Crystal Growth of High-Quality Protein Crystals under the Presence of an Alternant Electric Field in Pulse-Wave Mode, and a Strong Magnetic Field with Radio Frequency Pulses Characterized by X-ray Diffraction
Abstract
:1. Introduction
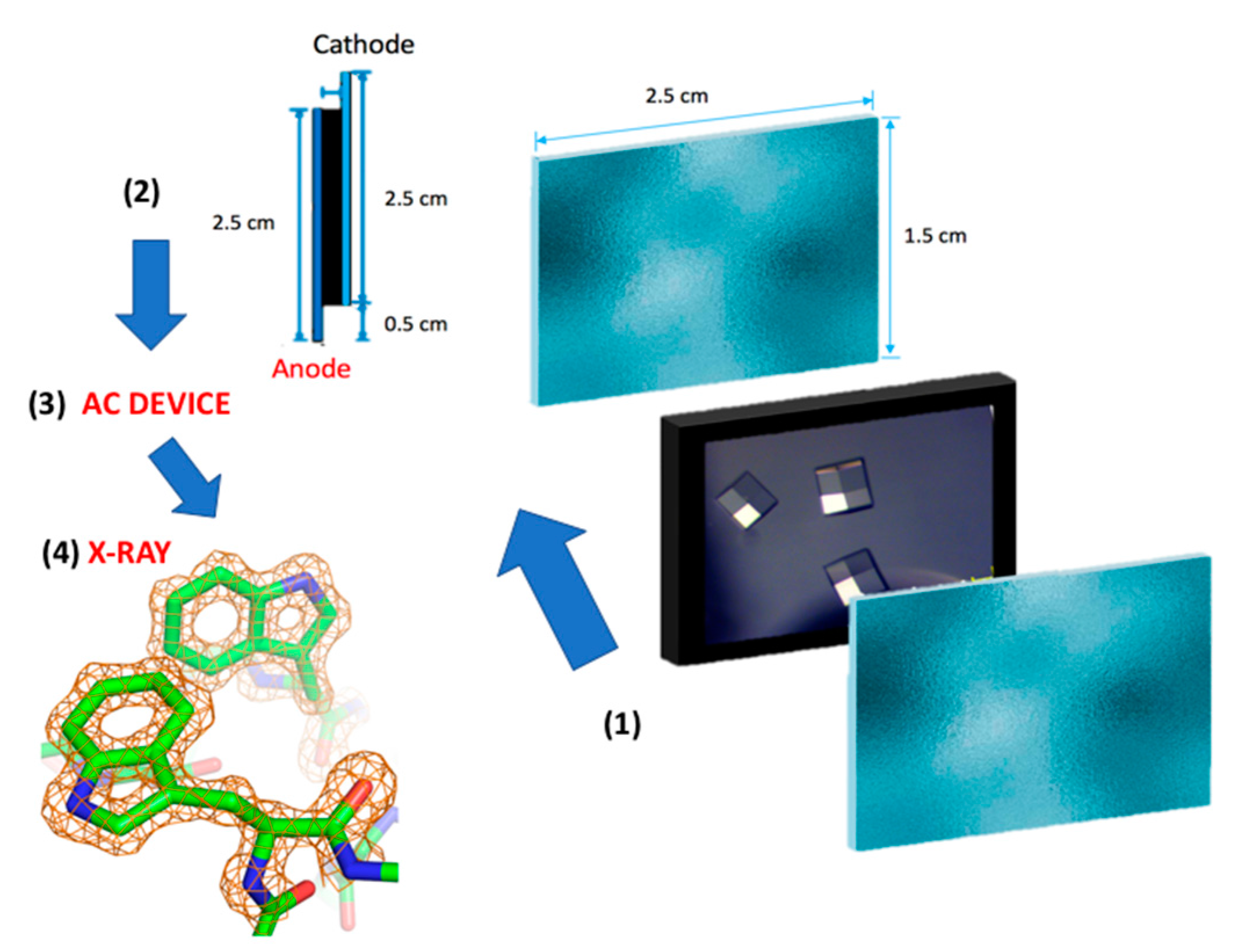
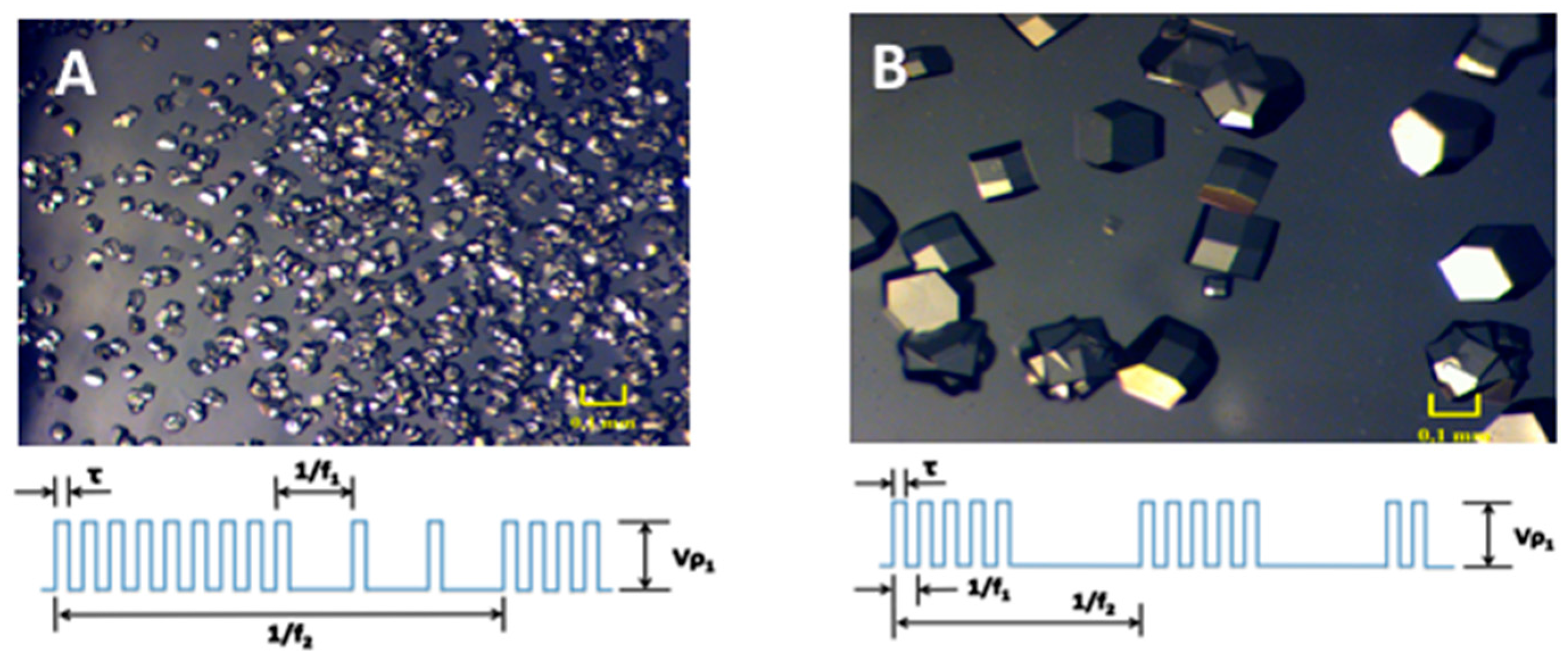
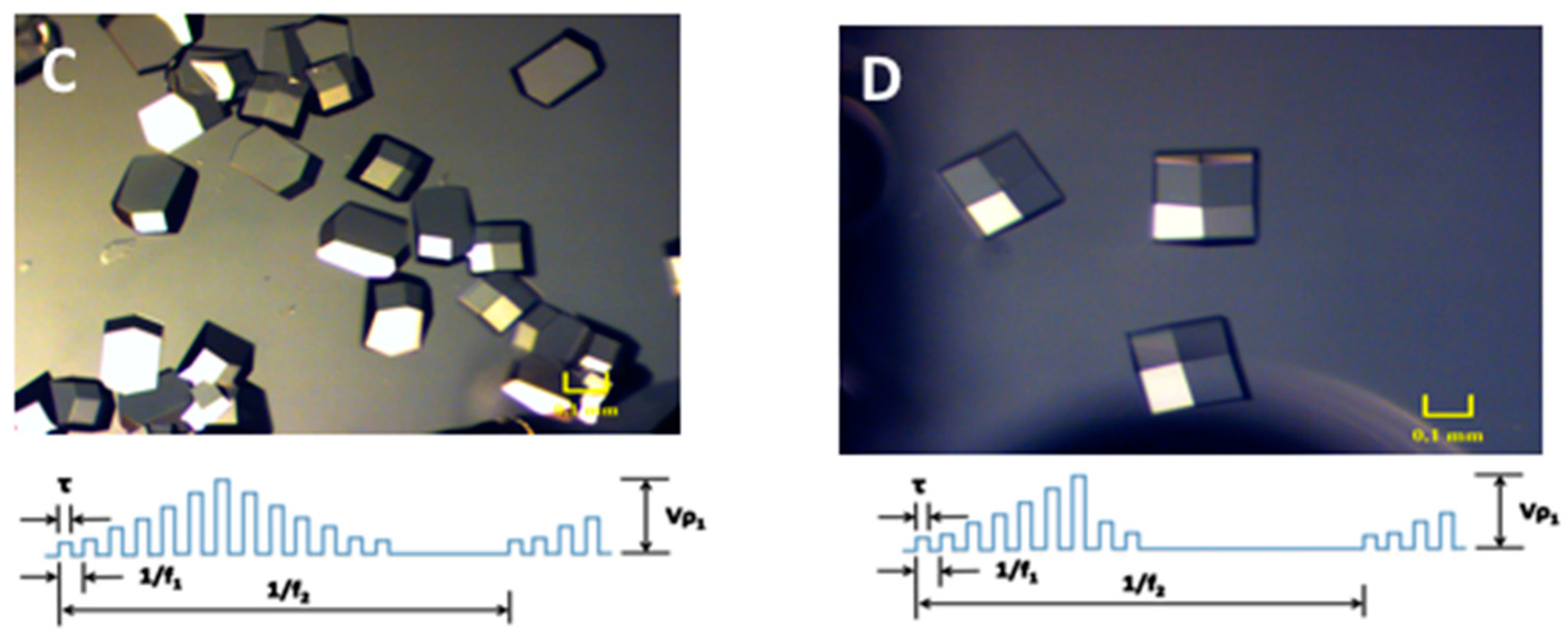
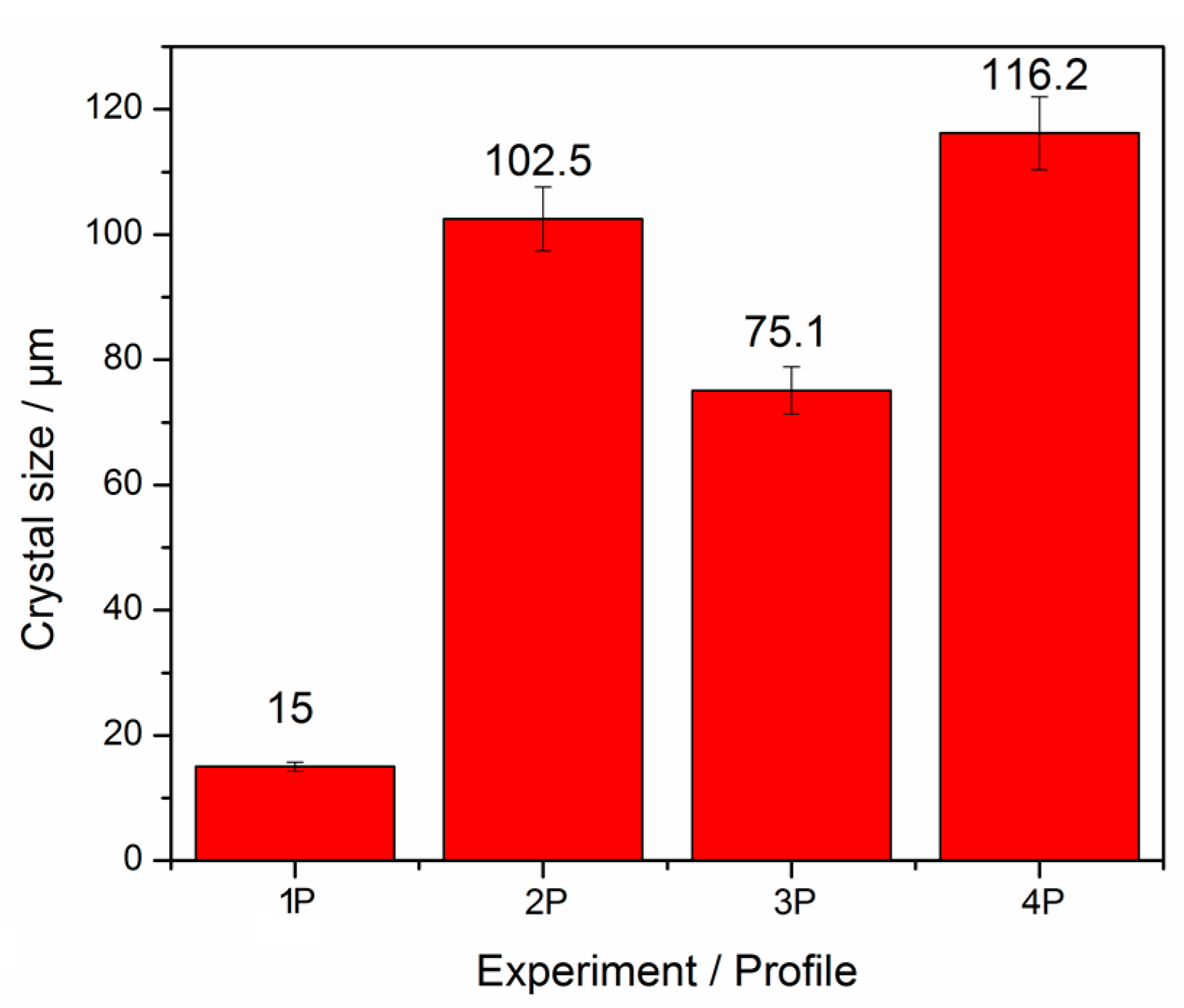
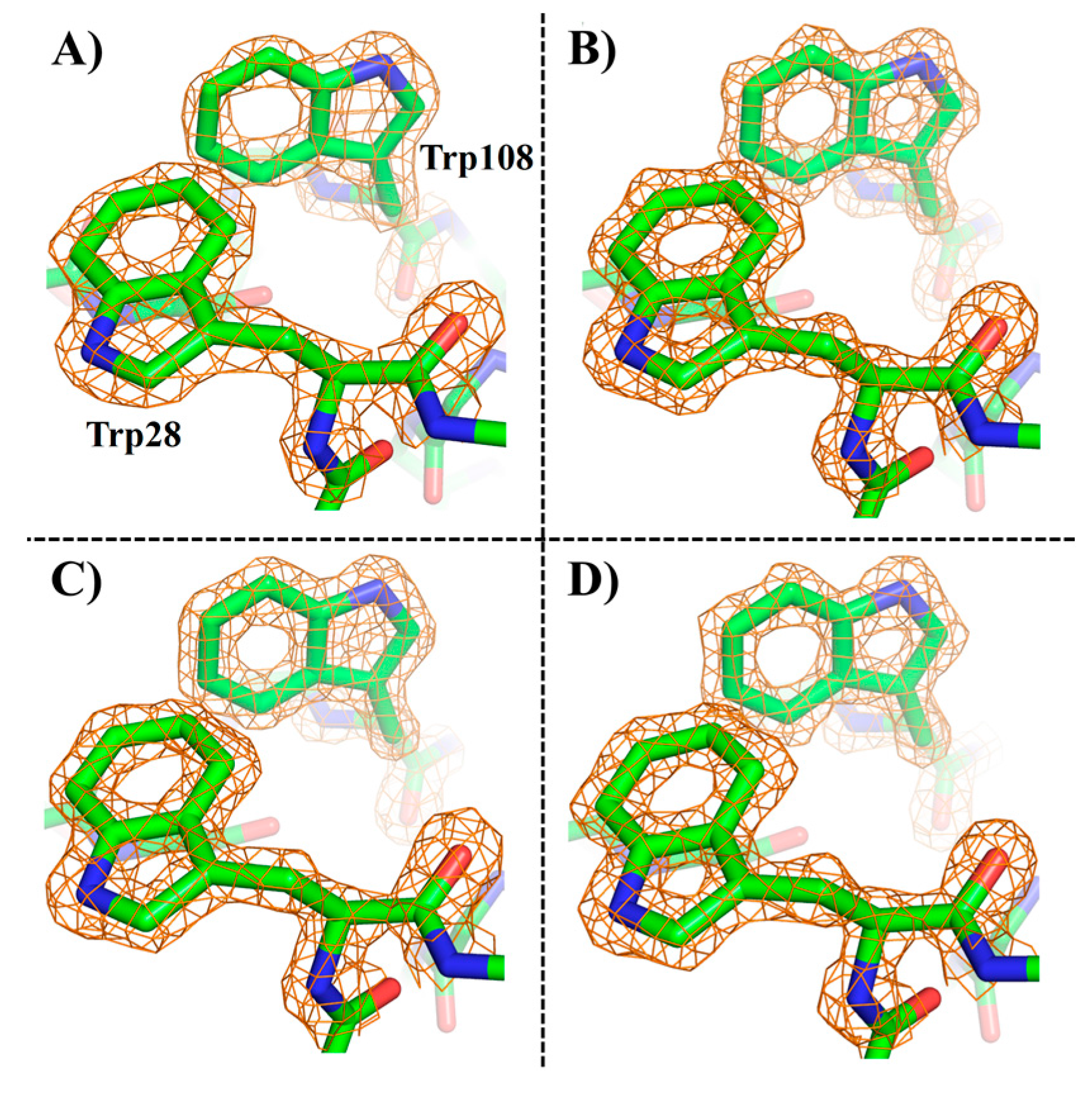
2. Results and Discussion
3. Materials and Methods
3.1. Gel Preparation
3.2. Electric Field Setup
3.3. Magnetic Field Setup
3.4. X-ray Data Diffraction and Data Processing
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Niimura, N.; Bau, R. Neutron protein crystallography: Beyond the folding structure of biological macromolecules. Acta Crystallogr. A 2008, 64, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Blakeley, M.P.; Langan, P.; Niimura, N.; Podjarny, A. Neutron crystallography: Opportunities, challenges, and limitations. Curr. Opin. Struct. Biol. 2008, 18, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Myles, D.A.A.; Dauvergne, F.; Blakeley, M.P.; Meilleur, F. Neutron protein crystallography at ultra-low (<15 k) temperatures. J. Appl. Cryst. 2012, 45, 686–692. [Google Scholar] [CrossRef]
- Yokoyama, T.; Ostermann, A.; Mizuguchi, M.; Niimura, N.; Schader, T.E.; Tanaka, I. Crystallization and preliminary neutron diffraction experiment of human farnesyl pyrophosphate synthase complexed with risedronate. Acta Crystallogr. F Struct. Biol. Commun. 2014, 70, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Blakeley, M.P.; Hasnain, S.S.; Antonyuk, S.V. Sub-atomic resolution X-ray crystallography and neutron crystallography: Promise, challenges and potential. IUCrJ 2015, 2, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Baxter, E.L.; Aguila, L.; Alonso-Mori, R.; Barnes, C.O.; Bonagura, C.A.; Brehmer, W.; Brunger, A.T.; Calero, G.; Caradoc-Davis, T.T.; Chaterjee, R.; et al. High-density grids for efficient data collection from multiple crystals. Acta Crystallogr. D Struct. Biol. 2016, 72, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.E.; Soltis, S.M.; González, A.; Aguila, L.; Alonso-Mori, R.; Barnes, C.O.; Baxter, E.L.; Brehmer, W.; Brewster, A.S.; Brunger, A.T.; et al. Goniometer-based femtosecond crystallography with X-ray free electron lasers. Proc. Natl. Acad. Sci. USA 2014, 111, 17122–17127. [Google Scholar] [CrossRef] [PubMed]
- Calero, G.; Cohen, A.E.; Luft, J.R.; Newman, J.; Snell, E.H. Identifying, studying and making good use of macromolecular crystals. Acta Crystallogr. F Struct. Biol. Commun. 2014, 70, 993–1008. [Google Scholar] [CrossRef] [PubMed]
- Boutet, S.; Lomb, L.; Williams, G.J.; Barends, T.R.M.; Aquil, A.; Doak, R.B.; Weierstall, U.; DePonte, D.P.; Steinbrener, J.; Shoeman, R.L.; et al. High-Resolution Protein Structure Determination by Serial Femtosecond Crystallography. Science 2012, 337, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.S.; Fromme, P. Toward structure determination using membrane-protein nanocrystals and microcrystals. Methods 2011, 55, 387–404. [Google Scholar] [CrossRef] [PubMed]
- Fromme, R.; Ishchenko, A.; Metz, M.; Chowdhury, S.R.; Basu, S.; Boutet, S.; Fromme, P.; White, T.A.; Barty, A.; Spence, J.C.H. Serial femtosecond crystallography of soluble proteins in lipidic cubic phase. IUCrJ 2015, 2, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Inui, Y.; Sekiguchi, Y.; Kobayashi, A.; Oroguchi, T.; Yamamoto, M.; Matsunaga, S.; Nakasako, M. Coherent X-ray Diffraction Imaging of Chloroplasts from Cyanidioschyzon merolae by Using X-ray Free Electron Laser. Plant Cell Physiol. 2015, 56, 1272–1286. [Google Scholar] [CrossRef] [PubMed]
- Sazaki, G.; Yoshida, E.; Komatsu, H.; Nakada, T.; Miyashita, S.; Watanabe, K. Effects of a magnetic field on the nucleation and growth of protein crystals. J. Cryst. Growth 1997, 173, 231–234. [Google Scholar] [CrossRef]
- Wakayama, N.I. Effects of a Strong Magnetic Field on Protein Crystal Growth. Cryst. Growth Des. 2003, 3, 17–24. [Google Scholar] [CrossRef]
- Yin, D.-C. Protein crystallization in a magnetic field. Prog. Cryst. Growth Charact. Mater. 2015, 61, 1–26. [Google Scholar] [CrossRef]
- Pareja-Rivera, C.; Cuéllar-Cruz, M.; Esturau-Escofet, N.; Demitri, N.; Polentarutti, M.; Stojanoff, V.; Moreno, A. Recent advances in the Understanding of the Influence of Electric and Magnetic Fields on Protein Crystal Growth. Cryst. Growth Des. 2017, 17, 135–145. [Google Scholar] [CrossRef]
- Yan, E.K.; Zhang, C.Y.; He, J.; Yin, D.-C. An Overview of Hardware for Protein Crystallization in a Magnetic Field. Int. J. Mol. Sci. 2016, 17, E1906. [Google Scholar] [CrossRef] [PubMed]
- Gavira, J.A.; García-Ruiz, J.M. Effects of a Magnetic Field on Lysozyme Crystal Nucleation and Growth in a Diffusive Environment. Cryst. Growth Des. 2009, 9, 2610–2615. [Google Scholar] [CrossRef]
- Hammadi, Z.; Veesler, S. New approaches on crystallization under electric fields. Prog. Biophys. Mol. Biol. 2009, 101, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, H.; Uda, S.; Fujiwara, K.; Tachibana, M.; Kojima, K.; Nozawa, J. Crystallization of high-quality protein crystals using an external electric field. J. Appl. Cryst. 2015, 48, 1507–1513. [Google Scholar] [CrossRef]
- Sazaki, G.; Moreno, A.; Nakajima, K. Novel coupling effects of the magnetic and electric fields on protein crystallization. J. Cryst. Growth 2004, 262, 499–502. [Google Scholar] [CrossRef]
- Gil-Alvaradejo, G.; Ruiz-Arellano, R.R.; Owen, C.; Rodríguez-Romero, A.; Rudiño-Piñera, E.; Antwi, M.K.; Stojanoff, V.; Moreno, A. Novel Protein Crystal Growth Electrochemical Cell For Applications In X-ray Diffraction and Atomic Force Microscopy. Cryst. Growth Des. 2011, 11, 3917–3922. [Google Scholar] [CrossRef]
- Nieto-Mendoza, E.; Frontana-Uribe, B.A.; Sazaki, G.; Moreno, A. Investigations on electromigration phenomena for protein crystallization using crystal growth cells with multiple electrodes: Effect of the potential control. J. Cryst. Growth 2005, 275, e1437–e1446. [Google Scholar] [CrossRef]
- Frontana-Uribe, B.A.; Moreno, A. On Electrochemically Assisted Protein Crystallization and Related Methods. Cryst. Growth Des. 2008, 8, 4194–4199. [Google Scholar] [CrossRef]
- Pérez, Y.; Eid, D.; Acosta, F.; Marin-Garcia, L.; Jakoncic, J.; Stojanoff, V.; Frontana-Uribe, B.A.; Moreno, A. Electrochemically Assisted Protein Crystallization of Commercial Cytochrome c without Previous Purification. Cryst. Growth Des. 2008, 8, 2493–2496. [Google Scholar] [CrossRef]
- Koizumi, H.; Uda, S.; Fujiwara, K.; Nozawa, J. Control of Effect on the Nucleation Rate for Hen Egg White Lysozyme Crystals under Application of an External AC Electric Field. Langmuir 2011, 27, 8333–8338. [Google Scholar] [CrossRef] [PubMed]
- Flores-Hernandez, E.; Stojanoff, V.; Arreguin-Espinosa, R.; Moreno, A.; Sanchez-Puig, N. An electrically assisted device for protein crystallization in a vapor-diffusion setup. J. Appl. Crystallogr. 2013, 46, 832–834. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, H.; Uda, S.; Fujiwara, K.; Tachibana, M.; Kojima, K.; Nozawa, J. Improvement of crystal quality for tetragonal hen egg white lysozyme crystals under application of an external alternating current electric field. J. Appl. Crystallogr. 2013, 46, 25–29. [Google Scholar] [CrossRef]
- Uda, S.; Koizumi, H.; Nozawa, J.; Fujiwara, K. Crystal Growth under External Electric Fields. In Proceedings of the 2014 International Conference of Computational Methods in Sciences and Engineering (ICCMSE 2014), Athens, Greece, 4–7 April 2014; Volume 1618, pp. 261–264. [Google Scholar]
- De la Mora, E.; Flores-Hernandez, E.; Jakoncic, J.; Stojanoff, V.; Siliqi, D.; Sanchez-Puig, N.; Moreno, A. SdsA polymorph isolation and improvement of their crystal quality using nonconventional crystallization techniques. J. Appl. Crystallogr. 2015, 48, 1551–1559. [Google Scholar] [CrossRef]
- Nanev, C.N.; Penkova, A. Nucleation of lysozyme crystals under external electric and ultrasonic fields. J. Cryst. Growth 2001, 232, 285–293. [Google Scholar] [CrossRef]
- Nanev, C.N.; Penkova, A. Nucleation and growth of lysozyme crystals under external electric field. Coll. Surf. A Physicochem. Eng. Asp. 2002, 209, 139–145. [Google Scholar] [CrossRef]
- Penkova, A.; Gliko, O.; Dimitrov, I.L.; Hodjaoglu, F.V.; Nanev, C.; Vekilov, P.G. Enhancement and suppression of protein crystal nucleation due to electrically driven convection. J. Cryst. Growth 2005, 275, e1527–e1532. [Google Scholar] [CrossRef]
- Taleb, M.; Didierjean, C.; Jelsch, C.; Mangeot, J.P.; Capelle, B.; Aubry, A. Crystallization of proteins under an external electric field. J. Cryst. Growth 1999, 200, 575–582. [Google Scholar] [CrossRef]
- Taleb, M.; Didierjean, C.; Jelsch, C.; Mangeot, J.P.; Aubry, A. Equilibrium kinetics of lysozyme crystallization under an external electric field. J. Cryst. Growth 2001, 232, 250–255. [Google Scholar] [CrossRef]
- Yoshikawa, H.Y.; Murai, R.; Sugiyama, S.; Sazaki, G.; Kitatani, T.; Takahashi, Y.; Adachi, H.; Matsumura, H.; Murakami, S.; Inoue, T.; et al. Femtosecond laser-induced nucleation of protein in agarose gel. J. Cryst. Growth 2009, 311, 956–959. [Google Scholar] [CrossRef]
- Yoshikawa, H.Y.; Murai, R.; Adachi, H.; Sugiyama, S.; Maruyama, M.; Takahashi, Y.; Takano, K.; Matsumura, H.; Inoue, T.; Murakami, S.; et al. Laser ablation for protein crystal nucleation and seeding. Chem. Soc. Rev. 2014, 43, 2147–2158. [Google Scholar] [CrossRef] [PubMed]
- Gavira, J.A.; Garcia-Ruiz, J.M. Agarose as crystallisation media for proteins II: Trapping of gel fibres into the crystals. Acta Crystallogr. D Biol. Crystallogr. 2002, D58, 1653–1656. [Google Scholar] [CrossRef]
- Charron, C.; Robert, M.C.; Capelle, B.; Kadri, A.; Jenner, G.; Giegé, R.; Lorber, B. X-ray diffraction properties of protein crystals prepared in agarose gel under hydrostatic pressure. J. Cryst. Growth 2002, 245, 321–333. [Google Scholar] [CrossRef]
- Sauter, C.; Balg, C.; Moreno, A.; Dhouib, K.; Theobald-Dietrich, A.; Chenevert, R.; Giege, R.; Lorber, B. Agarose gel facilitates enzyme crystal soaking with a ligand analog. J. Appl. Crystallogr. 2009, 42, 279–283. [Google Scholar] [CrossRef]
- Gonzalez-Ramirez, L.A.; Caballero, A.G.; Garcia-Ruiz, J.M. Investigation of the Compatibility of Gels with Precipitating Agents and Detergents in Protein Crystallization Experiments. Cryst. Growth Des. 2008, 8, 4291–4296. [Google Scholar] [CrossRef]
- Gavira, J.A.; Van Driessche, A.E.S.; Garcia-Ruiz, J.M. Growth of Ultrastable Protein-Silica Composite Crystals. Cryst. Growth Des. 2013, 13, 2522–2529. [Google Scholar] [CrossRef]
- Choquesillo-Lazarte, D.; Garcia-Ruiz, J.M. Poly (ethylene) oxide for small-molecule crystal growth in gelled organic solvents. J. Appl. Crystallogr. 2011, 44, 172–176. [Google Scholar] [CrossRef]
- Pietras, Z.; Lin, H.-T.; Surade, S.; Luisi, B.; Slattery, O.; Pos, K.M.; Moreno, A. The use of novel organic gels and hydrogels in protein crystallization. J. Appl. Crystallogr. 2010, 43, 58–63. [Google Scholar] [CrossRef]
- Chayen, N.E.; Saridakis, E. Protein crystallization: From purified protein to diffraction-quality crystal. Nat. Methods 2008, 5, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Saijo, Sh.; Yamada, Y.; Sato, T.; Tanaka, N.; Matsui, T.; Sazaki, G.; N.akajima, K.; Matsuura, Y. Structural consequences of the hen egg-white lysozyme orthorhombic crystal growth in a high magnetic field: Validation of X-ray diffraction intensity, conformational energy searching and quantitative analysis of B factors and mosaicity. Acta Crystallogr. D Biol. Crystallogr. 2005, D61, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Minor, W.; Cymborowski, M.; Otwinowski, Z.; Chruszcz, M. HKL-3000: The integration of data reduction and structure solution-from diffraction images to an initial model in minutes. Acta Crystallogr. D Biol. Crystallogr. 2006, D62, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Vagin, A.; Teplyakov, A. Molecular replacement with MOLREP. Acta Crystallogr. 2010, D66, 22–25. [Google Scholar]
- Mushudov, G.N.; Vagin, A.A.; Dodson, E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 1997, D53, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Emsley, P.; Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; MacArthur, M.W.; Thornton, J.M. PROCHECK: Validation of protein structure coordinates. In International Tables of Crystallography, Volume F. Crystallography of Biological Macromolecules; Rossmann, M.G., Arnold, E., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 722–725. [Google Scholar]
- De Lano, W.L. The PyMOL Molecular Graphics System; DeLano Scientific LLC: San Carlos, CA, USA, 2002. [Google Scholar]


| Control (Solution) | Control (gel) | Lys-Gel (16.5 Tesla) | Lys2HzP2 (Figure 2B) | Lys2HzP3 (Figure 2C) | Lys2HzP4 (Figure 2D) | |
|---|---|---|---|---|---|---|
| Data collection and processing statistics | ||||||
| Wavelength (Å) | 1.54 | 1.54 | 1.54 | 1.54 | 1.54 | 1.54 |
| Space group | P43212 | P43212 | P43212 | P43212 | P43212 | P43212 |
| Unit-cell parameters (Å, °) | a = 77.43 | a = 78.27 | a = 78.27 | a = 77.36 | a = 78.44 | a = 78.31 |
| b = 77.43 | b = 78.27 | b = 78.27 | b = 77.36 | b = 78.44 | b = 78.31 | |
| c = 37.38 | c = 37.21 | c = 37.21 | c = 37.85 | c = 37.08 | c = 37.44 | |
| Reflections (unique) | 10,721 | 19,481 | 22,482 | 18,167 | 22,596 | 25,225 |
| Resolution limits (Å) | 24.5–1.82 | 39.16–1.49 | 39.22–1.39 | 38.79–1.49 | 39.25–1.39 | 39.15–1.34 |
| Completeness (%) | 99.9 (100.0) | 99.40 (90.0) | 99.40 (86.0) | 99.9 (99.9) | 99.9 (99.2) | 100 (99.7) |
| Rmerge (%) | 3.1 (12) | 4.1 (19) | 4.3 (19) | 4.4 (39) | 3.5 (20) | 3.4 (34) |
| I/σ(I) | 77 (4.5) | 76.8 (3.6) | 79 (3.1) | 68 (3.9) | 70 (5.2) | 72 (4.2) |
| Average multiplicity | 13.7 (4.0) | 15.1 (7.3) | 15.1 (7.3) | 14.6 (8.1) | 3.3 (3.1) | 14 (4.1) |
| B factor from Wilson plot (Å2) | 18.7 | 18.2 | 15.1 | 17.2 | 14.6 | 14.1 |
| Refinement statistics | ||||||
| R/Rfree | 0.17/0.21 | 0.181/0.21 | 0.178/0.217 | 0.172/0.221 | 0.177/0.22 | 0.169/0.193 |
| No. of protein atoms | 1032 | 1002 | 1032 | 1197 | 1034 | 1199 |
| No. of water molecules | 129 | 127 | 163 | 190 | 176 | 181 |
| No. of other atoms | 3 | 12 | 6 | 12 | 19 | |
| R.m.s. deviations | ||||||
| Bonds lengths (Å) | 0.017 | 0.018 | 0.018 | 0.021 | 0.017 | 0.014 |
| Bonds angles (°) | 1.744 | 1.991 | 1.890 | 1.960 | 1.59 | 1.401 |
| Average B factor (Å2) | 18.70 | 20.00 | 17.43 | 21.28 | 16.60 | 15.70 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Romero, A.; Esturau-Escofet, N.; Pareja-Rivera, C.; Moreno, A. Crystal Growth of High-Quality Protein Crystals under the Presence of an Alternant Electric Field in Pulse-Wave Mode, and a Strong Magnetic Field with Radio Frequency Pulses Characterized by X-ray Diffraction. Crystals 2017, 7, 179. https://doi.org/10.3390/cryst7060179
Rodríguez-Romero A, Esturau-Escofet N, Pareja-Rivera C, Moreno A. Crystal Growth of High-Quality Protein Crystals under the Presence of an Alternant Electric Field in Pulse-Wave Mode, and a Strong Magnetic Field with Radio Frequency Pulses Characterized by X-ray Diffraction. Crystals. 2017; 7(6):179. https://doi.org/10.3390/cryst7060179
Chicago/Turabian StyleRodríguez-Romero, Adela, Nuria Esturau-Escofet, Carina Pareja-Rivera, and Abel Moreno. 2017. "Crystal Growth of High-Quality Protein Crystals under the Presence of an Alternant Electric Field in Pulse-Wave Mode, and a Strong Magnetic Field with Radio Frequency Pulses Characterized by X-ray Diffraction" Crystals 7, no. 6: 179. https://doi.org/10.3390/cryst7060179






