Helical Arrangement of 2-(4-hydroxy-3-methoxyphenyl)-Benzothiazole in Crystal Formation and Biological Evaluation on HeLa Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Molecular Structure
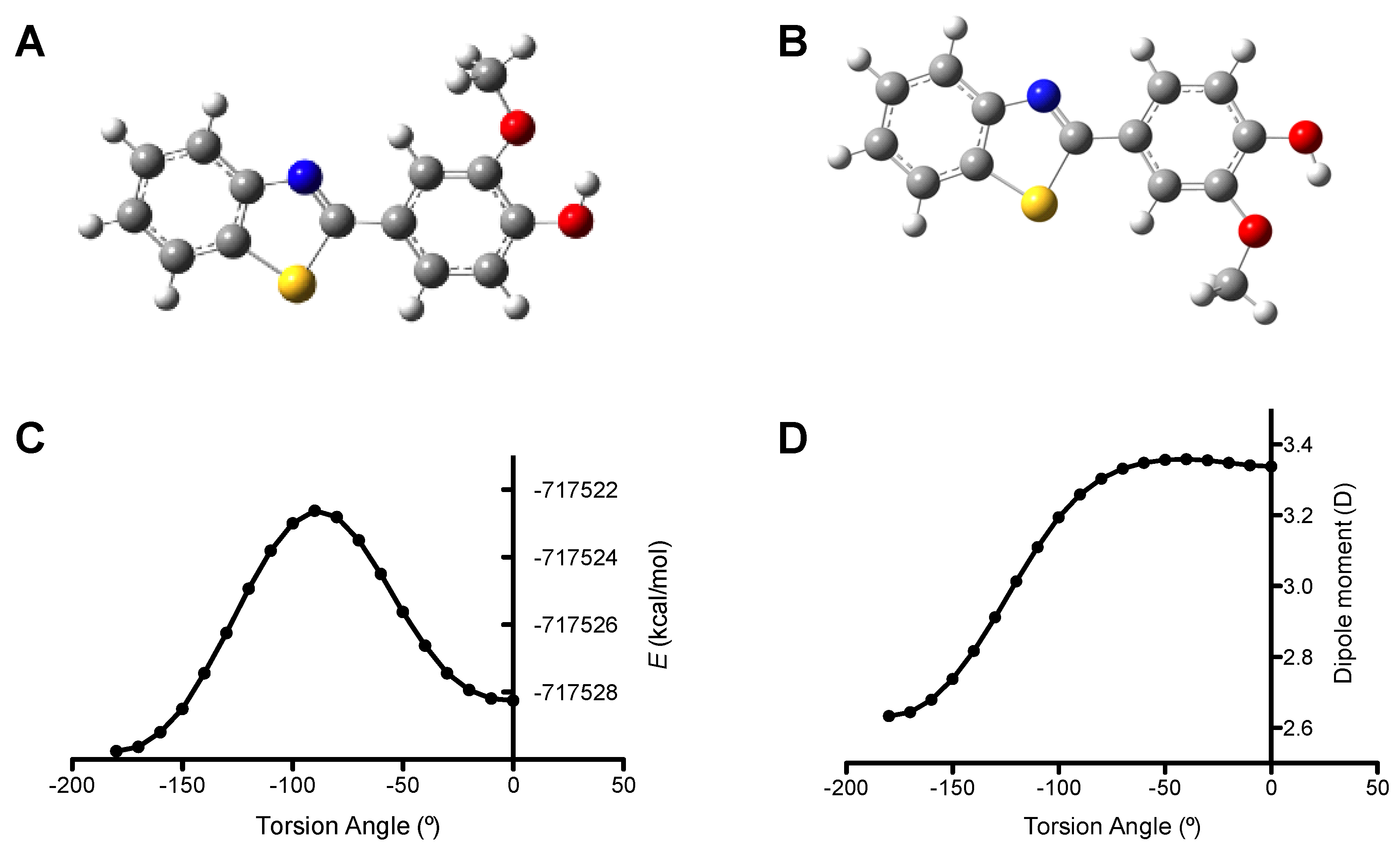
2.2. Theoretical Molecular Modeling
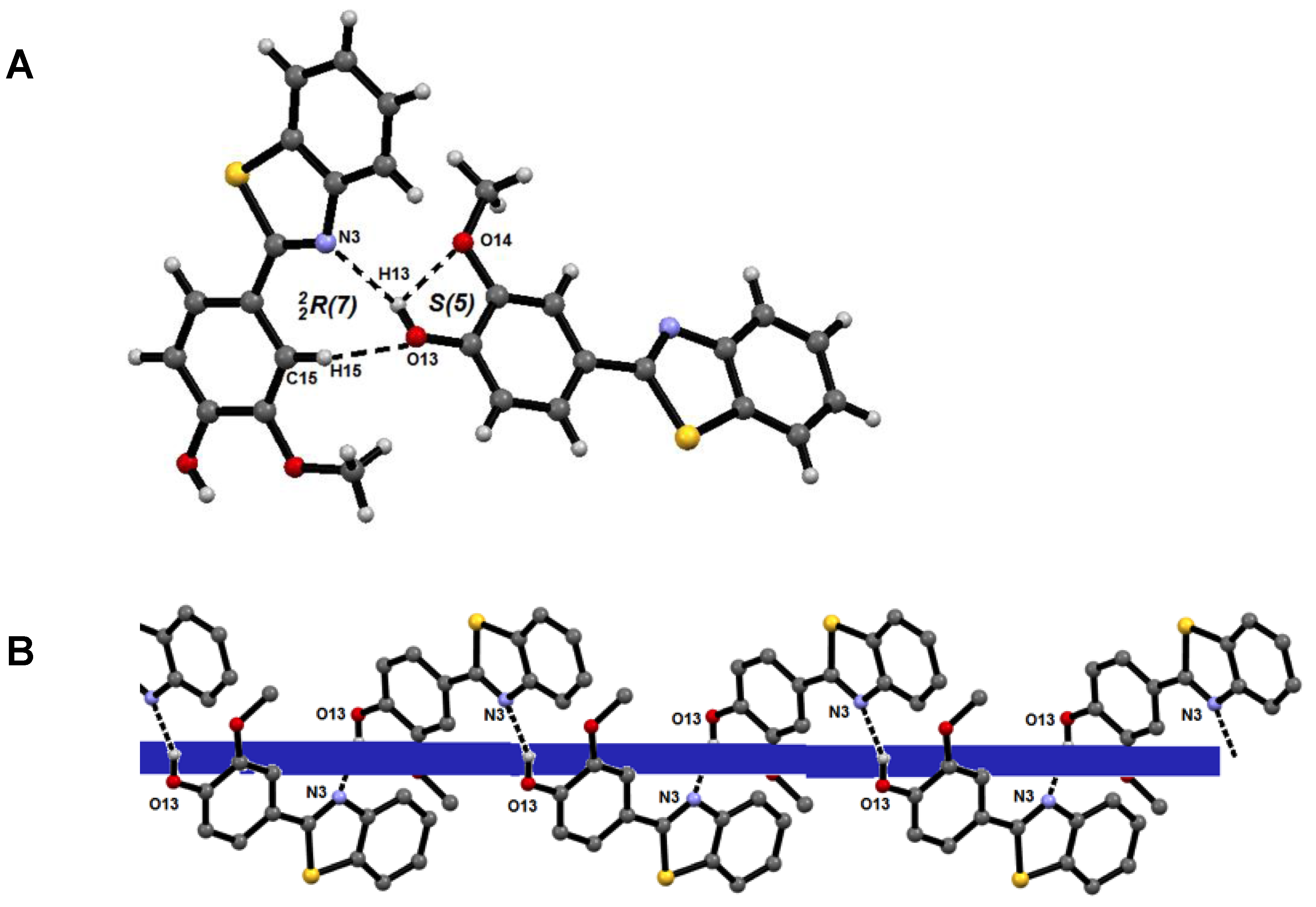
2.3. Supramolecular Structure
2.4. In Vitro Citotoxicity Assay on HeLa Cells
3. Materials and Methods
3.1. Instrumental
3.2. Chemical Synthesis and Crystallization
3.3. X-ray Diffraction Methods
3.4. Molecular Modeling
3.5. In Vitro Cytotoxicity Assay on HeLa Cells
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Benazzouz, A.; Boraud, T.; Dubédat, P.; Boireau, A.; Stutzmann, J.M.; Gross, C. Riluzole prevents MPTP-induced parkinsonism in the rhesus monkey: A pilot study. Eur. J. Pharmacol. 1995, 284, 299–307. [Google Scholar] [CrossRef]
- Bae, H.-J.; Lee, Y.-S.; Kang, D.-W.; Gu, J.-S.; Yoon, B.-W.; Roh, J.-K. Neuroprotective effect of low dose riluzole in gerbil model of transient global ischemia. Neurosci. Lett. 2000, 294, 29–32. [Google Scholar] [CrossRef]
- Delmas, F.; Avellaneda, A.; Di Giorgio, C.; Robin, M.; De Clercq, E.; Timon-David, P.; Galy, J.P. Synthesis and antileishmanial activity of (1,3-benzothiazol-2-yl) amino-9-(10H)-acridinone derivatives. Eur. J. Med. Chem. 2004, 39, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Sheng, C.; Zhu, J.; Zhang, W.; Zhang, M.; Ji, H.; Song, Y.; Xu, H.; Yao, J.; Miao, Z.; Zhou, Y.; et al. 3D-QSAR and molecular docking studies on benzothiazole derivatives as Candida albicans N-myristoyltransferase inhibitors. Eur. J. Med. Chem. 2007, 42, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Soni, B.; Ranawat, M.S.; Sharma, R.; Bhandari, A.; Sharma, S. Synthesis and evaluation of some new benzothiazole derivatives as potential antimicrobial agents. Eur. J. Med. Chem. 2010, 45, 2938–2942. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.K.; Sahu, P.K.; Gupta, S.K.; Thavaselvam, D.; Agarwal, D.D. Synthesis and evaluation of antimicrobial activity of 4H-pyrimido[2,1-b]benzothiazole, pyrazole and benzylidene derivatives of curcumin. Eur. J. Med. Chem. 2012, 54, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Cressier, D.; Prouillac, C.; Hernandez, P.; Amourette, C.; Diserbo, M.; Lion, C.; Rima, G. Synthesis, antioxidant properties and radioprotective effects of new benzothiazoles and thiadiazoles. Bioorg. Med. Chem. 2009, 17, 5275–5284. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Shrivastava, B.; Pandeya, S.N.; Tripathi, L.; Stables, J.P. Design, synthesis, and anticonvulsant evaluation of some novel 1, 3 benzothiazol-2-yl hydrazones/acetohydrazones. Med. Chem. Res. 2012, 21, 2428–2442. [Google Scholar] [CrossRef]
- Navale, A.; Pawar, S.; Deodhar, M.; Kale, A. Synthesis of substituted benzo[d]thiazol-2-ylcarbamates as potential anticonvulsants. Med. Chem. Res. 2013, 22, 4316–4321. [Google Scholar] [CrossRef]
- Mortimer, C.G.; Wells, G.; Crochard, J.P.; Stone, E.L.; Bradshaw, T.D.; Stevens, M.F.; Westwell, A.D. Antitumor benzothiazoles. 26.1 2-(3,4-dimethoxyphenyl)-5- fluorobenzothiazole (GW 610, NSC 721648), a simple fluorinated 2-arylbenzothiazole, shows potent and selective inhibitory activity against lung, colon, and breast cancer cell lines. J. Med. Chem. 2006, 49, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Lowe, P.R.; Stevens, M.F. Antitumor benzothiazoles. 13. (Diacetoxy)iodobenzene (DAIB) oxidation of 2-(4-hydroxy-3- methoxyphenyl)benzothiazole and related compounds in the presence of dienophiles. ARCHIVOC 2000, 779–797. [Google Scholar] [CrossRef]
- Ha, Y.M.; Park, J.Y.; Park, Y.J.; Park, D.; Choi, Y.J.; Kim, J.M.; Lee, E.K.; Han, Y.K.; Kim, J.A.; Lee, J.Y.; et al. Synthesis and biological activity of hydroxy substituted phenyl-benzo[d]thiazole analogues for antityrosinase activity in B16 cells. Bioorg. Med. Chem. Lett. 2011, 21, 2445–2449. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, I.; Chua, M.S.; Browne, H.L.; Trapani, V.; Bradshaw, T.D.; Westwell, A.D.; Stevens, M.F. Antitumor benzothiazoles. 14. Synthesis and in vitro biological properties of fluorinated 2-(4-aminophenyl)benzothiazoles. J. Med. Chem. 2001, 44, 1446–1455. [Google Scholar] [CrossRef] [PubMed]
- Loaiza-Pérez, A.I.; Trapani, V.; Hose, C.; Singh, S.S.; Trepel, J.B.; Stevens, M.F.; Bradshaw, T.D.; Sausville, E.A. Aryl hydrocarbon receptor mediates sensitivity of MCF-7 breast cancer cells to antitumor agent 2-(4-Amino-3-methylphenyl) benzothiazole. Mol. Pharmacol. 2002, 61, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Trapani, V.; Patel, V.; Leong, C.O.; Ciolino, H.P.; Yeh, G.C.; Hose, C.; Trepel, J.B.; Stevens, M.F.; Sausville, E.A.; Loaiza-Pérez, A.I. DNA damage and cell cycle arrest induced by 2-(4-amino-3-methylphenyl)-5- fluorobenzothiazole (5F 203, NSC 703786) is attenuated in aryl hydrocarbon receptor deficient MCF-7 cells. Br. J. Cancer 2003, 88, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Leong, C.O.; Suggitt, M.; Swaine, D.J.; Bibby, M.C.; Stevens, M.F.; Bradshaw, T.D. In vitro, in vivo, and in silico analyses of the antitumor activity of 2-(4-amino- 3-methylphenyl)-5-fluorobenzothiazoles. Mol. Cancer Ther. 2004, 3, 1565–1575. [Google Scholar] [PubMed]
- Hiyoshi, H.; Goto, N.; Tsuchiya, M.; Lida, K.; Nakajima, Y.; Hirata, N.; Kanda, Y.; Nawasawa, K.; Yanagisawa, J. 2-(4-Hydroxy-3-methoxyphenyl)-benzothiazole suppresses tumor progression and metastatic potential of breast cancer cells by inducing ubiquitin ligase CHIP. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.H.; Nikam, M.D.; Mahajan, P.S.; Chate, A.V.; Dabhade, S.K.; Badadhe, P.V. Lithium bromide catalyzed efficient and convenient synthesis of 2-arylbenzothiazole derivatives. Res. Chem. Intermed. 2015, 41, 7509–7516. [Google Scholar] [CrossRef]
- Banerjee, S.; Payra, S.; Saha, A.; Sereda, G. ZnO nanoparticles: A green efficient catalyst for the room temperature synthesis of biologically active 2-aryl-1,3-benzothiazole and 1,3-benzoxazole derivatives. Tetrahedron Lett. 2014, 55, 5515–5520. [Google Scholar] [CrossRef]
- Sudipto, D.; Suvendu, S.; Swarup, K.M.; Partha, K.K.S.; Amit, K.D.; Divesh, N.S.; Bibhutosh, A.; Papu, B. Visible-light-driven synthesis of 2-substituted benzothiazoles using CdS nanosphere as heterogenous recyclable catalyst. Tetrahedron Lett. 2013, 54, 1090–1096. [Google Scholar] [CrossRef]
- Chen, G.F.; Zhang, L.Y.; Jia, H.M.; Chen, B.H.; Li, J.T.; Wang, S.X.; Bai, G.Y. Eco-friendly synthesis of 2-substituted benzothiazoles catalyzed by silica sulfuric acid. Res. Chem. Intermed. 2013, 39, 2077–2086. [Google Scholar] [CrossRef]
- Chen, G.F.; Jia, H.M.; Zhang, L.Y.; Chen, B.H.; Li, J.T. An efficient synthesis of 2-substituted benzothiazoles in the presence of FeCl3/Montmorillonite K-10 under ultrasound irradiation. Ultrason. Sonochem. 2013, 20, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.F.; Xiao, N.; Jing, S.Y.; Li, H.Y.; Chen, B.H.; Han, L.F. A simple and eco-friendly process catalyzed by montmorillonite K-10, with air as oxidant, for synthesis of 2-substituted benzothiazoles. Res. Chem. Intermed. 2015, 41, 5159–5166. [Google Scholar] [CrossRef]
- Hyvl, J.; Srogl, J. Copper-Catalyzed Activation of Disulfides as a Key Step in the Synthesis of Benzothiazole Moieties. Eur. J. Org. Chem. 2010, 15, 2849–2851. [Google Scholar] [CrossRef]
- Saiwen, L.; Chen, R.; Guo, X.; Yang, H.; Deng, G.; Li, C.H. Iron-catalyzed arylation of benzoazoles with aromatic aldehydes using oxygen as oxidant. Green Chem. 2012, 14, 1577–1580. [Google Scholar] [CrossRef]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of bond lengths determined by X-ray and Neutron Diffraction. Part 1. Bond lengths in Organic compounds. J. Chem. Soc. Perkin Trans. II 1987, S1–S19. [Google Scholar] [CrossRef]
- Soon-Beng, T.; Okechuckwu, R.; Siang-Guan, T.; Hoong-Kun, F.; Chinnakali, K. 2-(4-Hydroxyphenyl)benzothiazole. Acta Cryst. 1995, C51, 1629–1630. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.L. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chem. Int. Ed. Engl. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Gómez-Castro, C.Z.; Padilla-Martínez, I.I.; García-Báez, E.V.; Castrejón-Flores, J.L.; Peraza-Campos, A.L.; Martínez-Martínez, F.J. Solid state structure and solution thermodynamics of three-centered hydrogen Bonds (O∙∙∙H∙∙∙O) using N-(2-benzoyl-phenyl) oxalyl derivatives as model compounds. Molecules 2014, 19, 14446–14460. [Google Scholar] [CrossRef] [PubMed]
- Weekes, A.; Bagley, M.C.; Westwell, A.D. An efficient synthetic route to biologically relevant 2- phenylbenzothiazoles substituted on the benzothiazole ring. Tetrahedron 2011, 67, 7743–7747. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Martínez-Ramos, F.; Luna-Palencia, G.R.; Vásquez-Moctezuma, I.; Méndez-Luna, D.; Fragoso-Vázquez, M.J.; Trujillo-Ferrara, J.; Meraz-Ríos, M.A.; Mendieta-Wejebe, J.E.; Correa-Basurto, J. Docking Studies of Glutamine Valproic Acid Derivative (S)-5- amino-2-(heptan-4-ylamino)-5-oxopentanoic Acid (Gln-VPA) on HDAC8 with Biological Evaluation in HeLa Cells. Anticancer Agents Med. Chem. 2016, 16, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
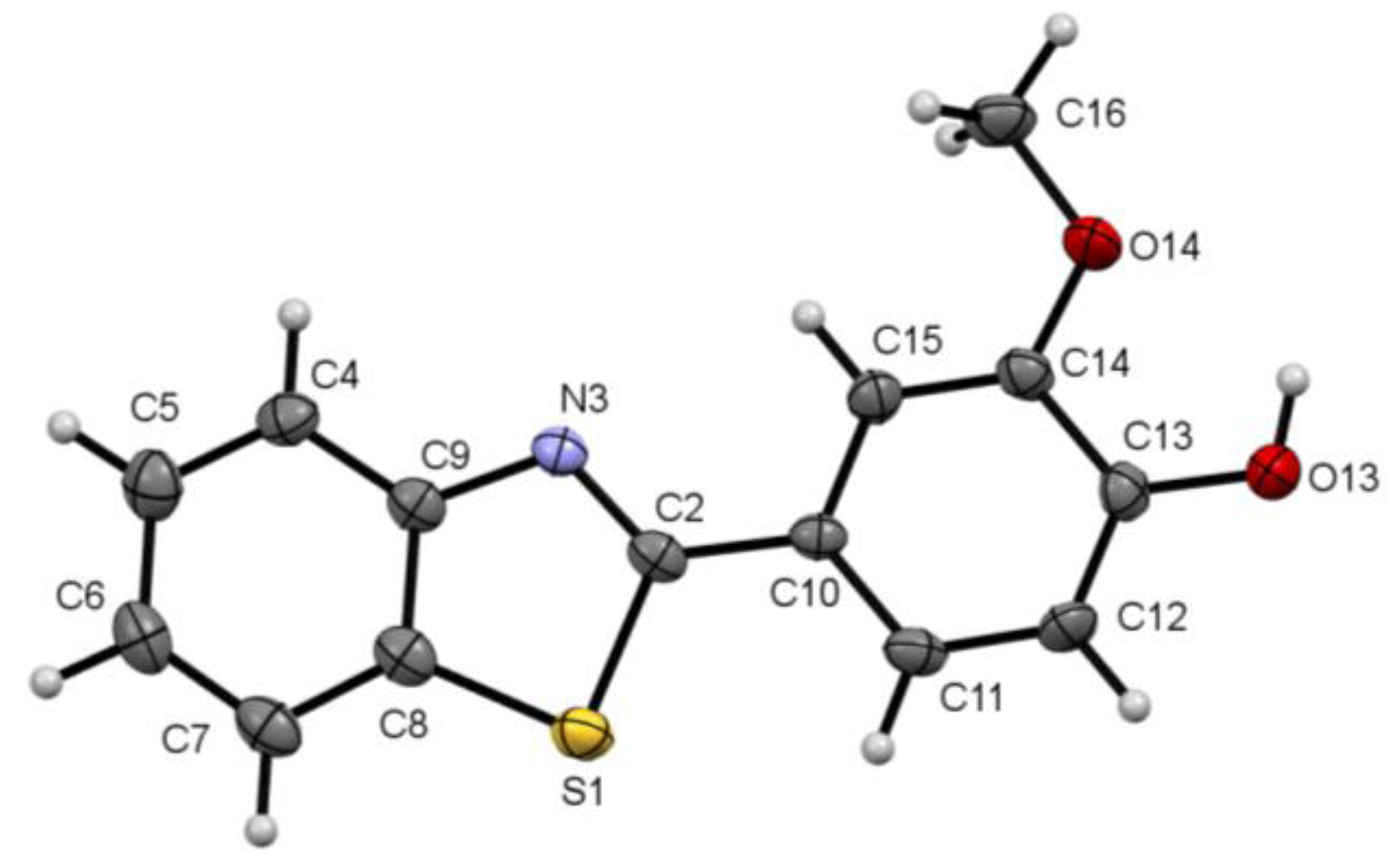

| Modeled Structure | Crystal Structure | |
|---|---|---|
| Energy (kcal/mol) | −717 529.76 | |
| EHOMO (kcal/mol) | −136.91 | |
| ELUMO (kcal/mol) | −41.60 | |
| GAP (kcal/mol) | −178.52 | |
| Bond lengths (Å) | ||
| N3—C9 | 1.398 | 1.368(4) |
| N3—C2 | 1.299 | 1.297(4) |
| C2—S1 | 1.877 | 1.735(3) |
| S1—C8 | 1.815 | 1.724(3) |
| C2—C10 | 1.456 | 1.453(4) |
| C13—C14 | 1.409 | 1.400(5) |
| O13—C13 | 1.381 | 1.337(4) |
| O14—C14 | 1.399 | 1.358(4) |
| Bond angles (°) | ||
| N3—C2—S1 | 113.5 | 115.0(2) |
| C2—C10—C11 | 122.7 | 122.1(3) |
| C2—C10—C15 | 118.1 | 118.7(3) |
| H—O13—C13 | 109.4 | 109 |
| C16—O14—C14 | 118.9 | 118.2(3) |
| Torsion angles (°) | ||
| N3—C2—C10—C11 | −179.987 | 174.7(3) |
| N3—C2—C10—C15 | 0.012 | −5.0(5) |
| C16—O14—C14—C13 | −179.995 | −179.2(3) |
| S1—C2—C10—C15 | −179.985 | 176.2(2) |
| S1—C2—C10—C11 | 0.016 | −4.1(4) |
| O13—C13—C14—O14 | 0.001 | -1.0(5) |
| D—H∙∙∙A | D—H (Å) | H∙∙∙A (Å) | D∙∙∙A (Å) | D—H∙∙∙A (°) |
|---|---|---|---|---|
| O13—H13∙∙∙O14 | 0.84 | 2.24 | 2.669(4) | 112 |
| O13—H13∙∙∙N3 i | 0.84 | 2.05 | 2.844(4) | 157 |
| C15—H15∙∙∙O13 ii | 0.95 | 2.40 | 3.339(4) | 169 |
| C16—H16A∙∙∙Ph iii | 0.95 | 2.77 | 3.571(4) | 140 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuevas-Hernández, R.I.; Padilla-Martínez, I.I.; Martínez-Cerón, S.; Vásquez-Moctezuma, I.; Trujillo-Ferrara, J.G. Helical Arrangement of 2-(4-hydroxy-3-methoxyphenyl)-Benzothiazole in Crystal Formation and Biological Evaluation on HeLa Cells. Crystals 2017, 7, 171. https://doi.org/10.3390/cryst7060171
Cuevas-Hernández RI, Padilla-Martínez II, Martínez-Cerón S, Vásquez-Moctezuma I, Trujillo-Ferrara JG. Helical Arrangement of 2-(4-hydroxy-3-methoxyphenyl)-Benzothiazole in Crystal Formation and Biological Evaluation on HeLa Cells. Crystals. 2017; 7(6):171. https://doi.org/10.3390/cryst7060171
Chicago/Turabian StyleCuevas-Hernández, Roberto I., Itzia I. Padilla-Martínez, Sarai Martínez-Cerón, Ismael Vásquez-Moctezuma, and José G. Trujillo-Ferrara. 2017. "Helical Arrangement of 2-(4-hydroxy-3-methoxyphenyl)-Benzothiazole in Crystal Formation and Biological Evaluation on HeLa Cells" Crystals 7, no. 6: 171. https://doi.org/10.3390/cryst7060171






