Effect of an External Electric Field on the Kinetics of Dislocation-Free Growth of Tetragonal Hen Egg White Lysozyme Crystals
Abstract
:1. Introduction
2. Experimental Procedure
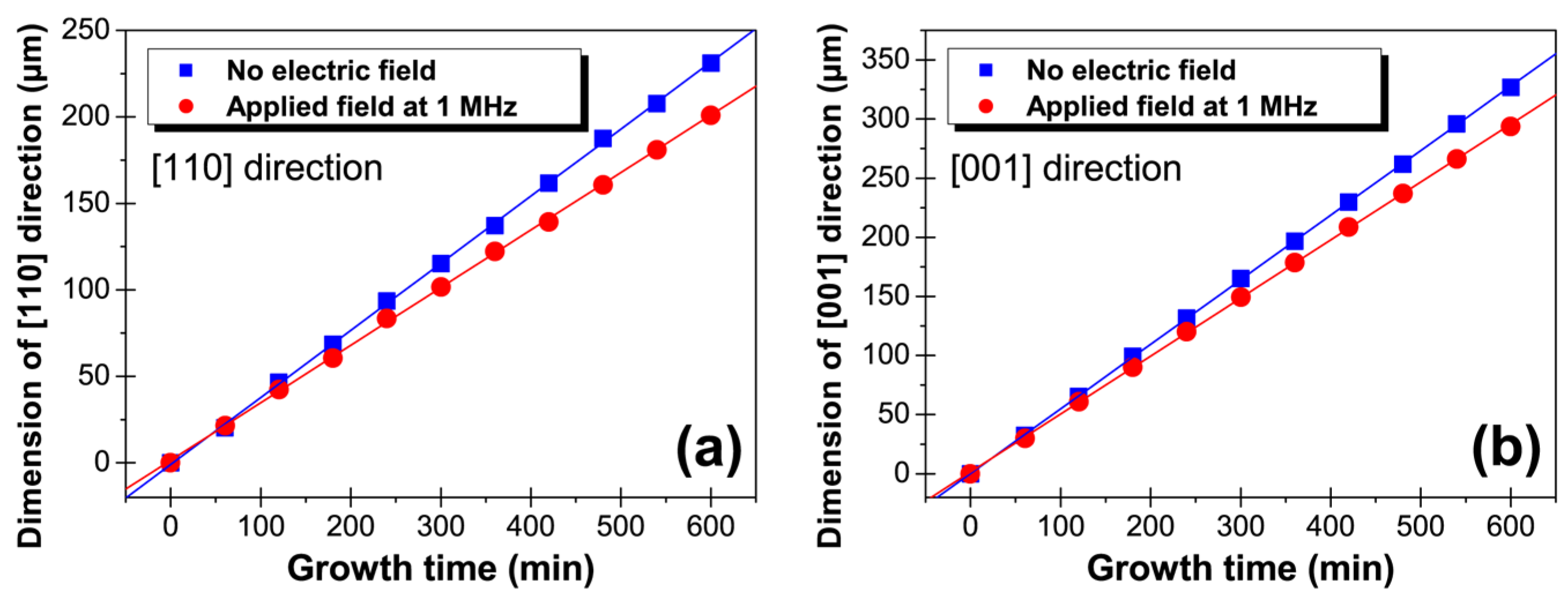
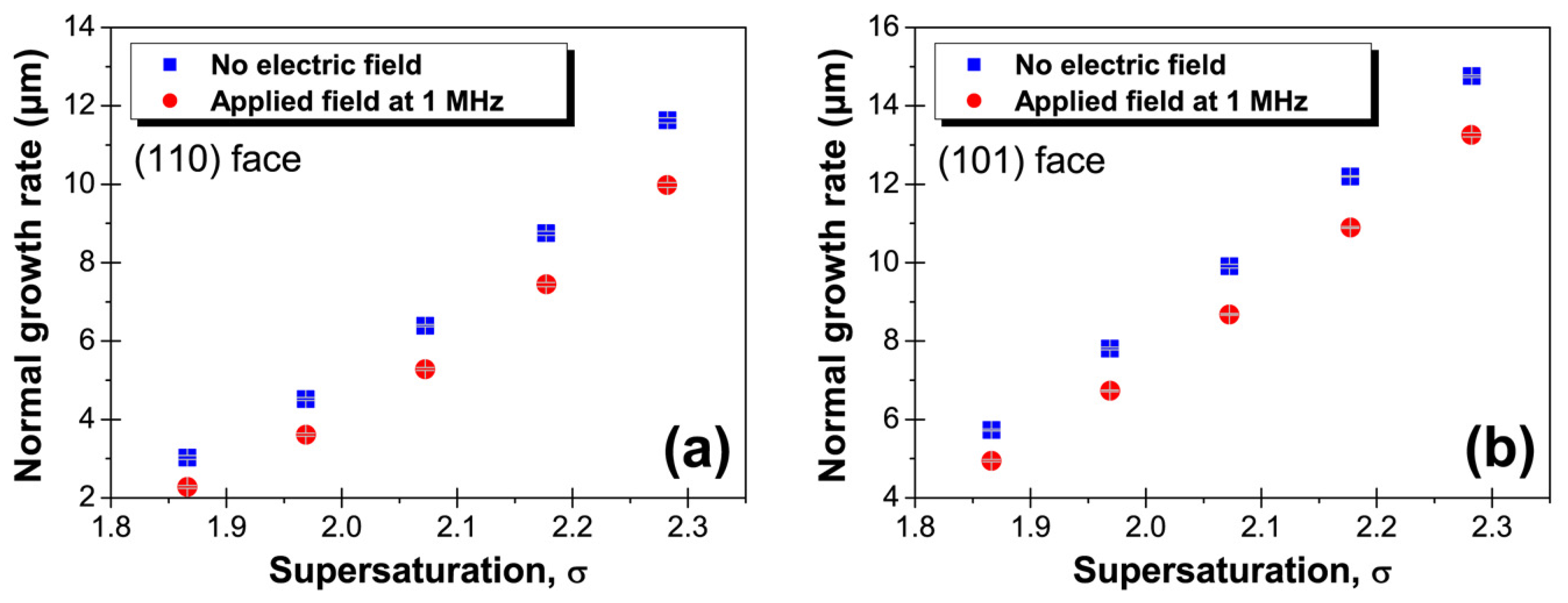
3. Results
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kuhn, P.; Wilson, K.; Patch, M.G.; Stevens, R.C. The genesis of high-throughput structure-based drug discovery using protein crystallography. Curr. Opin. Chem. Biol. 2002, 6, 704–710. [Google Scholar] [CrossRef]
- Chayen, N.E.; Helliwell, J.R.; Snell, E.H. Macromolecular Crystallization and Crystal Perfection; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Sato, T.; Yamada, Y.; Saijo, S.; Hori, T.; Hirose, R.; Tanaka, N.; Sazaki, G.; Nakajima, K.; Igarashi, N.; Tanaka, M.; et al. Enhancement in the perfection of orthorhombic lysozyme crystals grown in a high magnetic field (10 T). Acta Crystallogr. 2000, D56, 1079–1083. [Google Scholar] [CrossRef]
- Lin, S.; Zhou, M.; Azzi, A.; Xu, G.; Wakayama, N.; Ataka, M. Magnet used for protein crystallization: Novel attempts to improve the crystal quality. Biochem. Biophys. Res. Commun. 2000, 275, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Ataka, M.; Wakayama, N. Effects of a magnetic field and magnetization force on protein crystal growth. Why does a magnet improve the quality of some crystals? Acta Crystallogr. 2002, D58, 1708–1710. [Google Scholar] [CrossRef]
- Wakayama, N. Effects of a strong magnetic field on protein crystal growth. Cryst. Growth Des. 2003, 3, 17–24. [Google Scholar] [CrossRef]
- Kinoshita, T.; Ataka, M.; Warizaya, M.; Neya, M.; Fujii, T. Improving quality and harvest period of protein crystals for structure-based drug design: effects of a gel and a magnetic field on bovine adenosine deaminase crystals. Acta Crystallogr. 2003, D59, 1333–1335. [Google Scholar] [CrossRef]
- Lübbert, D.; Meents, A.; Weckert, E. Accurate rocking-curve measurements on protein crystals grown in a homogeneous magnetic field of 2.4 T. Acta Crystallogr. 2004, D60, 987–998. [Google Scholar]
- Moreno, A.; Quiroz-García, B.; Yokaichiya, F.; Stojanoff, V.; Rudolph, P. Protein crystal growth in gels and stationary magnetic fields. Cryst. Res. Technol. 2007, 42, 231–236. [Google Scholar] [CrossRef]
- DeLucas, L.; Smith, C.; Smith, H.; Vijay-Kumar, S.; Senadhi, S.; Ealick, S.; Carter, D.; Snyder, R.; Weber, P.; Salemme, F. Protein crystal growth in microgravity. Science 1989, 246, 651–654. [Google Scholar] [CrossRef] [PubMed]
- McPherson, A. Virus and protein crystal growth on earth and in microgravity. J. Phys. D 1993, 26, 104–112. [Google Scholar] [CrossRef]
- Snell, E.; Weisgerber, S.; Helliwell, J.; Weckert, E.; Holzer, K.; Schroer, K. Improvements in lysozyme protein crystal perfection through microgravity growth. Acta Crystallogr. 1995, D51, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Tanaka, H.; Inaka, K.; Shinozaki, S.; Yamanaka, A.; Takahashi, S.; Yamanaka, M.; Hirota, E.; Sugiyama, S.; Kato, M.; et al. JAXA-GCF project-high-quality protein crystals grown under microgravity environment for better understanding of protein structure. Microgravity Sci. Technol. 2006, 18, 184–189. [Google Scholar] [CrossRef]
- Takahashi, S.; Tsurumura, T.; Aritake, K.; Furubayashi, N.; Sato, M.; Yamanaka, M.; Hirota, E.; Sano, S.; Kobayashi, T.; Tanaka, T.; et al. High-quality crystals of human haematopoietic prostaglandin D synthase with novel inhibitors. Acta Crystallogr. 2010, F66, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Inaka, K.; Takahashi, S.; Aritake, K.; Tsurumura, T.; Furubayashi, N.; Yan, B.; Hirota, E.; Sano, S.; Sato, M.; Kobayashi, T.; et al. High-quality protein crystal growth of mouse lipocalin-type prostaglandin D synthase in microgravity. Cryst. Growth Des. 2011, 11, 2107–2111. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, S.; Kukimoto-Niino, M.; Parker, L.; Handa, N.; Terada, T.; Fujimoto, T.; Terazawa, Y.; Wakiyama, M.; Sato, M.; Sano, S.; et al. Structural basis for the altered drug sensitivities of non-small cell lung cancer-associated mutants of human epidermal growth factor receptor. Oncogene 2012, 32, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Vekilov, P.G.; Thomas, B.R.; Rosenberger, F. Effects of convective solute and impurity transport in protein crystal growth. J. Phys. Chem. B 1998, 102, 5208–5216. [Google Scholar] [CrossRef]
- Kadowaki, A.; Yoshizaki, I.; Adachi, S.; Komatsu, H.; Odawara, O.; Yoda, S. Effects of forced solution flow on protein-crystal quality and growth process. Cryst. Growth Des. 2006, 6, 2398–2403. [Google Scholar] [CrossRef]
- Otálora, F.; Gavira, J.A.; Ng, J.D.; García-Ruiz, J.M. Counterdiffusion methods applied to protein crystallization. Prog. Biophys. Mol. Biol. 2009, 101, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, M.; Kawahara, H.; Sazaki, G.; Maki, S.; Takahashi, Y.; Yoshikawa, H.Y.; Sugiyama, S.; Adachi, H.; Takano, K.; Matsumura, H.; et al. Effects of a forced solution flow on the step advancement on {110} faces of tetragonal lysozyme crystals: direct visualization of individual steps under a forced solution flow. Cryst. Growth Des. 2012, 12, 2856–2863. [Google Scholar] [CrossRef]
- Garcia-Ruiz, J.; Moreno, A. Investigations on protein crystal growth by the gel acupuncture method. Acta Crystallogr. 1994, D50, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Vidal, O.; Robert, M.; Arnoux, B.; Capelle, B. Crystalline quality of lysozyme crystals grown in agarose and silica gels studied by X-ray diffraction techniques. J. Cryst. Growth 1999, 196, 559–571. [Google Scholar] [CrossRef]
- Lorber, B.; Sauter, C.; Ng, J.; Zhu, D.; Giegé, R.; Vidal, O.; Robert, M.; Capelle, B. Characterization of protein and virus crystals by quasi-planar wave X-ray topography: A comparison between crystals grown in solution and in agarose gel. J. Cryst. Growth 1999, 204, 357–368. [Google Scholar] [CrossRef]
- Dong, J.; Boggon, T.J.; Chayen, N.E.; Raftery, J.; Bi, R.C.; Helliwell, J.R. Bound-solvent structures for microgravity-, ground control-, gel-and microbatch-grown hen egg-white lysozyme crystals at 1.8 A resolution. Acta Crystallogr. 1999, D55, 745–752. [Google Scholar] [CrossRef]
- Garcıa-Ruiz, J.; Novella, M.; Moreno, R.; Gavira, J. Agarose as crystallization media for proteins: I: Transport processes. J. Cryst. Growth 2001, 232, 165–172. [Google Scholar] [CrossRef]
- Gavira, J.A.; García-Ruiz, J.M. Agarose as crystallisation media for proteins II: Trapping of gel fibres into the crystals. Acta Crystallogr. 2002, D58, 1653–1656. [Google Scholar] [CrossRef]
- Sugiyama, S.; Maruyama, M.; Sazaki, G.; Hirose, M.; Adachi, H.; Takano, K.; Murakami, S.; Inoue, T.; Mori, Y.; Matsumura, H. Growth of protein crystals in hydrogels prevents osmotic shock. JACS 2012, 134, 5786–5789. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, M.; Hayashi, Y.; Yoshikawa, H.Y.; Okada, S.; Koizumi, H.; Tachibana, M.; Sugiyama, S.; Adachi, H.; Matsumura, H.; Inoue, T.; et al. A crystallization technique for obtaining large protein crystals with increased mechanical stability using agarose gel combined with a stirring technique. J. Cryst. Growth 2016, 452, 172–178. [Google Scholar] [CrossRef]
- Sleutel, M.; Sazaki, G.; Van Driessche, A.E. Spiral-mediated growth can lead to crystals of higher purity. Cryst. Growth Des. 2012, 12, 2367–2374. [Google Scholar] [CrossRef]
- Sleutel, M.; Van Driessche, A.E. On the self-purification cascade during crystal growth from solution. Cryst. Growth Des. 2013, 13, 688–695. [Google Scholar] [CrossRef]
- Hayashi, Y.; Maruyama, M.; Yoshimura, M.; Okada, S.; Yoshikawa, H.Y.; Sugiyama, S.; Adachi, H.; Matsumura, H.; Inoue, T.; Takano, K.; et al. Spiral growth can enhance both the normal growth rate and quality of tetragonal lysozyme crystals grown under a forced solution flow. Cryst. Growth Des. 2015, 15, 2137–2143. [Google Scholar] [CrossRef]
- Tominaga, Y.; Maruyama, M.; Yoshimura, M.; Koizumi, H.; Tachibana, M.; Sugiyama, S.; Adachi, H.; Tsukamoto, K.; Matsumura, H.; Takano, K.; et al. Promotion of protein crystal growth by actively switching crystal growth mode via femtosecond laser ablation. Nat. Photonics 2016, 10, 723–726. [Google Scholar] [CrossRef]
- Taleb, M.; Didierjean, C.; Jelsch, C.; Mangeot, J.; Capelle, B.; Aubry, A. Crystallization of proteins under an external electric field. J. Cryst. Growth 1999, 200, 575–582. [Google Scholar] [CrossRef]
- Taleb, M.; Didierjean, C.; Jelsch, C.; Mangeot, J.; Aubry, A. Equilibrium kinetics of lysozyme crystallization under an external electric field. J. Cryst. Growth 2001, 232, 250–255. [Google Scholar] [CrossRef]
- Nanev, C.; Penkova, A. Nucleation of lysozyme crystals under external electric and ultrasonic fields. J. Cryst. Growth 2001, 232, 285–293. [Google Scholar] [CrossRef]
- Charron, C.; Didierjean, C.; Mangeot, J.; Aubry, A. TheOctopus’ plate for protein crystallization under an electric field. J. Appl. Crystallogr. 2003, 36, 1482–1483. [Google Scholar] [CrossRef]
- Mirkin, N.; Frontana-Uribe, B.; Rodríguez-Romero, A.; Hernández-Santoyo, A.; Moreno, A. The influence of an internal electric field upon protein crystallization using the gel-acupuncture method. Acta Crystallogr. 2003, D59, 1533–1538. [Google Scholar] [CrossRef]
- Moreno, A.; Sazaki, G. The use of a new ad hoc growth cell with parallel electrodes for the nucleation control of lysozyme. J. Cryst. Growth 2004, 264, 438–444. [Google Scholar] [CrossRef]
- Penkova, A.; Gliko, O.; Dimitrov, I.; Hodjaoglu, F.; Nanev, C.; Vekilov, P. Enhancement and suppression of protein crystal nucleation due to electrically driven convection. J. Cryst. Growth 2005, 275, 1527–1532. [Google Scholar] [CrossRef]
- Penkova, A.; Pan, W.; Hodjaoglu, F.; Vekilov, P. Nucleation of protein crystals under the influence of solution shear flow. Ann. N. Y. Acad. Sci. 2006, 1077, 214–231. [Google Scholar] [CrossRef] [PubMed]
- Al-Haq, M.; Lebrasseur, E.; Choi, W.; Tsuchiya, H.; Torii, T.; Yamazaki, H.; Shinohara, E. An apparatus for electric-field-induced protein crystallization. J. Appl. Crystallogr. 2007, 40, 199–201. [Google Scholar] [CrossRef]
- Al-Haq, M.; Lebrasseur, E.; Tsuchiya, H.; Torii, T. Protein crystallization under an electric field. Crystallogr. Rev. 2007, 13, 29–64. [Google Scholar] [CrossRef]
- Hammadi, Z.; Astier, J.; Morin, R.; Veesler, S. Protein crystallization induced by a localized voltage. Cryst. Growth Des. 2007, 7, 1472–1475. [Google Scholar] [CrossRef]
- Pérez, Y.; Eid, D.; Acosta, F.; Marín-García, L.; Jakoncic, J.; Stojanoff, V.; Frontana-Uribe, B.; Moreno, A. Electrochemically assisted protein crystallization of commercial cytochrome c without previous purification. Cryst. Growth Des. 2008, 8, 2493–2496. [Google Scholar]
- Mirkin, N.; Jaconcic, J.; Stojanoff, V.; Moreno, A. High resolution X-ray crystallographic structure of bovine heart cytochrome c and its application to the design of an electron transfer biosensor. Proteins Struct. Funct. Bioinf. 2008, 70, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Chang, H. ac field enhanced protein crystallization. Appl. Phys. Lett. 2008, 92, 223902. [Google Scholar] [CrossRef]
- Revalor, E.; Hammadi, Z.; Astier, J.; Grossier, R.; Garcia, E.; Hoff, C.; Furuta, K.; Okustu, T.; Morin, R.; Veesler, S. Usual and unusual crystallization from solution. J. Cryst. Growth 2010, 312, 939–946. [Google Scholar] [CrossRef]
- Wakamatsu, T. Transparent cell for protein crystallization under low applied voltage. Jpn. J. Appl. Phys. 2011, 50, 048003. [Google Scholar] [CrossRef]
- Wakamatsu, T.; Toyoshima, S.; Shimizu, H. Observation of electric-field induced aggregation in crystallizing protein solutions by forward light scattering. Appl. Phys. Lett. 2011, 99, 153701. [Google Scholar] [CrossRef]
- Koizumi, H.; Fujiwara, K.; Uda, S. Control of nucleation rate for tetragonal hen-egg white lysozyme crystals by application of an electric field with variable frequencies. Cryst. Growth Des. 2009, 9, 2420–2424. [Google Scholar] [CrossRef]
- Koizumi, H.; Tomita, Y.; Uda, S.; Fujiwara, K.; Nozawa, J. Nucleation rate enhancement of porcine insulin by application of an external AC electric field. J. Cryst. Growth 2012, 352, 155–157. [Google Scholar] [CrossRef]
- Koizumi, H.; Fujiwara, K.; Uda, S. Role of the electric double layer in controlling the nucleation rate for tetragonal hen egg white lysozyme crystals by application of an external electric field. Cryst. Growth Des. 2010, 10, 2591–2595. [Google Scholar] [CrossRef]
- Koizumi, H.; Uda, S.; Fujiwara, K.; Tachibana, M.; Kojima, K.; Nozawa, J. Improvement of crystal quality for tetragonal hen-egg white lysozyme crystals under application of an external AC electric field. J. Appl. Crystallogr. 2013, 46, 25–29. [Google Scholar] [CrossRef]
- Koizumi, H.; Uda, S.; Fujiwara, K.; Tachibana, M.; Kojima, K.; Nozawa, J. Enhancement of crystal homogeneity of protein crystals under application of an external alternating current electric field. AIP Conf. Proc. 2014, 1618, 265–268. [Google Scholar]
- Koizumi, H.; Uda, S.; Fujiwara, K.; Tachibana, M.; Kojima, K.; Nozawa, J. Control of subgrain formation in protein crystals by the application of an external electric field. Cryst. Growth Des. 2014, 14, 5662–5667. [Google Scholar] [CrossRef]
- Iimura, Y.; Yoshizaki, I.; Rong, L.; Adachi, S.; Yoda, S.; Komatsu, H. Development of a reusable protein seed crystal processed by chemical cross-linking. J. Cryst. Growth 2005, 275, 554–560. [Google Scholar] [CrossRef]
- Koizumi, H.; Tachibana, M.; Yoshizaki, I.; Fukuyama, S.; Tsukamoto, K.; Suzuki, Y.; Uda, S.; Kojima, K. Dislocations in high-quality glucose isomerase crystals grown from seed crystals. Cryst. Growth Des. 2014, 14, 5111–5116. [Google Scholar] [CrossRef]
- Koizumi, H.; Uda, S.; Tachibana, M.; Tsukamoto, K.; Kojima, K.; Nozawa, J. Crystallization technique for strain-free protein crystals using cross-linked seed crystals. Cryst. Growth Des. 2016, 16, 6089–6094. [Google Scholar] [CrossRef]
- Cacioppo, E.; Pusey, M.L. The solubility of the tetragonal form of hen egg white lysozyme from pH 4.0 to 5.4. J. Cryst. Growth 1991, 114, 286–292. [Google Scholar] [CrossRef]
- Huang, X.; Uda, S.; Yao, X.; Koh, S. In situ observation of crystal growth process of YBCO superconductive oxide with an external electric field. J. Cryst. Growth 2006, 294, 420–426. [Google Scholar] [CrossRef]
- Huang, X.; Uda, S.; Koh, S. Effect of an external electric field on the crystal growth process of YBCO superconductive oxide. J. Cryst. Growth 2007, 307, 432–439. [Google Scholar] [CrossRef]
- Van Driessche, A.E.; Sazaki, G.; Otálora, F.; González-Rico, F.M.; Dold, P.; Tsukamoto, K.; Nakajima, K. Direct and noninvasive observation of two-dimensional nucleation behavior of protein crystals by advanced optical microscopy. Cryst. Growth Des. 2007, 7, 1980–1987. [Google Scholar] [CrossRef]
- Malkin, A.; Chernov, A.; Alexeev, I. Growth of dipyramidal face of dislocation-free ADP crystals; free energy of steps. J. Cryst. Growth 1989, 97, 765–769. [Google Scholar] [CrossRef]
- Sleutel, M.; Willaert, R.; Gillespie, C.; Evrard, C.; Wyns, L.; Maes, D. Kinetics and thermodynamics of glucose isomerase crystallization. Cryst. Growth Des. 2008, 9, 497–504. [Google Scholar] [CrossRef]
- Vekilov, P.G.; Alexander, J.I.D. Dynamics of layer growth in protein crystallization. Chem. Rev. 2000, 100, 2061–2090. [Google Scholar] [CrossRef] [PubMed]
- Durbin, S.; Feher, G. Studies of crystal growth mechanisms of proteins by electron microscopy. J. Mol. Biol. 1990, 212, 763–774. [Google Scholar] [CrossRef]
- Durbin, S.D.; Carlson, W.E. Lysozyme crystal growth studied by atomic force microscopy. J. Cryst. Growth 1992, 122, 71–79. [Google Scholar] [CrossRef]
- Konnert, J.H.; D’Antonio, P.; Ward, K. Observation of growth steps, spiral dislocations and molecular packing on the surface of lysozyme crystals with the atomic force microscope. Acta Crystallogr. 1994, D50, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Nadarajah, A.; Pusey, M.L. Determining the molecular-growth mechanisms of protein crystal faces by atomic force microscopy. Acta Crystallogr. 1999, D55, 1036–1045. [Google Scholar] [CrossRef]
- Markov, I.V. Crystal Growth for Beginners: Fundamentals of Nucleation, Crystal Growth and Epitaxy; World Scientific: Singapore, 2003. [Google Scholar]
- Rashkovich, L.; Smirnov, V.; Petrova, E. Some dielectric properties of monoclinic lysozyme crystals. Phys. Solid State 2008, 50, 631–637. [Google Scholar] [CrossRef]

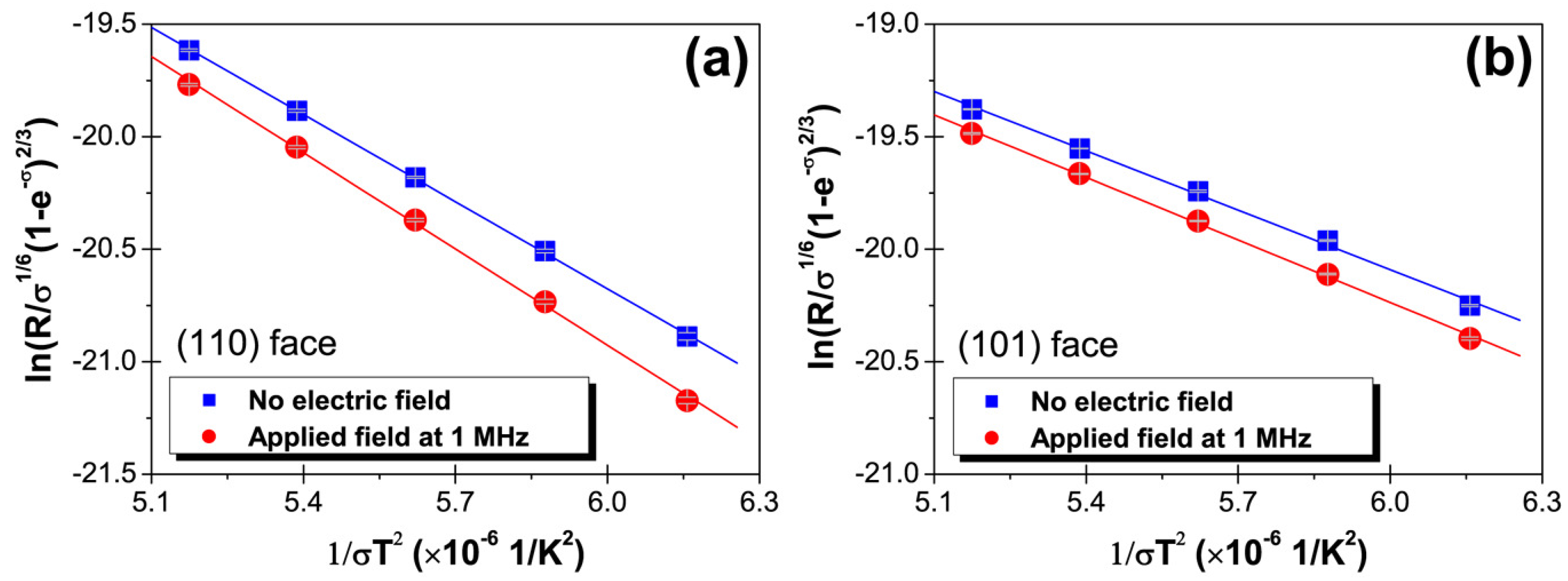
| Surface Energy, (mJ/) | ||
|---|---|---|
| (110) Face | (101) Face | |
| No electric field | 1.189 ± 0.009 | 1.329 ± 0.037 |
| Applied field at 1 MHz | 1.249 ± 0.023 | 1.364 ± 0.027 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koizumi, H.; Uda, S.; Fujiwara, K.; Okada, J.; Nozawa, J. Effect of an External Electric Field on the Kinetics of Dislocation-Free Growth of Tetragonal Hen Egg White Lysozyme Crystals. Crystals 2017, 7, 170. https://doi.org/10.3390/cryst7060170
Koizumi H, Uda S, Fujiwara K, Okada J, Nozawa J. Effect of an External Electric Field on the Kinetics of Dislocation-Free Growth of Tetragonal Hen Egg White Lysozyme Crystals. Crystals. 2017; 7(6):170. https://doi.org/10.3390/cryst7060170
Chicago/Turabian StyleKoizumi, Haruhiko, Satoshi Uda, Kozo Fujiwara, Junpei Okada, and Jun Nozawa. 2017. "Effect of an External Electric Field on the Kinetics of Dislocation-Free Growth of Tetragonal Hen Egg White Lysozyme Crystals" Crystals 7, no. 6: 170. https://doi.org/10.3390/cryst7060170




