Halogen-Bonded Co-Crystals of Aromatic N-oxides: Polydentate Acceptors for Halogen and Hydrogen Bonds
Abstract
:1. Introduction
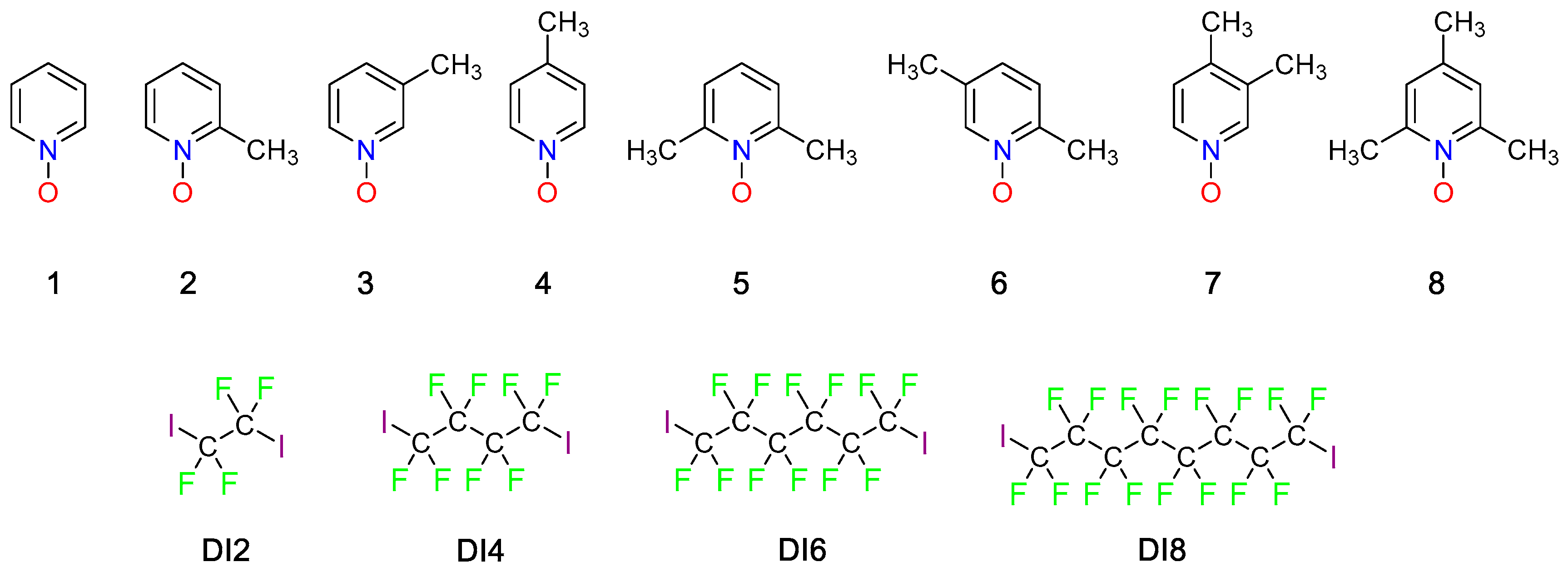
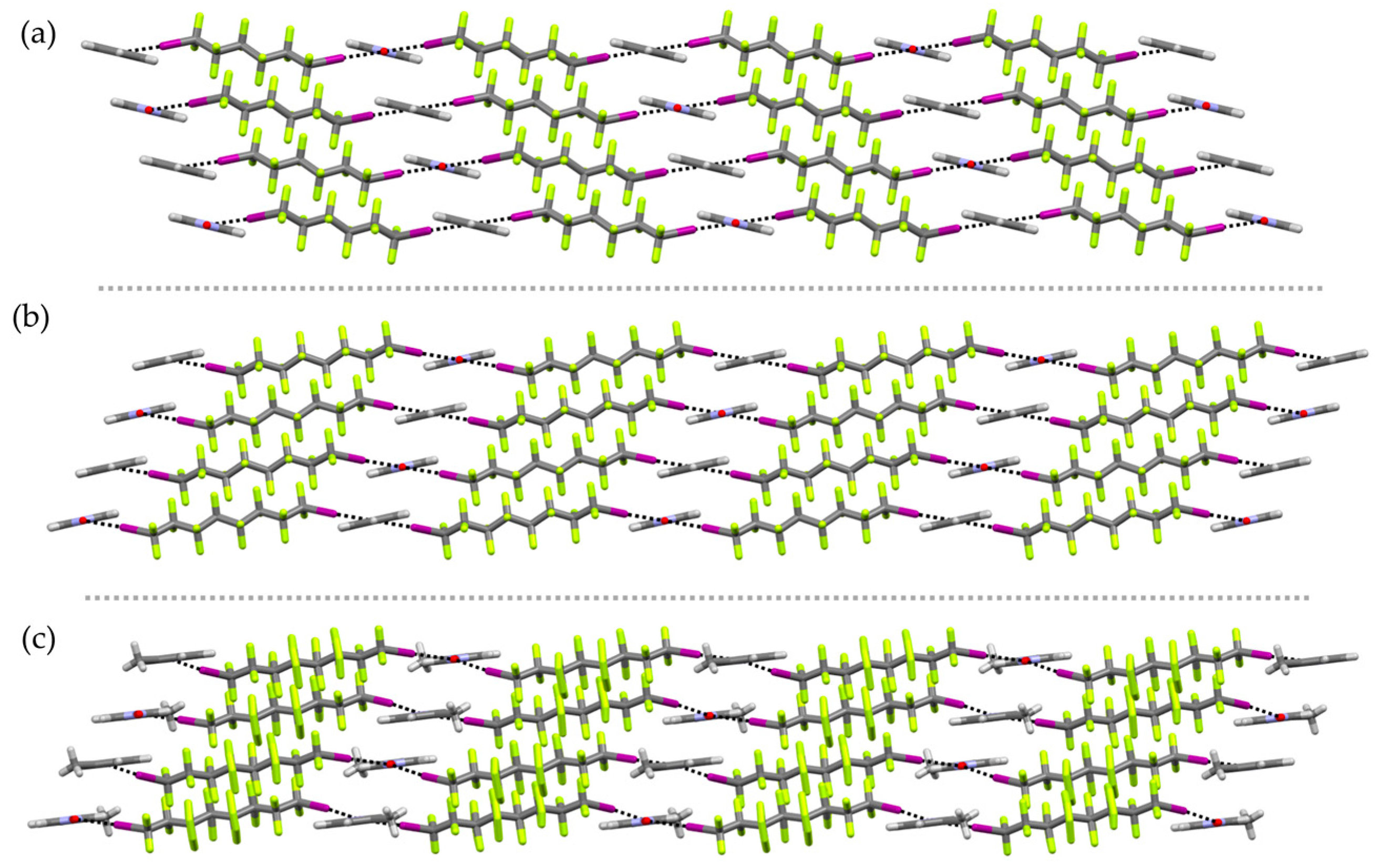
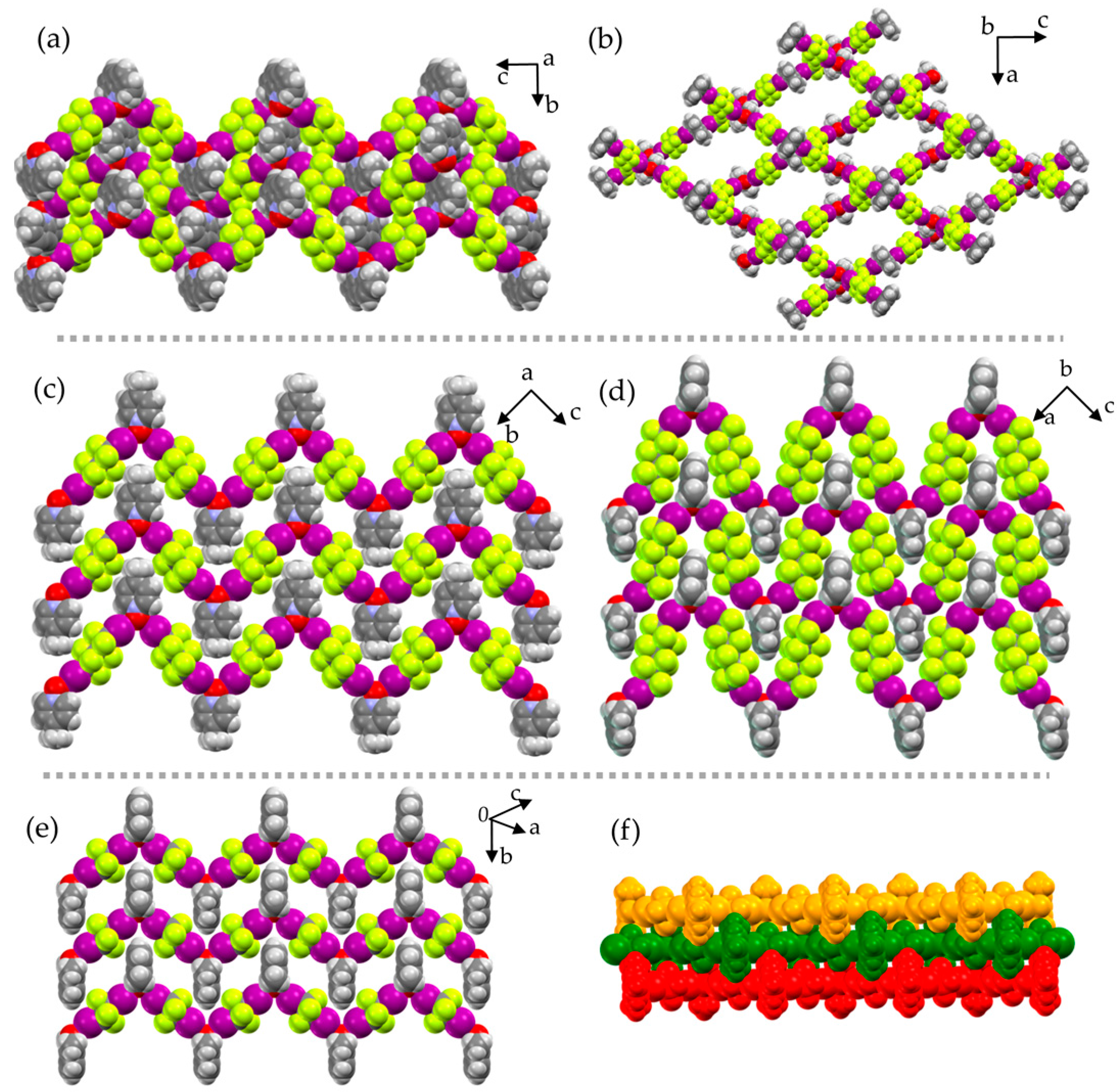
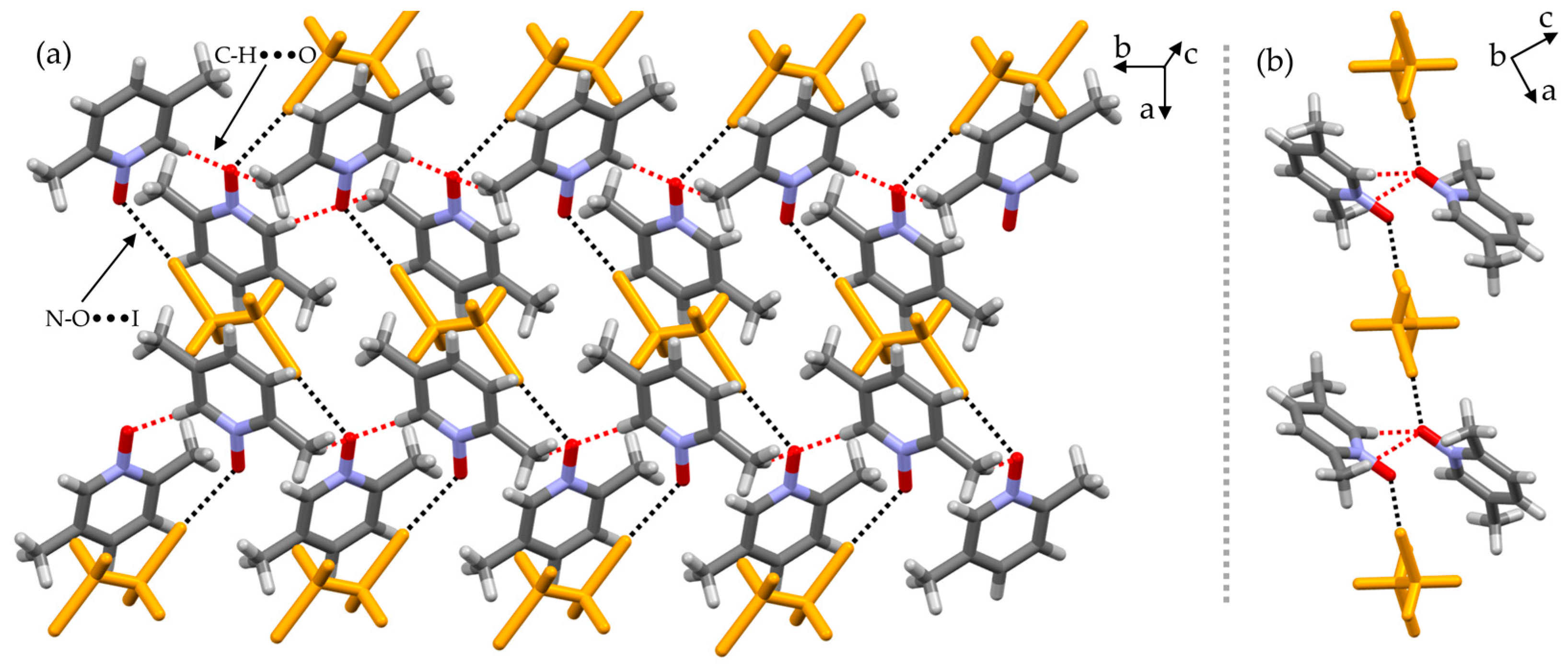
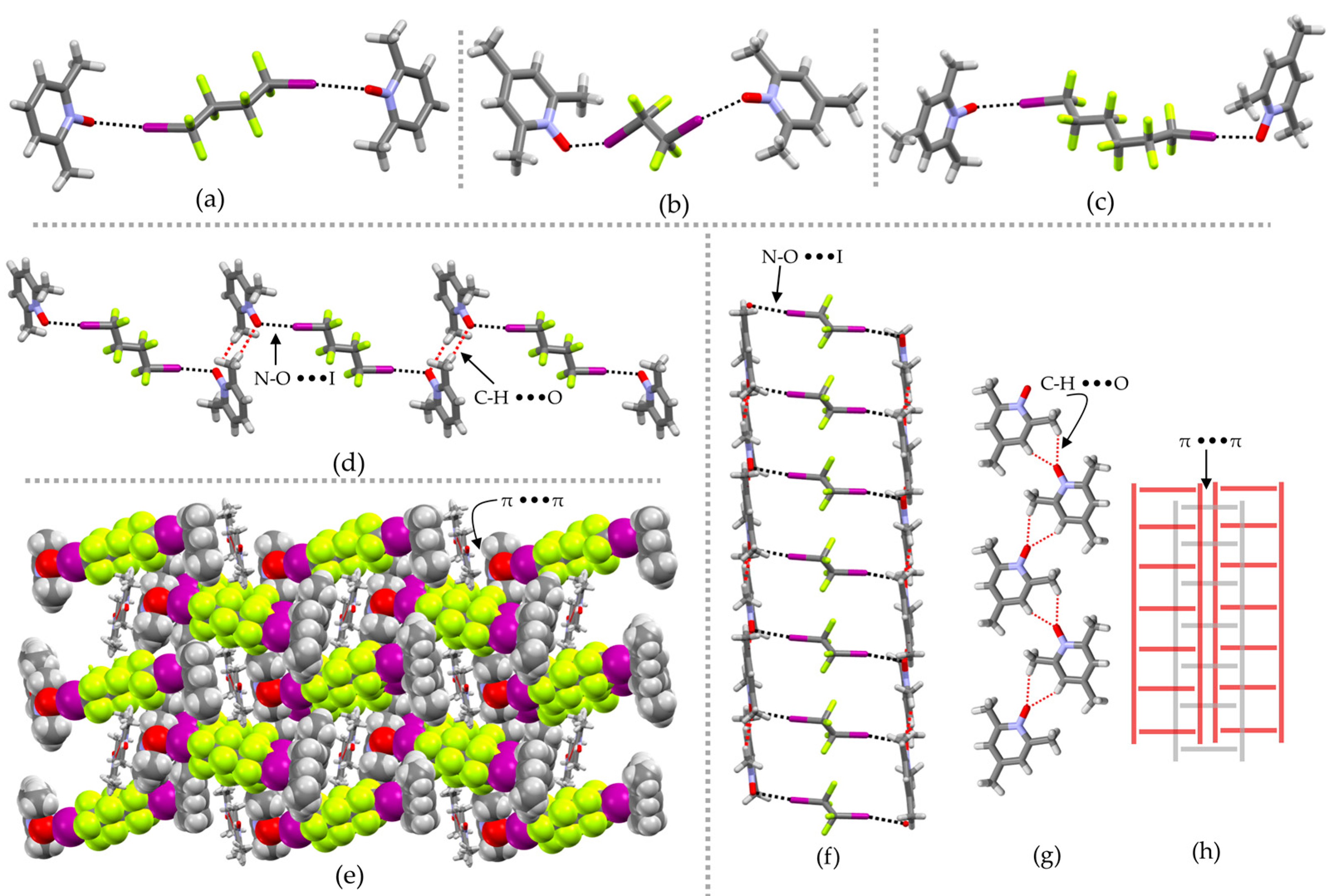
2. Results and Discussion
3. Conclusions
4. Materials and Instrumentation
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Desiraju, R.G.; Ho, S.P.; Kloo, L.; Legon, C.A.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the halogen bond (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Halogen Bonding, 2008th ed.; Metrangolo, P.; Resnati, G. (Eds.) Springer: Berlin, Germany, 2008; Volume 126. [Google Scholar]
- Politzer, P.; Murray, J.S. Halogen bonding: An interim discussion. ChemPhysChem 2013, 14, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding and other [sigma]-hole interactions: A perspective. Phys. Chem. Chem. Phys. 2013, 15, 11178–11189. [Google Scholar] [CrossRef] [PubMed]
- Rissanen, K. Halogen bonded supramolecular complexes and networks. CrystEngComm 2008, 10, 1107–1113. [Google Scholar] [CrossRef]
- Troff, R.W.; Mäkelä, T.; Topić, F.; Valkonen, A.; Raatikainen, K.; Rissanen, K. Alternative motifs for halogen bonding. Eur. J. Org. Chem. 2013, 2013, 1617–1637. [Google Scholar] [CrossRef]
- Metrangolo, P.; Meyer, F.; Pilati, T.; Resnati, G.; Terraneo, G. Halogen bonding in supramolecular chemistry. Angew. Chem. Int. Ed. 2008, 47, 6114–6127. [Google Scholar] [CrossRef] [PubMed]
- Priimagi, A.; Cavallo, G.; Metrangolo, P.; Resnati, G. The halogen bond in the design of functional supramolecular materials: Recent advances. Acc. Chem. Res. 2013, 46, 2686–2695. [Google Scholar] [CrossRef] [PubMed]
- Voth, A.R.; Khuu, P.; Oishi, K.; Ho, P.S. Halogen bonds as orthogonal molecular interactions to hydrogen bonds. Nat. Chem. 2009, 1, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Bedin, M.; Karim, A.; Reitti, M.; Carlsson, A.-C.C.; Topić, F.; Cetina, M.; Pan, F.; Havel, V.; Al-Ameri, F.; Sindelar, V.; et al. Counterion influence on the [N-I-N]+ halogen bond. Chem. Sci. 2015, 6, 3746–3756. [Google Scholar] [CrossRef]
- Carlsson, A.-C.C.; Gräfenstein, J.; Budnjo, A.; Laurila, J.L.; Bergquist, J.; Karim, A.; Kleinmaier, R.; Brath, U.; Erdélyi, M. Symmetric halogen bonding is preferred in solution. J. Am. Chem. Soc. 2012, 134, 5706–5715. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, A.-C.C.; Veiga, A.; Erdélyi, M. Halogen bonding in solution. In Halogen Bonding II SE-607; Metrangolo, P., Resnati, G., Eds.; Springer: Berlin, Germany, 2015; Volume 359, pp. 49–76. [Google Scholar]
- Rissanen, K.; Haukka, M. Halonium Ions as Halogen Bond Donors in the Solid State [XL2]Y Complexes. In Halogen Bonding II SE-587; Metrangolo, P., Resnati, G., Eds.; Springer: Berlin, Germany, 2015; Volume 359, pp. 77–90. [Google Scholar]
- Barluenga, J.; González, J.M.; Campos, P.J.; Asensio, G. I(Py)2BF4, a new reagent in organic synthesis: General method for the 1,2-Iodofunctionalization of olefins. Angew. Chem. Int. Ed. 1985, 24, 319–320. [Google Scholar] [CrossRef]
- Turunen, L.; Warzok, U.; Puttreddy, R.; Beyeh, N.K.; Schalley, C.A.; Rissanen, K. [N⋅⋅⋅I+⋅⋅⋅N] halogen-bonded dimeric capsules from tetrakis(3-pyridyl)ethylene cavitands. Angew. Chem. Int. Ed. 2016, 55, 14033–14036. [Google Scholar] [CrossRef] [PubMed]
- Fourmigué, M. Halogen bonding: Recent advances. Curr. Opin. Solid State Mater. Sci. 2009, 13, 36–45. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Horton, P.N.; Hursthouse, M.B.; Legon, A.C.; Bruce, D.W. Halogen bonding: A new interaction for liquid crystal formation. J. Am. Chem. Soc. 2004, 126, 16–17. [Google Scholar] [CrossRef] [PubMed]
- Metrangolo, P.; Prasang, C.; Resnati, G.; Liantonio, R.; Whitwood, A.C.; Bruce, D.W. Fluorinated liquid crystals formed by halogen bonding. Chem. Commun. 2006, 3290–3292. [Google Scholar] [CrossRef] [PubMed]
- Bull, J.A.; Mousseau, J.J.; Pelletier, G.; Charette, A.B. Synthesis of pyridine and dihydropyridine derivatives by regio- and stereoselective addition to N-activated pyridines. Chem. Rev. 2012, 112, 2642–2713. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Liu, Z.-J.; Liu, L.; Fu, Y. Palladium-catalyzed C–H activation/cross-coupling of pyridine N-oxides with nonactivated secondary alkyl bromides. J. Am. Chem. Soc. 2013, 135, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cui, X.; Chen, L.; Jiang, G.; Wu, Y. Palladium-catalyzed alkenylation of quinoline-N-oxides via C−H activation under external-oxidant-free conditions. J. Am. Chem. Soc. 2009, 131, 13888–13889. [Google Scholar] [CrossRef] [PubMed]
- Campeau, L.-C.; Schipper, D.J.; Fagnou, K. Site-selective sp2 and benzylic sp3 palladium-catalyzed direct arylation. J. Am. Chem. Soc. 2008, 130, 3266–3267. [Google Scholar] [CrossRef] [PubMed]
- Campeau, L.-C.; Bertrand-Laperle, M.; Leclerc, J.-P.; Villemure, E.; Gorelsky, S.; Fagnou, K. C2, C5, and C4 azole N-oxide direct arylation including room-temperature reactions. J. Am. Chem. Soc. 2008, 130, 3276–3277. [Google Scholar] [CrossRef] [PubMed]
- Pool, J.A.; Scott, B.L.; Kiplinger, J.L. A new mode of reactivity for pyridine N-oxide: C−H activation with uranium(IV) and thorium(IV) bis(alkyl) complexes. J. Am. Chem. Soc. 2005, 127, 1338–1339. [Google Scholar] [CrossRef] [PubMed]
- Campeau, L.-C.; Rousseaux, S.; Fagnou, K. A solution to the 2-Pyridyl organometallic cross-coupling problem: Regioselective catalytic direct arylation of pyridine N-oxides. J. Am. Chem. Soc. 2005, 127, 18020–18021. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, L.; Ballester, P. Hydrogen bonded supramolecular capsules with functionalized interiors: The controlled orientation of included guests. Chem. Soc. Rev. 2013, 42, 3261–3277. [Google Scholar] [CrossRef] [PubMed]
- Raynal, M.; Ballester, P.; Vidal-Ferran, A.; van Leeuwen, P.W. Supramolecular catalysis. Part 1: Non-covalent interactions as a tool for building and modifying homogeneous catalysts. Chem. Soc. Rev. 2014, 43, 1660–1733. [Google Scholar] [CrossRef] [PubMed]
- Albini, A. Heterocyclic N-oxides; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Katritzky, A.R.; Lagowski, J.M. Chemistry of the Heterocyclic N-oxides; Academic Press: Cambridge, MA, USA, 1971. [Google Scholar]
- Puttreddy, R.; Steel, P.J. 4-Methoxypyridine N-oxide: An electron-rich ligand that can simultaneously bridge three silver atoms. Inorg. Chem. Commun. 2014, 41, 33–36. [Google Scholar] [CrossRef]
- Puttreddy, R.; Steel, P.J. Pyridine N-oxide: A hyperdentate argentophile. CrystEngComm 2014, 16, 556–560. [Google Scholar] [CrossRef]
- Pang, X.; Jin, W.J. Exploring the halogen bond specific solvent effects in halogenated solvent systems by ESR probe. New J. Chem. 2015, 39, 5477–5483. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Wijethunga, T.K.; Desper, J. Constructing molecular polygons using halogen bonding and bifurcated N-oxides. CrystEngComm 2014, 16, 28–31. [Google Scholar] [CrossRef]
- Messina, M.T.; Metrangolo, P.; Panzeri, W.; Pilati, T.; Resnati, G. Intermolecular recognition between hydrocarbon oxygen-donors and perfluorocarbon iodine-acceptors: The shortest O⋯I non-covalent bond. Tetrahedron 2001, 57, 8543–8550. [Google Scholar] [CrossRef]
- Puttreddy, R.; Jurček, O.; Bhowmik, S.; Makela, T.; Rissanen, K. Very strong -N-X+···−O-N+ halogen bonds. Chem. Commun. 2016, 11, 2338–2341. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.S. Halogen Bonding I. Top. Curr. Chem. 2015, 358, 241–276. [Google Scholar] [PubMed]
- Aakeröy, C.B.; Wijethunga, T.K.; Benton, J.; Desper, J. Stabilizing volatile liquid chemicals using co-crystallization. Chem. Commun. 2015, 51, 2425–2428. [Google Scholar] [CrossRef] [PubMed]
- Omorodion, H.; Twamley, B.; Platts, J.A.; Baker, R.J. Further evidence on the importance of fluorous–fluorous interactions in supramolecular chemistry: A combined structural and computational study. Cryst. Growth Des. 2015, 15, 2835–2841. [Google Scholar] [CrossRef]
- Baker, R.J.; Colavita, P.E.; Murphy, D.M.; Platts, J.A.; Wallis, J.D. Fluorine–fluorine interactions in the solid state: An experimental and theoretical study. J. Phys. Chem. A 2012, 116, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Reichenbacher, K.; Suss, H.I.; Hulliger, J. Fluorine in crystal engineering—“The little atom that could”. Chem. Soc. Rev. 2005, 34, 22–30. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 2008, 37, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.; Resnati, G.; Metrangolo, P.; Weber, E.; Hulliger, J. Organic fluorine compounds: A great opportunity for enhanced materials properties. Chem. Soc. Rev. 2011, 40, 3496–3508. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R. It Isn’t, It Is: The C–H···X (X = O, N, F, Cl) interaction really is significant in crystal packing. Cryst. Growth Des. 2016, 16, 4165–4168. [Google Scholar] [CrossRef]
- Dragelj, J.L.; Stanković, I.M.; Božinovski, D.M.; Meyer, T.; Veljković, D.Ž.; Medaković, V.B.; Knapp, E.-W.; Zarić, S.D. C–H/O interactions of aromatic CH donors within proteins: A crystallographic study. Cryst. Growth Des. 2016, 16, 1948–1957. [Google Scholar] [CrossRef]
- Ji, W.; Liu, G.; Li, Z.; Feng, C. Influence of C–H···O hydrogen bonds on macroscopic properties of supramolecular assembly. ACS Appl. Mater. Interfaces 2016, 8, 5188–5195. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.R.; Baruah, P.K.; Thompson, A.L.; Scheiner, S.; Smith, M.D. Can a C–H···O interaction be a determinant of conformation? J. Am. Chem. Soc. 2012, 134, 12064–12071. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Kar, T.; Scheiner, S. Fundamental properties of the C–H···O interaction: Is it a true hydrogen bond? J. Am. Chem. Soc. 1999, 121, 9411–9422. [Google Scholar] [CrossRef]
- Jiang, L.; Lai, L. C–H···O hydrogen bonds at protein-protein interfaces. J. Biol. Chem. 2002, 277, 37732–37740. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Dissection of the factors affecting formation of a C–H∙∙∙O–H-bond. A Case Study. Crystals 2015, 5, 327–345. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Vittal, J.J.; Ramanan, A. Crystal Engineering: A Textbook; World Scientific: Singapore, 2011. [Google Scholar]
- Babu, N.J.; Reddy, L.S.; Nangia, A. AmideN-oxide heterosynthon and amide dimer homosynthon in cocrystals of carboxamide drugs and pyridine N-oxides. Mol. Pharm. 2007, 4, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Goud, N.R.; Babu, N.J.; Nangia, A. Sulfonamide-pyridinen-N-oxide cocrystals. Cryst. Growth Des. 2011, 11, 1930–1939. [Google Scholar] [CrossRef]
- Reddy, L.S.; Babu, N.J.; Nangia, A. Carboxamide-pyridine N-oxide heterosynthon for crystal engineering and pharmaceutical cocrystals. Chem. Commun. 2006, 1369–1371. [Google Scholar] [CrossRef] [PubMed]
- Puttreddy, R.; Cottam, J.R.A.; Steel, P.J. Anion dependent silver(I) complexes of pyrazine mono-N-oxide. RSC Adv. 2014, 4, 22449–22454. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffr. 2016; Version 1.171.38.41.
- Bruker AXS BV. Madison, WI, USA, 1997–2004.
- Otwinowski, Z.; Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997, 276, 307–326. [Google Scholar] [PubMed]
- Blessing, R.H. Outlier treatment in data merging. J. Appl. Crystallogr. 1997, 30, 421–426. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]


| S.No | Code | Monodentate | μ-O,O | ||
|---|---|---|---|---|---|
| ca. d(I···O−N)/Å * | Ð(C−I···O)/° | ca. d(I···O−N)/Å * | Ð(C−I···O)/° | ||
| 1 | 1•DI6 | 2.834 [0.81] | 177.1 | ** | ** |
| 2 | 1•DI8 | 2.833 [0.81] | 177.7 | ** | ** |
| 3 | 2•DI4 | 2.747 [0.79] | 174.2 | 2.861 [0.82] | 178.9 |
| 4 | 3•DI4 | 2.840 [0.81] | 172.1 | 2.875 [0.82] | 168.8 |
| 5 | 3•DI8 | 2.809 [0.80] | 174.8 | 2.817 [0.81] | 174.7 |
| 6 | 4•DI4_I | 2.808 [0.80] *** | 172.3 *** | 2.813 [0.80] *** | 174.1 *** |
| 7 | 4•DI4_II | 2.766 [0.79] | 177.5 *** | ** | ** |
| 8 | 5•DI2 | 2.743 [0.78] | 161.2 *** | ** | ** |
| 9 | 5•DI4 | 2.669 [0.76] | 171.3 *** | -- | -- |
| 10 | 5•DI6 | 2.733 [0.78] | 175.5 | 2.774 [0.80] | 169.0 |
| 2.764 [0.79] | 174.9 | 2.813 [0.804] | 170.3 | ||
| 11 | 6•DI2 | 2.714 [0.78] | 171.9 | -- | -- |
| 12 | 7•DI2 | 2.703 [0.77] | 174.6 | -- | -- |
| 13 | 7•DI4 | 2.835 [0.81] | 167.5 *** | 2.906 [0.83] | 170.0 *** |
| 14 | 7•DI6 | 2.825 [0.81] | 167.9 *** | 2.827 [0.81] | 178.2 *** |
| 15 | 7•DI8 | 2.715 [0.78] | 176.5 | -- | -- |
| 16 | 8•DI2 | 2.702 [0.78] | 166.4 | -- | -- |
| 2.775 [0.79] | 170.8 | -- | -- | ||
| 17 | 8•DI6 | 2.649 [0.76] | 174.3 | -- | -- |
| 2.682 [0.77] | 176.1 | -- | -- | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puttreddy, R.; Topić, F.; Valkonen, A.; Rissanen, K. Halogen-Bonded Co-Crystals of Aromatic N-oxides: Polydentate Acceptors for Halogen and Hydrogen Bonds. Crystals 2017, 7, 214. https://doi.org/10.3390/cryst7070214
Puttreddy R, Topić F, Valkonen A, Rissanen K. Halogen-Bonded Co-Crystals of Aromatic N-oxides: Polydentate Acceptors for Halogen and Hydrogen Bonds. Crystals. 2017; 7(7):214. https://doi.org/10.3390/cryst7070214
Chicago/Turabian StylePuttreddy, Rakesh, Filip Topić, Arto Valkonen, and Kari Rissanen. 2017. "Halogen-Bonded Co-Crystals of Aromatic N-oxides: Polydentate Acceptors for Halogen and Hydrogen Bonds" Crystals 7, no. 7: 214. https://doi.org/10.3390/cryst7070214






