1. Introduction
Porous Si (PSi) is a versatile material that has generated interest because of its compatibility with biological materials and its different applications, such as in medicine [
1,
2] and molecular electronics [
3,
4,
5]. Cytochrome C is a protein whose solid-state electrical properties have been studied in recent years [
6,
7,
8]. Thin films of cytochrome C in its solid state have conduction mechanisms both inside the molecule and at the macroscopic level in the form of a thin film [
9,
10]. The possibility of using these conduction mechanisms, using PSi as a base for infiltrating cytochrome C, allows the study of the thin films’ electrical properties as a hybrid material—that is, an effective medium formed by PSi and cytochrome C.
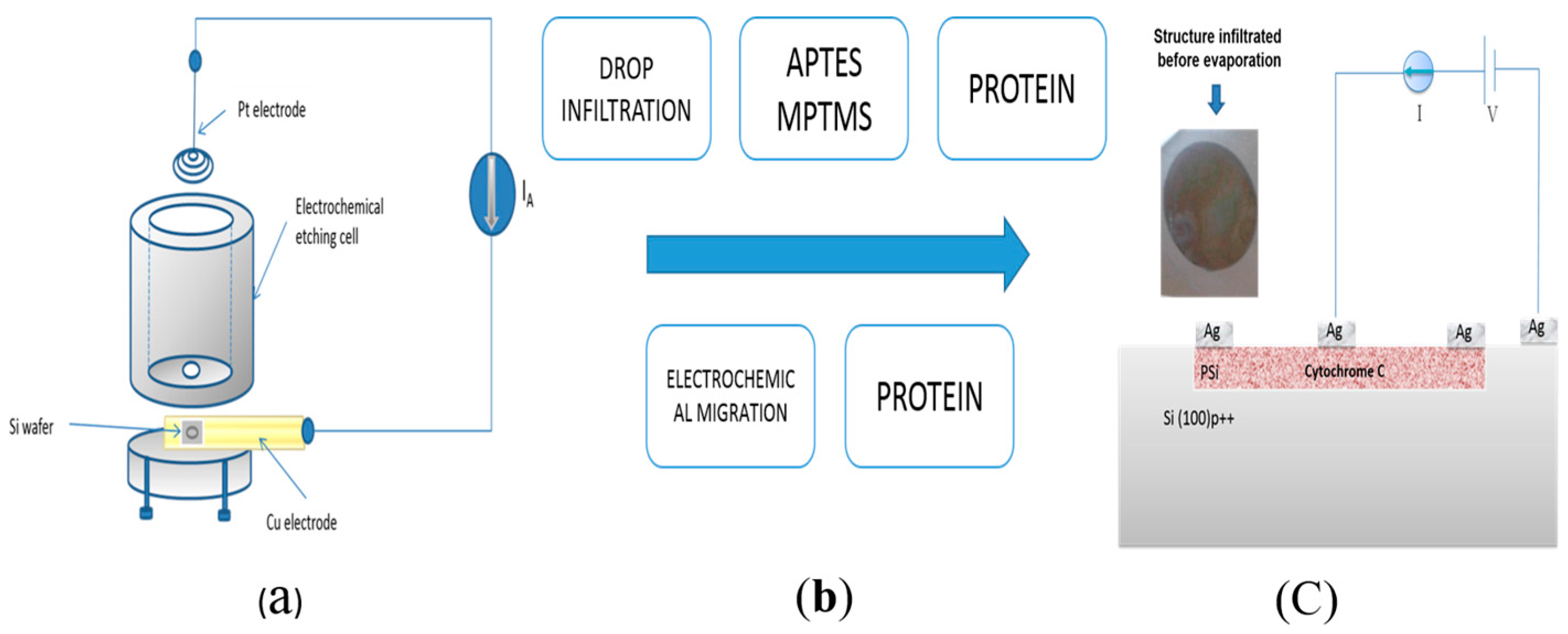
Different protein immobilization methods have been reported. The most common is the drop infiltration method or covalent bond immobilization [
11,
12], which consists of modifying the functional group of the PSi surface by thermal oxidation and subsequently with two silane groups. This method serves as a binder to immobilize the protein, thereby forming covalent bonds of the functional groups of the PSi surface with some of the functional groups of the protein. This method also allows its position to be identified with respect to the metallic contact or the substrate, thereby allowing electrical measurements to be carried out.
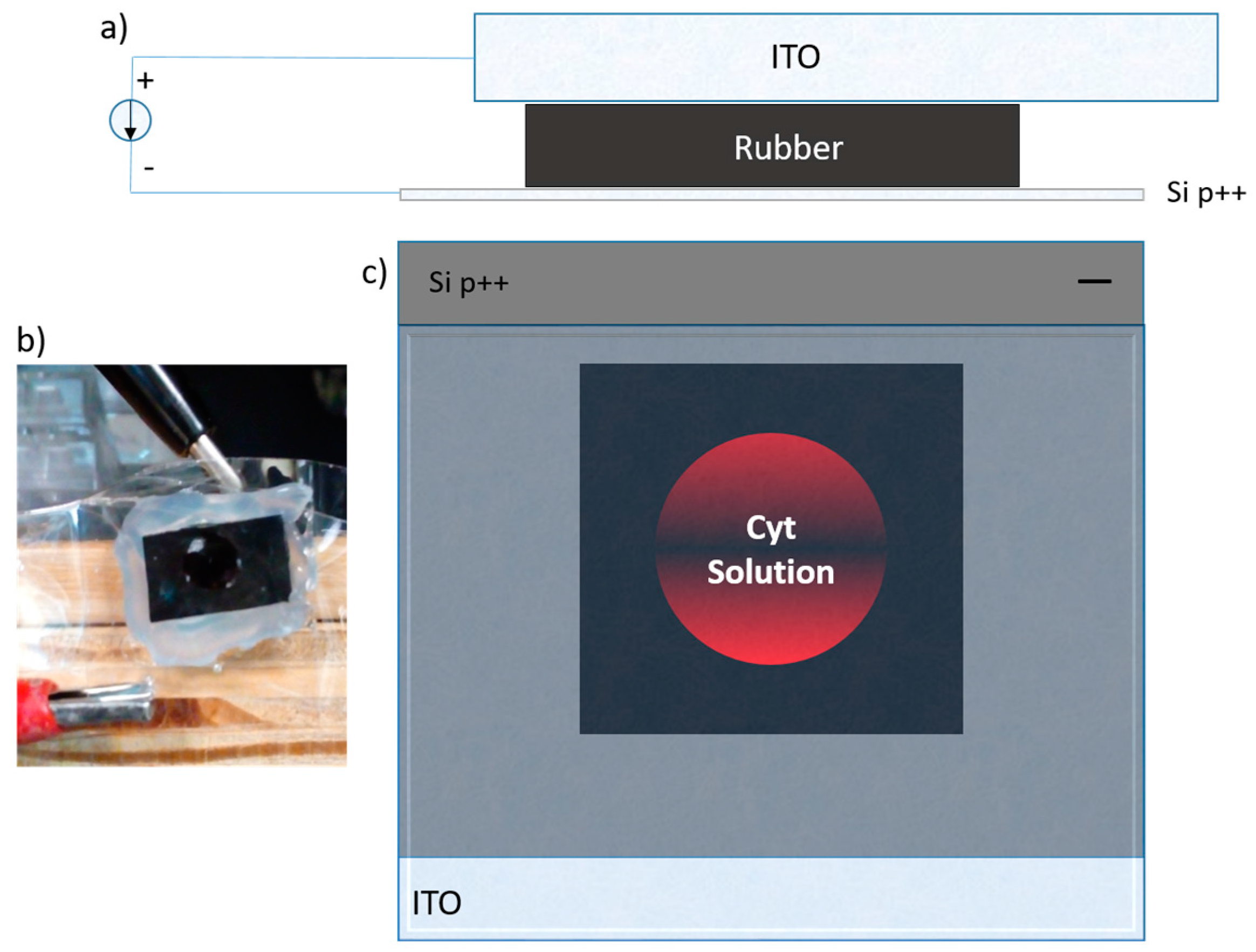
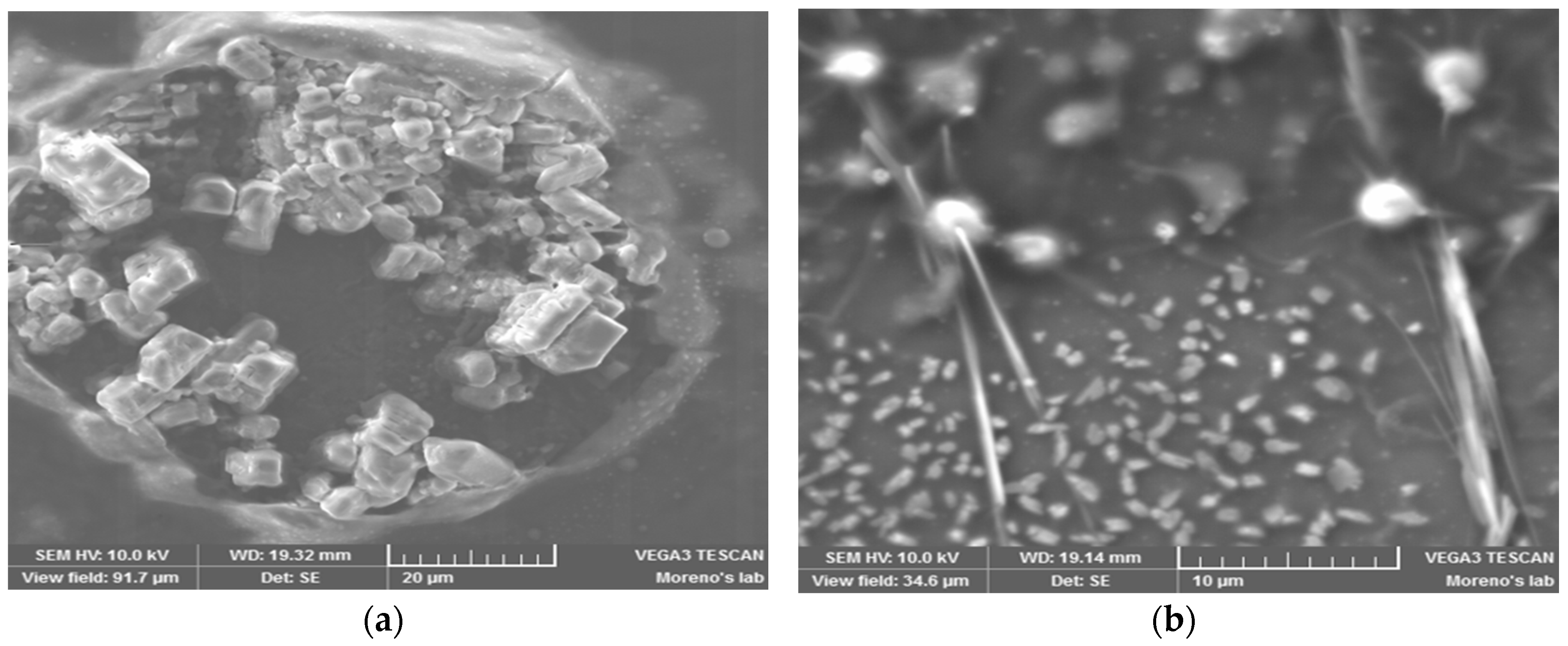
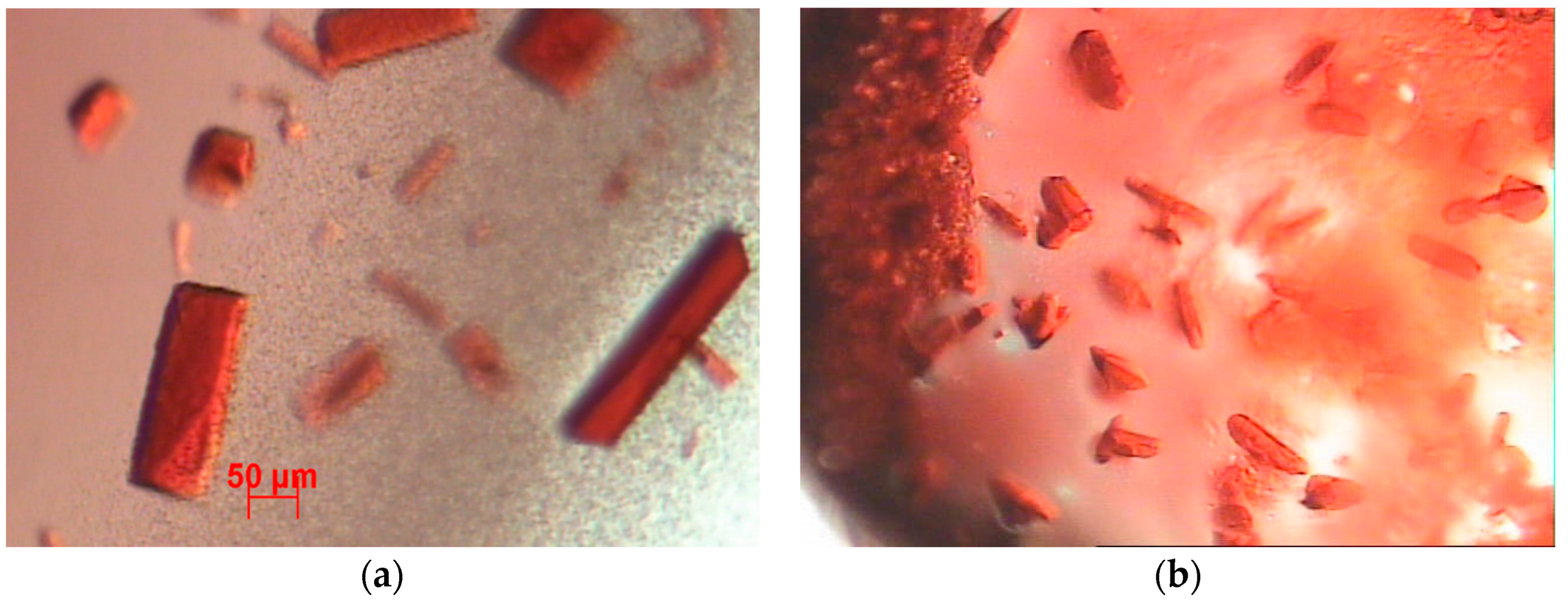
In this work, we used an ad hoc method for protein infiltration, based on preceding work [
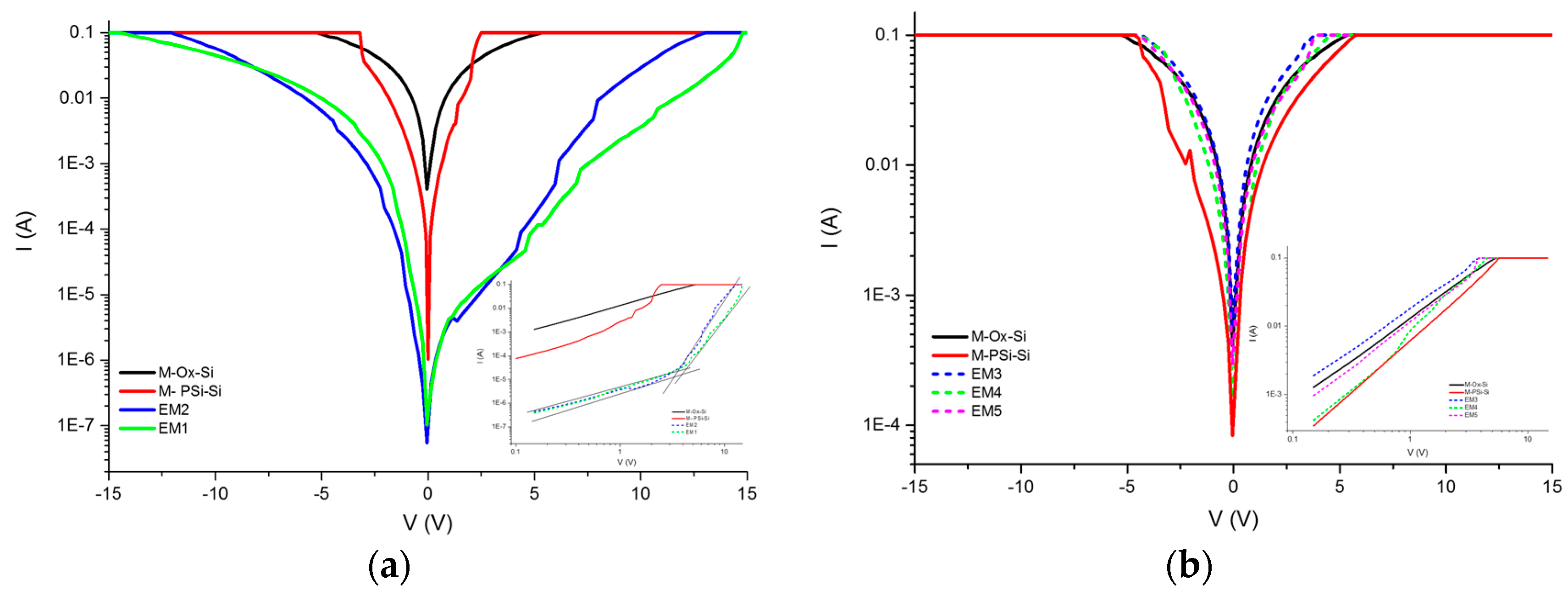
13] in which the protein crystallization was induced by employing magnetic and electric fields (DC) as nucleation-inductor factors. With this method, it is not necessary to do thermal oxidation or silane impregnation of the PSi; only an anodizing current is applied that causes the protein to infiltrate into the porous silicone due to the isoelectric point of the protein and the PSi. Subsequently, we continued with the protein crystal growth through a vapor diffusion set-up. We applied both forward and reverse bias currents only to the infiltrated Cyt C. Finally, the electrical characteristics were compared to the M/Ox/Si and M/PSi/Si structures, as well as to the control (where the protein molecules were not infiltrated) in the case of the crystallized samples.
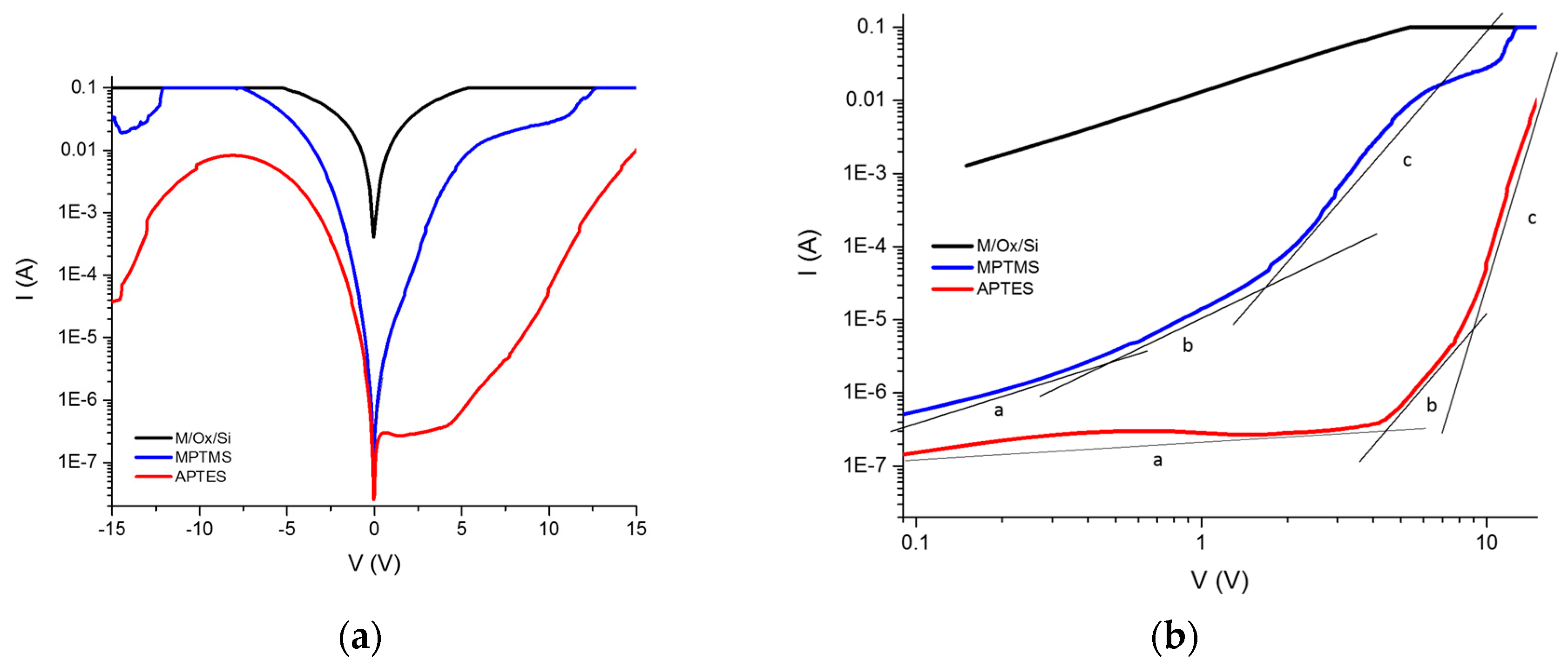
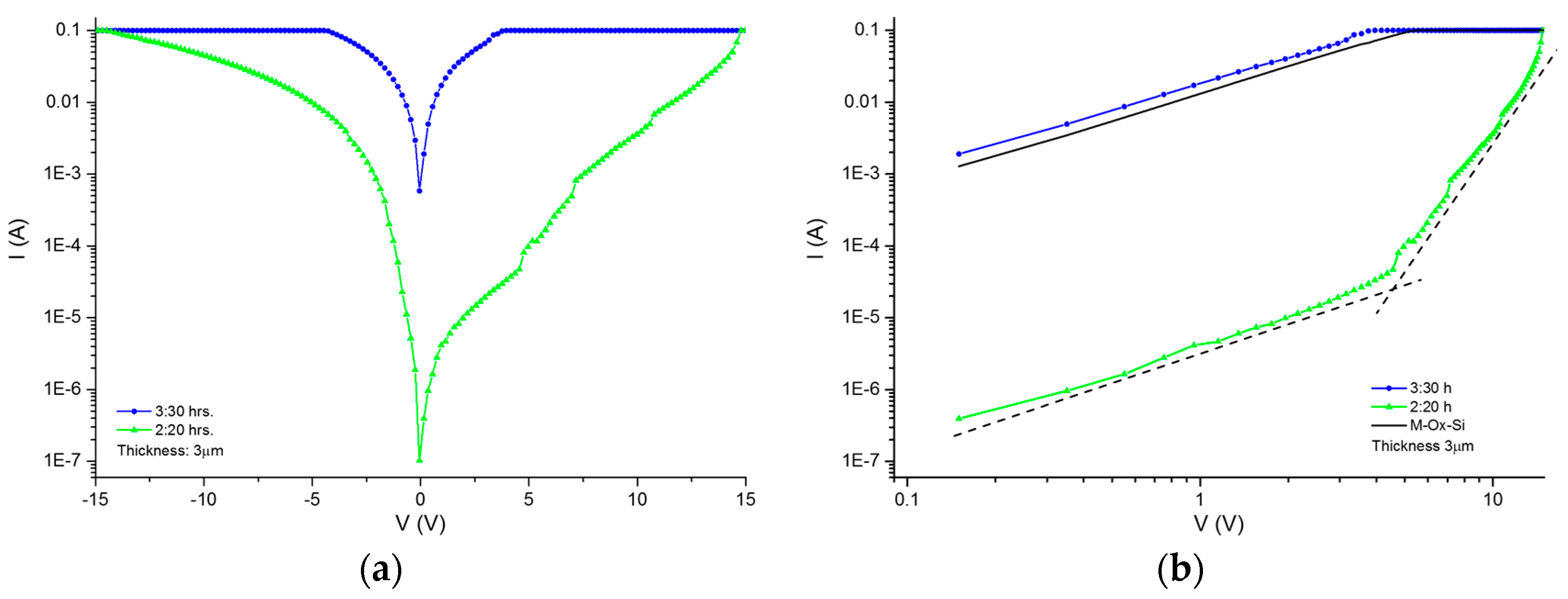
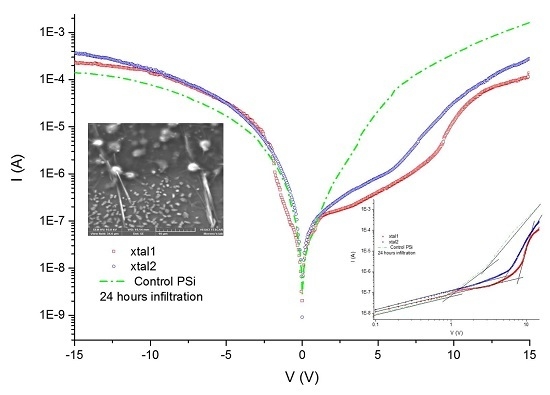
The electrical properties can be studied through the measurement of current–voltage (I–V) curves as a first approximation, to determine whether the material modifies its conductivity according to the method of infiltration. Once the protein is infiltrated by anodization, the I–V curves of the material are obtained. In comparison with the I–V curves of the immobilization, infiltration is larger by two orders of magnitude. The I–V curves of the crystallized proteins inside the PSi show a change in the order of magnitude and the current shows an asymmetric form. We then compared the level of current obtained for the crystallized protein with respect to the non-crystallized proteins. Our first approximation was based on the Schottky diode model, where the I–V curves let us determine the Schottky barrier height, considering the structure of the porous material as an effective medium.
The porous layer is considered as in the model proposed by Vikulov et al. [
14], in the equation for current density. In order to determine the height of the barrier we assume the structure to be as in the Schottky diode and use the relation of specific contact resistance (Rc), the relation for a rectifying contact in our case, according to the experimental I–V curves. This allows us to obtain the relations for Rc [
15]:
where k is the Boltzmann constant, T is the temperature, A* is the Richardson constant and
ϕB is the Schottky height barrier. Following the idea of the Schottky diode model, the ideality factor (n) can be calculated using the exponential relations:
where n is the ideality factor, determined by evaluating the slope in the I–V curve’s exponential section.
4. Conclusions
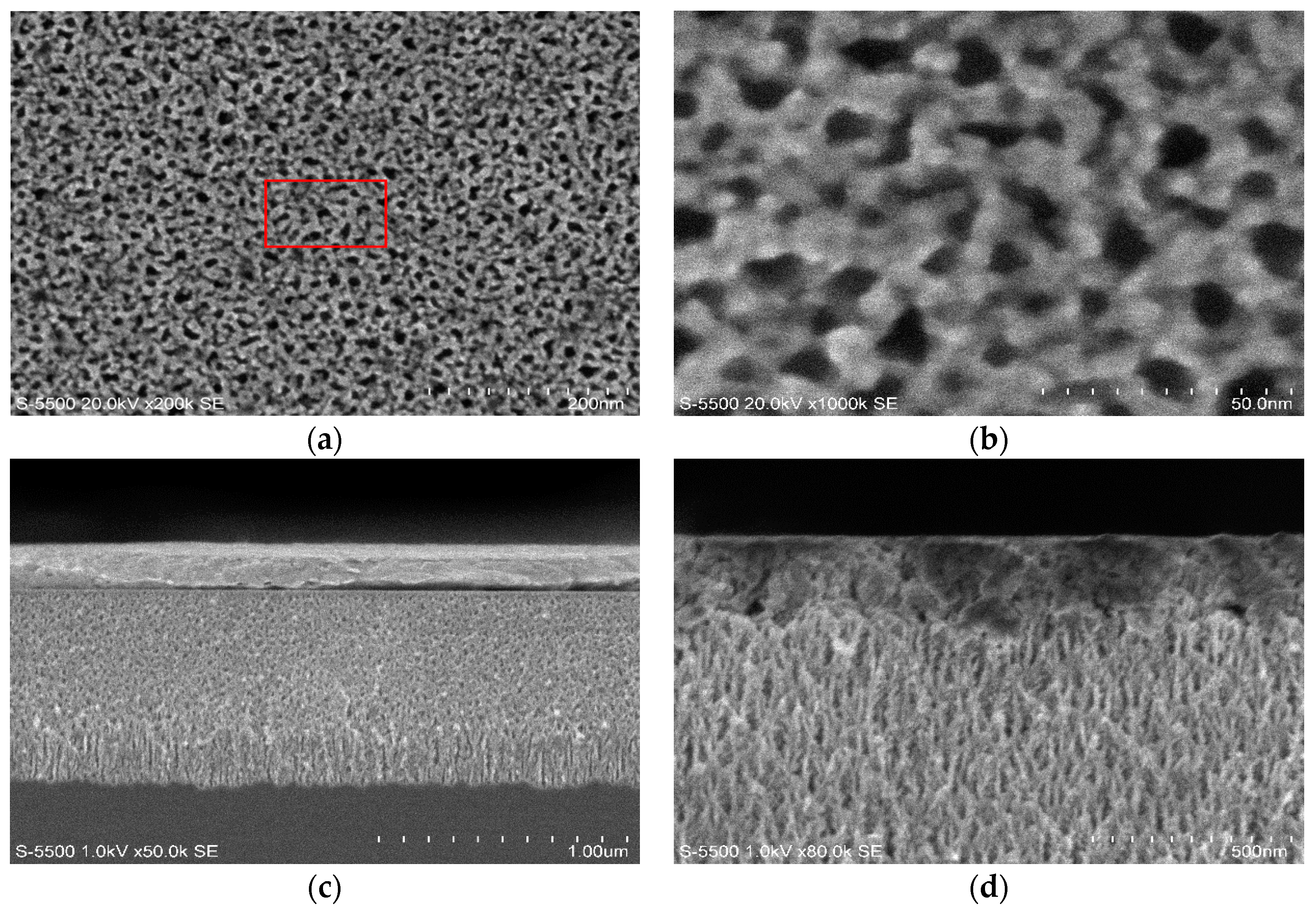
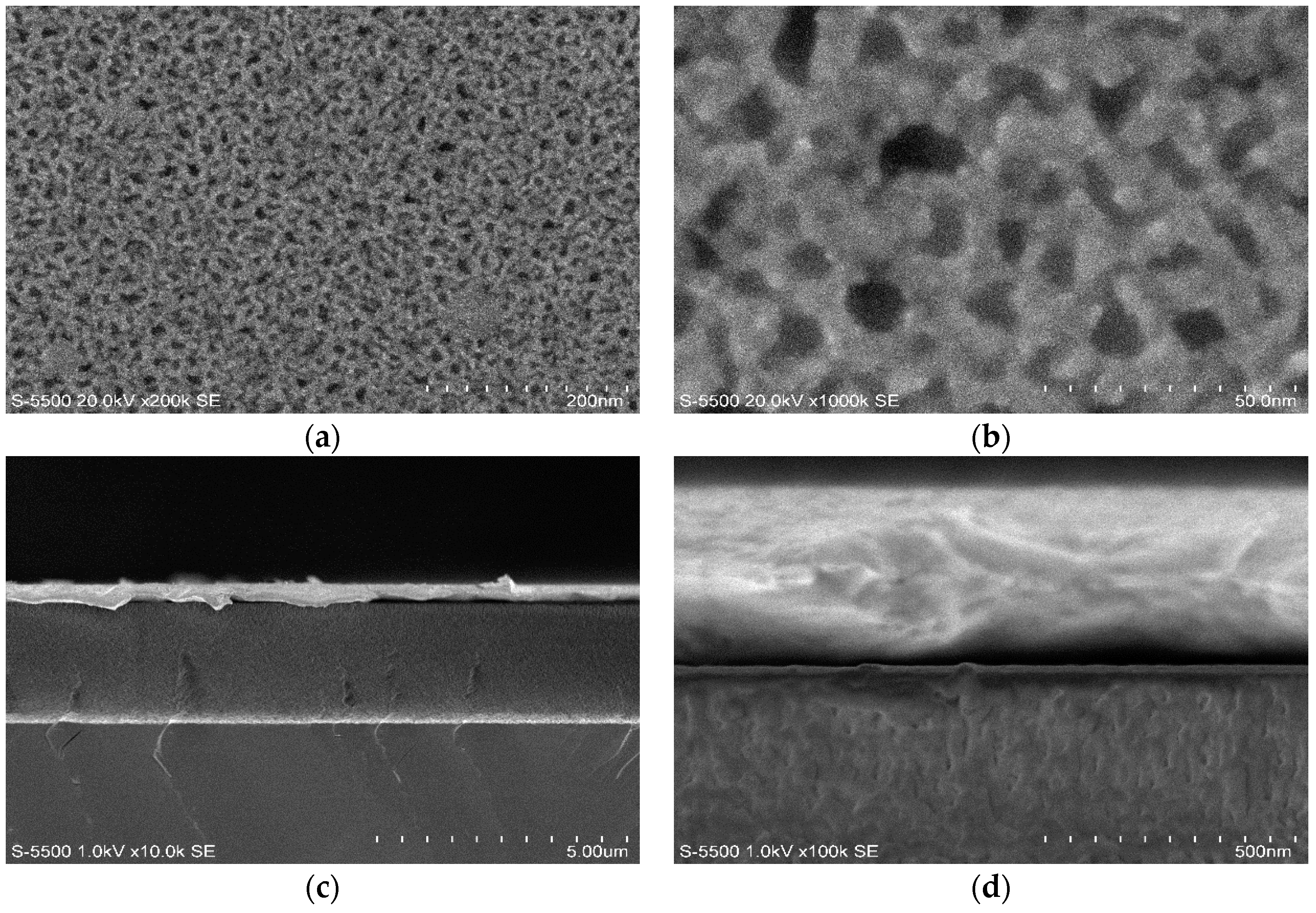
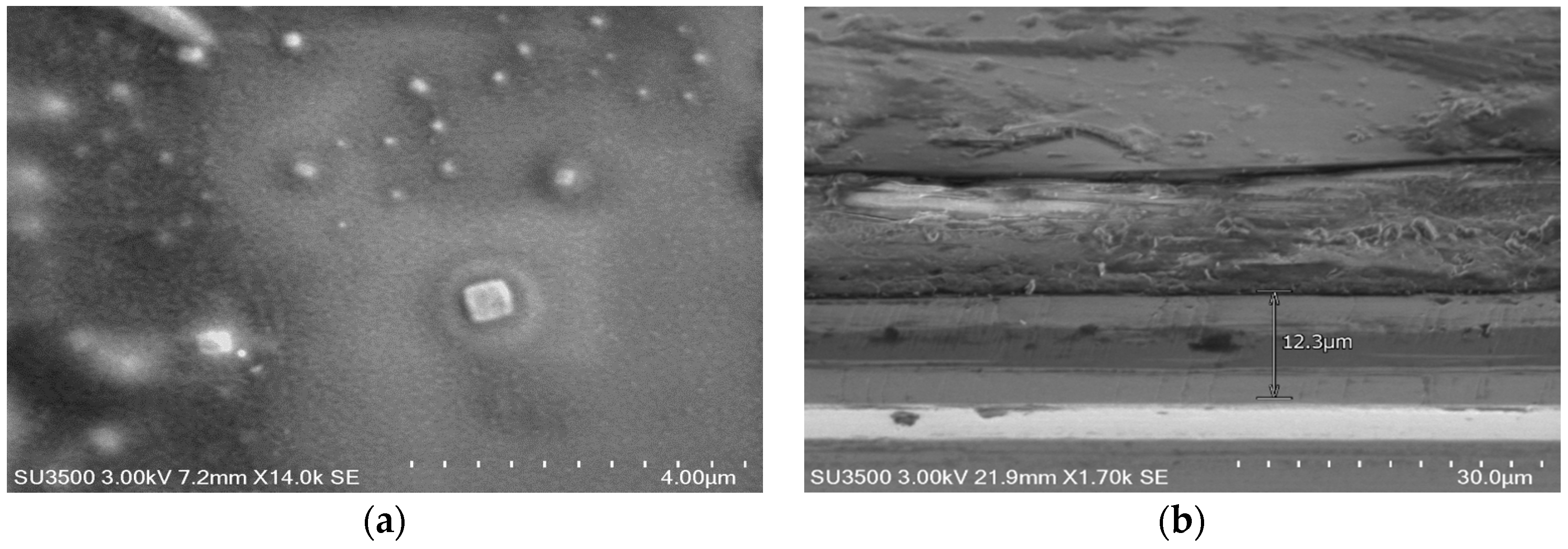
The PSi layer was obtained by the electrochemical etching of p-type Si with 60% porosity determined by the gravimetric method. This layer was oxidized by thermal oxidation to generate Si–O bonds. These bonds were employed to pin up the silane groups obtained by the infiltration of the silane-coupling agents APTES and MPTMS. The silane groups allow the interaction with the protein and the different structures used in this work. We used two protein infiltration techniques: the sitting drop technique and electrochemical migration method. Cyt C was infiltrated into the PSi by using purified and non-purified forms. With the M/Psi-silane-coupling agent-Cyt C/Si and M/PSi-Cyt C/Si structures were obtained. The crystallization of proteins was found to be feasible on the surface of PSi. I–V curve measurements were made of these structures, which determined the Schottky barrier height. The basic equations of the Schottky diode model were used as a first approximation to determine the effect of the infiltrated protein (purified and non–purified) on conductivity into the structures. The model, according to the electrical parameters, is not the most appropriate for carrying out analysis of the structures, so the next step is to perform the I–V measurements at different temperatures and to obtain the C–V curves. However, the information obtained from this first approximation allows us to consider that a PSi layer infiltrated with a protein forms an effective media that is an alternative procedure for the building of photovoltaic devices. The electrical parameters of structures with crystallized proteins do not help to improve the transport properties within the structures, so the crystallization process can be avoided in the case of photovoltaic structures. According to the I–V curves, the most efficient method, in terms of electrical properties, is electro-migration.