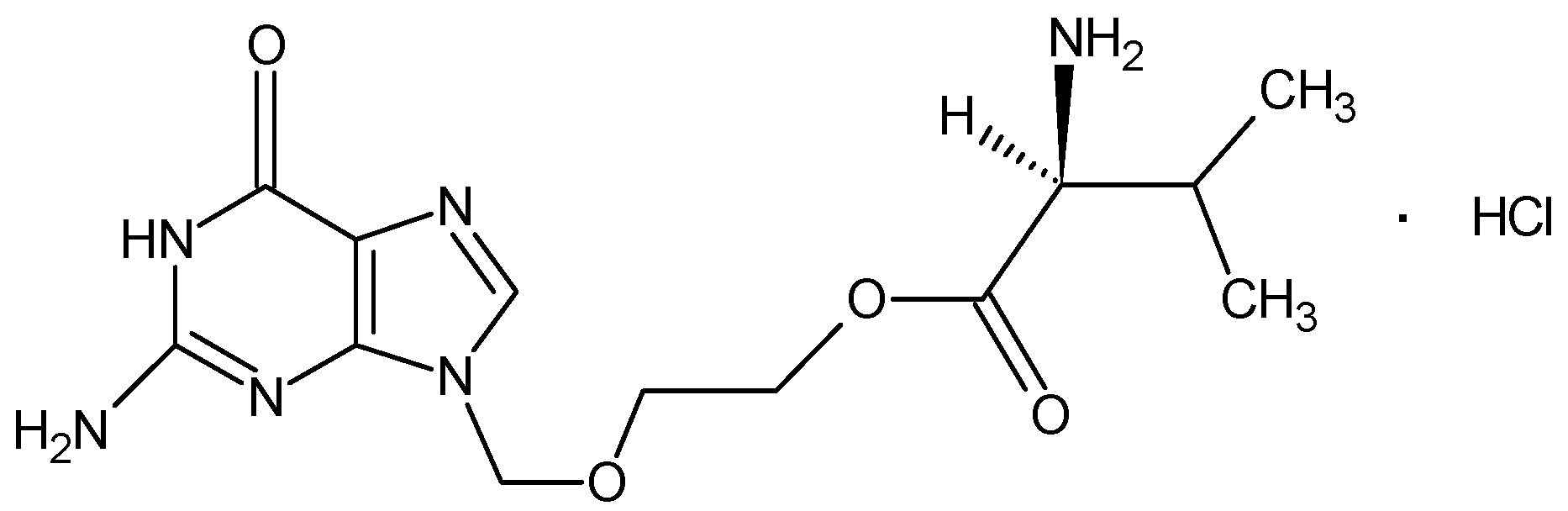
A New Hemihydrate of Valacyclovir Hydrochloride
Abstract
:1. Introduction
2. Results and Discussion
2.1. X-ray Diffraction
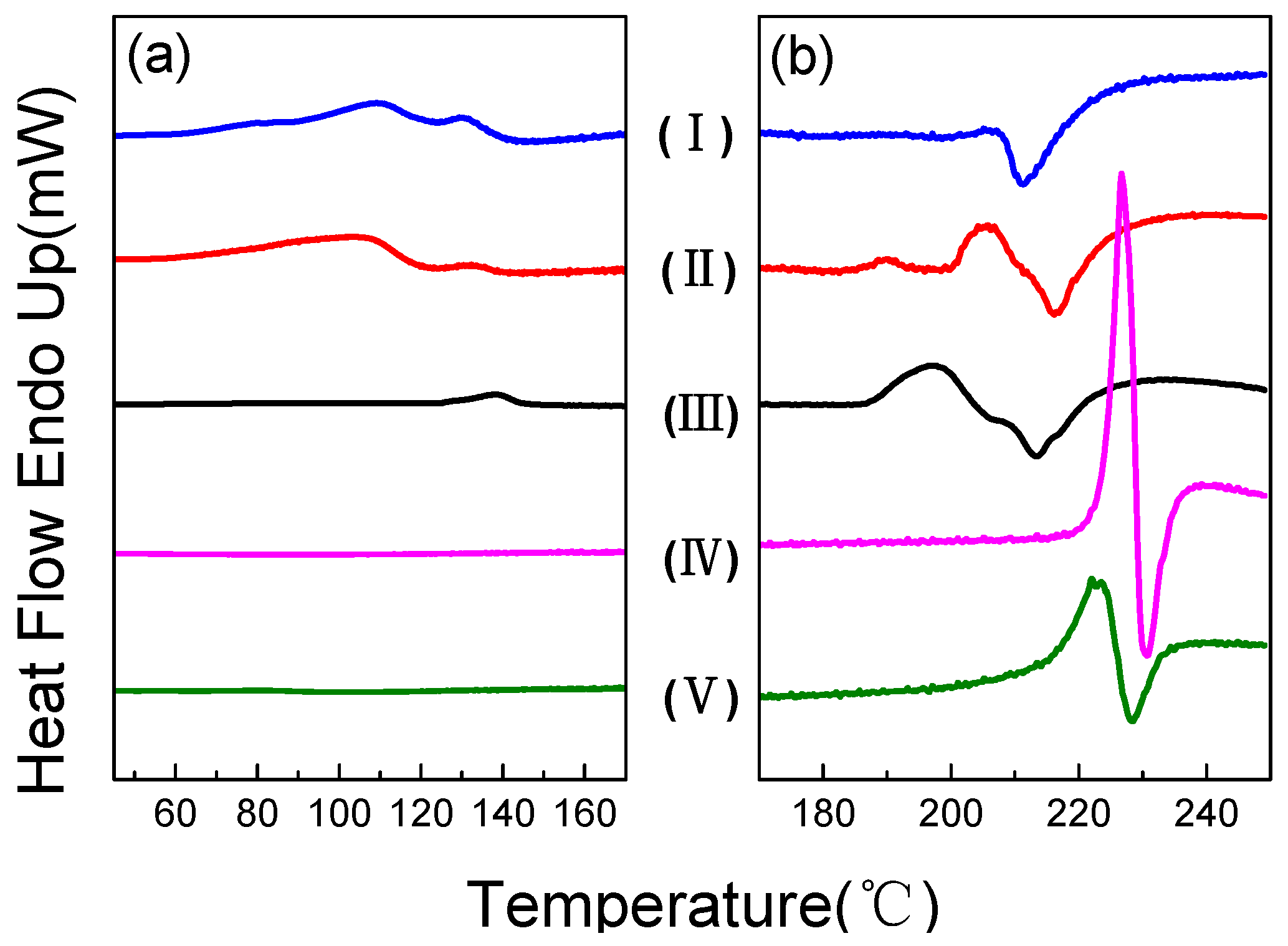
2.2. Differential Scanning Calorimetry
2.3. Thermogravimetric Analysis
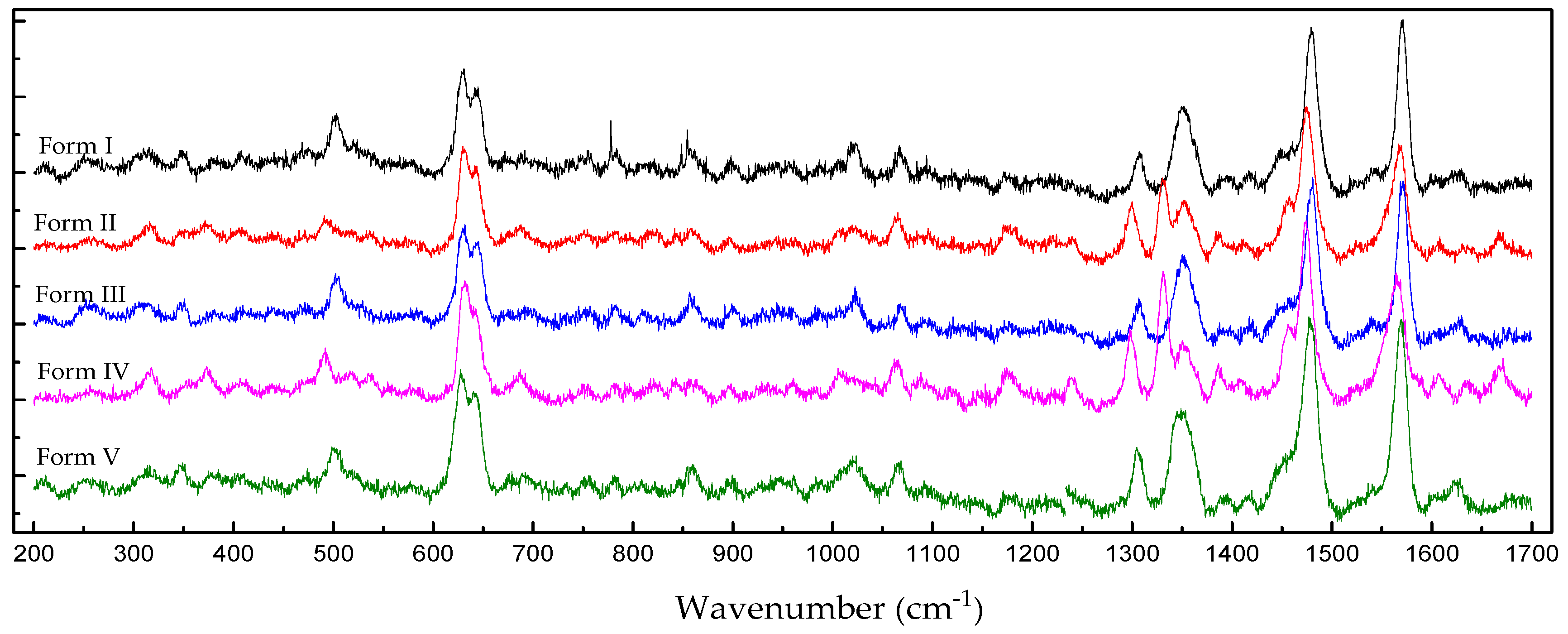
2.4. Raman Spectroscopy
2.5. Apparent Solubility
2.6. Stability Studies
3. Materials and Methods
3.1. Materials
3.2. Preparation of Polymorphs and Hydrates
3.3. Powder X-ray Diffraction Measurements
3.4. Thermal Analysis
3.5. Raman Measurement
3.6. Apparent Solubility Measurement
3.7. Stability Testing
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vega, D.; Petragalli, A.; Fernández, D.; Ellena, J.A. Polymorphism on Leflunomide: Stability and Crystal Structures. J. Pharm. Sci. 2005, 95, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Ushio, T.; Miura, H.; Nakamura, T.; Moribe, K.; Yamamoto, K. Four new polymorphic forms of suplatast tosilate. Int. J. Pharm. 2014, 460, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Sohn, Y.T.; Kim, S.H. Polymorphism and pseudopolymorphism of acyclovir. Arch. Pharm. Res. 2008, 31, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Brittain, H.G. Polymorphism in Pharmaceutical Solids; Drugs and the Pharmaceutical Science: New York, NY, USA, 1999; pp. 76–81. ISBN 9781420073218. [Google Scholar]
- Haleblian, J.K. Characterization of habits and crystalline modification of solids and their pharmaceutical applications. J. Pharm. Sci. 1975, 64, 1269–1288. [Google Scholar] [CrossRef] [PubMed]
- Lutker, K.M.; Quinones, R.; Xu, J.; Ramamoorthy, A.; Matzger, A.J. Polymorphs and hydrates of acyclovir. J. Pharm. Sci. 2010, 100, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Haleblian, J.; McCrone, W. Pharmaceutical Applications of Polymorphism. J. Pharm. Sci. 1969, 58, 911–929. [Google Scholar] [CrossRef] [PubMed]
- Granero, G.E.; Amidon, G.L. Stability of valacyclovir: Implications for its oral bioavailability. Int. J. Pharm. 2006, 317, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Shinkai, I.; Ohta, Y. Valacyclovir. Bioorgan. Med. Chem. 1996, 4, 1–2. [Google Scholar] [CrossRef]
- Beutner, K.R. Valacyclovir: A review of its antiviral activity, pharmacokinetic properties, and clinical efficacy. Antivir. Res. 1995, 28, 281–290. [Google Scholar] [CrossRef]
- Yadav, M.; Upadhyay, V.; Singhal, P.; Goswami, S.; Shrivastav, P.S. Stability evaluation and sensitive determination of antiviral drug, valacyclovir and its metabolite acyclovir in human plasma by a rapid liquid chromatography–tandem mass spectrometry method. J. Chromatogr. B 2009, 877, 680–688. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. Milestones in the discovery of antiviral agents: Nucleosides and nucleotides. Acta Pharm. Sin. B 2012, 2, 535–548. [Google Scholar] [CrossRef]
- Jadhav, A.S.; Pathare, D.B.; Shingare, M.S. Development and validation of enantioselective high performance liquid chromatographic method for Valacyclovir, an antiviral drug in drug substance. J. Pharm. Biomed. Anal. 2007, 43, 1568–1572. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.H.; Partin, J.M.; Varlashkin, P.G. Guanine Derivative. U.S. Patent 6,107,302, 22 August 2000. [Google Scholar]
- Wizel, S.; Aronhime, J.; Niddam-Hildesheim, V.; Dolitzky, B.Z. Crystalline Forms of Valacyclovir Hydrochloride. U.S. Patent 6,849,736 B2, 1 February 2005. [Google Scholar]
- Shepherd, M.; Mckay, B. Valacyclovir Polymorphs and a Process for the Preparation Thereof. U.S. Patent 0,021,444 A1, 25 January 2007. [Google Scholar]
- Shefter, E.; Higuchi, T. Dissolution behavior of crystalline solvated and nonsolvated forms of some pharmaceuticals. J. Pharm. Sci. 1963, 52, 781–791. [Google Scholar] [CrossRef] [PubMed]

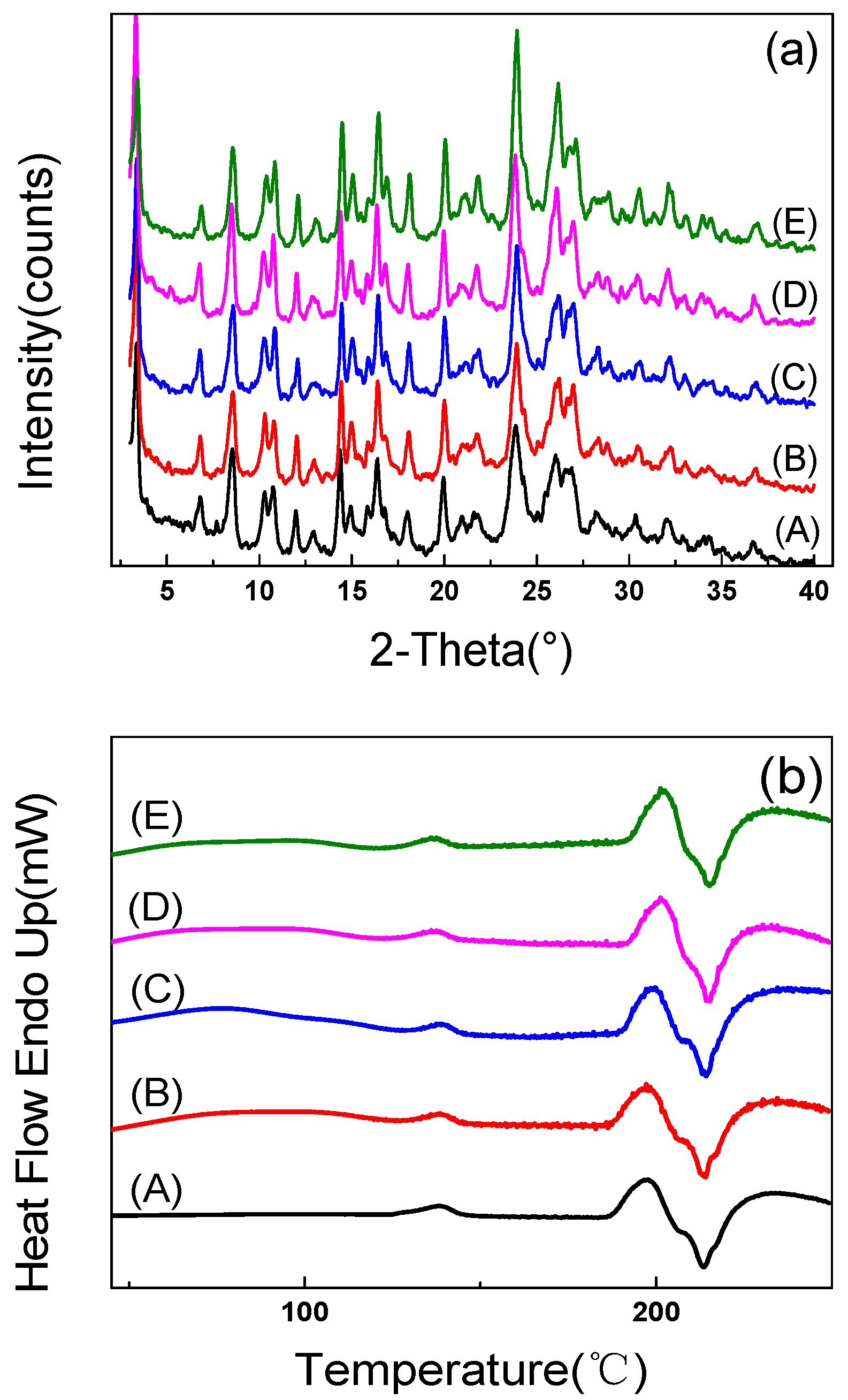
| Form I | Form II | Form III | Form IV | Form V |
|---|---|---|---|---|
| 2θ(°) Intensity (%) | 2θ(°) Intensity (%) | 2θ(°) Intensity (%) | 2θ(°) Intensity (%) | 2θ(°) Intensity (%) |
| 3.66 100 | 3.56 100 | 3.46 100 | 3.66 63.1 | 3.50 33.6 |
| 8.58 2.6 | 8.58 5.2 | 6.94 93.1 | 8.66 41.1 | 6.58 58.7 |
| 9.48 6.0 | 9.38 5.2 | 7.88 13.2 | 10.62 13.8 | 9.18 100 |
| 10.60 9.8 | 10.52 8.6 | 8.46 10.9 | 10.92 48.5 | 11.32 18.8 |
| 10.86 24.4 | 10.78 15.8 | 9.30 13.3 | 12.18 18.3 | 15.34 13.3 |
| 13.36 7.8 | 12.08 0.9 | 10.46 9.5 | 13.32 12.1 | 15.62 16.9 |
| 16.44 3.9 | 13.26 6.1 | 11.64 8.4 | 14.56 51.1 | 16.16 10.4 |
| 20.20 2.0 | 14.42 2.5 | 13.02 11.5 | 15.20 14.3 | 17.02 16.1 |
| 21.42 2.9 | 16.36 5.3 | 14.44 12.9 | 16.48 52.2 | 19.14 18.8 |
| 21.80 7.2 | 20.04 2.5 | 15.34 9.2 | 16.96 22.5 | 19.66 9.0 |
| 24.00 3.2 | 21.34 2.2 | 16.25 11 | 18.16 33.8 | 21.26 19.6 |
| 26.76 2.1 | 23.92 9.3 | 20.78 11.4 | 20.10 34.1 | 22.86 15.0 |
| 27.28 5.2 | 25.94 1.8 | 23.47 6.6 | 20.62 7.1 | 24.16 10.8 |
| 26.82 2.4 | 23.90 9.9 | 21.28 15.3 | 26.30 8.4 | |
| 27.22 4.1 | 24.54 19.5 | 21.88 25.7 | 27.34 17.8 | |
| 26.18 11.0 | 22.72 8.8 | 27.86 15.3 | ||
| 27.16 19.6 | 24.02 100 | 28.76 3.4 | ||
| 27.80 11.6 | 24.46 15.9 | |||
| 25.13 6.8 | ||||
| 25.94 21.1 | ||||
| 26.26 60.8 | ||||
| 26.92 10.6 | ||||
| 27.26 8.4 | ||||
| 28.10 7.9 | ||||
| 28.38 6.5 | ||||
| 29.00 16.2 | ||||
| 29.70 8.8 | ||||
| 30.64 18.1 |
| Items | Form I | Form II | Form III | Form IV | Form V |
|---|---|---|---|---|---|
| Melting point (°C) | 205 | 206 | 209 | 227 | 223 |
| Transition 1 (°C) | 53–139 | 60–140 | 124–141 | / | / |
| Transition 2 (°C) | / | 183–196 | 186–206 | / | / |
| Raman Shift (cm−1) | Form I | Form II | Form III | Form IV | Form V |
|---|---|---|---|---|---|
| 400–600 | Three peaks at 409.4, 472.6 and 501.8 | Four peaks at 409.9, 470.4, 493.1 and 536.4 | Three peaks at 471.5, 504.0 and 527.7 | Four peaks at 409.4, 439.9, 490.3 and 538.1 | Five peaks at 409.4, 438.1, 470.9, 503.5 and 578.6 |
| 600–800 | Four peaks at 630.7, 644.8, 754.3 and 777.3 | Five peaks at 630.2, 642.1, 687.9, 751.2 and 785.2 | Five peaks at 629.6, 642.6, 691.1, 752.7 and 782.5 | Five peaks at 634.5, 643.2, 685.7, 754.8 and 783.1 | Five peaks at 626.9, 940.9, 692.2, 751.7 and 780.4 |
| 1000–1200 | Five peaks at 1023.0, 1067.8, 1097.1 and 1171.3 | Three peaks at 1019.4, 1065.3 and 1177.8 | Four peaks at 1021.9, 1067.8, 1096.6 and 1176.3 | Four peaks at 1010.1, 1064.2, 1089.4 and 1176.3 | Four peaks at 1019.9, 1067.3, 1091.5 and 1172.3 |
| 1200–1400 | Three peaks at 1308.5, 1351.3 and 1392.3 | Four peaks at 1299.4, 1331.7, 1352.3 and 1387.3 | Two peaks at 1307.1 and 1353.3 | Four peaks at 1298.4, 1331.7, 1352.8 and 1386.8 | Three peaks at 1306.1, 1348.8 and 1393.3 |
| 1400–1700 | Five peaks at 1416.7, 1450.0, 1479.7, 1571.0 and 1629.3 | Six peaks at 1411.3, 1454.5, 1473.8, 1569.5, 1606.6 and 1666.5 | Five peaks at 1420.7, 1452.5, 1480.7, 1570.6 and 1629.3 | Six peaks at 1457.4, 1473.8, 1565.1, 1607.0, 1633.6 and 1667.0 | Five peaks at 1416.2, 1451.0, 1477.7, 1568.5 and 1625.9 |
| Crystals | Apparent Solubility (mg/mL) | Crystal form after Solubility Determination |
|---|---|---|
| Form I | 145 | Form I |
| Form II | 143 | Form II |
| Form III | 118 | Form III |
| Form IV | 147 | Form I |
| Form V | 161 | Form V |
| Number | Form I | Form II | Form III | Form IV | Form V |
|---|---|---|---|---|---|
| Crystal form | Sesquihydrate | Monohydrate | Hemihydrate | Anhydrous one | Anhydrous two |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Zheng, M.; Zhou, M.; Chen, T.; Wang, N.; Zhang, Z.; Zhang, G. A New Hemihydrate of Valacyclovir Hydrochloride. Crystals 2017, 7, 140. https://doi.org/10.3390/cryst7050140
Zhang S, Zheng M, Zhou M, Chen T, Wang N, Zhang Z, Zhang G. A New Hemihydrate of Valacyclovir Hydrochloride. Crystals. 2017; 7(5):140. https://doi.org/10.3390/cryst7050140
Chicago/Turabian StyleZhang, Shuai, Meiqi Zheng, Mengqing Zhou, Tian Chen, Naixing Wang, Zhaoxia Zhang, and Guoqing Zhang. 2017. "A New Hemihydrate of Valacyclovir Hydrochloride" Crystals 7, no. 5: 140. https://doi.org/10.3390/cryst7050140





