1. Introduction
Stereochemistry is important in biofunctional molecules [
1]. Therefore, methods that facilitate the elucidation of absolute configurations and the preparation of single enantiomers are highly desired [
2]. Based on stereochemical studies of biofunctional molecules, we synthesized a chiral resolving agent, MαNP acid (acid
1,
Figure 1) [
3,
4]. Acid
1 is superior to Mosher’s 3,3,3-trifluoro-2-methoxy-2-phenylpropanoic acid (MTPA,
2) [
5] for the enantioresolution of secondary alcohols [
2].
Goto et al. reported the enantioresolution of
rac-
1 via diastereomeric salt formation with (
R)-
3 [
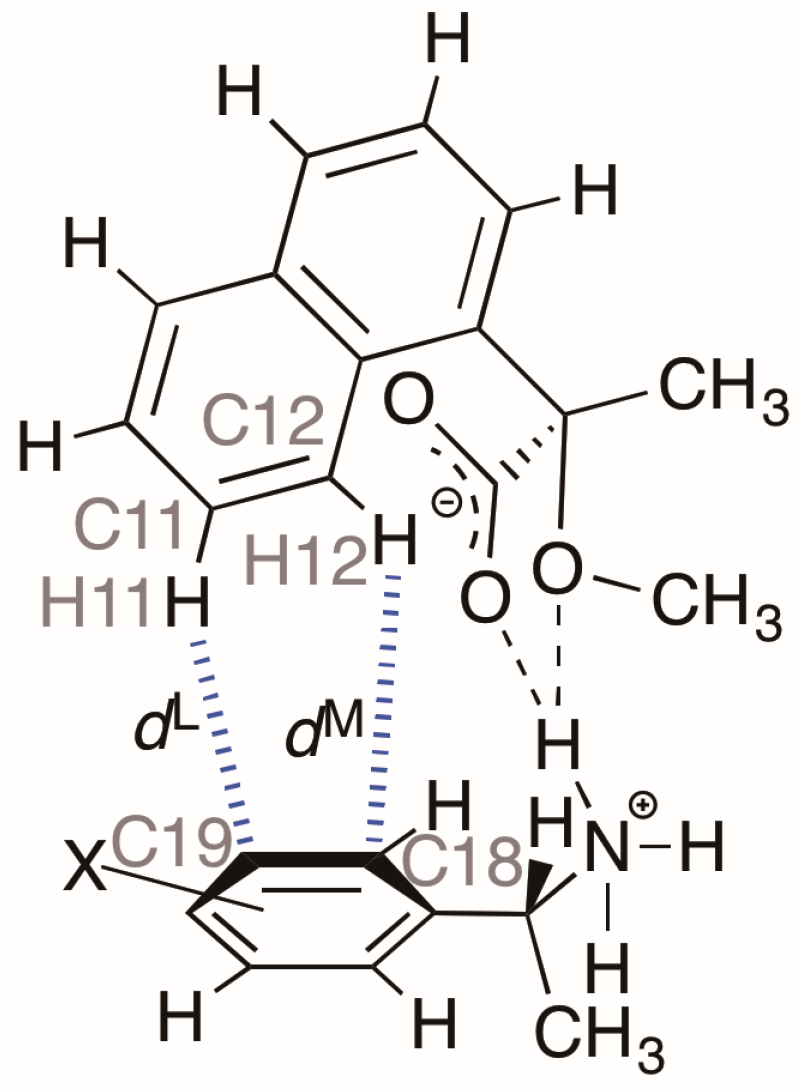
3]. In 2011, we examined the crystal structures of the less-soluble salt
4 [(
R)-
3∙(
R)-
1] and the more-soluble diastereomeric salt (
R)-
3∙(
S)-
1 by X-ray crystallography [
6]. Those crystal structures revealed a chiral recognition mechanism during the enantioresolution process. With the less-soluble salt
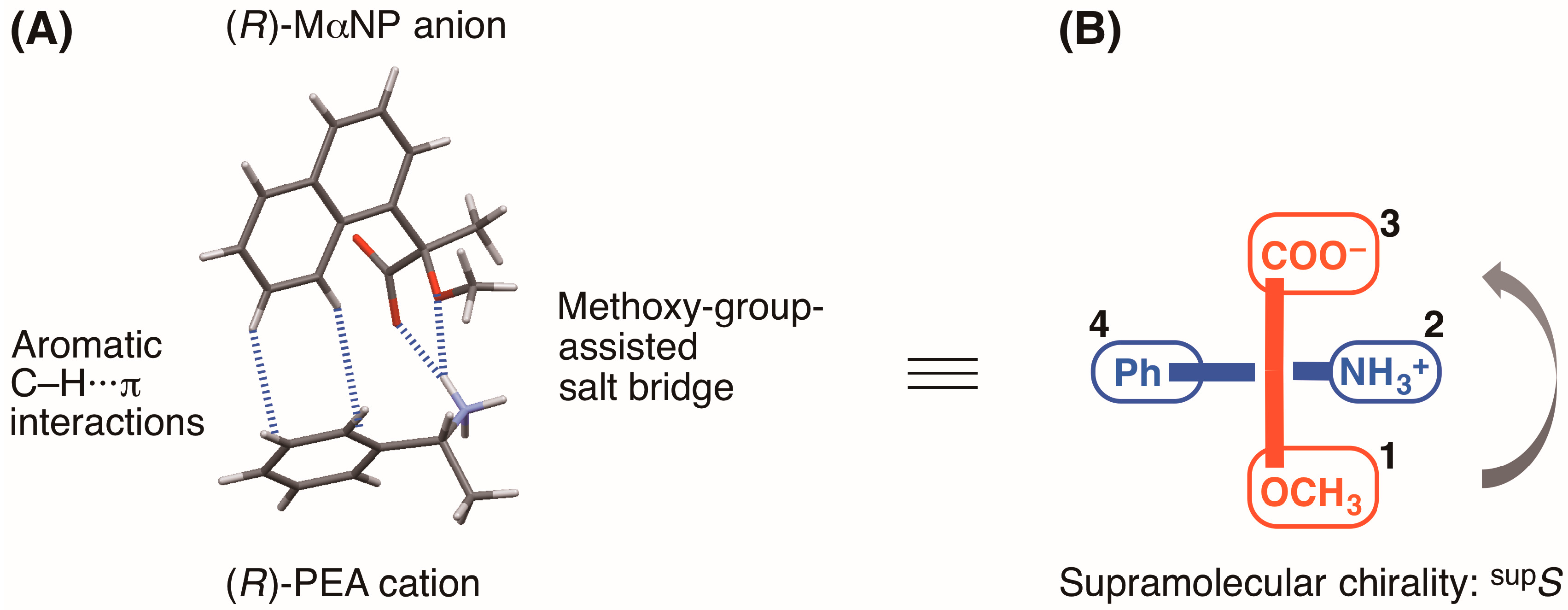
4, the (
R)-MαNP anion and the (
R)-PEA cation form a close ion-pair via a methoxy-group-assisted salt bridge and aromatic C–H∙∙∙π interactions (
Figure 2A). The close ion-pairs then join with the salt bridges to form 2
1 columns. Additionally, results have shown that intercolumnar aromatic C–H∙∙∙π interactions [
7,
8,
9] are more effective with the less-soluble salt
4.
Recently, we introduced the concept of supramolecular chirality as a means of defining the association of close ion-pairs in the solid state [
10]. Considering the virtual chiral center of the carboxylate and methoxy groups of the (
R)-MαNP anion and the phenyl and ammonium groups of the (
R)-PEA cation, the supramolecular chirality of salt
4 was assigned as
supS (
Figure 2B) [
10].
Salts
4 and
5 [(
R)-1-(
p-tolyl)ethylamine∙(
R)-
1] yielded space groups
P2
1 and
C2, respectively [
6,
11]. This implied an effect of
para-substitution of the PEA cation on the latter stage of hierarchical assembly [
12,
13].
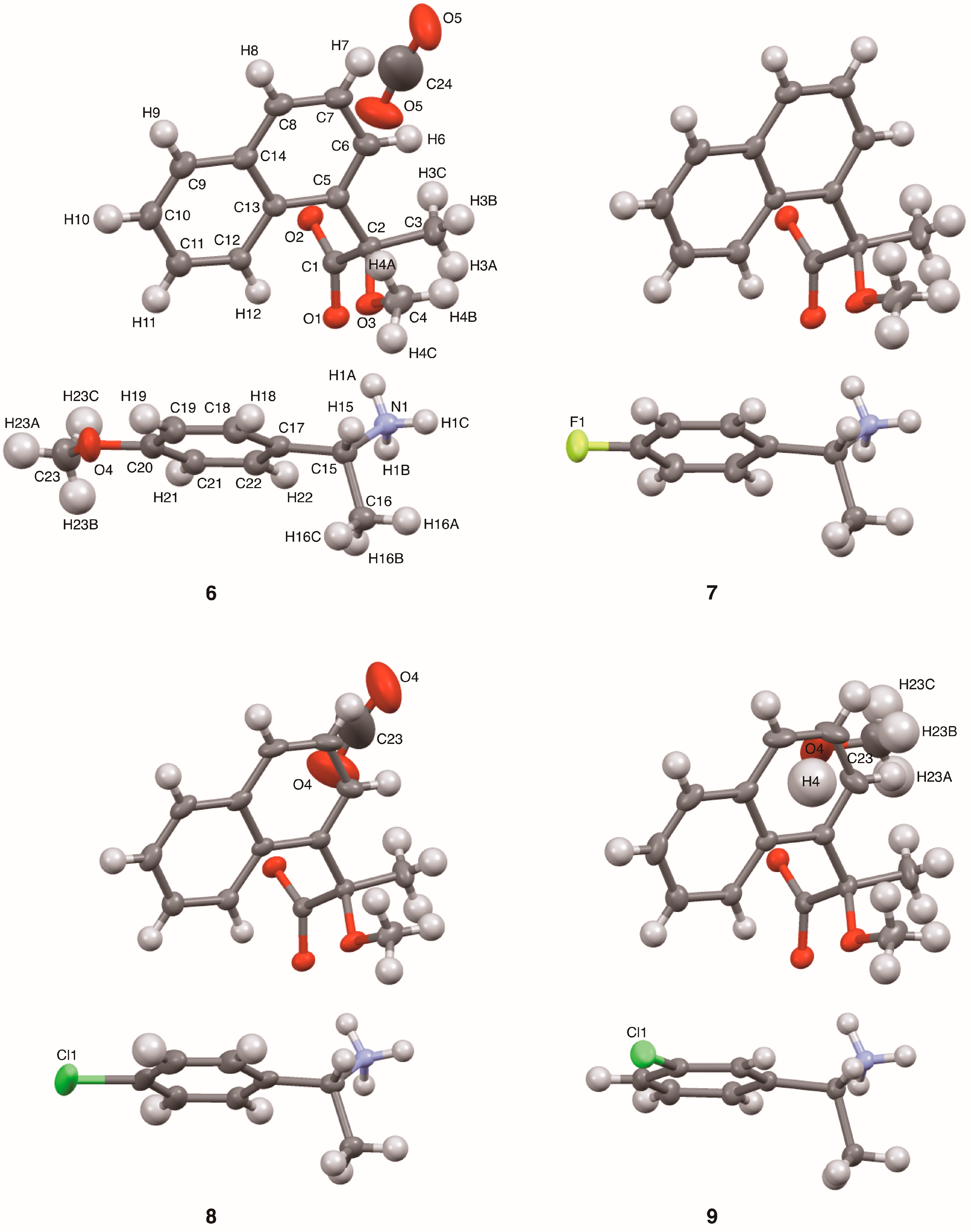
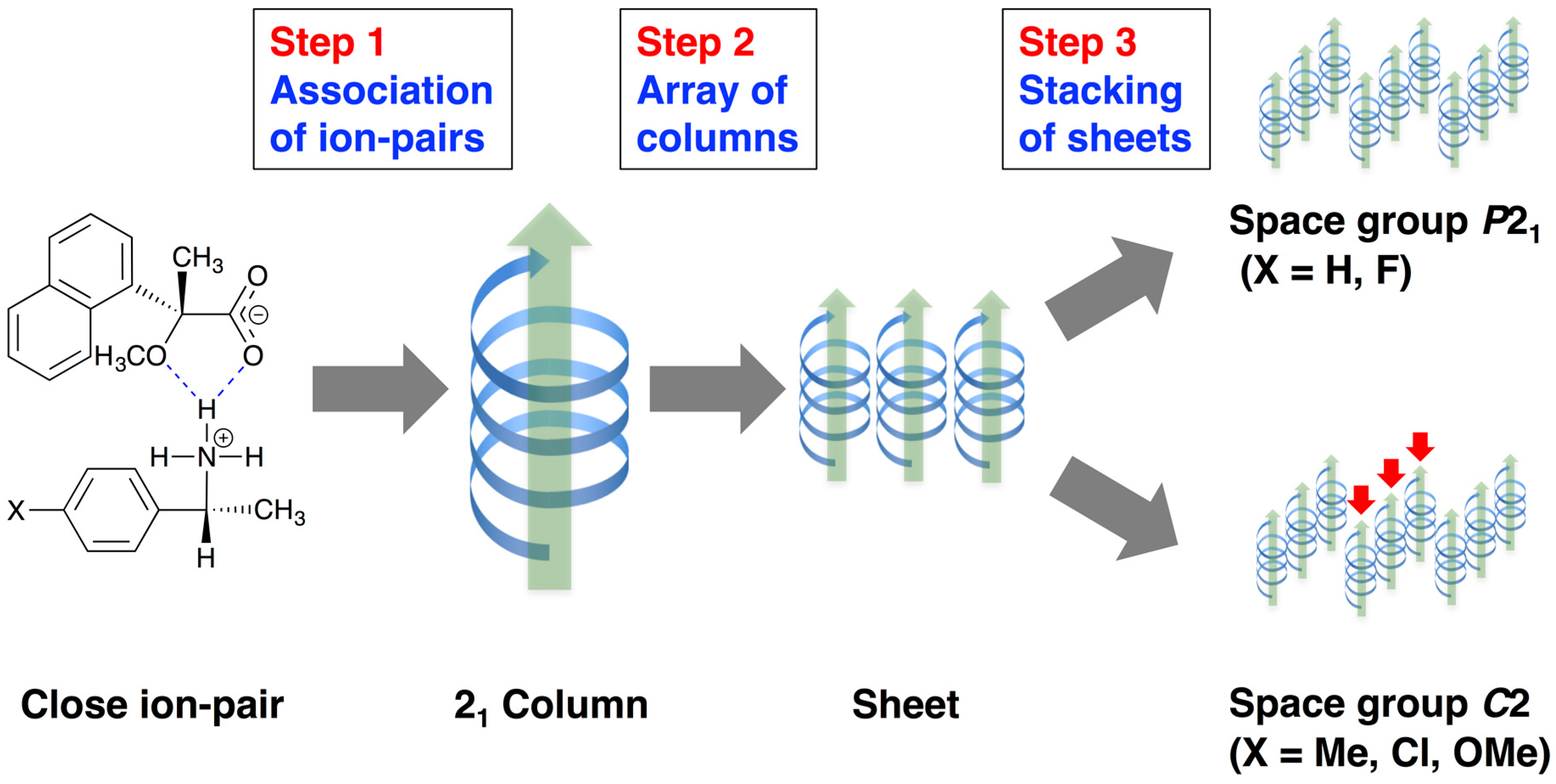
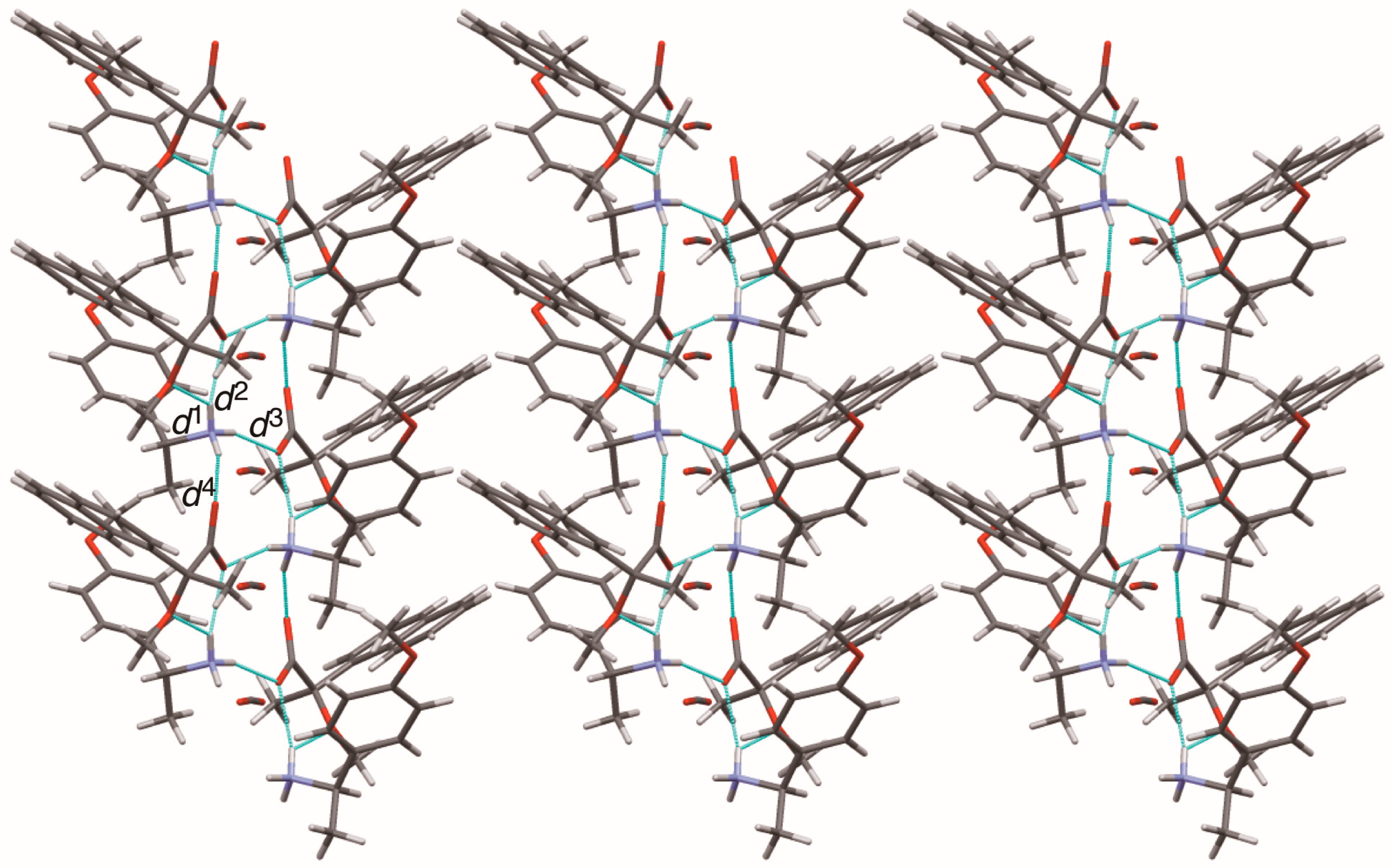
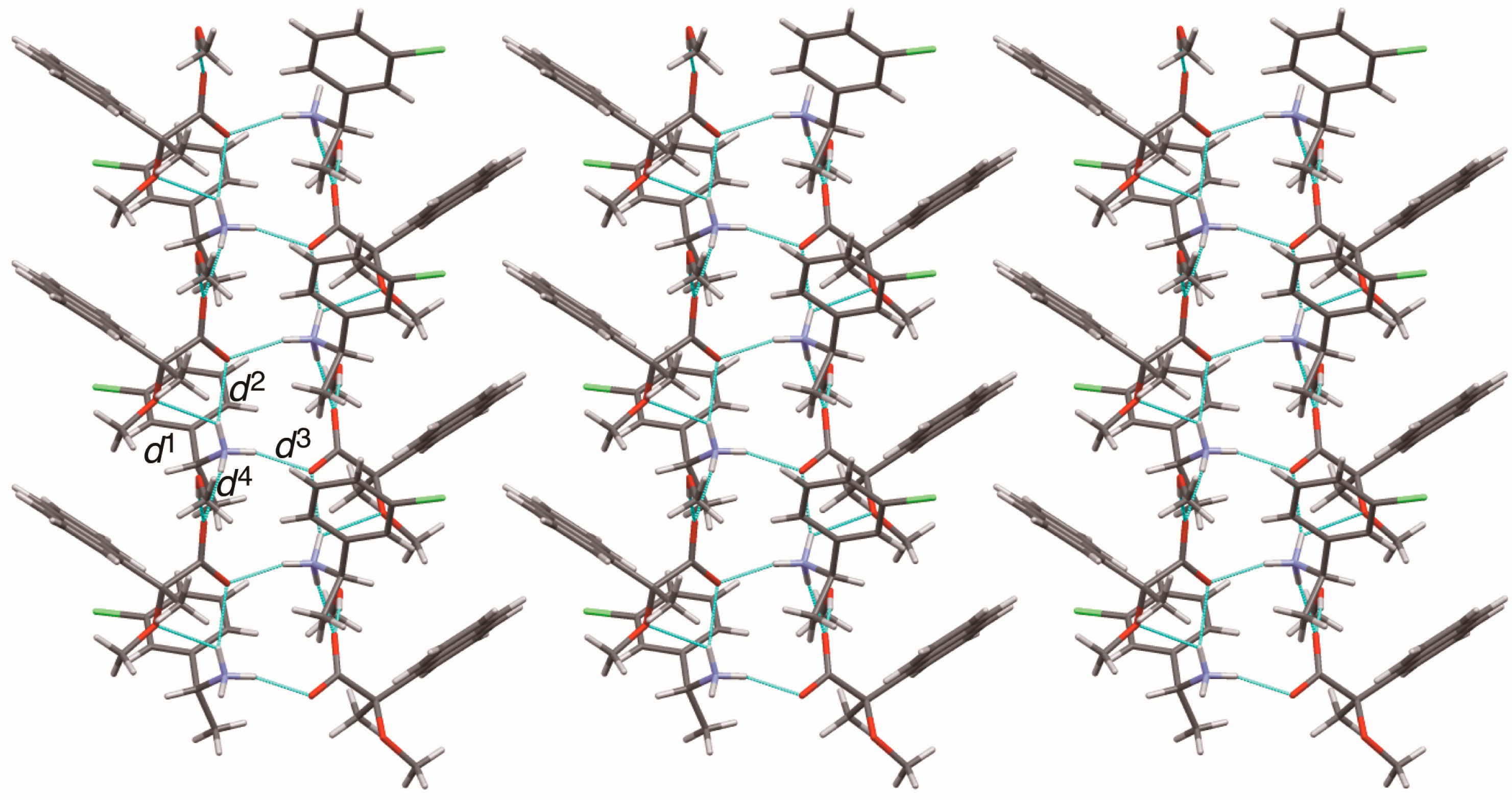
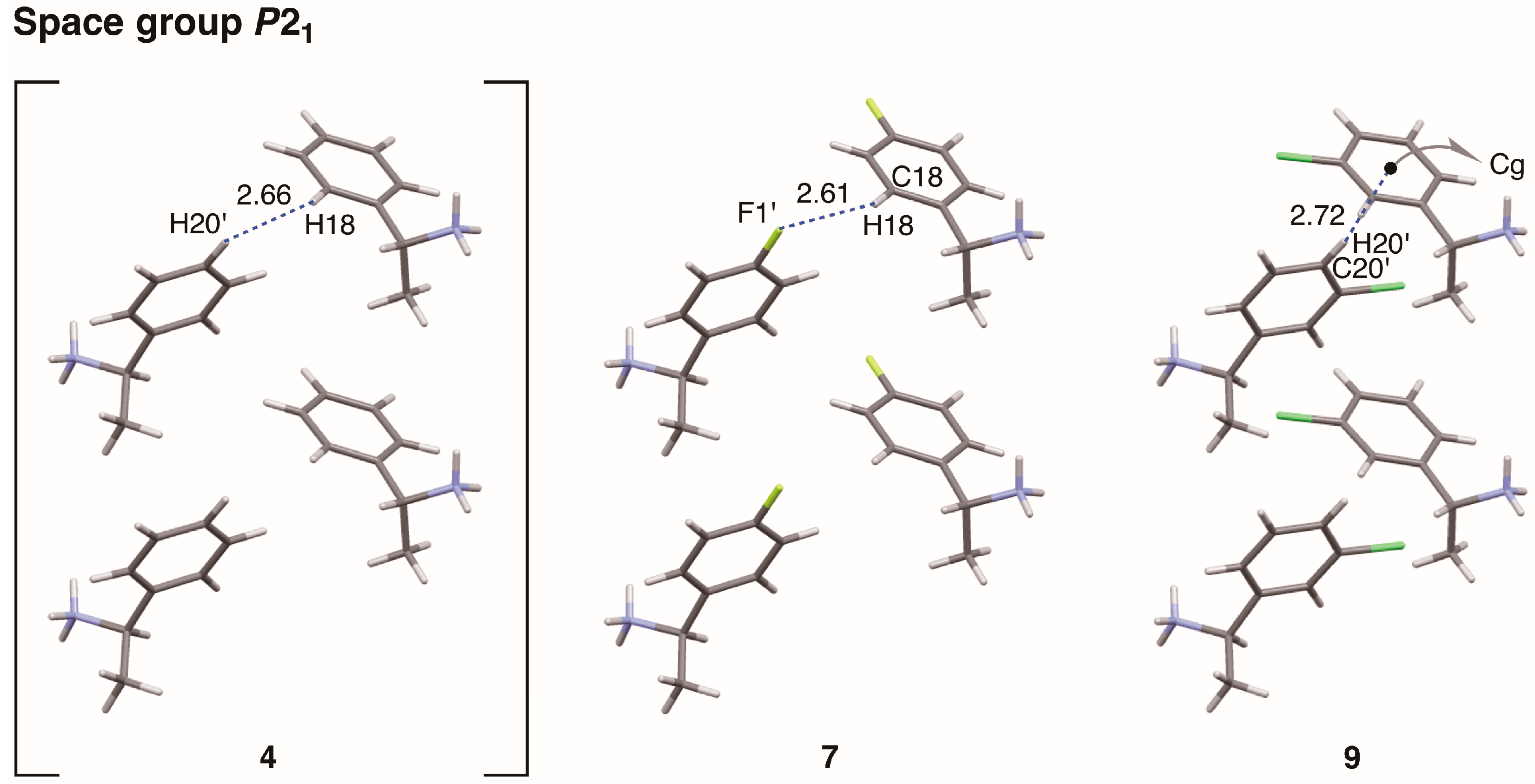
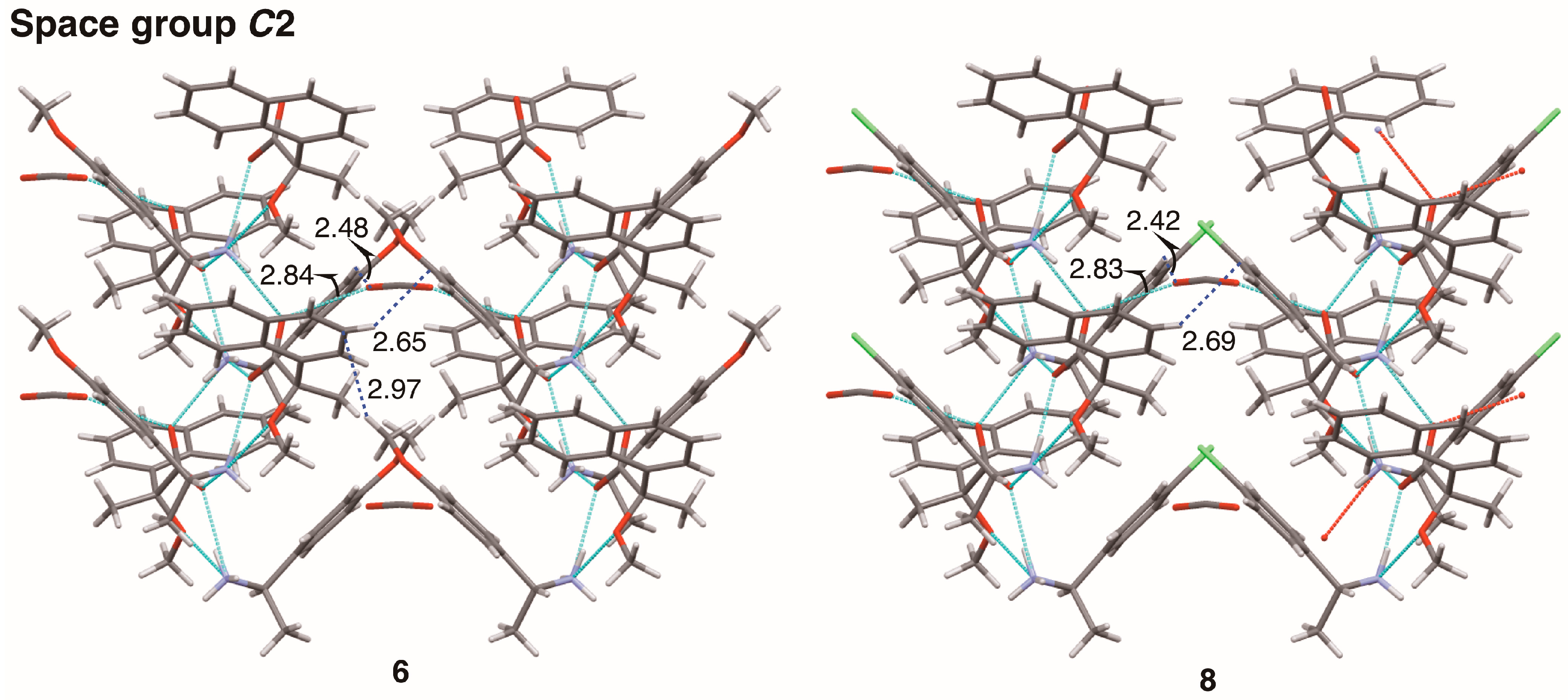
This report describes substituent effects on the crystal structures of salts
6–
9. The 4-methoxyphenyl- and 3-chlrorophenyl groups were fixed in the crystal lattice. Thus, the planar chirality [
14] of the (
R)-1-arylethylammonium cations was assigned in salts
6 and
9. The molecular packing of salts
6–
9 was interpreted as the three-step hierarchical assembly [
12,
13].
A large number of agrochemicals and pharmaceuticals are halogenated compounds. In 2007, Müller et al. reported that ca. 20% of all pharmaceuticals, and even more agrochemicals (up to 30%), contained fluorine atoms [
15]. The ratio of chlorinated drugs was next to the fluorinated drugs in all halogenated drugs [
16]. However, the effects of halogen atoms, especially fluorine, are ambivalent. Organic crystals are now considered a type of supermolecule [
7]. Therefore, the elucidation of substitution effects in organic crystals will contribute to an overall understanding of weak intermolecular interactions. The current study explores aromatic C–H···π, C–H···F, and C–H···O interactions in crystalline salts
6–
9.
Crystal engineering of organic salts is important as a means of enantioresolution [
17,
18,
19]. Investigations of crystal structures provide information on weak intermolecular interactions that are useful in the design of biofunctional molecules.
3. Conclusions
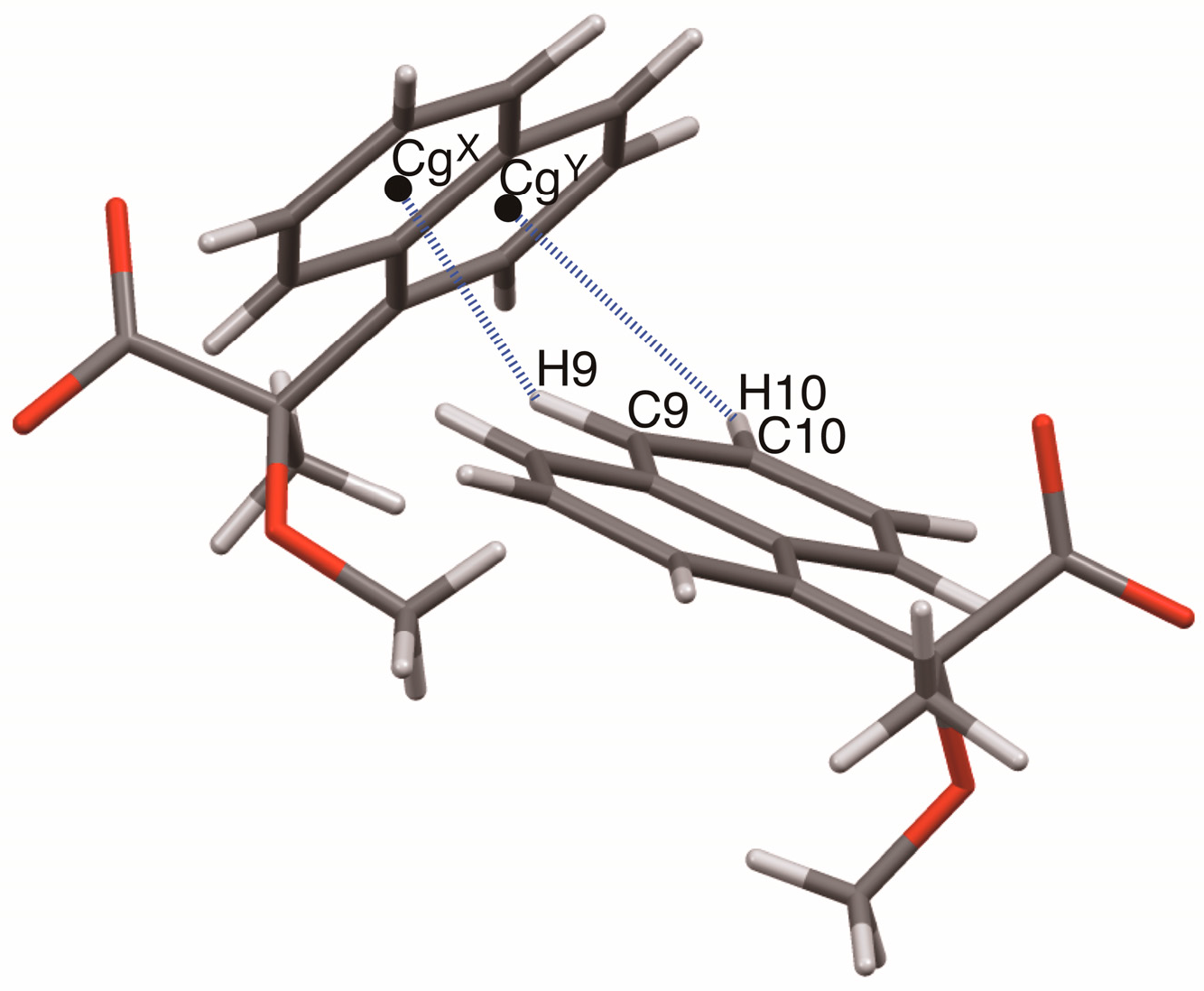
This study clarified the crystal structures of MαNP salts 6–9 prepared from (R)-1 and (R)-1-arylethylamines. Using the concepts of supramolecular chirality, the solid-state associations of close ion-pairs were elucidated as supS in all salts. In addition, the solid-state planar chirality of the (R)-1-(4-methoxyphenyl)ethylammonium cation of salt 6 and the (R)-1-(3-chlorophenyl)ethylammonium cation of salt 9 were assigned as plR and plS, respectively. It should be noted that these are not genuine planar chirality due to unrestricted rotations. The crystal structures of MαNP salts 6–9 were interpreted as three-step hierarchical assemblies. Para-substituents of phenyl groups were positioned on sheet structures consisting of 21 columns and thereby affected the sheet stacking. Smaller para-substituents, that is, salt 7 (p-F) and salt 9 (p-H), and larger para-substituents, that is, salt 6 (p-OMe) and salt 8 (p-Cl), yielded space groups P21 and C2, respectively. For MαNP salts, p-H and p-OMe groups were isosteric with p-F and p-Cl groups, respectively. The 3-chlorophenyl groups of salt 9 exhibited homo-aromatic C–H···π interactions. For (R)-1-arylethylamines with larger substituents, methanol molecules filled the space in the crystal lattice. These results provide information on supramolecular chemistry for the design and preparation of single-enantiomer biofunctional molecules.
4. Materials and Methods
4.1. X-ray Crystallography
The single crystals (salt
6, 0.300 × 0.100 × 0.060 mm; salt
7, 0.200 × 0.200 × 0.200 mm; salt
8, 0.550 × 0.150 × 0.100 mm; salt
9, 0.600 × 0.600 × 0.500 mm) were covered with paraffin oil and mounted on a glass fiber, respectively. All measurements were made on a Rigaku Mercury70 diffractometer using graphite monochromated Mo-Kα radiation, operating at 50 kV/40 mA. Data were processed on a PC using CrystalClear Software (Rigaku, Tokyo, Japan). Structures were solved using direct methods and refined by full-matrix least-squares methods on
F2 (SHELXL-97). CCDC 1442523 (salt
6), CCDC 1442524 (salt
7), CCDC 1442525 (salt
8), and CCDC 1442526 (salt
9) contain the supplementary crystallographic data for this study, which can be obtained from The Cambridge Crystallographic Data Centre via
www.ccdc.cam.ac.uk. Each crystal was dried in vacuo overnight at 80 °C prior to elemental analysis.
4.2. Preparation of Salt 6
A mixture of (R)-1 (29.2 mg, 127 μmol) and (R)-1-(4-methoxyphenyl)ethylamine (21.7 mg, 144 μmol) was dissolved in CHCl3 and MeOH (0.5 mL and 1.5 mL, respectively). The solution was warmed in a water bath at 45 °C and concentrated to ca. 1 mL in vacuo. Then, the solution was allowed to stand at RT for 5 days to give colorless crystals of salt 6 (MeOH solvate; 13.7 mg, 34 μmol) in 27% yield.
(R)-1-(4-Methoxyphenyl)ethylammonium (R)-2-methoxy-2-(1-naphthyl)propanoate (6). Elemental analysis calculated (%) for C23.5H29NO4.5 (6·0.5MeOH): C 71.01, H 7.35, N 3.52; found: C 71.21, H 7.37, N 3.51.
4.3. Preparation of Salt 7
A mixture of (R)-1 (31.0 mg, 135 μmol) and (R)-1-(4-fluorophenyl)ethylamine (18.7 mg, 134 μmol) was dissolved in CHCl3 and MeOH (2 mL and 1 mL, respectively). Then, the solution was allowed to stand at RT for three days to give colorless crystals of salt 7 (32.6 mg, 88 μmol) in 66% yield.
(R)-1-(4-Fluorophenyl)ethylammonium (R)-2-methoxy-2-(1-naphthyl)propanoate (7). Elemental analysis calculated (%) for C22H24FNO3: C 71.53, H 6.55, N 3.79; found: C 71.52, H 6.22, N 3.72.
4.4. Preparation of Salt 8
A mixture of (R)-1 (31.5 mg, 137 μmol) and (R)-1-(4-chlorophenyl)ethylamine (32.7 mg, 210 μmol) was dissolved in CHCl3 and MeOH (0.5 mL and 1.5 mL, respectively). The solution was warmed in a water bath at 45 °C and allowed to stand at RT for two days to give crude crystals of salt 8. Recrystallization from CHCl3/MeOH (1mL and 0.5 mL, respectively) gave colorless crystals of salt 8 (MeOH solvate; 18.3 mg, 46 μmol) with a total yield of 33%.
(R)-1-(4-Chlorophenyl)ethylammonium (R)-2-methoxy-2-(1-naphthyl)propanoate (8). Elemental analysis calculated (%) for C22.5H26ClNO3.5 (8·0.5MeOH): C 67.24, H 6.52, N 3.49; found: C 67.55, H 6.28, N 3.53.
4.5. Preparation of Salt 9
A mixture of (R)-1 (29.9 mg, 130 μmol) and (R)-1-(3-chlorophenyl)ethylamine (25.9 mg, 166 μmol) was dissolved in CHCl3 and MeOH (1 mL and 2 mL, respectively). The solution was warmed in a water bath at 47 °C and allowed to stand at RT for 10 days to give colorless crystals of salt 9 (MeOH solvate; 33.1 mg, 79 μmol) in 61% yield.
(R)-1-(3-Chlorophenyl)ethylammonium (R)-2-methoxy-2-(1-naphthyl)propanoate (9). Elemental analysis calculated (%) for C22H24ClNO3: C 68.48, H 6.27, N 3.63; found: C 68.50, H 6.24, N 3.56.