Microstructure and Electrical Properties of Fe,Cu Substituted (Co,Mn)3O4 Thin Films
Abstract
:1. Introduction
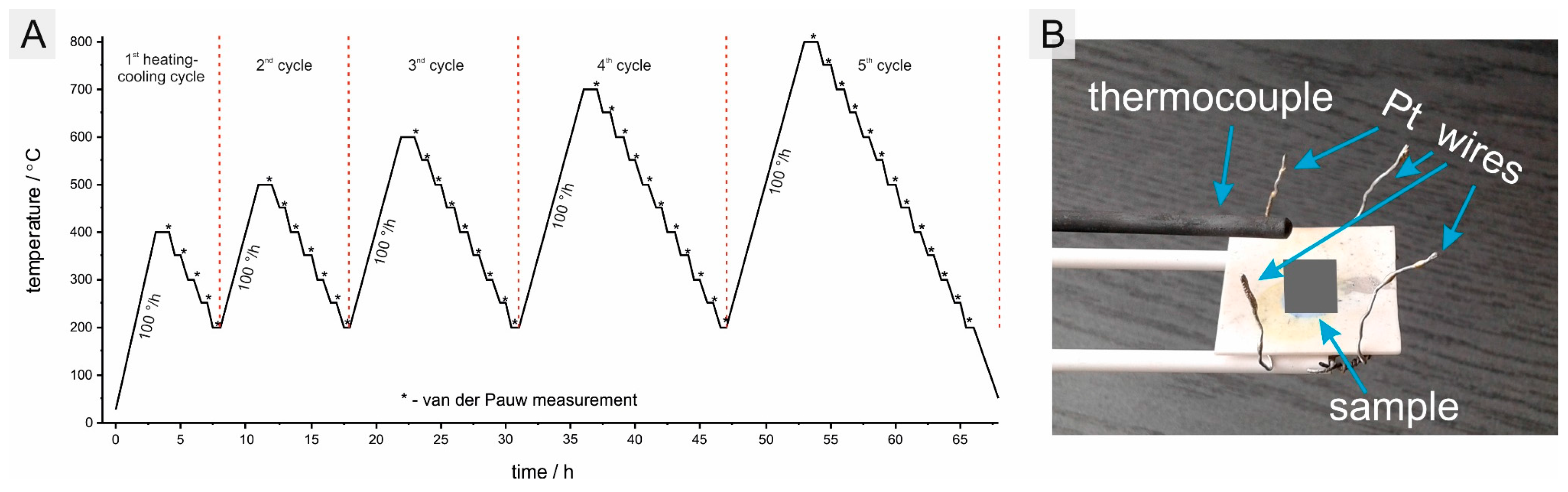
2. Materials and Methods
3. Results and Discussion
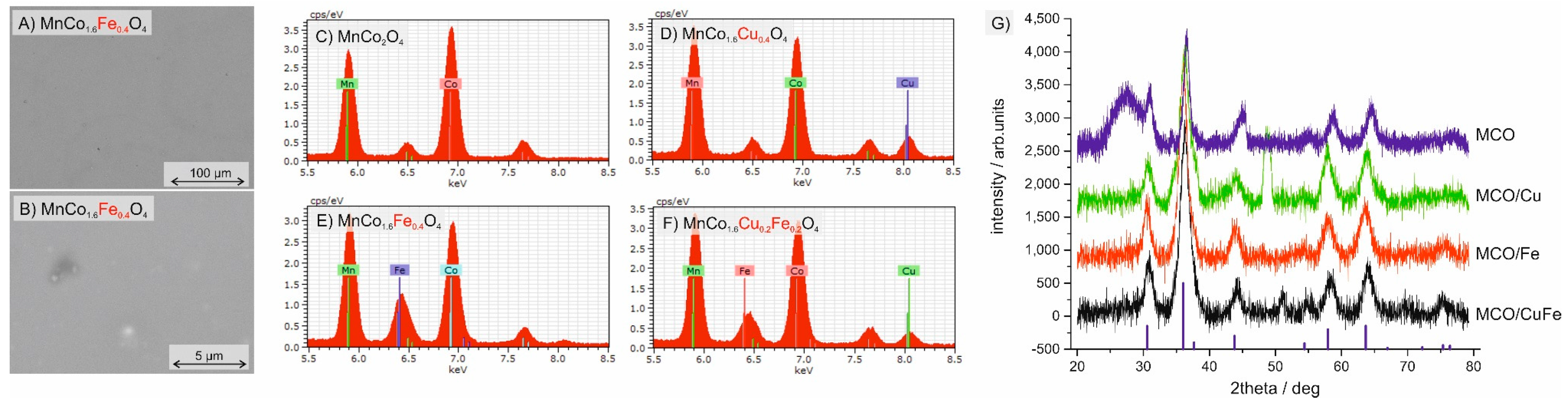
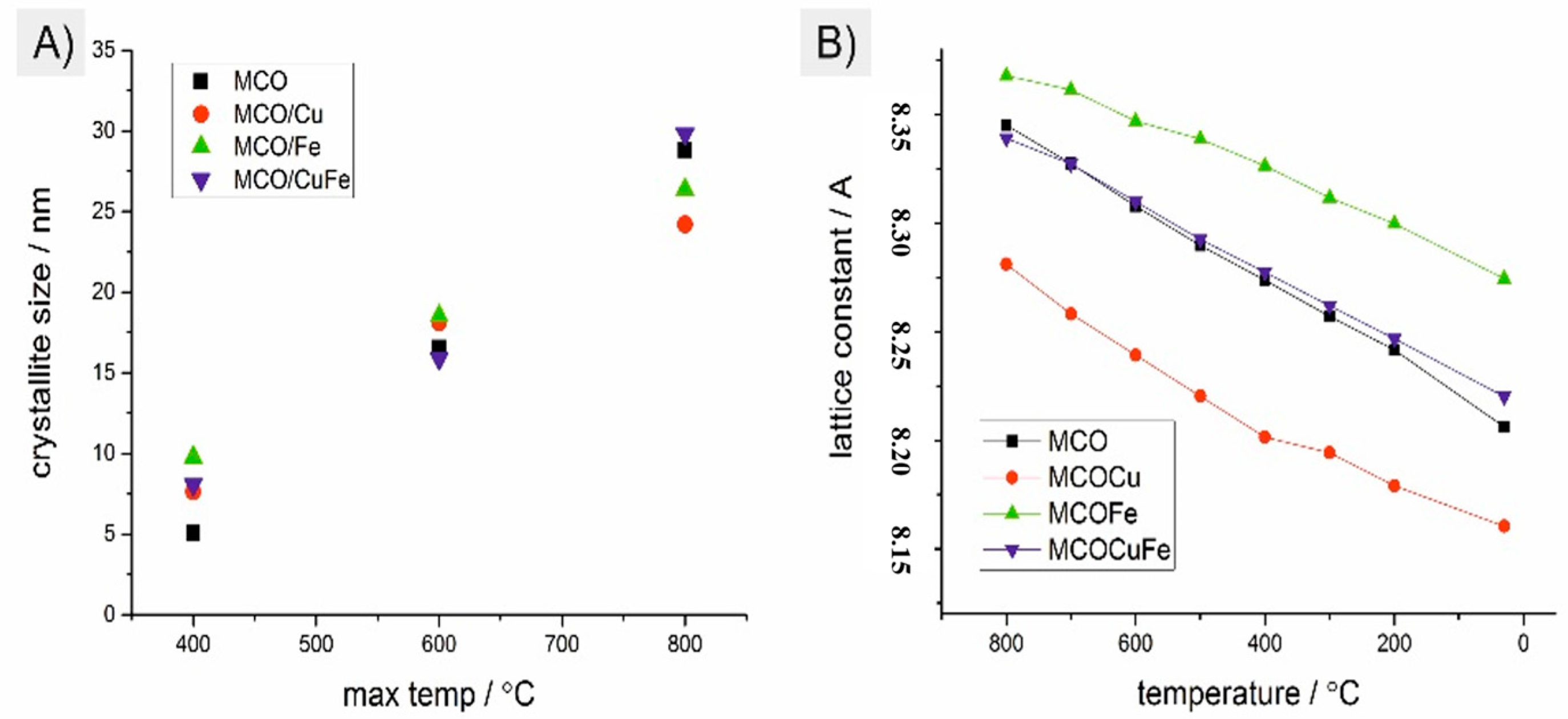
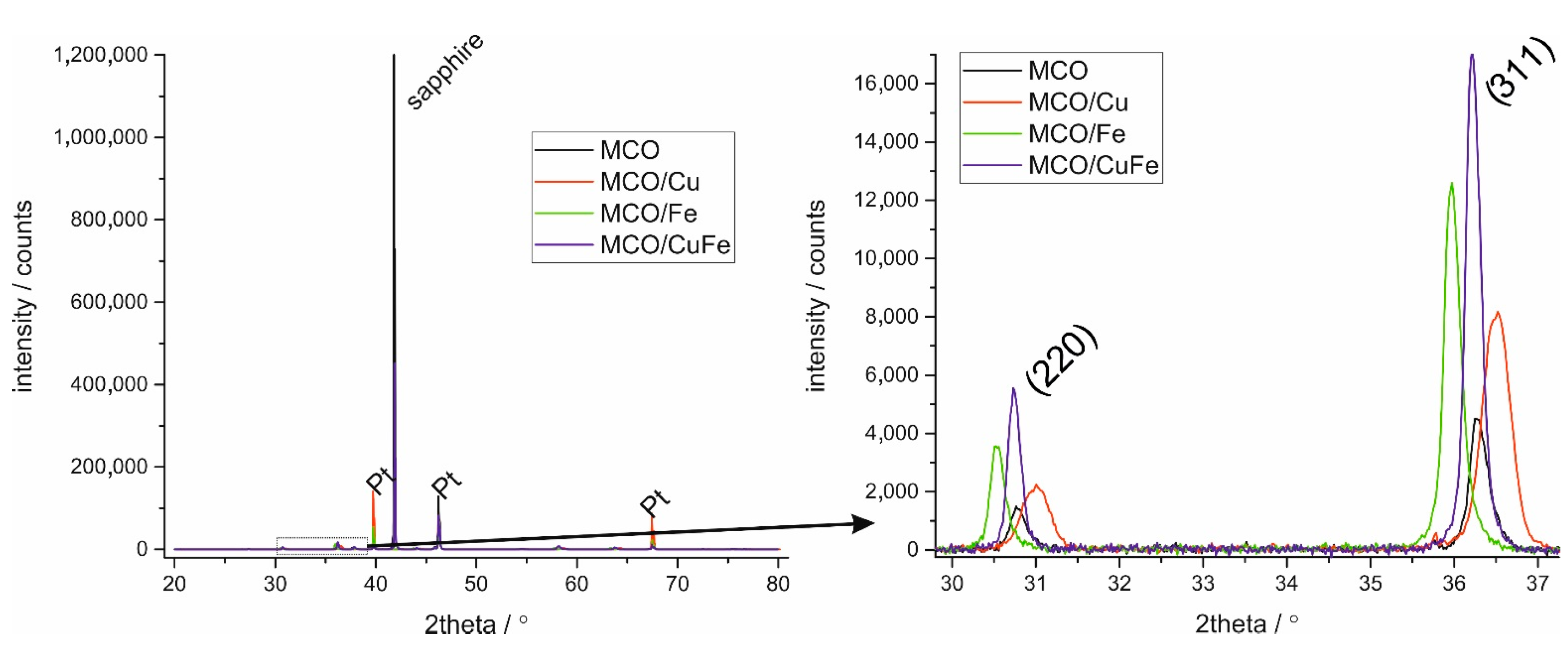
3.1. Analysis of the Produced Coatings
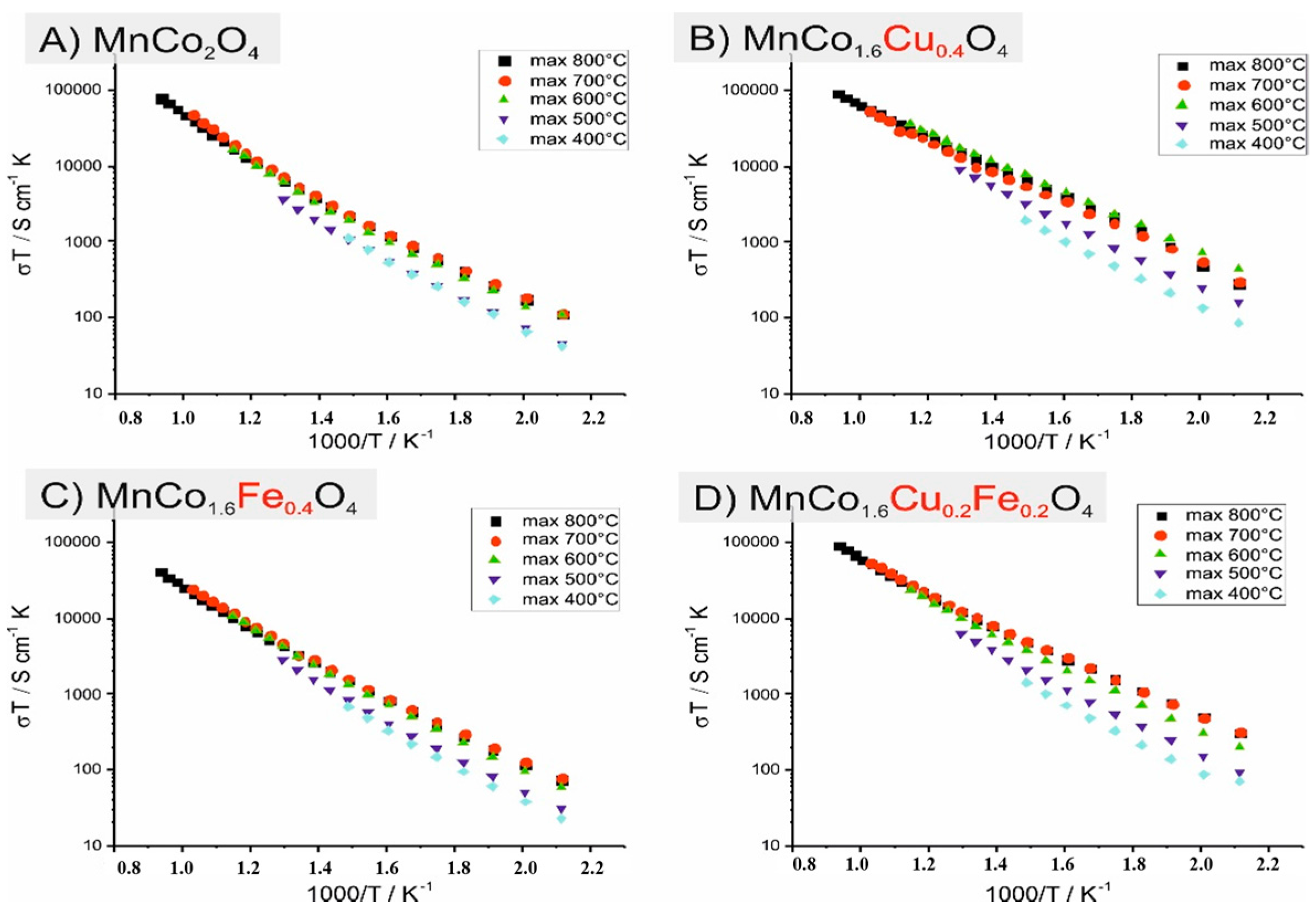
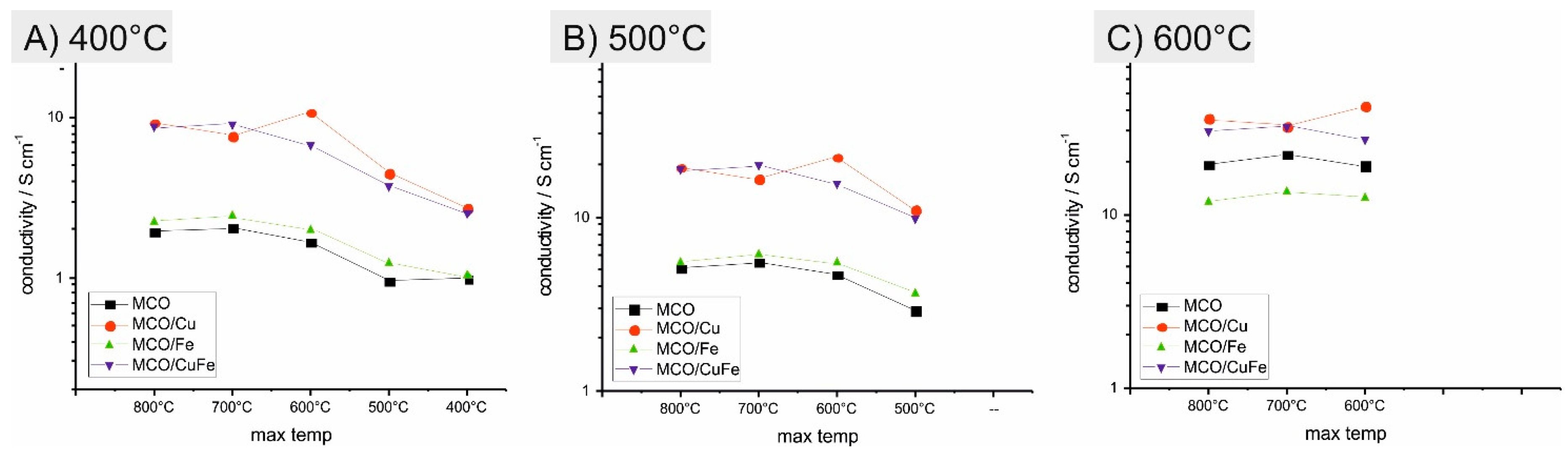
3.2. Electrical Characterization
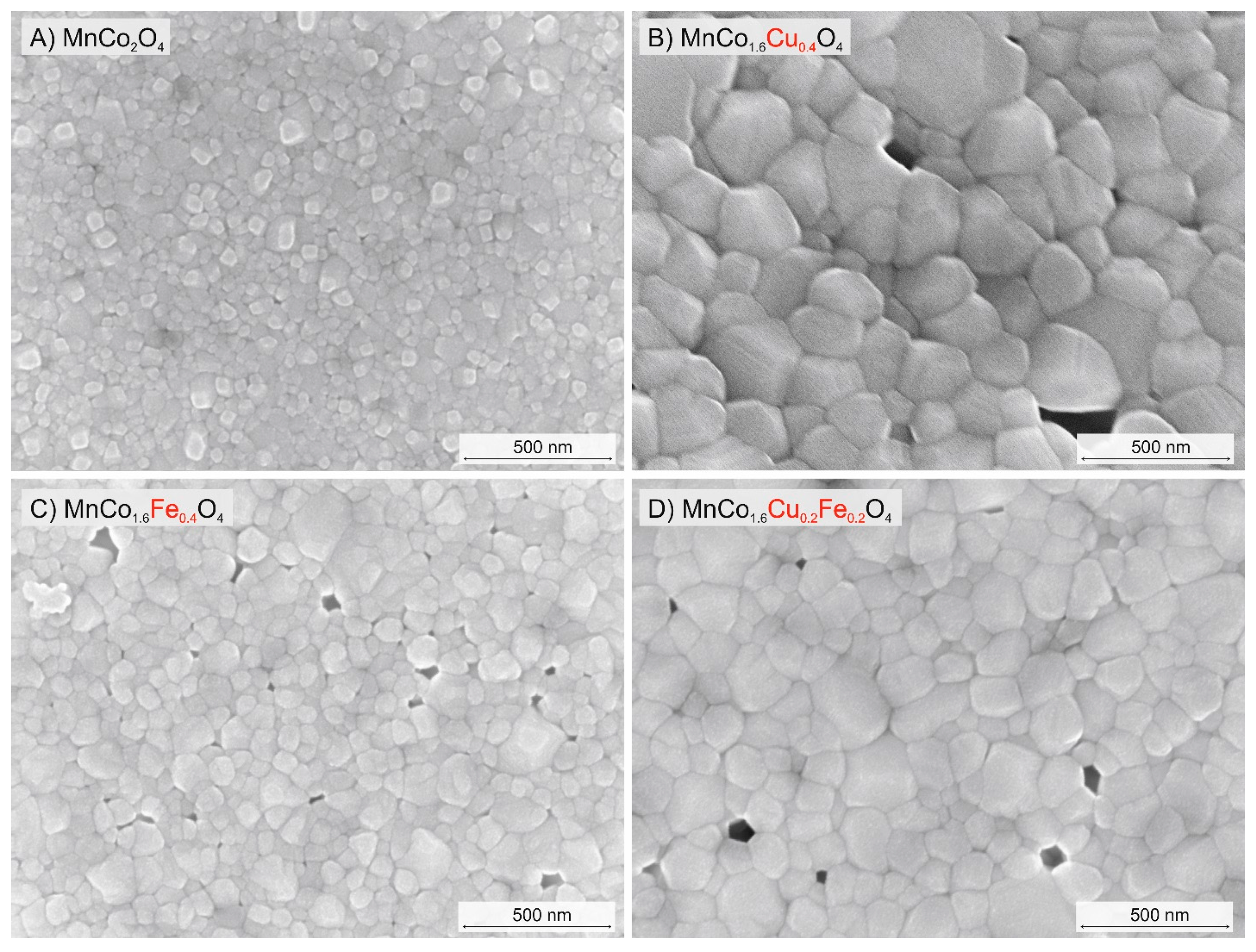
3.3. Microstructural Characterization
4. Summary and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ginley, T.; Wang, Y.; Law, S. Topological insulator film growth by molecular beam epitaxy: A review. Crystals 2016, 6, 154. [Google Scholar] [CrossRef]
- Deng, L.; Wang, K.; Zhao, C.X.; Yan, H.; Britten, J.F.; Xu, G. Phase and texture of solution-processed copper phthalocyanine thin films investigated by two-dimensional grazing incidence X-ray diffraction. Crystals 2011, 1, 112–119. [Google Scholar] [CrossRef]
- Lin, D.; Li, Z.; Li, F.; Cai, C.; Liu, W.; Zhang, S. Tetragonal-to-tetragonal phase transition in lead-free (kxna1−x) nbo3 (x = 0.11 and 0.17) crystals. Crystals 2014, 4, 113–122. [Google Scholar] [CrossRef]
- Chen, Y.; Santos, D.M.F.; Sequeira, C.A.C.; Lobo, R.F.M. Studies of modified hydrogen storage intermetallic compounds used as fuel cell anodes. Crystals 2012, 2, 22–33. [Google Scholar] [CrossRef]
- Molenda, J.; Kupecki, J.; Baron, R.; Blesznowski, M.; Brus, G.; Brylewski, T.; Bucko, M.; Chmielowiec, J.; Cwieka, K.; Gazda, M.; et al. Status report on high temperature fuel cells in Poland—Recent advances and achievements. Int. J. Hydrogen Energy 2017, 42, 4366–4403. [Google Scholar] [CrossRef]
- Wu, X.; Li, S.; Wang, B.; Liu, J.; Yu, M. Controllable synthesis of micro/nano-structured MnCo2O4 with multiporous core–shell architectures as high-performance anode materials for lithium-ion batteries. New J. Chem. 2015, 39, 8416–8423. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, L.; Jiang, Q.; Du, X.; Ji, C.; He, X. Mesoporous MnCo2O4 microflower constructed by sheets for lithium ion batteries. Mater. Lett. 2016, 177, 85–88. [Google Scholar] [CrossRef]
- Akhtar, M.A.; Sharma, V.; Biswas, S.; Chandra, A. Tuning porous nanostructures of MnCo2O4 for application in supercapacitors and catalysis. RSC. Adv. 2016, 6, 96296–96305. [Google Scholar] [CrossRef]
- Larring, Y.; Norby, T. Spinel and perovskite functional layers between plansee metallic interconnect (Cr-5 wt % Fe-1 wt % Y2O3) and ceramic (La0.85Sr0.15)0.91MnO3 cathode materials for solid oxide fuel cells. J. Electrochem. Soc. 2000, 147, 3251–3256. [Google Scholar] [CrossRef]
- Talic, B.; Falk-Windisch, H.; Venkatachalam, V.; Hendriksen, P.V.; Wiik, K.; Lein, H.L. Effect of coating density on oxidation resistance and Cr vaporization from solid oxide fuel cell interconnects. J. Power Sources 2017, 354, 57–67. [Google Scholar] [CrossRef]
- Molin, S.; Jasinski, P.; Mikkelsen, L.; Zhang, W.; Chen, M.; Hendriksen, P.V. Low temperature processed MnCo2O4 and MnCo1.8Fe0.2O4 as effective protective coatings for solid oxide fuel cell interconnects at 750 °C. J. Power Sources 2016, 336, 408–418. [Google Scholar] [CrossRef]
- Montero, X.; Tietz, F.; Sebold, D.; Buchkremer, H.P.; Ringuede, A.; Cassir, M.; Laresgoiti, A.; Villarreal, I. MnCo1.9Fe0.1O4 spinel protection layer on commercial ferritic steels for interconnect applications in solid oxide fuel cells. J. Power Sources 2008, 184, 172–179. [Google Scholar] [CrossRef]
- Masi, A.; Bellusci, M.; McPhail, S.J.; Padella, F.; Reale, P.; Hon, J.E.; Wilckens, R.S.; Carlini, M. The effect of chemical composition on high temperature behaviour of Fe and Cu doped Mn-Co spinels. Ceram. Int. 2017, 43, 2829–2835. [Google Scholar] [CrossRef]
- Shaigan, N.; Qu, W.; Ivey, D.G.; Chen, W. A review of recent progress in coatings, surface modifications and alloy developments for solid oxide fuel cell ferritic stainless steel interconnects. J. Power Sources 2010, 195, 1529–1542. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Y.; Fergus, J.W. Interactions between SOFC interconnect coating materials and chromia. J. Am. Ceram. Soc. 2011, 94, 4490–4495. [Google Scholar] [CrossRef]
- Jasinski, P. Electrical properties of nanocrystalline Sm-doped ceria ceramics. Solid State Ion. 2006, 177, 2509–2512. [Google Scholar] [CrossRef]
- Rupp, J.L.M.; Gauckler, L.J. Microstructures and electrical conductivity of nanocrystalline ceria-based thin films. Solid State Ion. 2006, 177, 2513–2518. [Google Scholar] [CrossRef]
- Rupp, J.L.M.; Infortuna, A.; Gauckler, L.J. Microstrain and self-limited grain growth in nanocrystalline ceria ceramics. Acta Mater. 2006, 54, 1721–1730. [Google Scholar] [CrossRef]
- Molin, S.; Jasinski, P.Z. Improved performance of LaNi0.6Fe0.4O3 solid oxide fuel cell cathode by application of a thin interface cathode functional layer. Mater. Lett. 2017, 189, 252–255. [Google Scholar] [CrossRef]
- Molin, S.; Chrzan, A.; Karczewski, J.; Szymczewska, D.; Jasinski, P. The role of thin functional layers in solid oxide fuel cells. Electrochim. Acta 2016, 204, 136–145. [Google Scholar] [CrossRef]
- Jasinski, P.; Molin, S.; Gazda, M.; Petrovsky, V.; Anderson, H.U. Applications of spin coating of polymer precursor and slurry suspensions for Solid Oxide Fuel Cell fabrication. J. Power Sources 2009, 194, 10–15. [Google Scholar] [CrossRef]
- Szymczewska, D.; Molin, S.; Chen, M.; Hendriksen, P.V.; Jasinski, P. Ceria based protective coatings for steel interconnects prepared by spray pyrolysis. Proc. Eng. 2014, 98, 93–100. [Google Scholar] [CrossRef]
- Brylewski, T.; Kucza, W.; Adamczyk, A.; Kruk, A.; Stygar, M.; Bobruk, M.; Dąbrowa, J. Microstructure and electrical properties of Mn1+xCo2−xO4 (0 ≤ x ≤ 1.5) spinels synthesized using EDTA-gel processes. Ceram. Int. 2014, 40, 13873–13882. [Google Scholar] [CrossRef]
- Brylewski, T.; Kruk, A.; Bobruk, M.; Adamczyk, A.; Partyka, J.; Rutkowski, P. Structure and electrical properties of Cu-doped Mn-Co-O spinel prepared via soft chemistry and its application in intermediate-temperature solid oxide fuel cell interconnects. J. Power Sources 2016, 333, 145–155. [Google Scholar] [CrossRef]
- Maier, J. Physical Chemistry of Ionic Materials: Ions and Electrons in Solids; Wiley: Stuttgart, Germany, 2004. [Google Scholar]
- Masi, A.; Bellusci, M.; McPhail, S.J.; Padella, F.; Reale, P.; Hong, J.; Robert, S.-W.; Carlini, M. Cu-Mn-Co oxides as protective materials in SOFC technology: The effect of chemical composition on mechanochemical synthesis, sintering behaviour, thermal expansion and electrical conductivity. J. Eur. Ceram. Soc. 2017, 37, 661–669. [Google Scholar] [CrossRef]
- Talic, B. Metallic Interconnects for Solid Oxide Fuel Cells: High Temperature Corrosion and Protective Spinel Coatings. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2016. Available online: http://hdl.handle.net/11250/2404554 (accessed on 19 May 2017).
- Chen, G.; Xin, X.; Luo, T.; Liu, L.; Zhou, Y.; Yuan, C.; Lin, C.; Zhan, Z.; Wang, S. Mn1.4Co1.4Cu0.2O4 spinel protective coating on ferritic stainless steels for solid oxide fuel cell interconnect applications. J. Power Sources 2015, 278, 230–234. [Google Scholar] [CrossRef]
- Bobruk, M.; Durczak, K.; Dąbek, J.; Brylewski, T. Structure and electrical properties of Mn-Cu-O Spinels. J. Mater. Eng. Perform. 2017, 26, 1598–1604. [Google Scholar] [CrossRef]
- Szymczewska, D.; Chrzan, A.; Karczewski, J.; Molin, S.; Jasinski, P. Spray pyrolysis of doped-ceria barrier layers for solid oxide fuel cells. Surf. Coat. Technol. 2017, 313, 168–176. [Google Scholar] [CrossRef]
- Szymczewska, D.; Karczewski, J.; Chrzan, A.; Jasinski, P. CGO as a barrier layer between LSCF electrodes and YSZ electrolyte fabricated by spray pyrolysis for solid oxide fuel cells. Solid State Ion. 2017, 302, 113–117. [Google Scholar] [CrossRef]



| MnCo2O4 | MnCo1.6Cu0.4O4 | MnCo1.6Fe0.4O4 | MnCo1.6Cu0.2Fe0.2O4 | |
|---|---|---|---|---|
| MCO | MCO/Cu | MCO/Fe | MCO/CuFe | |
| O | 60.0 | 56.7 | 60.2 | 58.5 |
| Mn | 12.3 | 13.4 | 12.7 | 12.6 |
| Co | 27.7 | 23.9 | 21.6 | 22.9 |
| Cu | 0 | 6.0 | 0 | 3.2 |
| Fe | 0 | 0 | 5.5 | 2.8 |
| (Co + Fe + Cu)/Mn | 2.25 | 2.23 | 2.14 | 2.30 |
| EDS determined stoichiometry | Mn0.92Co2.08 | Mn0.93Co1.66Cu0.42 | Mn0.96Co1.63Fe0.41 | Mn0.91Co1.66Cu0.23Fe0.20 |
| σ 800 °C | EA (max 800 °C) | EA (max 700 °C) | EA (max 600 °C) | EA (max 500 °C) | EA (max 400 °C) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Name/S cm−1/eV | HT | LT | HT | LT | HT | LT | HT | LT | ||
| MCO | 73.0 | 0.58 | 0.40 | 0.59 | 0.40 | 0.57 | 0.41 | 0.54 | 0.42 | 0.45 |
| MCO/Cu | 85.2 | 0.43 | 0.46 | 0.44 | 0.41 | 0.40 | 0.38 | 0.44 | 0.40 | 0.44 |
| MCO/Fe | 37.9 | 0.52 | 0.41 | 0.52 | 0.40 | 0.54 | 0.41 | 0.54 | 0.43 | 0.44 |
| MCO/CuFe | 86.6 | 0.48 | 0.38 | 0.48 | 0.38 | 0.47 | 0.40 | 0.48 | 0.41 | 0.44 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymczewska, D.; Molin, S.; Hendriksen, P.V.; Jasiński, P. Microstructure and Electrical Properties of Fe,Cu Substituted (Co,Mn)3O4 Thin Films. Crystals 2017, 7, 185. https://doi.org/10.3390/cryst7070185
Szymczewska D, Molin S, Hendriksen PV, Jasiński P. Microstructure and Electrical Properties of Fe,Cu Substituted (Co,Mn)3O4 Thin Films. Crystals. 2017; 7(7):185. https://doi.org/10.3390/cryst7070185
Chicago/Turabian StyleSzymczewska, Dagmara, Sebastian Molin, Peter Vang Hendriksen, and Piotr Jasiński. 2017. "Microstructure and Electrical Properties of Fe,Cu Substituted (Co,Mn)3O4 Thin Films" Crystals 7, no. 7: 185. https://doi.org/10.3390/cryst7070185






