Polymorphism and Structural Distortions of Mixed-Metal Oxide Photocatalysts Constructed with α-U3O8 Types of Layers
Abstract
:1. Introduction
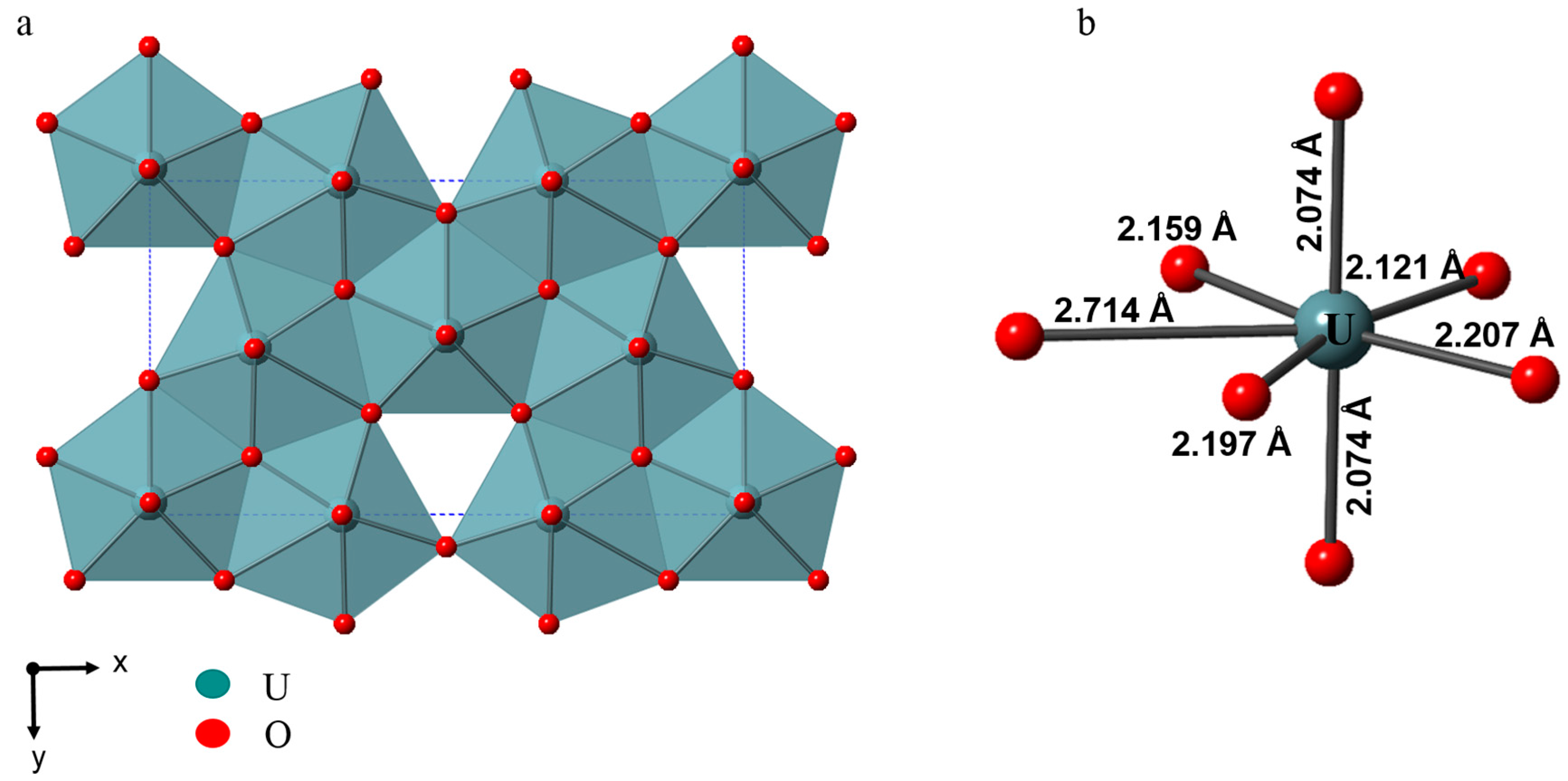
2. α-U3O8 Type Structural Layers
2.1. Single Layers
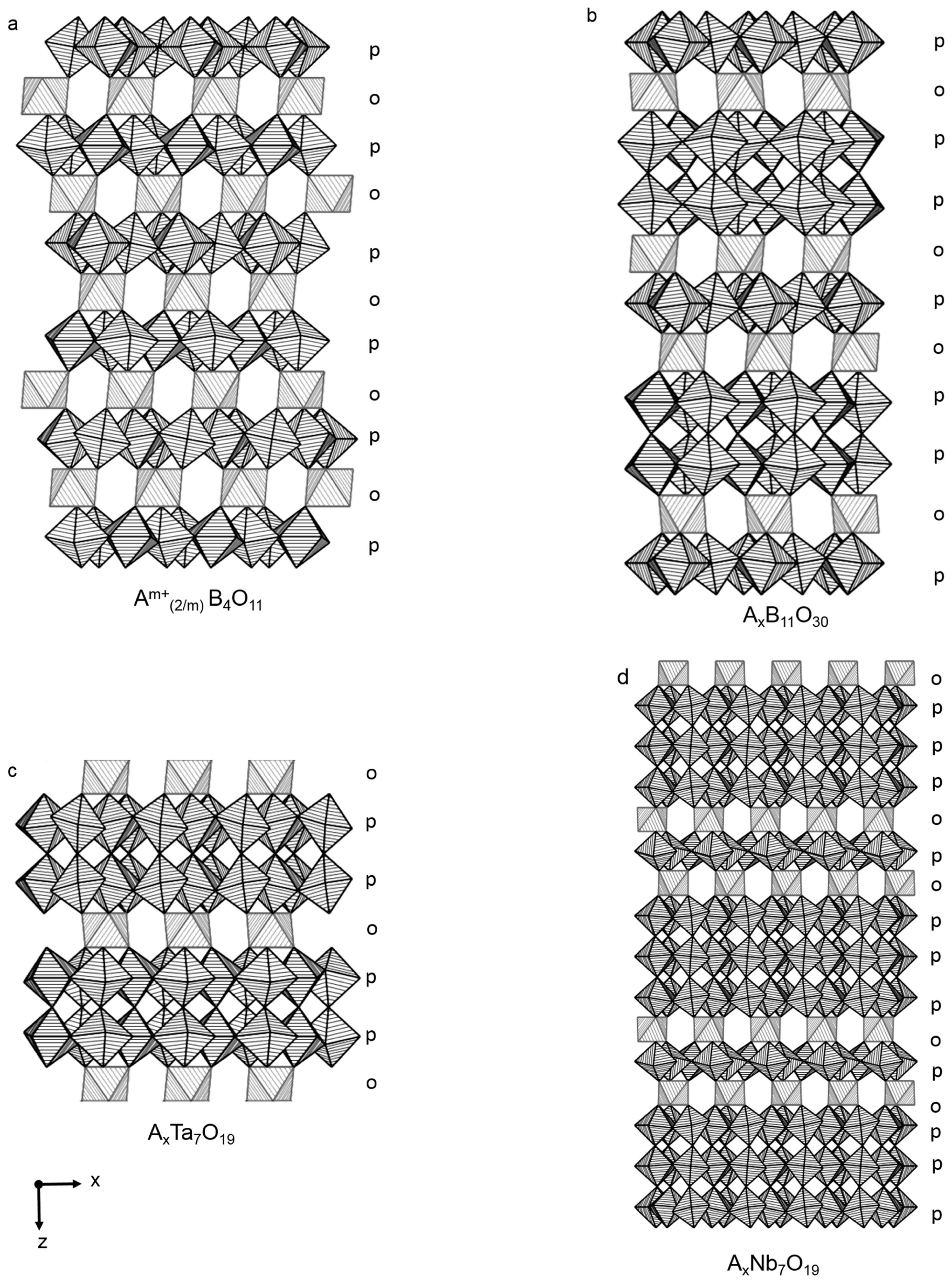
2.2. Alternating Single and Double Layers
2.3. Double Layers
2.4. Alternating Single and Triple Layers
3. Structural Distortion and Polymorphism
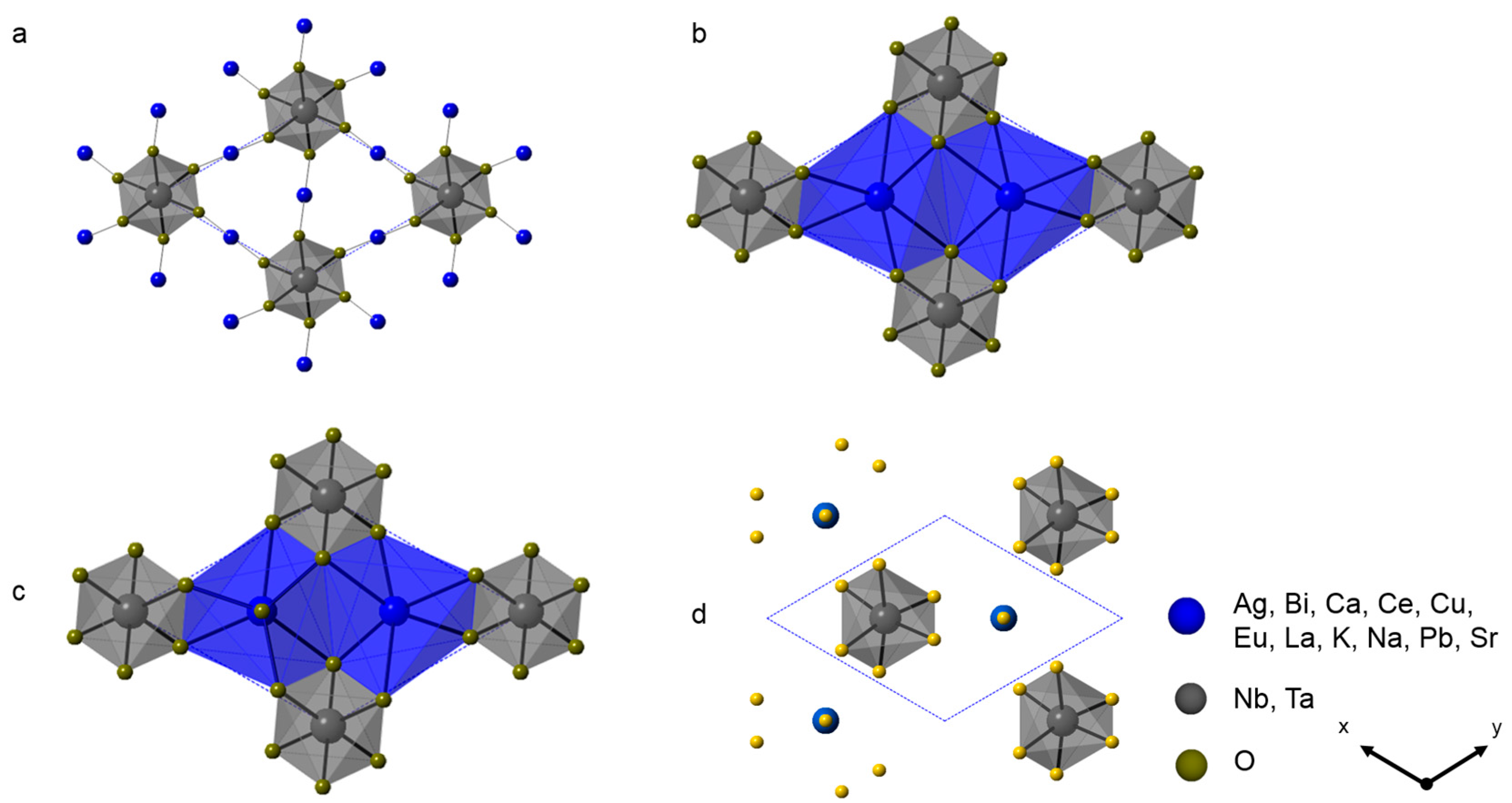
3.1. Niobates
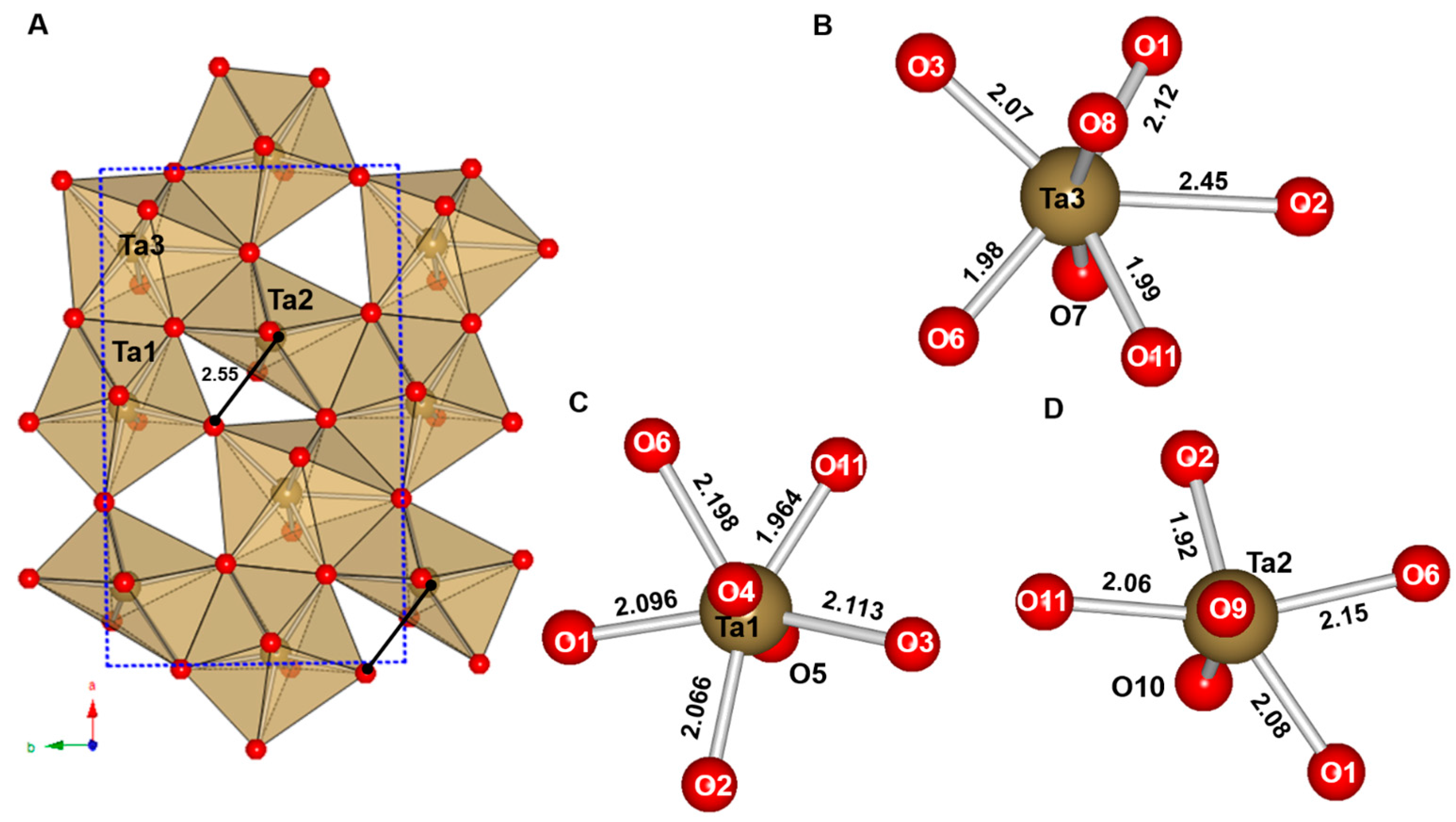
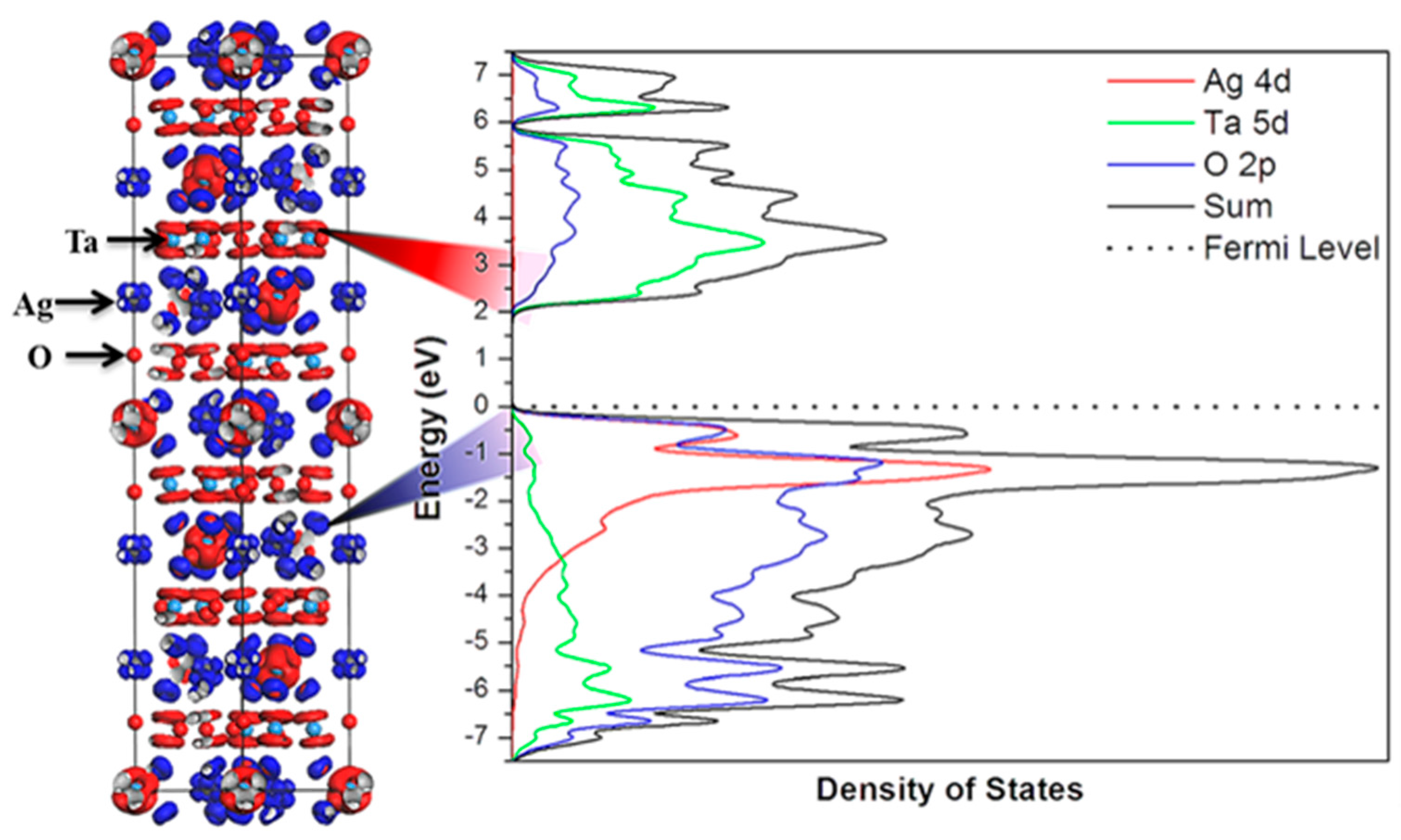
3.2. Tantalates
3.2.1. Cu2Ta4O11
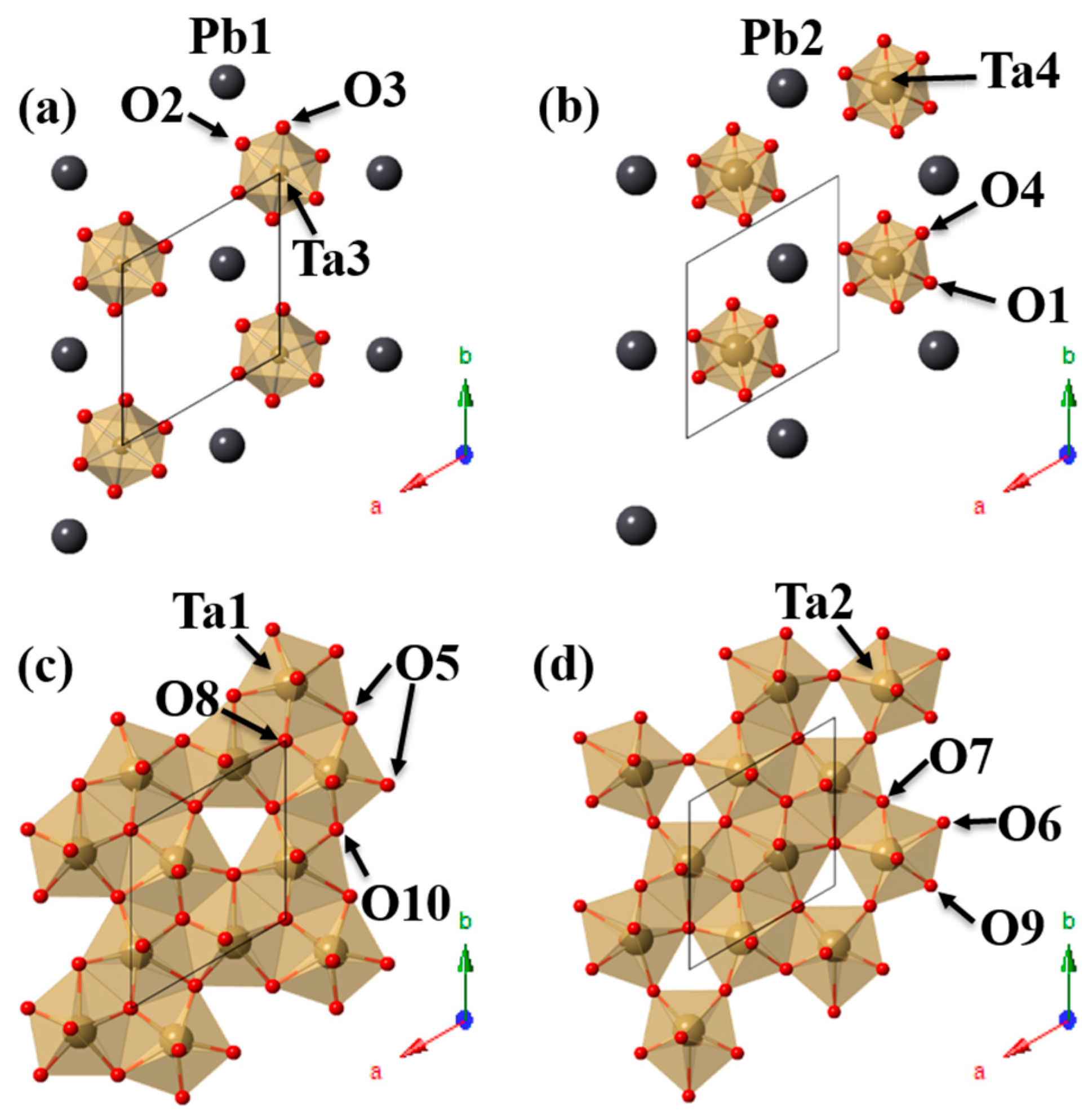
3.2.2. PbTa4O11
3.3. Niobates/Tantalates Solid Solution
4. Photocatalysis
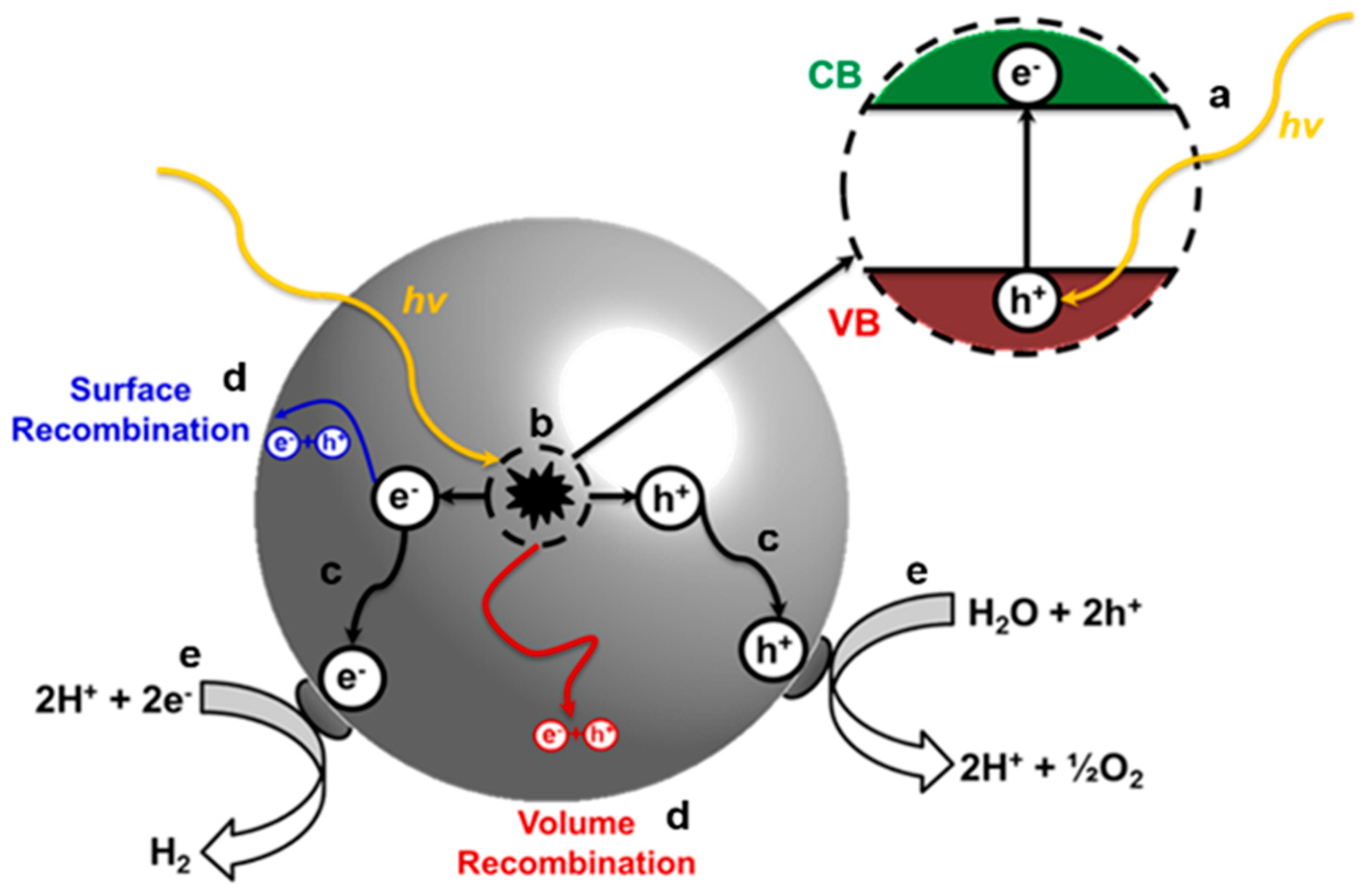
4.1. Background
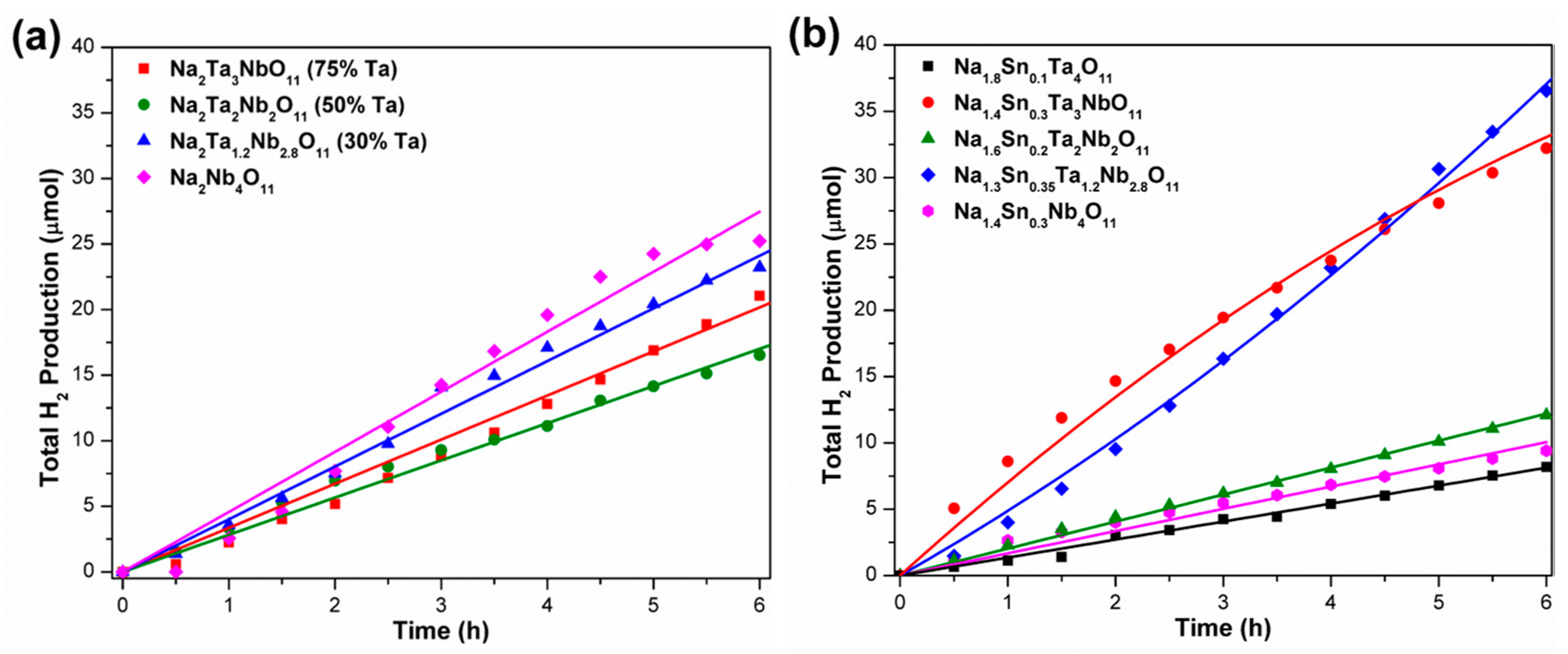
4.2. Photocatalysts with Single MO7 Layers
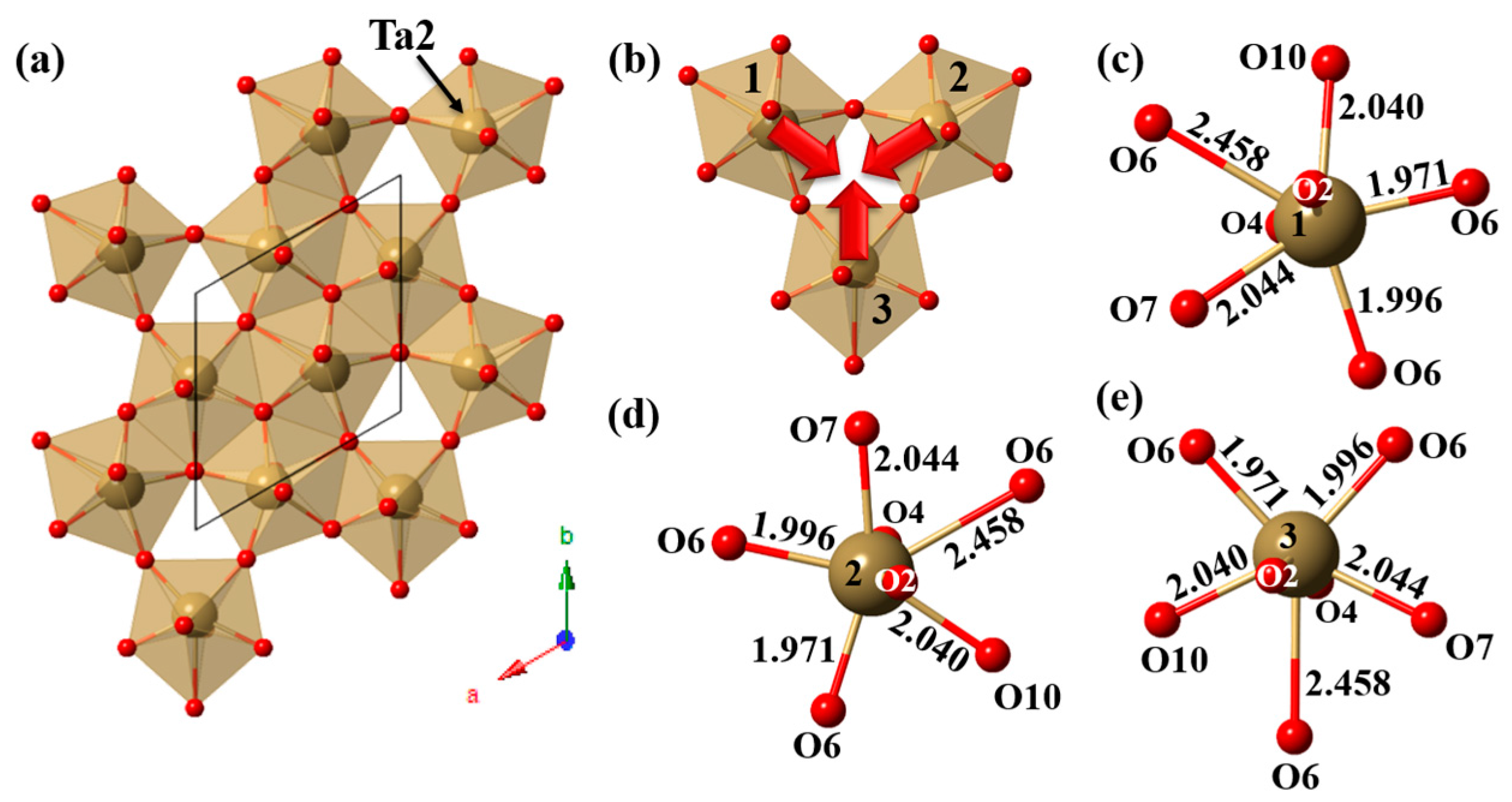
4.3. Double MO7 Layered Photocatalysts
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhu, J.; Li, H.; Zhong, L.; Xiao, P.; Xu, X.; Yang, X.; Zhao, Z.; Li, J. Perovskite oxides: Preparation, Characterizations, and Applications in Heterogeneous Catalysis. ACS Catal. 2014, 4, 2917–2940. [Google Scholar] [CrossRef]
- Zeng, Z.; Calle-Vallejo, F.; Mogensen, M.B.; Rossmeisl, J. Generalized trends in the formation energies of perovskite oxides. Phys. Chem. Chem. Phys. 2013, 15, 7526–7533. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Yuan, L.; Feng, S. Crystal Facet Tailoring Art in Perovskite Oxides. Inorg. Chem. Front. 2015, 2, 965–981. [Google Scholar] [CrossRef]
- Bhalla, A.S.; Guo, R.; Roy, R. The perovskite structure—A review of its role in ceramic science and technology. Mater. Res. Innov. 2000, 4, 3–26. [Google Scholar] [CrossRef]
- Pena, M.A.; Fierro, J.L.G. Chemical Structures and Performance of Perovskite Oxides. Chem. Rev. 2001, 101, 1981–2017. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, P.V.; Puggioni, D.; Rondinelli, J.M. Crystal-Chemistry Guidelines for Noncentrosymmetric A2BO4 Ruddlesden-Popper Oxides. Inorg. Chem. 2014, 53, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Bhuvanesh, N.S.P.; Crossnier-Lopez, M.P.; Duroy, H.; Fourquet, J.L. Synthesis and Structure of Novel Layered perovskite oxides. J. Chem. Soc. 1999, 9, 3093–3100. [Google Scholar] [CrossRef]
- Crosnier-Lopez, M.-P.; Le Berre, F.; Fourquet, J.L. The layered perovskite K2SrTa2O7 : Hydration and K+/H+ Ion exchange. J. Mater. Chem. 2001, 11, 1146–1151. [Google Scholar] [CrossRef]
- Zhang, P.; Li, X.; Zhao, Q.; Liu, S. Synthesis and optical property of one-dimensional spinel ZnMn2O4 nanorods. Nanoscale Res. Lett. 2011, 6, 323. [Google Scholar] [CrossRef] [PubMed]
- Zerarga, F.; Bouhemadou, A.; Khenata, R.; Bin-Omran, S. Structural, electronic and optical properties of spinel oxides ZnAl2O4, ZnGa2O4 and ZnIn2O4. Solid State Sci. 2011, 13, 1638–1648. [Google Scholar] [CrossRef]
- O’Neill, H.S.C.; Navrotsky, A. Simple spinels: Crystallographic parameters, cation radii, lattice energies, and cation distribution. Am. Mineral. 1983, 68, 181–194. [Google Scholar]
- Vivanco, H.K.; Rodriguez, E.E. The intercalation chemistry of layered iron chalcogenide superconductors. J. Solid State Chem. 2016, 242, 3–21. [Google Scholar] [CrossRef]
- Pickett, W.E. Electronic structure of the high-temperature oxide superconductors. Rev. Mod. Phys. 1989, 61, 433–512. [Google Scholar] [CrossRef]
- Ren, Z.A.; Zhao, Z.X. Research and prospects of iron-based superconductors. Adv. Mater. 2009, 21, 4584–4592. [Google Scholar] [CrossRef]
- Cava, R.J. Oxide Superconductors. J. Am. Ceram. Soc. 2000, 83, 5–28. [Google Scholar] [CrossRef]
- Zhang, K.H.L.; Xi, K.; Blamire, M.G.; Egdell, R.G. p-Type transparent conducting oxides. J. Phys. Condens. Matter 2016, 28, 383002. [Google Scholar] [CrossRef] [PubMed]
- Exarhos, G.J.; Zhou, X.-D. Discovery-based design of transparent conducting oxide films. Thin Solid Films 2007, 515, 7025–7052. [Google Scholar] [CrossRef]
- Hosono, H. Recent progress in transparent oxide semiconductors: Materials and device application. Thin Solid Films 2007, 515, 6000–6014. [Google Scholar] [CrossRef]
- Hoel, C.A.; Mason, T.O.; Gaillard, J.F.; Poeppelmeier, K.R. Transparent Conducting Oxides in the ZnO-In2O3-SnO2 System. Chem. Mater. 2010, 22, 3569–3579. [Google Scholar] [CrossRef]
- Dixon, S.C.; Scanlon, D.O.; Carmalt, C.J.; Parkin, I.P. n-Type doped transparent conducting binary oxides: An overview. J. Mater. Chem. 2016, 419, 462–465. [Google Scholar] [CrossRef]
- Maeda, K. Photocatalytic water splitting using semiconductor particles: History and recent developments. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 237–268. [Google Scholar] [CrossRef]
- Abe, R. Recent progress on photocatalytic and photoelectrochemical water splitting under visible light irradiation. J. Photochem. Photobiol. C Photochem. Rev. 2010, 11, 179–209. [Google Scholar] [CrossRef]
- Kudo, A. Development of photocatalyst materials for water splitting. Int. J. Hydrogen Energy 2006, 31, 197–202. [Google Scholar] [CrossRef]
- Osterloh, F.E. Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Chem. Soc. Rev. 2013, 42, 2294–2320. [Google Scholar] [CrossRef] [PubMed]
- Martsinovich, N. Theory of materials for solar energy conversion. J. Phys. Condens. Matter 2016, 28, 70301. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Guo, L. ABO3-based photocatalysts for water splitting. Prog. Nat. Sci. Mater. Int. 2012, 22, 592–615. [Google Scholar] [CrossRef]
- Zachariasen, W.H. Crystal chemical studies of the 5f-series of elements. XII. New compounds representing known structure types. Acta Crystallogr. 1949, 2, 388–390. [Google Scholar] [CrossRef]
- Chodura, B.; Maly, J. A Contribution to the solution of the Structure of U3O8. J. Inorg. Nucl. Chem. 1958, 7, 177–178. [Google Scholar]
- Andresen, A.F. The Structure of U3O8 Determined by Neutron Diffraction. Acta Crystallogr. 1958, 11, 612–614. [Google Scholar] [CrossRef]
- Loopstra, B.O. Neutron Diffraction Investigation of U3O8. Acta Crystallogr. 1964, 17, 651–654. [Google Scholar] [CrossRef]
- Loopstra, B.O. The structure of β-U3O8. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1970, 26, 656–657. [Google Scholar] [CrossRef]
- Momin, A.C.; Deshapande, V.V.; Karkhanavala, M.D. Phase Transition Studies of U3O8. J. Nucl. Mater. 1973, 48, 360–364. [Google Scholar] [CrossRef]
- Loopstra, B.O. On The Existence of delta U3O8: A comment on papers by Karkhanavalla. J. Nucl. Mater. 1969, 29, 354–355. [Google Scholar] [CrossRef]
- Loopstra, B.O. The Phase Transition in alpha U3O8 at 210. J. Appl. Cryst. 1970, 3, 94–96. [Google Scholar] [CrossRef]
- Boltersdorf, J.; Maggard, P.A. Structural and electronic investigations of PbTa4O11 and BiTa7O19 constructed from α-U3O8 types of layers. J. Solid State Chem. 2015, 229, 310–321. [Google Scholar] [CrossRef]
- Jahnberg, L. Hexa- and Hepta-Coordination in Niobium and Tantalum Oxides and Oxide Flourides and Structurally Related Compounds.pdf. Chem. Comm. 1971, 1–41. [Google Scholar]
- Jahnberg, L. A series of Strucutres based on the stacking of alpha U3O8 Layers of pentagonal bipyramids. Mat. Res. Bull. 1981, 16, 513–518. [Google Scholar] [CrossRef]
- Jahnberg, L. Crystal structures of Na2Nb4O11 and CaTa4O11. J. Solid State Chem. 1970, 3–4, 454–462. [Google Scholar] [CrossRef]
- HeuNen, G.W.J.C.; IJdo, D.J.W. SrTa4O11: A Rietveld Refinement using Neutron Powder Diffraction Data. Acta Cryst. 1995, C51, 1723–1725. [Google Scholar] [CrossRef]
- Jahnberg, L.; Sundberg, M. HREM studies of phases based on alpha-U3O8-type layers in the Cu2O-Ta2O5 system. J. Solid State Chem. 1992, 100, 212–219. [Google Scholar] [CrossRef]
- Babaryk, A.A.; Odynets, I.V.; Khainakov, S.; Slobodyanik, N.S.; Garcia-Granda, S. K2Ta4O11 (“kalitantite”): A wide band gap semiconductor synthesized in molybdate flux medium. CrystEngComm 2013, 15, 5539–5544. [Google Scholar] [CrossRef]
- Masó, N.; Woodward, D.I.; Várez, A.; West, A.R. Polymorphism, structural characterisation and electrical properties of Na2Nb4O11. J. Mater. Chem. 2011, 21, 12096–12102. [Google Scholar] [CrossRef]
- Masó, N.; Woodward, D.I.; Thomas, P.A.; Várez, A.; West, A.R. Structural characterization of ferroelectric Ag2Nb4O11 and dielectric Ag2Ta4O11. J. Mater. Chem. 2011, 21, 2715–2722. [Google Scholar] [CrossRef]
- Jahnberg, L. Structure of the Copper (I) Tantalum Oxide, Cu5Ta11O30. J. Solid State Chem. 1982, 41, 286–292. [Google Scholar] [CrossRef]
- Kwentus, Y.; Willson, N. Synthesis and Crystal Strucutre of Th2Th6O19, the first example of a Jahnberg-Structure. Z. Anorg. Allg. Chem. 1996, 622, 67–75. [Google Scholar] [CrossRef]
- Stemmer, W.; Gruehn, R. Tantalated and Niobate Metlts. Z. Anorg. Allg. Chem. 1993, 619, 409–415. [Google Scholar] [CrossRef]
- Palasyuk, O.; Palasyuk, A.; Maggard, P.A. Syntheses, optical properties and electronic structures of copper(I) tantalates: Cu5Ta11O30 and Cu3Ta7O19. J. Solid State Chem. 2010, 183, 814–822. [Google Scholar] [CrossRef]
- Jahnberg, L. Phase Analysis Studies in the System Cu2O-Ta2O5. Acta Chem. Scand. 1987, 41, 527–532. [Google Scholar] [CrossRef]
- Eibenstein, U.; Jung, W. A New Praseodymiumniobate Pr2Nb11O30. Z. Anorg. Allg. Chem. 1998, 624, 802–806. [Google Scholar] [CrossRef]
- Gatehouse, B.M. Crystal Structures Part V: CeTa7O19 of Some Niobium and Tantalum. J. Solid State Chem. 1979, 27, 209–213. [Google Scholar] [CrossRef]
- Guo, G.C.; Zhuang, J.N.; Wang, Y.G.; Chen, J.T.; Zhuang, H.H.; Huang, J.S.; Zhang, Q.E. Dysprosium Tantalum Oxide, DyTa7O19. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1996, 52, 5–7. [Google Scholar] [CrossRef]
- Langebach-Kuttert, B.; Sturm, J.; Gruehn, R. On the Structure of LaTa7O19 Xray and Electron Microscopy. Z. Anorg. Allg. Chem. 1986, 543, 117–128. [Google Scholar]
- Rossell, H.J.; Scott, H.G. The unit cell of YTa7O19, A new compound, and its isomorphism with the corresponding rare earth tantalates. Mater. Res. Bull. 1976, 11, 1231–1235. [Google Scholar] [CrossRef]
- Cretien, A.; Bodit, D. Synthesis of Rare Earth Niobates. C. R. Acad. Sci. Paris 1966, 263C, 882. [Google Scholar]
- Kubota, S.; Endo, T. Energy migration in EuTa7O19, TbTa7O19 and La0.86Tmo.14Ta7O19. J. Alloys Compd. 1996, 241, 16–21. [Google Scholar] [CrossRef]
- Johnson, A.W.S.; Gatehouse, B.M. Convergent-Beam Electron Diffraction Symmetry from a Disordered Structure (Ce,Ta)Ta6O19. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1980, 36, 523–526. [Google Scholar] [CrossRef]
- Hofmann, R.; Gruehn, R. Zur Darstellung und Struktur neuer Niobate LnNb7O19 (Ln = La, Ce). Z. Anorg. Allg. Chem. 1991, 602, 105–117. [Google Scholar] [CrossRef]
- Hofmann, R. Synthesis of Rare Earth Solid State Compounds by Using Chemical Transport. Ph.D. Thesis, University Giessen, Gießen, Germany, 1993. [Google Scholar]
- Halasyamani, P.S.; Poeppelmeier, K.R. Noncentrosymmetric Oxides. Chem. Mater. 1998, 10, 2753–2769. [Google Scholar] [CrossRef]
- Woodward, P.M.; Mizoguchi, H.; Kim, Y.I.; Stoltzfus, M.W. Metal Oxides: Chemistry and Applications; CRC Press: Boca Raton, FL, USA, 2005; Volume 108, pp. 133–194. [Google Scholar] [CrossRef]
- Kunz, M.; Brown, I.D. Out-of-Center Distortions around Octahedrally Coordinated d0 Transition Metals. J. Solid State Chem. 1995, 115, 395–406. [Google Scholar] [CrossRef]
- Masó, N.; West, A.R. A new family of ferroelectric materials: Me2Nb4O11 (Me = Na and Ag). J. Mater. Chem. 2010, 20, 2082–2084. [Google Scholar] [CrossRef]
- Ercit, T.S.; Hawthorne, C.; Cerny, P. Crystal Structure of synthetic natrotantite. Bull. Miner. 1985, 108, 541–549. [Google Scholar]
- King, N.; Sullivan, I.; Watkins-Curry, P.; Chan, J.Y.; Maggard, P.A. Flux-mediated syntheses, structural characterization and low-temperature polymorphism of the p-type semiconductor Cu2Ta4O11. J. Solid State Chem. 2016, 236, 10–18. [Google Scholar] [CrossRef]
- King, N.; Sommer, R.D.; Watkins-Curry, P.; Chan, J.Y.; Maggard, P.A. Synthesis, Structure, and Thermal Instability of the Cu2Ta4O11 Phase. Cryst. Growth Des. 2015, 15, 552–558. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Longo, J.M. ABX Perowskit-Struktur. In Magnetic and Other Properties of Oxides and Related Compounds; Springer-Verlag: Berlin/Heidelberg, Germany, 1970; pp. 142–144. ISBN 978-3-540-04898-5. [Google Scholar]
- Kang, S.K.; Tang, H.; Albright, T.A. Structures for d0 ML6 and ML5 complexes. J. Am. Chem. Soc. 1993, 115, 1971–1981. [Google Scholar] [CrossRef]
- Wheeler, R.A.; Whangbo, M.H.; Hughbanks, T.; Hoffman, R. Symmetric vs. Asymmetric Linear M-X-M Linkages in Molecules, Polymers, and Extended Networks. J. Am. Chem. Soc. 1986, 108, 2222–2236. [Google Scholar] [CrossRef] [PubMed]
- Woodward, D.I.; Lees, M.R.; Thomas, P.A. Structural phase transitions in the Ag2Nb4O11-Na2Nb4O11 solid solution. J. Solid State Chem. 2012, 192, 385–389. [Google Scholar] [CrossRef]
- Masó, N.; West, A.R. Dielectric properties, polymorphism, structural characterisation and phase diagram of Na2Nb4O11-Ag2Nb4O11 solid solutions. J. Solid State Chem. 2015, 225, 438–449. [Google Scholar] [CrossRef]
- Palasyuk, O.; Palasyuk, A.; Maggard, P.A. Site-Differentiated Solid Solution in Na1 − xCuxTa4O11 and Its Electronic Structure and Optical Properties. Inorg. Chem. 2010, 49, 10571–10578. [Google Scholar] [CrossRef] [PubMed]
- Nocera, D.G.; Lewis, S.N. Powering the Planet: Chemical challages in solar energy utillization. PNAS 2006, 103, 15729–15735. [Google Scholar] [CrossRef]
- Yuan, L.; Han, C.; Yang, M.-Q.; Xu, Y.-J. Photocatalytic water splitting for solar hydrogen generation: Fundamentals and recent advancements. Int. Rev. Phys. Chem. 2016, 35, 1–36. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar Water Splitting Cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef] [PubMed]
- Pan, H. Principles on design and fabrication of nanomaterials as photocatalysts for water-splitting. Renew. Sustain. Energy Rev. 2016, 57, 584–601. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, D.; Song, D.; Jiang, J.; Ma, A.; Hu, M.Z.; Ni, C. Recent progress in enhancing solar-to-hydrogen efficiency. J. Power Sources 2015, 280, 649–666. [Google Scholar] [CrossRef]
- Rimoldi, L.; Meroni, D.; Falletta, E.; Ferretti, A.M.; Gervasini, A.; Cappelletti, G.; Ardizzone, S. The role played by different TiO2 features on the photocatalytic degradation of paracetamol. Appl. Surf. Sci. 2017. [Google Scholar] [CrossRef]
- Chen, C.; Ma, W.; Zhao, J. Semiconductor-mediated photodegradation of pollutants under visible-light irradiation. Chem. Soc. Rev. 2010, 39, 4206–4219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.Y.; Long, L.L.; Liu, C.; Li, W.W.; Yu, H.Q. Chemical recycling of the waste anodic electrolyte from the TiO2 nanotube preparation process to synthesize facet-controlled TiO2 single crystals as an efficient photocatalyst. Green Chem. 2014, 16, 2745–2753. [Google Scholar] [CrossRef]
- Rajamanickam, D.; Shanthi, M. Photocatalytic degradation of an organic pollutant by zinc oxide—Solar process. Arab. J. Chem. 2012, 9, S1858–S1868. [Google Scholar] [CrossRef]
- Bowker, M. Sustainable hydrogen production by the application of ambient temperature photocatalysis. Green Chem. 2011, 13, 2235–2246. [Google Scholar] [CrossRef]
- Serpone, N.; Sauvé, G.; Koch, R.; Tahiri, H.; Pichat, P.; Piccinini, P.; Pelizzetti, E.; Hidaka, H. Standardization protocol of process efficiencies and activation parameters in heterogeneous photocatalysis: Relative photonic efficiencies ζr. J. Photochem. Photobiol. A Chem. 1996, 94, 191–203. [Google Scholar] [CrossRef]
- Shi, X.; Cai, L.; Ma, M.; Zheng, X.; Park, J.H. General Characterization Methods for Photoelectrochemical Cells for Solar Water Splitting. ChemSusChem 2015, 8, 3192–3203. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J. Photelectrochemistry and Heterogeneous Photocatalysis At Semiconductors. J. Photochem. 1979, 10, 59–75. [Google Scholar] [CrossRef]
- Ismail, A.A.; Bahnemann, D.W. Photochemical splitting of water for hydrogen production by photocatalysis: A review. Sol. Energy Mater. Sol. Cells 2014, 128, 85–101. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based Photocatalytic Hydrogen Generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Niu, P.; Cheng, H.M. Visible-light-active elemental photocatalysts. ChemPhysChem 2013, 14, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.Z. Hydrogen generation from photoelectrochemical water splitting based on nanomaterials. Laser Photonics Rev. 2010, 4, 517–528. [Google Scholar] [CrossRef]
- Bae, D.; Seger, B.; Vesborg, P.C.K.; Hansen, O.; Chorkendorff, I. Strategies for stable water splitting via protected photoelectrodes. Chem. Soc. Rev. 2017, 46, 1933–1954. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yan, H.; Zong, X.; Wen, F.; Liu, M.; Li, C. Roles of cocatalysts in semiconductor-based photocatalytic hydrogen production. Philos. Trans. R. Soc. London A Math. Phys. Eng. Sci. 2013, 371, 20110430. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, I.; Zoellner, B.; Maggard, P.A. Copper(I)-Based p -Type Oxides for Photoelectrochemical and Photovoltaic Solar Energy Conversion. Chem. Mater. 2016, 28, 5999–6016. [Google Scholar] [CrossRef]
- Chen, Z.; Huyen, D.; Miller, E. Photoelectrochemical Water Splitting Standards, Experimental Methods, and Protocols; Springer: New York, NY, USA, 2013; ISBN 9781461482970. [Google Scholar]
- Li, Z.; Luo, W.; Zhang, M.; Feng, J.; Zou, Z. Photoelectrochemical cells for solar hydrogen production: Current State of promissing photoelectrodes, methods to improve their properties, and outlook. Energy Environ. Sci. 2013, 6, 347–370. [Google Scholar] [CrossRef]
- Zhang, Z.; Yates, J.T. Band bending in semiconductors: Chemical and physical consequences at surfaces and interfaces. Chem. Rev. 2012, 112, 5520–5551. [Google Scholar] [CrossRef] [PubMed]
- Rimoldi, L.; Ambrosi, C.; Di Liberto, G.; Lo Presti, L.; Ceotto, M.; Oliva, C.; Meroni, D.; Cappelli, S.; Cappelletti, G.; Soliveri, G. Impregnation versus Bulk Synthesis: How the Synthetic Route Affects the Photocatalytic Efficiency of Nb/Ta:N Codoped TiO2 Nanomaterials. J. Phys. Chem. 2015, 119, 24104–24115. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Z. Bismuth—Based Photocatalytic Semiconductors : Introduc-tion, Challenges and Possible Approaches. J. Mol. Catal. A Chem. 2016, 423, 533–549. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, H.; Ma, W.; Chen, C.; Song, W.; Zhao, J. Doping-promoted solar water oxidation on hematite photoanodes. Molecules 2016, 21, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Minggu, L.J.; Wan Daud, W.R.; Kassim, M.B. An overview of photocells and photoreactors for photoelectrochemical water splitting. Int. J. Hydrogen Energy 2010, 35, 5233–5244. [Google Scholar] [CrossRef]
- Nowotny, J.; Sorrell, C.C.; Sheppard, L.R.; Bak, T. Solar-hydrogen: Environmentally safe fuel for the future. Int. J. Hydrogen Energy 2005, 30, 521–544. [Google Scholar] [CrossRef]
- Sivula, K.; Van de Krol, R. Semiconducting materials for photoelectrochemical energy conversion. Nat. Rev. Mater. 2016, 1, 15010. [Google Scholar] [CrossRef]
- Gratzel, M. Photoelectrochemical Cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Liu, G.; Wang, L. Recent advances in 2D materials for photocatalysis. Nanoscale 2016, 8, 6904–6920. [Google Scholar] [CrossRef] [PubMed]
- Satsangi, V.R. Metal Oxide Semiconductors in PEC Splitting of Water. Sol. Hydrog. Nanotechnol. 2007, 6650, 66500D-66500D-13. [Google Scholar] [CrossRef]
- Xu, D.; Hai, Y.; Zhang, X.; Zhang, S.; He, R. Bi2O3 cocatalyst improving photocatalytic hydrogen evolution performance of TiO2. Appl. Surf. Sci. 2017, 400, 530–536. [Google Scholar] [CrossRef]
- Paracchino, A.; Laporte, V.; Sivula, K.; Grätzel, M.; Thimsen, E. Highly active oxide photocathode for photoelectrochemical water reduction. Nat. Mater. 2011, 10, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhang, D.; Tang, G.; Ji, X.; Li, W.; Li, C.; Yang, X. Hydrothermal synthesis and visible-light photocatalytic activity of α-Fe2O3/TiO2 composite hollow microspheres. Ceram. Int. 2013, 39, 8633–8640. [Google Scholar] [CrossRef]
- Yourey, J.E.J.; Pyper, K.J.K.; Kurtz, J.B.; Bartlett, B.M. Chemical Stability of CuWO4 for Photoelectrochemical Water Oxidation. J. Phys. Chem. 2013, 117, 8708–8718. [Google Scholar] [CrossRef]
- Horie, H.; Iwase, A.; Kudo, A. Photocatalytic Properties of Layered Metal Oxides Substituted with Silver by a Molten AgNO3 Treatment. ACS Appl. Mater. Interfaces 2015, 14638–14643. [Google Scholar] [CrossRef] [PubMed]
- Abdi, F.F.; Firet, N.; Van de Krol, R. Efficient BiVO4 Thin Film Photoanodes Modified with Cobalt Phosphate Catalyst and W-doping. ChemCatChem 2013, 5, 490–496. [Google Scholar] [CrossRef]
- Gu, J.; Wuttig, A.; Krizan, J.W.; Hu, Y.; Detweiler, Z.M.; Cava, R.J.; Bocarsly, A.B. Mg-Doped CuFeO2 photocathodes for photoelectrochemical reduction of carbon dioxide. J. Phys. Chem. 2013, 117, 12415–12422. [Google Scholar] [CrossRef]
- Arney, D.; Maggard, P.A. Effect of Platelet-Shaped Surfaces and Silver-Cation Exchange on the Photocatalytic Hydrogen Production of RbLaNb2O7. J. Phys. Chem. 2012, 116, 14022–14030. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, L. Rapid preparation of perovskite lead niobate nanosheets by ultrasonic-assisted exfoliation for enhanced visible-light-driven photocatalytic hydrogen production. ChemCatChem 2015, 7, 584–587. [Google Scholar] [CrossRef]
- Kanhere, P.; Chen, Z. A review on visible light active perovskite-based photocatalysts. Molecules 2014, 19, 19995–20022. [Google Scholar] [CrossRef] [PubMed]
- Joshi, U.A.; Palasyuk, A.; Arney, D.; Maggard, P.A. Semiconducting oxides to facilitate the conversion of solar energy to chemical fuels. J. Phys. Chem. Lett. 2010, 1, 2719–2726. [Google Scholar] [CrossRef]
- Kudo, A. Photocatalytic decomposition of water over NiO-Kb4Nb6O17 catalyst. J. Catal. 1988, 111, 67–76. [Google Scholar] [CrossRef]
- Machida, M.; Mitsuyama, T.; Ikeue, K. Photocatalytic Property and Electronic Structure of Triple-Layered Perovskite Tantalates. J. Phys. Chem. 2005, 109, 7801–7806. [Google Scholar] [CrossRef] [PubMed]
- Kulischow, N.; Ladasiu, C.; Marschall, R. Layered Dion-Jacobson type niobium oxides for photocatalytic hydrogen production prepared via molten salt synthesis. Catal. Today 2016, 287, 65–69. [Google Scholar] [CrossRef]
- Arney, D.; Porter, B.; Greve, B.; Maggard, P.A. New molten-salt synthesis and photocatalytic properties of La2Ti2O7 particles. J. Photochem. Photobiol. A Chem. 2008, 199, 230–235. [Google Scholar] [CrossRef]
- Arney, D.; Fuoco, L.; Boltersdorf, J.; Maggard, P.A. Flux Synthesis of Na2Ca2Nb4O13 : The Influence of Particle Shapes, Surface Features, and Surface Areas on Photocatalytic Hydrogen Production. J. Am. Ceram. Soc. 2013, 96, 1158–1162. [Google Scholar] [CrossRef]
- Fuoco, L.; Joshi, U.A.; Maggard, P.A. Preparation and Photoelectrochemical Properties of p-type Cu5Ta11O30 and Cu3Ta7O19 Semiconducting Polycrystalline Films. J. Phys. Chem. 2012, 116, 10490–10497. [Google Scholar] [CrossRef]
- Dong, H.; Sun, J.; Chen, G.; Li, C.; Hu, Y.; Lv, C. An advanced Ag-based photocatalyst Ag2Ta4O11 with outstanding activity, durability and universality for removing organic dyes. Phys. Chem. Chem. Phys. 2014, 16, 23915–23921. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Chen, G.; Sun, J.; Li, C. Durability, inactivation and regeneration of silver tetratantalate in photocatalytic H2 evolution. Phys. Chem. Chem. Phys. 2014, 17, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Boltersdorf, J.; Zoellner, B.; Fancher, C.; Jones, J.L.; Maggard, P.A. Single and Double-Site Substitutions in Mixed-Metal Oxides: Adjusting the Band Edges Towards the Water Redox Couples. J. Phys. Chem. 2016, 120, 19175–19188. [Google Scholar] [CrossRef]
- Boltersdorf, J.; Wong, T.; Maggard, P.A. Synthesis and optical properties of Ag(I), Pb(II), and Bi(III) tantalate-based photocatalysts. ACS Catal. 2013, 3, 2943–2953. [Google Scholar] [CrossRef]
- McLamb, N.; Sahoo, P.P.; Fuoco, L.; Maggard, P.A. Flux growth of single-crystal Na2Ta4O11 particles and their photocatalytic hydrogen production. Cryst. Growth Des. 2013, 13, 2322–2326. [Google Scholar] [CrossRef]
- Harb, M.; Masih, D.; Ould-Chikh, S.; Sautet, P.; Basset, J.M.; Takanabe, K. Determination of the electronic structure and UV-Vis absorption properties of (Na2−xCux)Ta4O11 from first-principle calculations. J. Phys. Chem. 2013, 117, 17477–17484. [Google Scholar] [CrossRef]
- Dong, H.; Chen, G.; Sun, J.; Feng, Y.; Li, C.; Lv, C. Stability, durability and regeneration ability of a novel Ag-based photocatalyst Ag2Nb4O11. Chem. Comm. 2014, 50, 6596–6599. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.; Iwase, A.; Kobayashi, H.; Kudo, A. Water Splitting over CaTa4O11 and LaZrTa3O11 Photocatalysts with Laminated Structure Consisting of Layers of TaO6 Octahedra and TaO7 Decahedra. Chem. Lett. 2014, 43, 396–398. [Google Scholar] [CrossRef]
- Cohen, R.E. Origin of ferroelectricity in perovskite oxides. Nature 1992, 358, 136–138. [Google Scholar] [CrossRef]
- Li, L.; Salvador, P.A.; Rohrer, G.S. Photocatalysts with internal electric fields. Nanoscale 2014, 6, 24–42. [Google Scholar] [CrossRef] [PubMed]
- Boltersdorf, J.; Maggard, P.A. Silver-Exchange of Layered Metal Oxides and their Photocatalytic Activities Silver-Exchange of Layered Metal Oxides and their Photocatalytic Activities. ACS Catal. 2013, 3, 2547–2555. [Google Scholar] [CrossRef]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
King, N.; Boltersdorf, J.; Maggard, P.A.; Wong-Ng, W. Polymorphism and Structural Distortions of Mixed-Metal Oxide Photocatalysts Constructed with α-U3O8 Types of Layers. Crystals 2017, 7, 145. https://doi.org/10.3390/cryst7050145
King N, Boltersdorf J, Maggard PA, Wong-Ng W. Polymorphism and Structural Distortions of Mixed-Metal Oxide Photocatalysts Constructed with α-U3O8 Types of Layers. Crystals. 2017; 7(5):145. https://doi.org/10.3390/cryst7050145
Chicago/Turabian StyleKing, Nacole, Jonathan Boltersdorf, Paul A. Maggard, and Winnie Wong-Ng. 2017. "Polymorphism and Structural Distortions of Mixed-Metal Oxide Photocatalysts Constructed with α-U3O8 Types of Layers" Crystals 7, no. 5: 145. https://doi.org/10.3390/cryst7050145






