Morphology of Diamond Layers Grown on Different Facets of Single Crystal Diamond Substrates by a Microwave Plasma CVD in CH4-H2-N2 Gas Mixtures
Abstract
:1. Introduction
2. Experimental
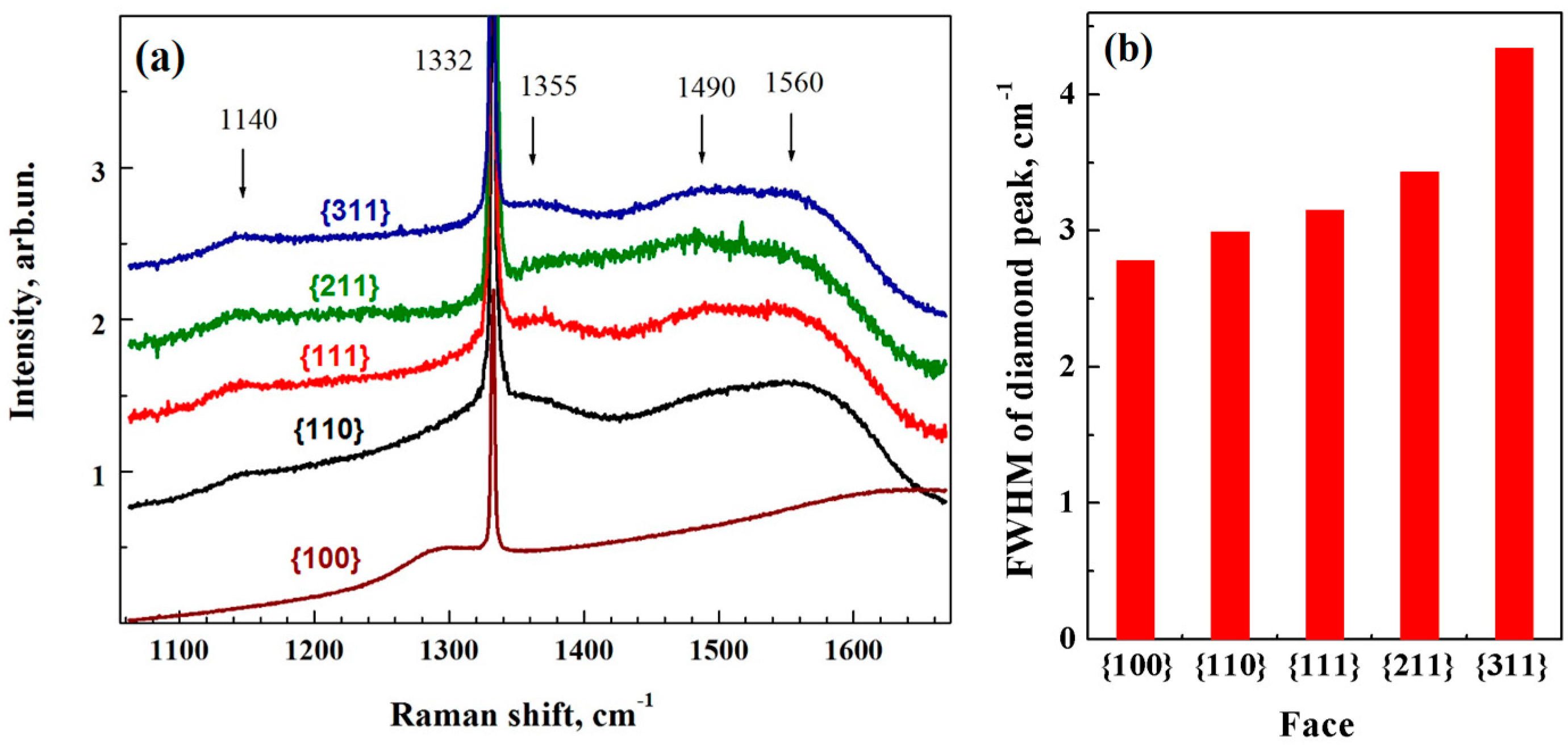
3. Results and Discussion
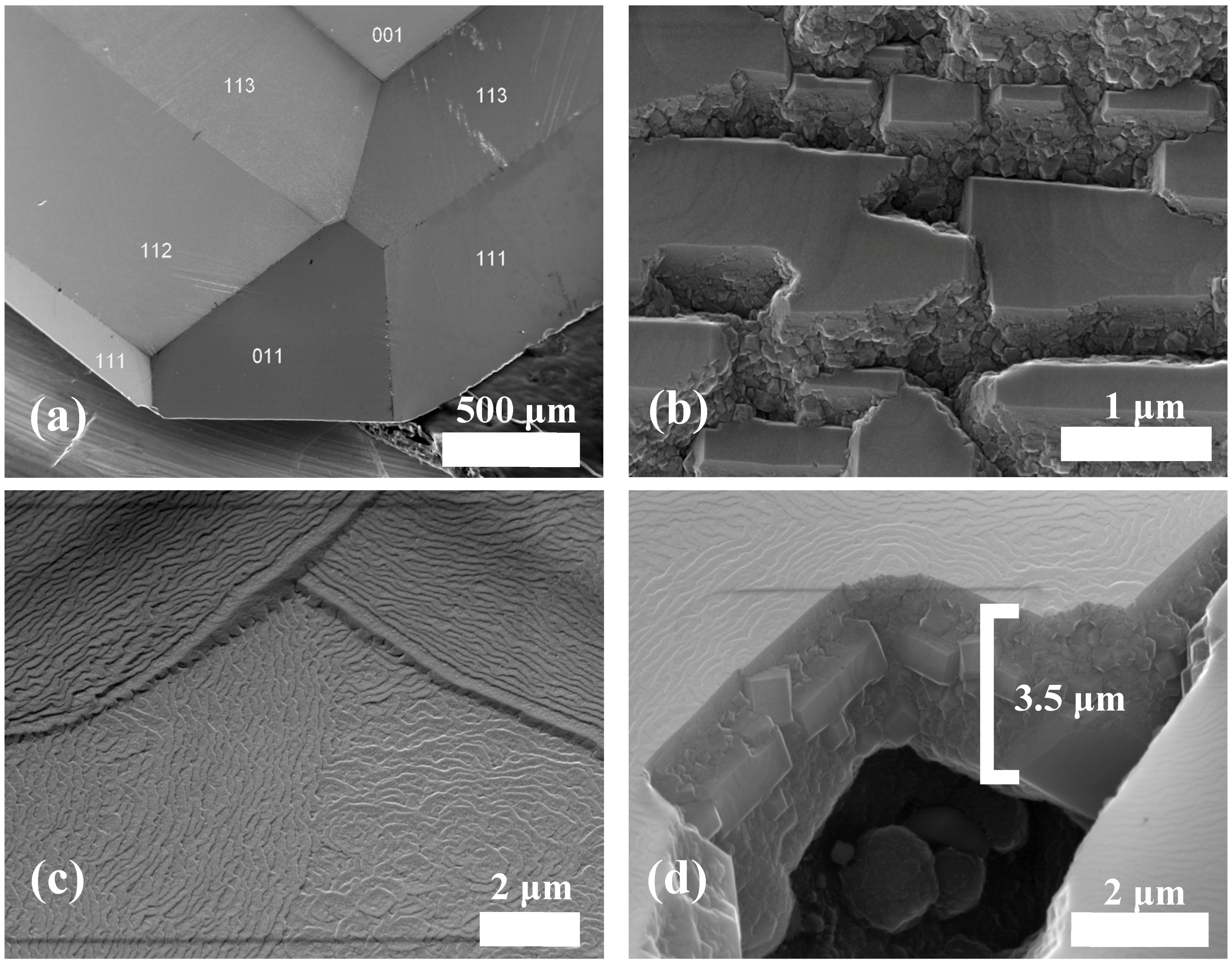
3.1. Diamond Deposition on Low-Index Facets of the Single Crystal Substrate
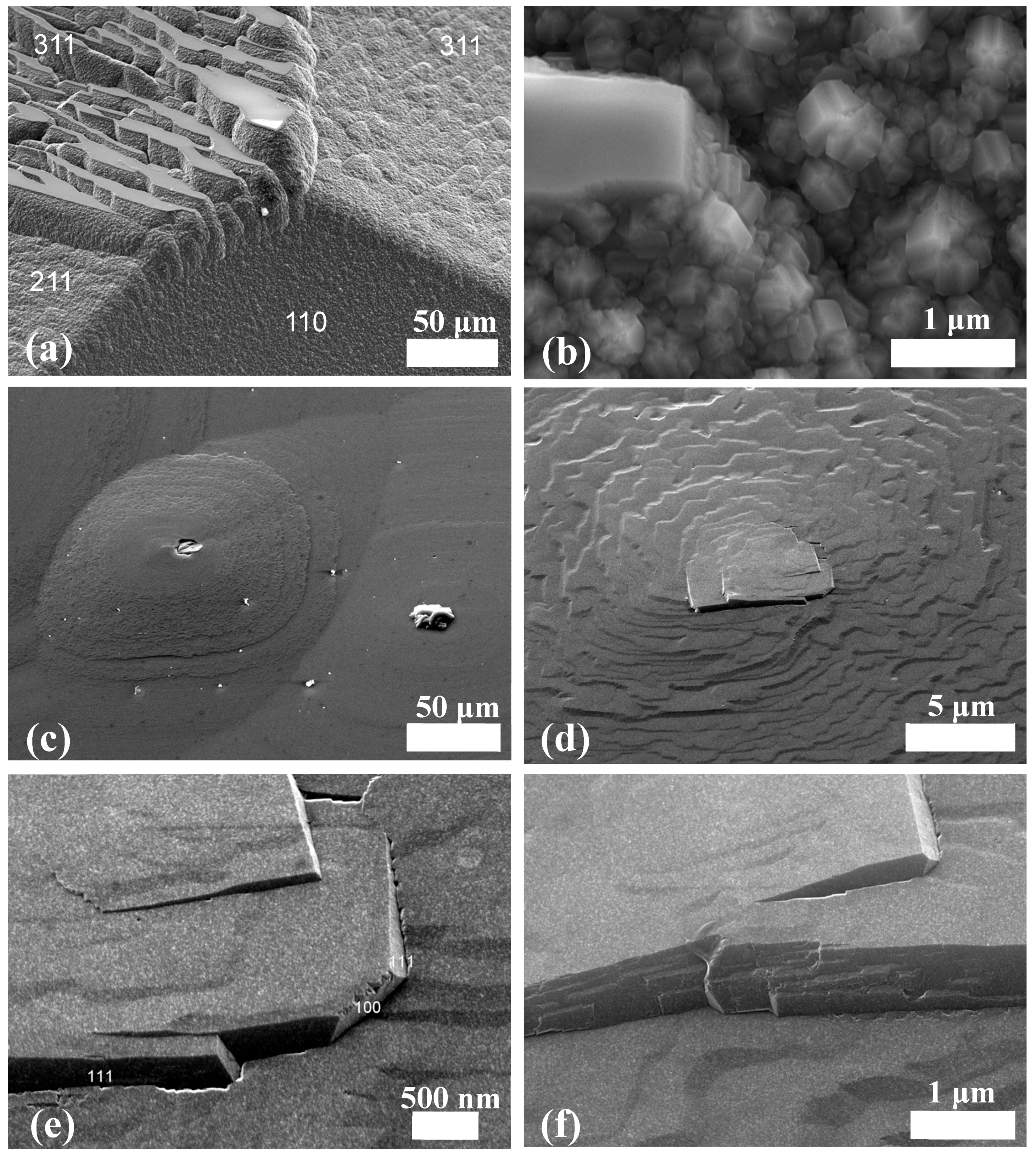
3.2. Polycrystalline Aggregations at {100} Face
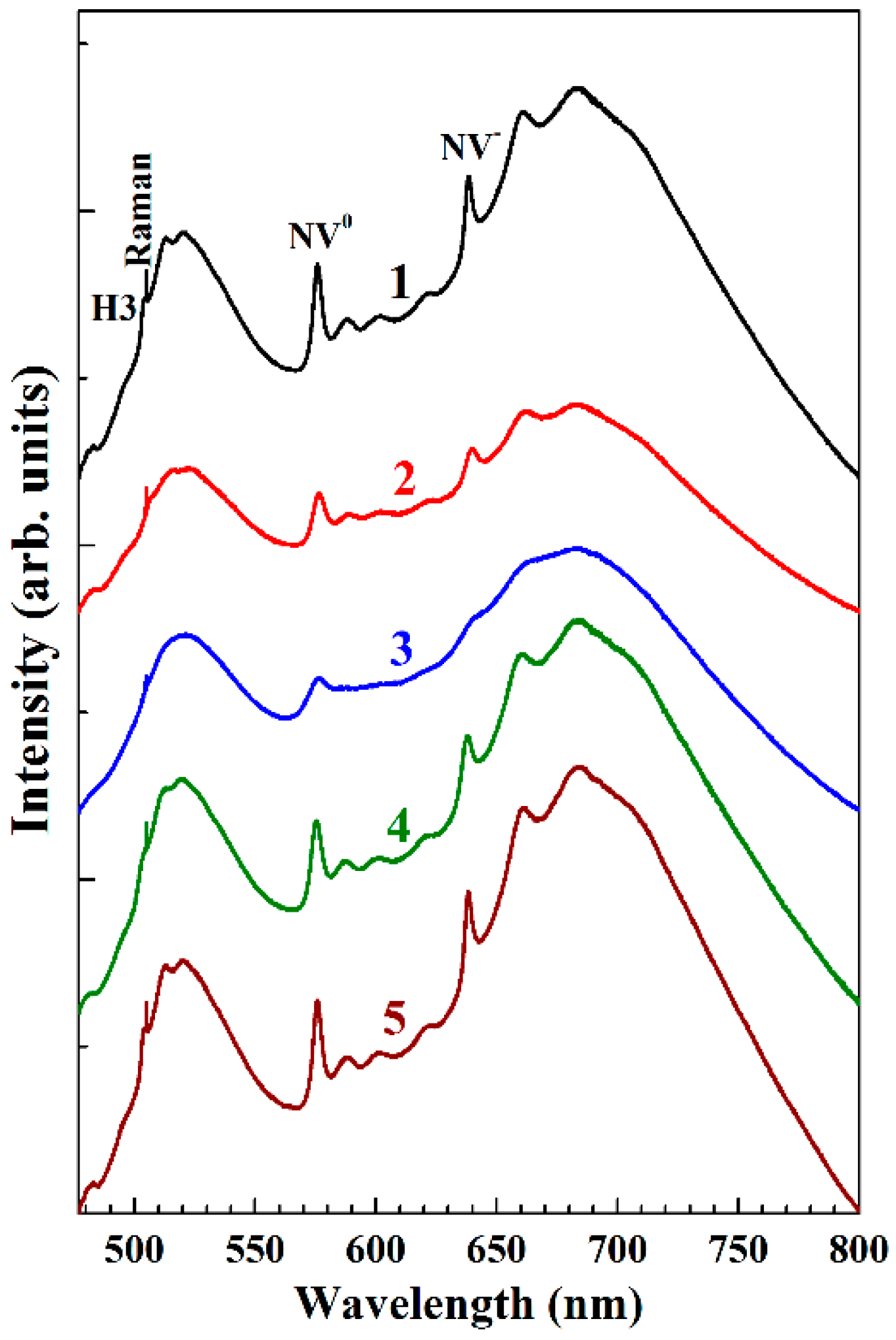
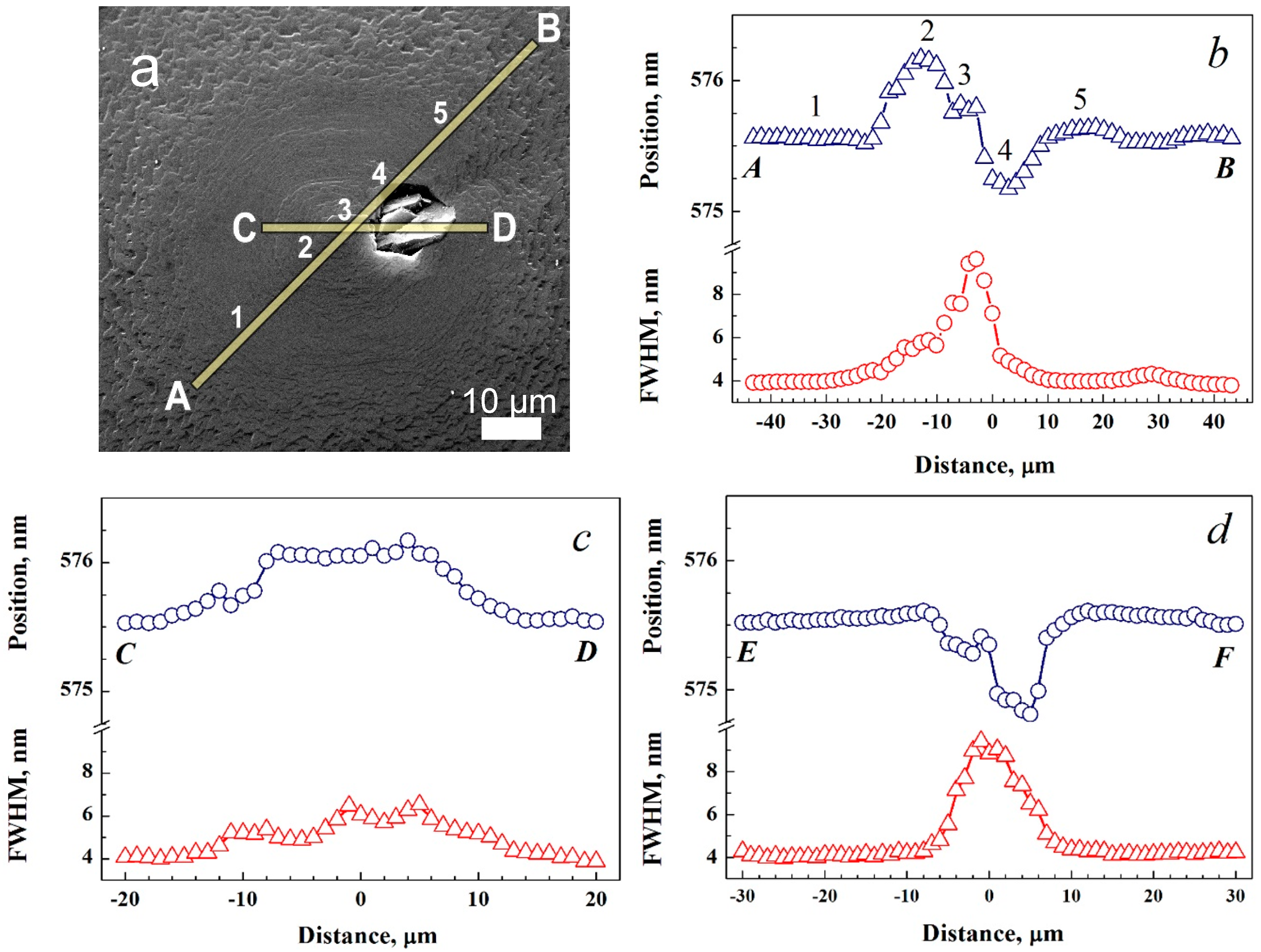
3.3. Photoluminescence Scanning Spectroscopy on Growth Defects on {100} Face
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Polini, R.; Barletta, M.; Rubino, G.; Vesco, S. Advances in the Deposition of Diamond Coatings on Co-Cemented Tungsten Carbides. Adv. Mater. Sci. Eng. 2012, 2012, 151629. [Google Scholar] [CrossRef]
- Dumpala, R.; Chandran, M.; Rao, M.S.R. Engineered CVD Diamond Coatings for Machining and Tribological Applications. J. Miner. Met. Mater. Soc. 2015, 67, 1565–1577. [Google Scholar] [CrossRef]
- Khomich, A.A.; Ashkinazi, E.E.; Ralchenko, V.G.; Sedov, V.S.; Khmelnitskii, R.A.; Poklonskaya, O.N.; Kozlova, M.V.; Khomich, A.V. Application of Raman spectroscopy for the analysis of the structure of diamond coatings on a hard alloy. J. Appl. Spectrosc. 2017, 84, 297–304. [Google Scholar] [CrossRef]
- Chromik, R.R.; Winfrey, A.L.; Lüning, J.; Nemanich, R.J.; Wahl, K.J. Run-in behavior of nanocrystalline diamond coatings studied by in situ tribometry. Wear 2008, 265, 477–489. [Google Scholar] [CrossRef]
- Sun, F.; Ma, Y.; Shen, B.; Zhang, Z.; Chen, M. Fabrication and application of nano-microcrystalline composite diamond films on the interior hole surfaces of Co cemented tungsten carbide substrates. Diam. Relat. Mater. 2009, 18, 276–279. [Google Scholar] [CrossRef]
- Shafer, L.; Hofer, M.; Kroger, R. The versatility of hot-filament activated chemical vapor deposition. Thin Solid Films 2006, 515, 1017–1024. [Google Scholar] [CrossRef]
- Chayahara, A.; Mokuno, Y.; Horino, Y.; Takasu, Y.; Kato, H.; Yoshikawa, H.; Fujimori, N. The effect of nitrogen addition during high-rate homoepitaxial growth of diamond by microwave plasma CVD. Diam. Relat. Mater. 2004, 13, 1954–1958. [Google Scholar] [CrossRef]
- Tallaire, A.; Achard, J.; Silva, F.; Brinza, O.; Gicquel, A. Growth of large size diamond single crystals by plasma assisted chemical vapour deposition: Recent achievements and remaining challenges. Comptes Rendus Phys. 2013, 14, 169–184. [Google Scholar] [CrossRef]
- Lloret, F.; Fiori, A.; Araujo, D.; Eon, D.; Villar, M.P.; Bustarret, E. Stratigraphy of a diamond epitaxial three-dimensional overgrowth using doping superlattices. Appl. Phys. Lett. 2016, 108. [Google Scholar] [CrossRef]
- Tallaire, A.; Brinza, O.; Mille, V.; William, L.; Achard, J. Reduction of dislocations in single crystal diamond by lateral growth over a macroscopic hole. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Koji, K. Diamond Films: Chemical Vapor Deposition for Oriented and Heteroepitaxial Growth; Elsevier: Amsterdam, The Netherlands, 2005; Chapter 5; pp. 31–50. [Google Scholar]
- Ralchenko, V.; Pimenov, S.; Konov, V.; Khomich, A.; Saveliev, A.; Popovich, A.; Vlasov, I.; Zavedeev, E.; Bozhko, A.; Loubnin, N.; et al. Nitrogenatednanocrystalline diamond films: Thermal and optical properties. Diam. Relat. Mater. 2007, 16, 2067–2073. [Google Scholar] [CrossRef]
- Bolshakov, A.P.; Ralchenko, V.G.; Yurov, V.Y.; Popovich, A.F.; Antonova, I.A.; Khomich, A.A.; Vlasov, I.I.; Ashkinazi, E.E.; Ryzhkov, S.G.; Vlasov, A.V.; et al. High-rate growth of single crystal diamond in microwave plasma in CH4/H2 and CH4/H2/Ar gas mixtures in presence of intensive soot formation. Diam. Relat. Mater. 2016, 62, 49–57. [Google Scholar] [CrossRef]
- Godbole, V.P.; Sumant, A.V.; Kshirsagar, R.B.; Dharmadhikari, C.V. Evidence for layered growth of (100) textured diamond films. Appl. Phys. Lett. 1997, 71, 2626–2628. [Google Scholar] [CrossRef]
- Silva, F.; Bonnin, X.; Achard, J.; Brinza, O.; Michau, A.; Secroun, A.; De Corte, K.; Felton, S.; Newton, M.; Gicquel, A. Single crystal CVD diamond growth strategy by the use of a 3D geometrical model: Growth on (113) oriented substrates. J. Cryst. Growth 2008, 310, 1067–1075. [Google Scholar] [CrossRef]
- Müller-Sebert, W.; Wörner, E.; Fuchs, F.; Wild, C.; Koidl, P. Nitrogen induced increase of growth rate in chemical vapor deposition of diamond. Appl. Phys. Lett. 1996, 68, 759–760. [Google Scholar] [CrossRef]
- Butler, J.E.; Oleynik, I. A mechanism for crystal twinning in the growth of diamond by chemical vapour deposition. Philos. Trans. R. Soc. A. 2008, 366, 295–311. [Google Scholar] [CrossRef] [PubMed]
- May, P.W.; Ashfold, M.N.R.; Mankelevich, Y.A. Microcrystalline, nanocrystalline, and ultrananocrystalline diamond chemical vapor deposition: Experiment and modeling of the factors controlling growth rate, nucleation, and crystal size. J. Appl. Phys. 2007, 101, 053115. [Google Scholar] [CrossRef]
- Richley, J.C.; Harvey, J.N.; Ashfold, M.N.R. CH2 Group Migration between H-Terminated 2 × 1 Reconstructed {100} and {111} Surfaces of Diamond. J. Phys. Chem. C 2012, 116, 7810–7816. [Google Scholar] [CrossRef]
- Barbosa, D.C.; Hammer, P.; Trava-Airoldi, V.J.; Corat, E.J. The valuable role of renucleation rate in ultrananocrystalline diamond growth. Diam. Relat. Mater. 2012, 23, 112–119. [Google Scholar] [CrossRef]
- Van der Putte, P.; Van Enckevort, W.J.P.; Giling, L.J.; Bloem, J. Surface morphology of HCl etched silicon wafers: II. Bunch formation. J. Cryst. Growth 1978, 43, 659–675. [Google Scholar] [CrossRef]
- Hayashi, K.; Yamanaka, S.; Okushi, H.; Kajimura, K. Homoepitaxial diamond films with large terraces. Appl. Phys. Lett. 1996, 68, 1220–1222. [Google Scholar] [CrossRef]
- Bushuev, E.V.; Yurov, V.Y.; Bolshakov, A.P.; Ralchenko, V.G.; Khomich, A.A.; Antonova, I.A.; Ashkinazi, E.E.; Shershulin, V.A.; Pashinin, V.P.; Konov, V.I. Express in situ measurement of epitaxial CVD diamond film growth kinetics. Diam. Relat. Mater. 2017, 72, 61–70. [Google Scholar] [CrossRef]
- Tardieu, A.; Cansell, F.; Petitet, J.P. Pressure and temperature dependence of the first-order Raman mode of diamond. J. Appl. Phys. 1990, 68, 3243–3245. [Google Scholar] [CrossRef]
- Zaitsev, A.M. Optical Properties of Diamond: A Data Handbook; Springer: Berlin, Germany, 2001. [Google Scholar]
- Sunagawa, I. Crystals: Growth, Morphology, and Perfection; Cambridge University Press: Cambridge, UK, 2005; pp. 44–45. [Google Scholar]
- Palyanov, Y.N.; Kupriyanov, I.N.; Khokhryakov, A.F.; Ralchenko, V.G. Crystal Growth of Diamond. In Handbook of Crystal Growth: Bulk Crystal Growth, 2nd ed.; Rudolph, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Samlenski, R.; Haug, C.; Brenn, R.; Wild, C.; Locher, R.; Koidl, P. Incorporation of nitrogen in chemical vapor deposition diamond. Appl. Phys. Lett. 1995, 67, 2798–2800. [Google Scholar] [CrossRef]
- Khomich, A.A.; Kudryavtsev, O.S.; Bolshakov, A.P.; Khomich, A.V.; Ashkinazi, E.E.; Ralchenko, V.G.; Vlasov, I.I.; Konov, V.I. Use of Optical Spectroscopy Methods to Determine the Solubility Limit for Nitrogen in Diamond Single Crystals Synthesized by Chemical Vapor Deposition. J. Appl. Spectrosc. 2015, 82, 242–247. [Google Scholar] [CrossRef]
- Yamada, H.; Chayahara, A.; Mokuno, Y. Effects of intentionally introduced nitrogen and substrate temperature on growth of diamond bulk single crystals. Jpn. J. Appl. Phys. 2016, 55. [Google Scholar] [CrossRef]
- Tallaire, A.; Kasu, M.; Ueda, K.; Makimoto, T. Origin of growth defects in CVD diamond epitaxial films. Diam. Relat. Mater. 2008, 17, 60–65. [Google Scholar] [CrossRef]
- Friel, I.; Clewes, S.L.; Dhillon, H.K.; Perkins, N.; Twitchen, D.J.; Scarsbrook, G.A. Control of surface and bulk crystalline quality in single crystal diamond grown by chemical vapor deposition. Diam. Relat. Mater. 2009, 18, 808–815. [Google Scholar] [CrossRef]
- Mccormick, T.L.; Jackson, W.E.; Nemanich, R.J. Strain and Impurity Content of Synthetic Diamond Crystals. In Materials Research Society Symposia Proceedings; Materials Research Society: Pittsburgh, PA, USA, 1994; Volume 349, pp. 445–450. [Google Scholar]
- Inyushkin, A.V.; Taldenkov, A.N.; Ralchenko, V.G.; Vlasov, I.I.; Konov, V.I.; Khomich, A.V.; Khmelnitskii, R.A.; Trushin, A.S. Thermal conductivity of polycrystalline CVD diamond: Effect of annealing-induced transformations of defects and grain boundaries. Phys. Status Solidi A 2008, 205, 2226–2232. [Google Scholar] [CrossRef]
- Jones, R.; Goss, J.P.; Pinto, H.; Palmer, D.W. Diffusion of nitrogen in diamond and the formation of A-centres. Diam. Relat. Mater. 2015, 53, 35–39. [Google Scholar] [CrossRef]
- Okushi, H.; Watanabe, H.; Ri, S.; Yamanaka, S.; Takeuchi, D. Device-grade homoepitaxial diamond film growth. J. Cryst. Growth 2002, 237–239, 1269–1276. [Google Scholar] [CrossRef]
- Deak, P.; Aradi, B.; Kaviani, M.; Frauenheim, T.; Gali, A. Formation of NV centers in diamond: A theoretical study based on calculated transitions and migration of nitrogen and vacancy related defects. Phys. Rev. B 2014, 89, 075203. [Google Scholar] [CrossRef]
- Fukura, S.; Nakagawa, T.; Kagi, H. High spatial resolution photoluminescence and Raman spectroscopic measurements of a natural polycrystalline diamond, carbonado. Diam. Relat. Mater. 2005, 14, 1950–1954. [Google Scholar] [CrossRef]
- Bolshakov, A.; Ralchenko, V.; Sedov, V.; Khomich, A.; Vlasov, I.; Khomich, A.; Trofimov, N.; Krivobok, V.; Nikolaev, S.; Khmelnitskii, R.; et al. Photoluminescence of SiV centers in single crystal CVD diamond in situ doped with Si from silane. Phys. Status Solidi A 2015, 212, 2525–2532. [Google Scholar] [CrossRef]
- Orlov, Y.L. The Mineralogy of Diamond; Wiley: New York, NY, USA, 1977; p. 235. [Google Scholar]
- Khokhryakov, A.F.; Palyanov, Y.N. Effect of crystal defects on diamond morphology during dissolution in the mantle. Am. Mineral. 2015, 100, 1528–1532. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashkinazi, E.E.; Khmelnitskii, R.A.; Sedov, V.S.; Khomich, A.A.; Khomich, A.V.; Ralchenko, V.G. Morphology of Diamond Layers Grown on Different Facets of Single Crystal Diamond Substrates by a Microwave Plasma CVD in CH4-H2-N2 Gas Mixtures. Crystals 2017, 7, 166. https://doi.org/10.3390/cryst7060166
Ashkinazi EE, Khmelnitskii RA, Sedov VS, Khomich AA, Khomich AV, Ralchenko VG. Morphology of Diamond Layers Grown on Different Facets of Single Crystal Diamond Substrates by a Microwave Plasma CVD in CH4-H2-N2 Gas Mixtures. Crystals. 2017; 7(6):166. https://doi.org/10.3390/cryst7060166
Chicago/Turabian StyleAshkinazi, Evgeny E., Roman A. Khmelnitskii, Vadim S. Sedov, Andrew A. Khomich, Alexander V. Khomich, and Viktor G. Ralchenko. 2017. "Morphology of Diamond Layers Grown on Different Facets of Single Crystal Diamond Substrates by a Microwave Plasma CVD in CH4-H2-N2 Gas Mixtures" Crystals 7, no. 6: 166. https://doi.org/10.3390/cryst7060166






