RhBr | AlCl () | [84] | 3.62, 3.62 | 6.00 | – | – | – | – |
| RhI | AlCl () | [84] | 3.91, 3.90 | 6.45 | – | – | – | – |
| IrCl | AlCl () | [85] | 3.46, 3.45 | 5.64 | – | – | – | – |
| IrBr | AlCl () | [86] | 3.67, 3.64 | 6.01 | – | – | – | – |
| IrI | AlCl () | [86] | – | 6.54 | – | – | – | – |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGuire, M.A. Crystal and Magnetic Structures in Layered, Transition Metal Dihalides and Trihalides. Crystals 2017, 7, 121. https://doi.org/10.3390/cryst7050121
McGuire MA. Crystal and Magnetic Structures in Layered, Transition Metal Dihalides and Trihalides. Crystals. 2017; 7(5):121. https://doi.org/10.3390/cryst7050121
Chicago/Turabian StyleMcGuire, Michael A. 2017. "Crystal and Magnetic Structures in Layered, Transition Metal Dihalides and Trihalides" Crystals 7, no. 5: 121. https://doi.org/10.3390/cryst7050121






