Synthesis and Thermoelectric Properties of Copper Sulfides via Solution Phase Methods and Spark Plasma Sintering
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. Powder Synthesis and Characterization
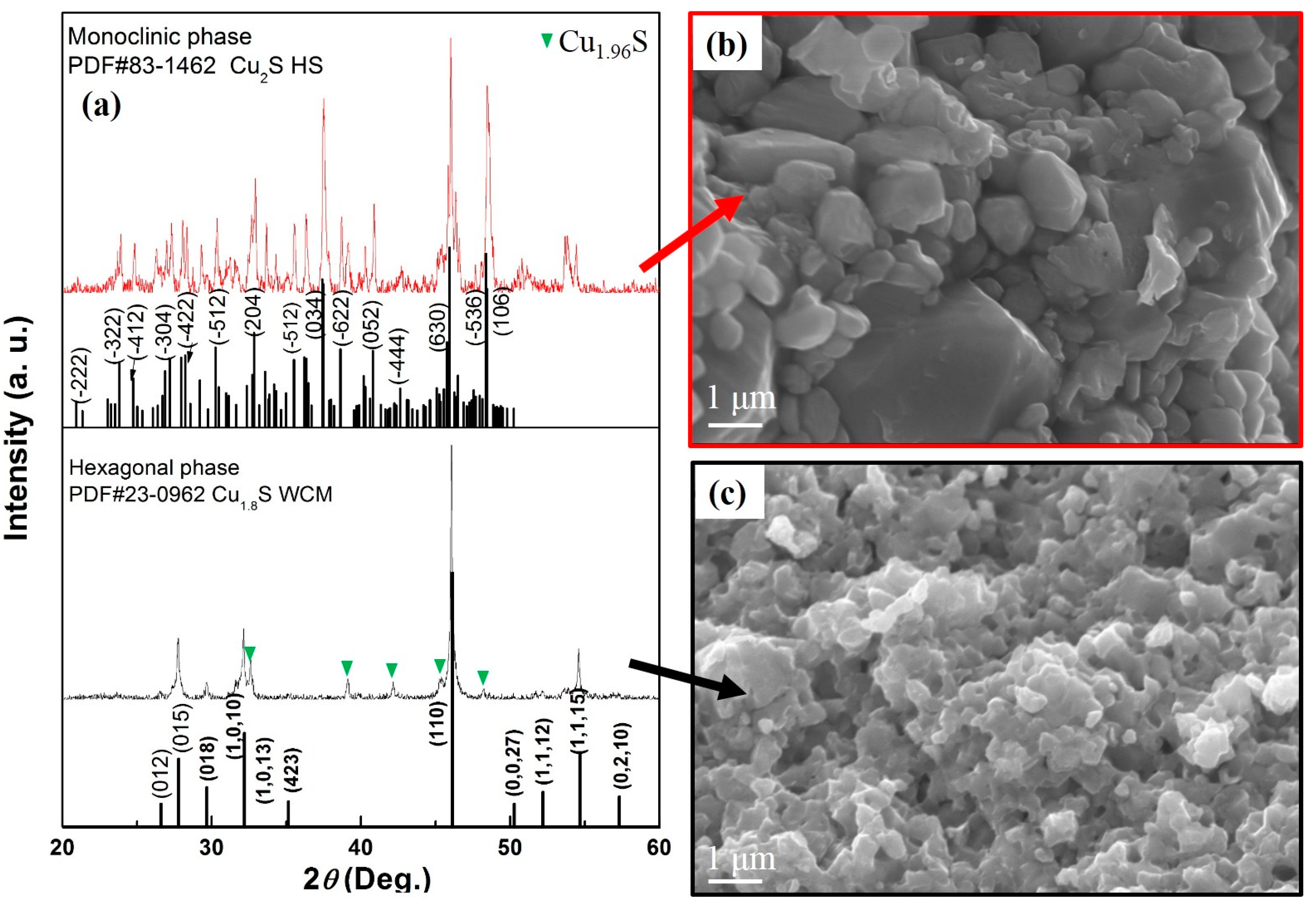
3.1.1. XRD Analysis
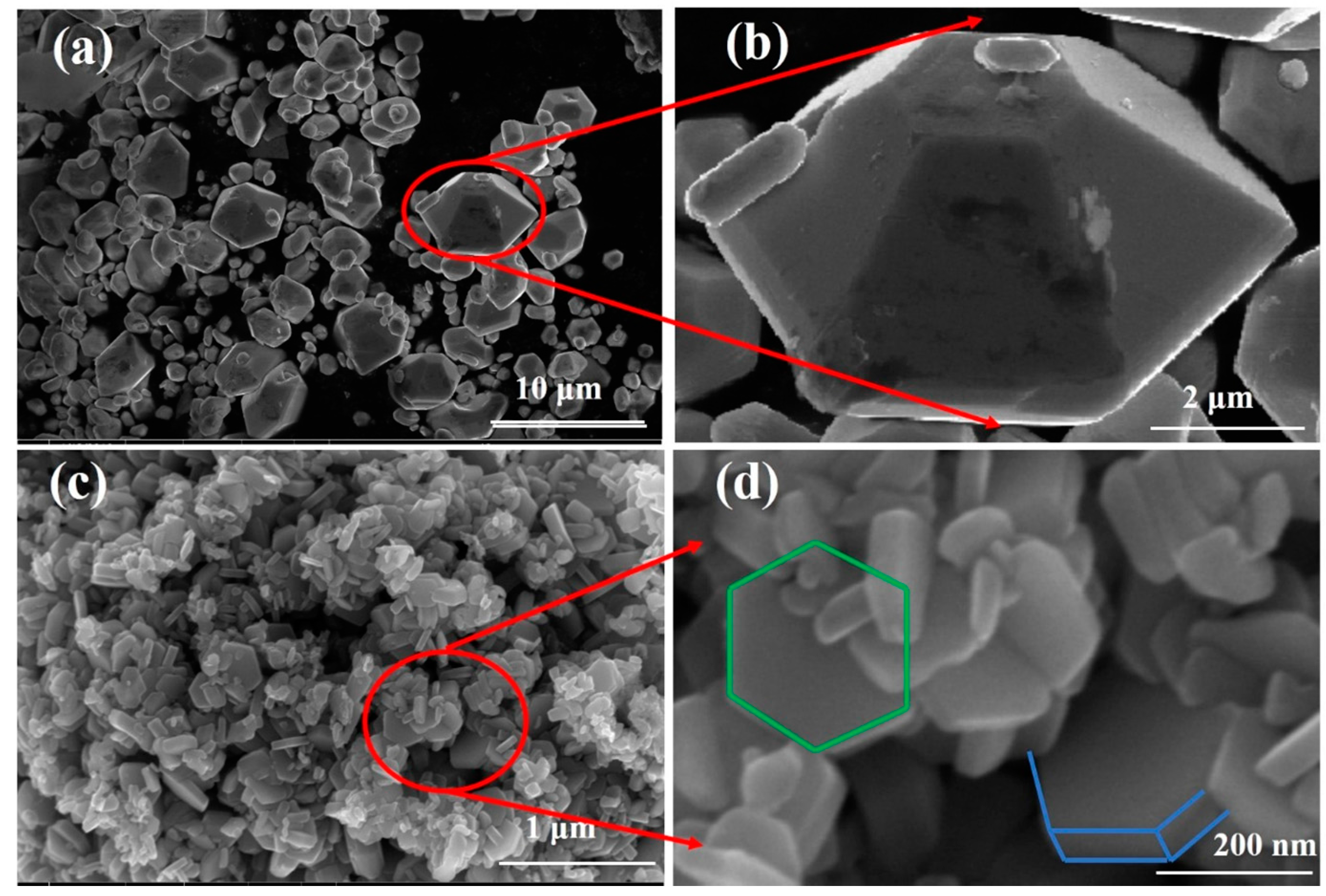
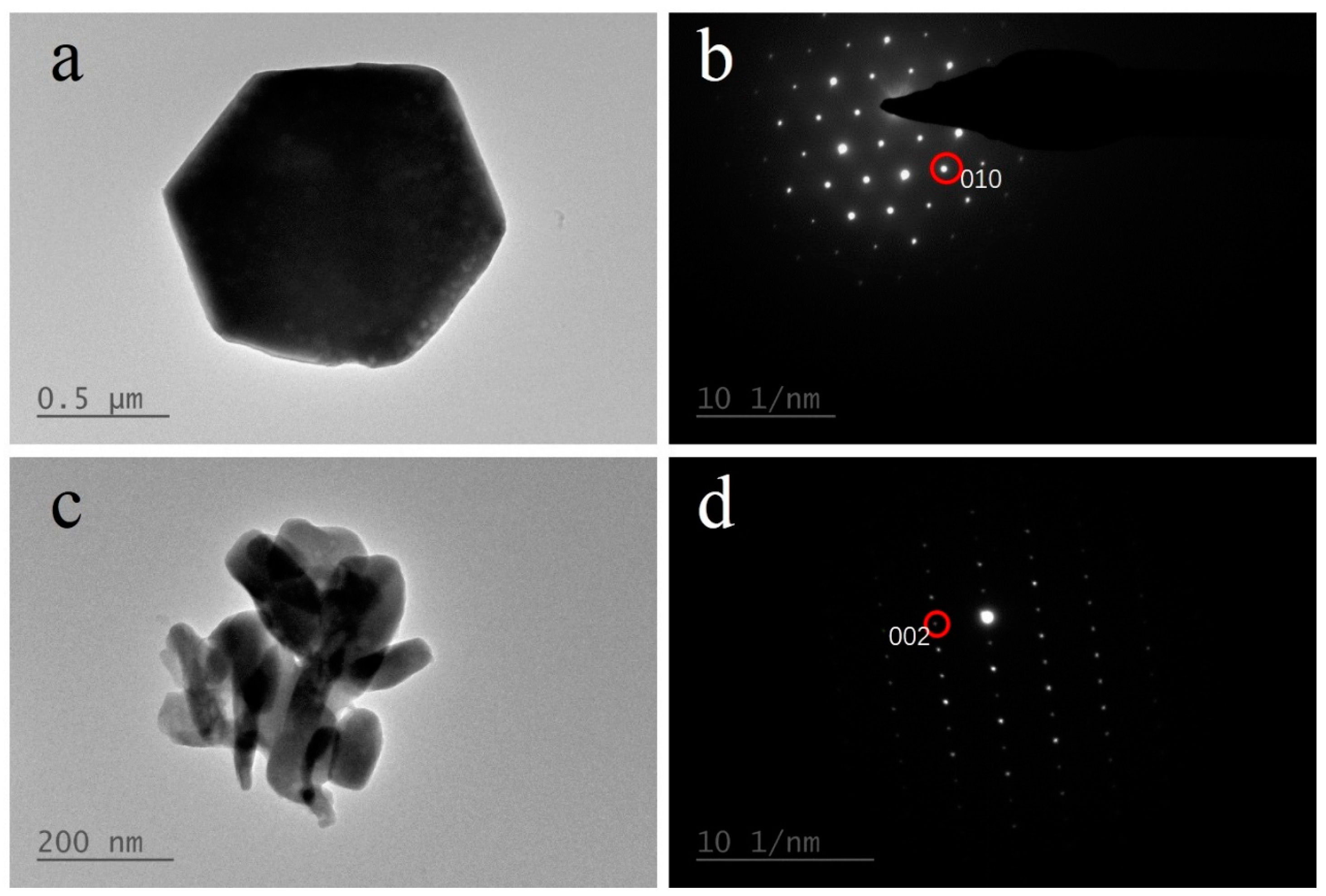
3.1.2. FESEM and TEM Analysis
3.1.3. Synthesis Mechanism
3.2. Bulk Characterization
3.2.1. XRD and FESEM Analysis
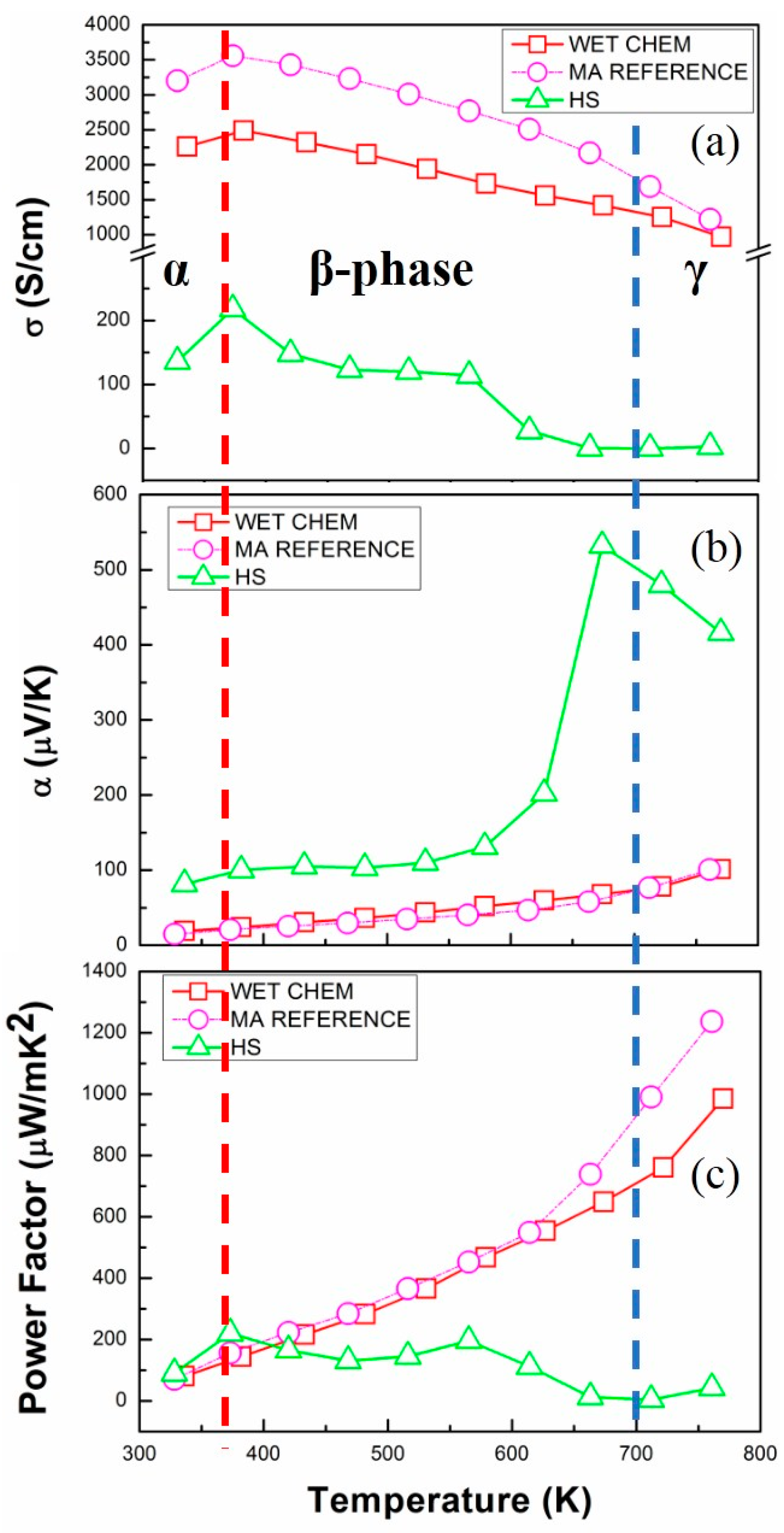
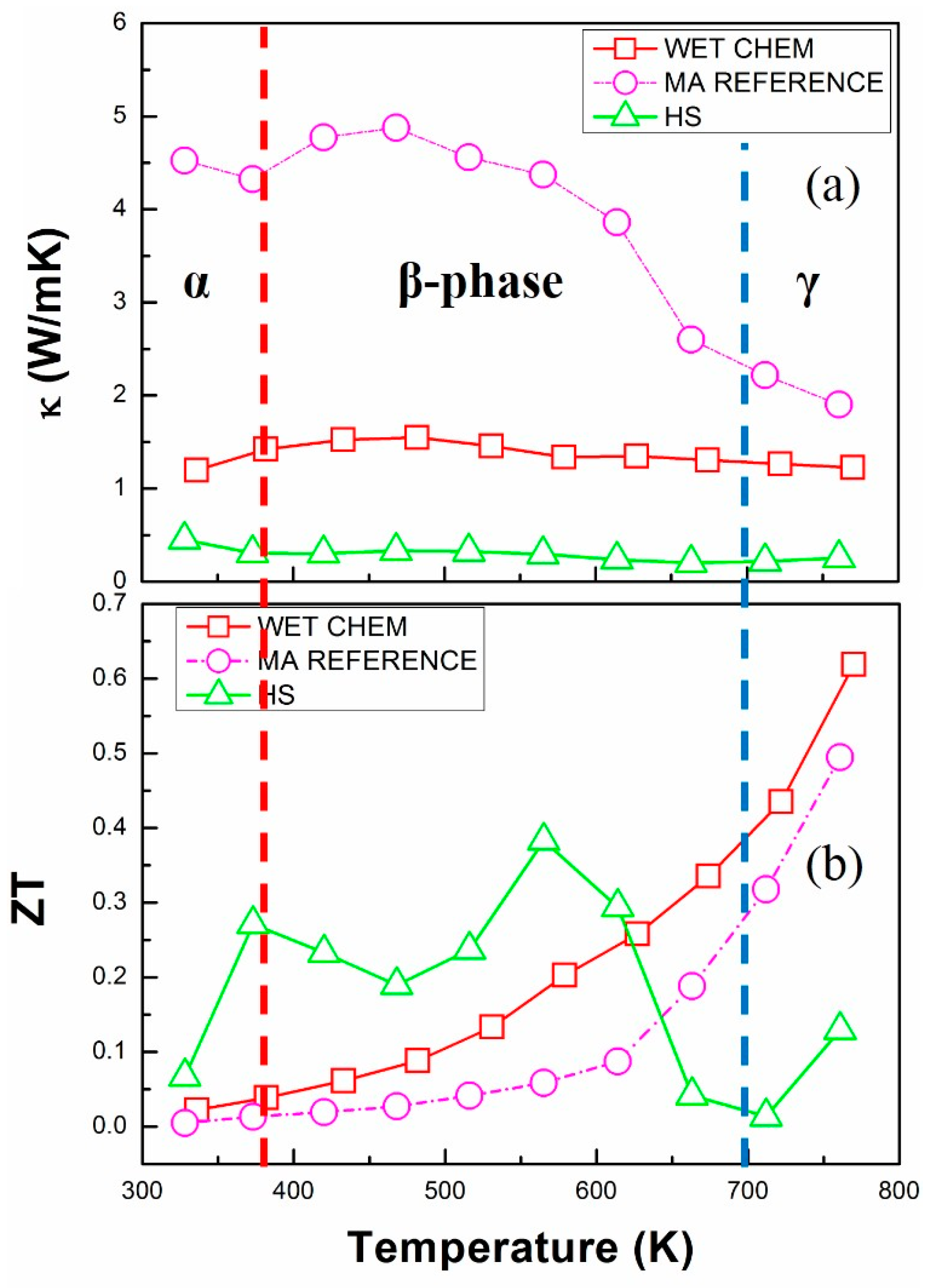
3.2.2. Thermoelectric Transport Properties
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sales, B.C. Smaller is cooler. Science 2001, 295, 1248–1249. [Google Scholar] [CrossRef] [PubMed]
- Sootsman, J.R.; Chung, D.Y.; Kanatzidis, M.G. New and old concepts in thermoelectric materials. Angew. Chem. Int. Ed. 2009, 48, 8616–8639. [Google Scholar] [CrossRef] [PubMed]
- Snyder, G.J.; Eric, S.T. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, F.J. Thermoelectric Cooling and Power Generation. Science 1999, 285, 703–706. [Google Scholar] [CrossRef]
- Zhao, X.B.; Ji, X.H.; Zhang, Y.H.; Zhu, T.J.; Tu, J.P.; Zhang, X.B. Bismuth telluride nanotubes and the effects on the thermoelectric properties of nanotube-containing nanocomposites. Appl. Phys. Lett. 2005, 86, 062111. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Wang, H.; Kraemer, S.; Shi, Y.F.; Zhang, F.; Snedaker, M.; Ding, K.; Moskovits, M.; Snyder, G.J.; Stucky, G.D. Surfactant-Free Synthesis of Bi2Te3-Te Micro-Nano Heterostructure with Enhanced Thermoelectric Figure of Merit. ACS Nano 2011, 5, 3158–3165. [Google Scholar] [CrossRef] [PubMed]
- Heremans, J.P.; Jovovic, V.; Toberer, E.S.; Saramat, A.; Kurosaki, K.; Charoenphakdee, A.; Yamanaka, S.; Snyder, G.J. Enhancement of thermoelectric efficiency in PbTe by distortion of the electronic density of states. Science 2008, 321, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Li, J.F.; Zhao, W.Y.; Tan, Q.; Wei, T.R.; Wu, C.F.; Xing, Z.B. PbTe-based thermoelectric nanocomposites with reduced thermal conductivity by SiC nanodispersion. Appl. Phys. Lett. 2014, 104, 113905. [Google Scholar] [CrossRef]
- Gao, M.R.; Xu, Y.F.; Jiang, J.; Yu, S.H. Nanostructured metal chalcogenides: Synthesis, modification, and applications in energy conversion and storage devices. Chem. Soc. Rev. 2013, 42, 2986–3017. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.H.; Nolas, G.S. Controllable Synthesis of Bismuth Chalcogenide Core–shell Nanorods. Cryst. Growth Des. 2014, 14, 533–536. [Google Scholar] [CrossRef]
- Liu, H.L.; Shi, X.; Xu, F.F.; Zhang, L.L.; Zhang, W.Q.; Chen, L.D.; Uher, C.; Day, T.; Snyder, G.J. Copper ion liquid-like thermoelectrics. Nat. Mater. 2012, 11, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Qin, X.Y.; Liu, Y.F.; Song, C.J.; Wang, L.; Zhang, J.; Xin, H.X.; Guo, G.L.; Zou, T.H.; Sun, G.L.; et al. Chemical synthesis of nanostructured Cu2Se with high thermoelectric performance. RSC Adv. 2014, 4, 8638–8644. [Google Scholar] [CrossRef]
- Shi, X.; Xi, L.; Fan, J.; Zhang, W.; Chen, L. Cu-Se bond network and thermoelectric compounds with complex diamondlike structure. Chem. Mater. 2010, 22, 6029–6031. [Google Scholar] [CrossRef]
- Ge, Z.H.; Zhang, B.P.; Chen, Y.X.; Yu, Z.X.; Liu, Y.; Li, J.F. Synthesis and transport property of Cu1.8S as a promising thermoelectric compound. Chem. Commun. 2011, 47, 12697–12699. [Google Scholar] [CrossRef] [PubMed]
- Dennler, G.; Chmielowski, R.; Jacob, S.; Capet, F.; Roussel, P.; Zastrow, S.; Nielsch, K.; Opahle, I.; Madsen, G.K.H. Are binary copper sulfides/selenides really new and promising thermoelectric materials? Adv. Energy Mater. 2014, 4, 1301581. [Google Scholar] [CrossRef]
- Abdullaev, G.B.; Aliyarova, Z.A.; Zamanova, E.H.; Asadov, G.A. Investigation of the electric properties of Cu2S single crystals. Phys. Status Solidi 1968, 26, 65–68. [Google Scholar] [CrossRef]
- Lu, X.; Morelli, D.T.; Xia, Y.; Zhou, F.; Ozolins, V.; Chi, H.; Zhou, X.Y.; Uher, C. High Performance Thermoelectricity in Earth-Abundant Compounds Based on Natural Mineral Tetrahedrites. Adv. Energy Mater. 2013, 3, 342–348. [Google Scholar] [CrossRef]
- Miller, T.A.; Wittenberg, J.S.; Wen, H.; Connor, S.; Cui, Y.; Lindenberg, A.M. The mechanism of ultrafast structural switching in superionic copper (I) sulphide nanocrystals. Nat. Commun. 2013, 4, 1369. [Google Scholar] [CrossRef] [PubMed]
- El Akkad, F.; Mansour, B.; Hendeya, T. Electrical and thermoelectric properties of Cu2Se and Cu2S. Mater. Res. Bull. 1981, 16, 535–539. [Google Scholar] [CrossRef]
- Rothwarf, A. The CdS/Cu2S solar cell: Basic operation and anomalous effects. Sol. Cells 1980, 2, 115–140. [Google Scholar] [CrossRef]
- Hall, R.B.; Meakin, J.D. The design and fabrication of high efficiency thin film CdS/Cu2S solar cells. Thin Solid Films 1979, 63, 203–211. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S.; Kim, Y.H.; Ku, B.C.; Jeong, Y. Enhancement of electrical conductivity of carbon nanotube fibers by copper sulfide plating. Fibers Polym. 2015, 16, 769–773. [Google Scholar] [CrossRef]
- Fernandez, A.M.; Nair, M.T.S.; Nair, P.K. Chemically deposited ZnS-NiS-CuS optical filters with wide range solar control characteristics. Mater. Manuf. Process 1993, 8, 535–548. [Google Scholar] [CrossRef]
- Lai, C.H.; Huang, K.W.; Cheng, J.H.; Lee, C.Y.; Hwang, B.J.; Chen, L.J. Direct growth of high-rate capability and high capacity copper sulfide nanowire array cathodes for lithium-ion batteries. J. Mater. Chem. 2010, 20, 6638–6645. [Google Scholar] [CrossRef]
- Wu, S.X.; Jiang, J.; Liang, Y.G.; Yang, P.; Niu, Y.; Chen, Y.D.; Xia, J.F.; Wang, C. Chemical Precipitation Synthesis and Thermoelectric Properties of Copper Sulfide. J. Electron. Mater. 2017, 46, 2432–2437. [Google Scholar] [CrossRef]
- Sigman, M.B.; Ghezelbash, A.; Hanrath, T.; Saunders, A.E.; Lee, F.; Korgel, B.A. Solventless synthesis of monodisperse Cu2S nanorods, nanodisks, and nanoplatelets. J. Am. Chem. Soc. 2003, 125, 16050–16057. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.B.; Peng, Q.; Zhang, B.; Li, Y.D. Controllable synthesis of Cu2S nanocrystals and their assembly into a superlattice. J. Am. Chem. Soc. 2008, 130, 10482–10483. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.P.; Xu, D.; Liang, J.B.; Shen, J.M.; Zhang, S.Y.; Qian, Y.T. Growth of Cu2S ultrathin nanowires in a binary surfactant solvent. J. Phys. Chem. 2005, 109, 10699–10704. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.Y.; Minakshi, M.; Ralph, D.E.; Jiang, Z.T.; Xie, Z.H.; Guo, H. Hydrothermal synthesis of cubic α-fe2O3, microparticles using glycine: Surface characterization, reaction mechanism and electrochemical activity. J. Alloys Compd. 2011, 509, 9821–9825. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Zhang, B.P.; Ge, Z.H.; Zhu, L.F.; Li, Y. Preparation by solvothermal synthesis, growth mechanism, and photocatalytic performance of CuS nanopowders. Eur. J. Inorg. Chem. 2014, 2014, 2368–2375. [Google Scholar] [CrossRef]
- Barmi, M.J.; Minakshi, M. Tuning the redox properties of the nanostructured CoMoO4 electrode: Effects of surfactant content and synthesis temperature. ChemPlusChem 2016, 81, 964–977. [Google Scholar] [CrossRef]
- Penki, T.R.; Shivakumara, S.; Minakshi, M.; Munichandraiah, N. Porous Flower-like α-Fe2O3 Nanostructure: A High Performance Anode Material for Lithium-ion Batteries. Electrochim. Acta 2015, 167, 330–339. [Google Scholar] [CrossRef]
- He, Y.; Day, T.; Zhang, T.S.; Liu, H.L.; Shi, X.; Chen, L.D.; Snyder, G.J. High Thermoelectric Performance in Non-Toxic Earth-Abundant Copper Sulfide. Adv. Mater. 2014, 26, 3974–3978. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.H.; Liu, X.Y.; Feng, D.; Lin, J.Y.; He, J.Q. High-Performance Thermoelectricity in Nanostructured Earth-Abundant Copper Sulfides Bulk Materials. Adv. Energy Mater. 2016, 6, 1600607. [Google Scholar] [CrossRef]
- Li, B.; Huang, L.; Zhao, G.Y.; Wei, Z.M.; Dong, H.L.; Hu, W.P.; Wang, L.W.; Li, J.B. Large-Size 2D β-Cu2S Nanosheets with Giant Phase Transition Temperature Lowering (120 K) Synthesized by a Novel Method of Super-Cooling Chemical-Vapor-Deposition. Adv. Mater. 2016, 28, 8271–8276. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Kawai, S. Electrical conduction and phase transition of copper sulfides. Jpn. J. Appl. Phys. 1973, 12, 1130. [Google Scholar] [CrossRef]
- Ge, Z.H.; Zhao, L.D.; Wu, D.; Zhang, B.P.; Li, J.F.; He, J.Q. Low cost, abundant binary sulfides as promising thermoelectric. Mater. Today 2016, 19, 227–239. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.-Q.; Ge, Z.-H.; Feng, J. Synthesis and Thermoelectric Properties of Copper Sulfides via Solution Phase Methods and Spark Plasma Sintering. Crystals 2017, 7, 141. https://doi.org/10.3390/cryst7050141
Tang Y-Q, Ge Z-H, Feng J. Synthesis and Thermoelectric Properties of Copper Sulfides via Solution Phase Methods and Spark Plasma Sintering. Crystals. 2017; 7(5):141. https://doi.org/10.3390/cryst7050141
Chicago/Turabian StyleTang, Yun-Qiao, Zhen-Hua Ge, and Jing Feng. 2017. "Synthesis and Thermoelectric Properties of Copper Sulfides via Solution Phase Methods and Spark Plasma Sintering" Crystals 7, no. 5: 141. https://doi.org/10.3390/cryst7050141





