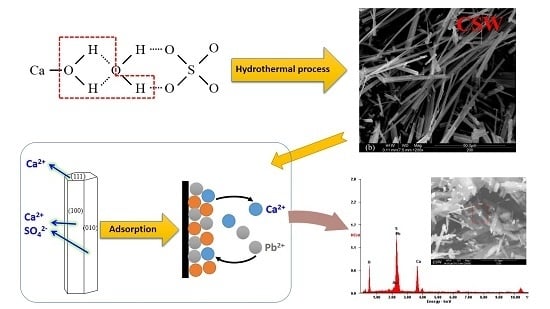
Calcium Sulfate Hemihydrate Whiskers Obtained from Flue Gas Desulfurization Gypsum and Used for the Adsorption Removal of Lead
Abstract
:1. Introduction
2. Experiment and Characterization
2.1. Chemicals
2.2. Synthesis of CSHW
2.3. Adsorption Study of Lead Ions
2.4. Characterization
3. Results and Discussion
3.1. Basic Characteristics of CSHW
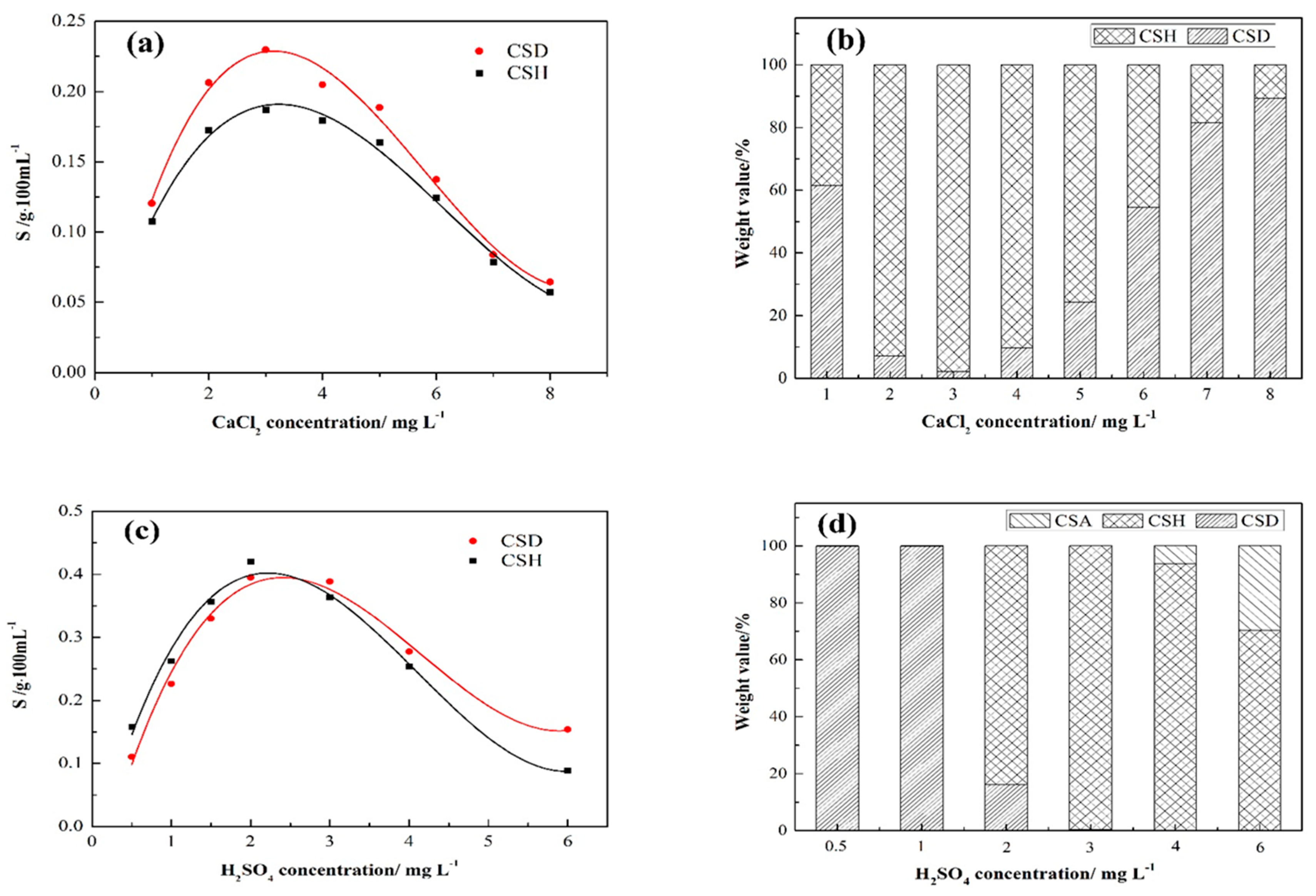
3.2. Metastable Lifetime Zone of CSHW during the Hydrothermal Reaction
3.3. Sorption of Lead Ions
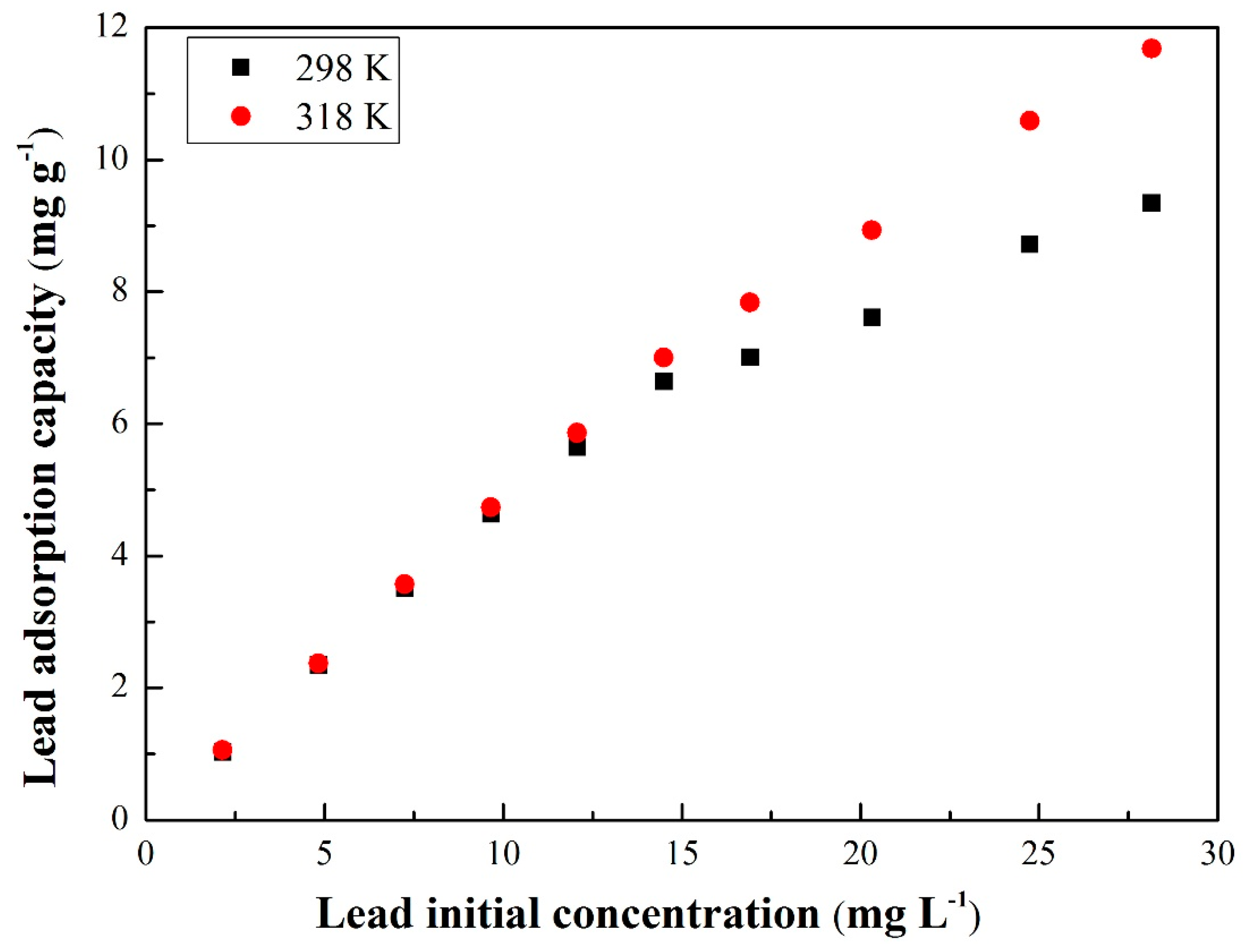
3.3.1. Equilibrium Isotherms
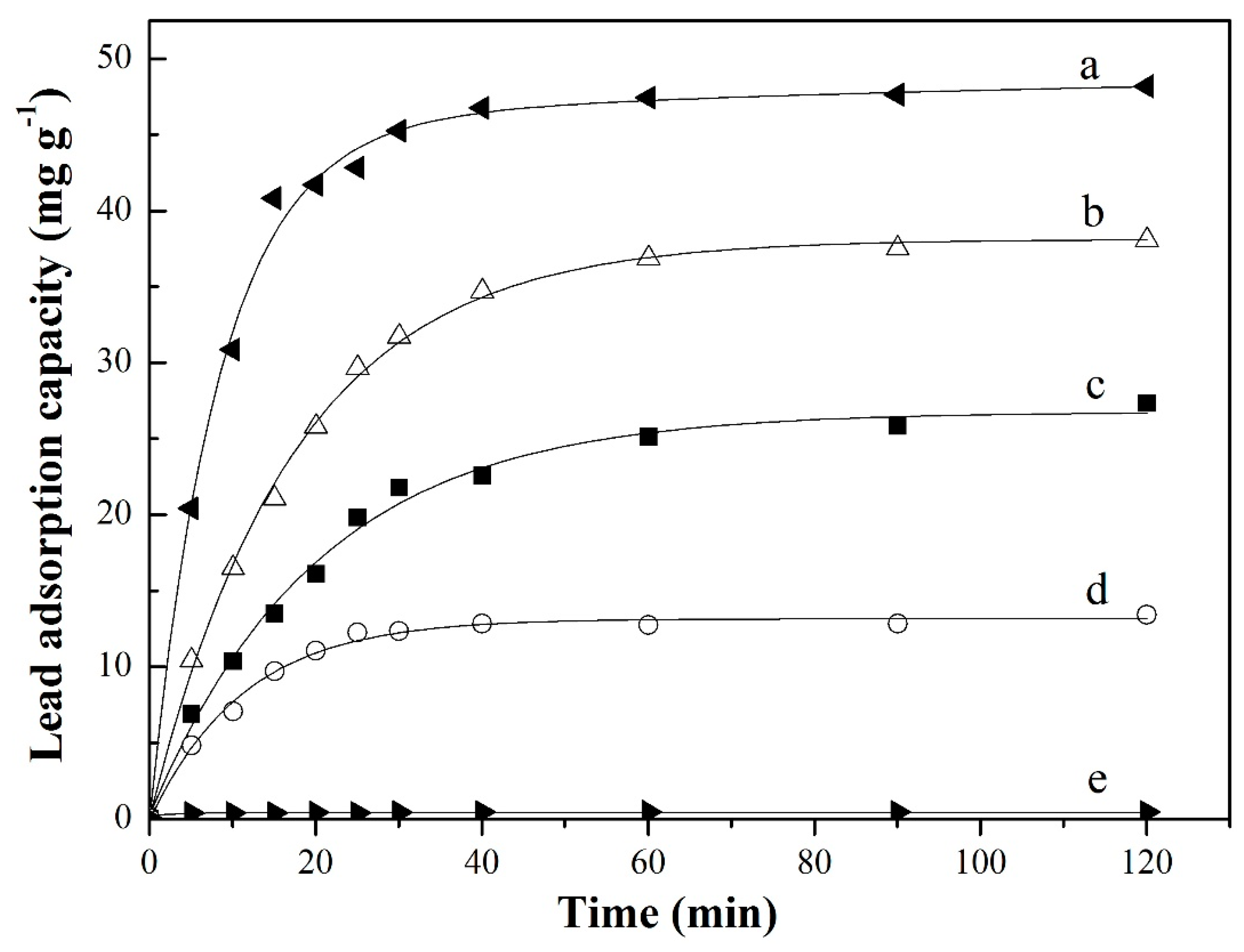
3.3.2. Adsorption Kinetics
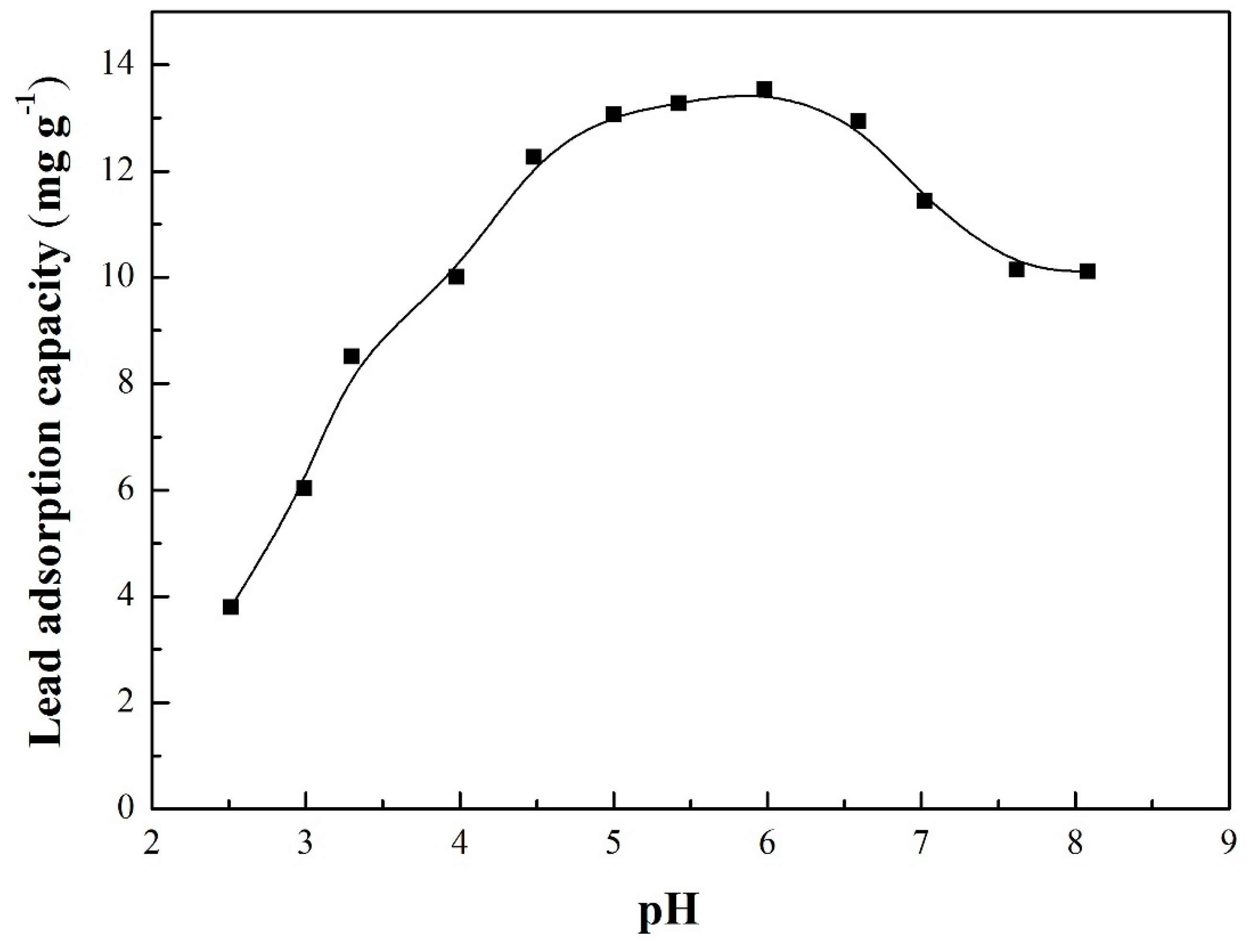
3.4. Adsorption Envelopes
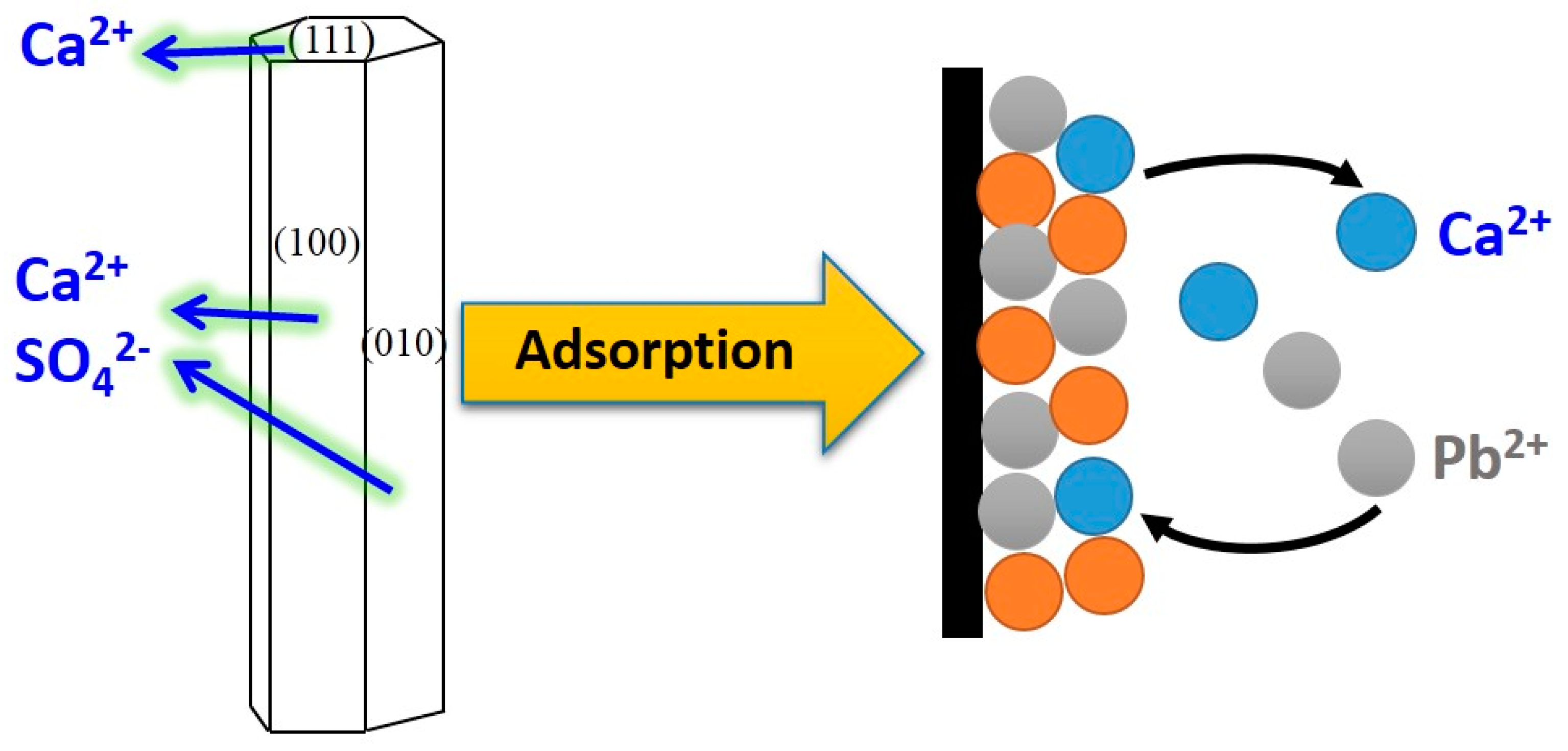
3.5. Adsorption Mechanism
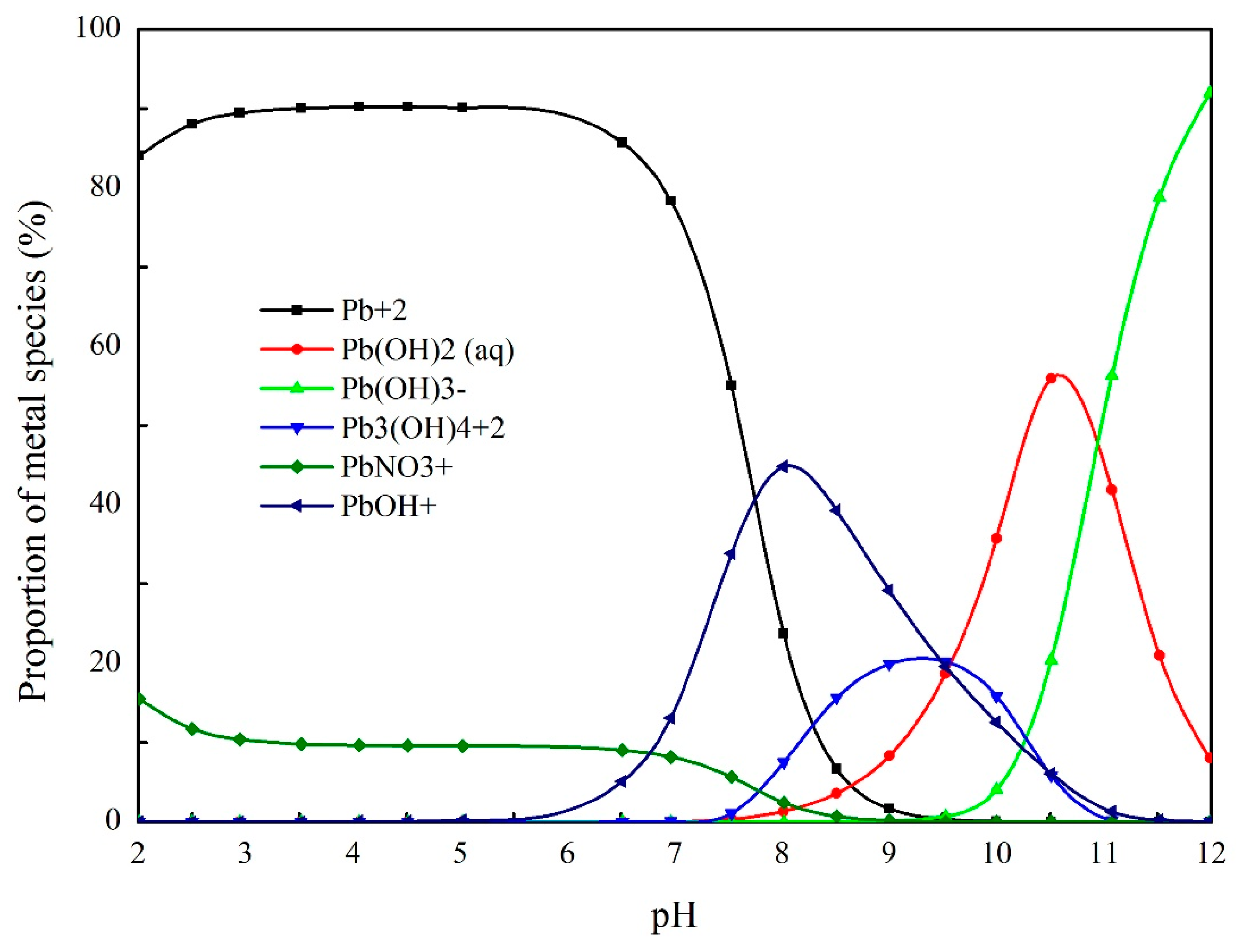
3.5.1. Lead Ion Speciation and Chemical Reaction
3.5.2. Adsorption Property of Lead Ions on CSHW Surface
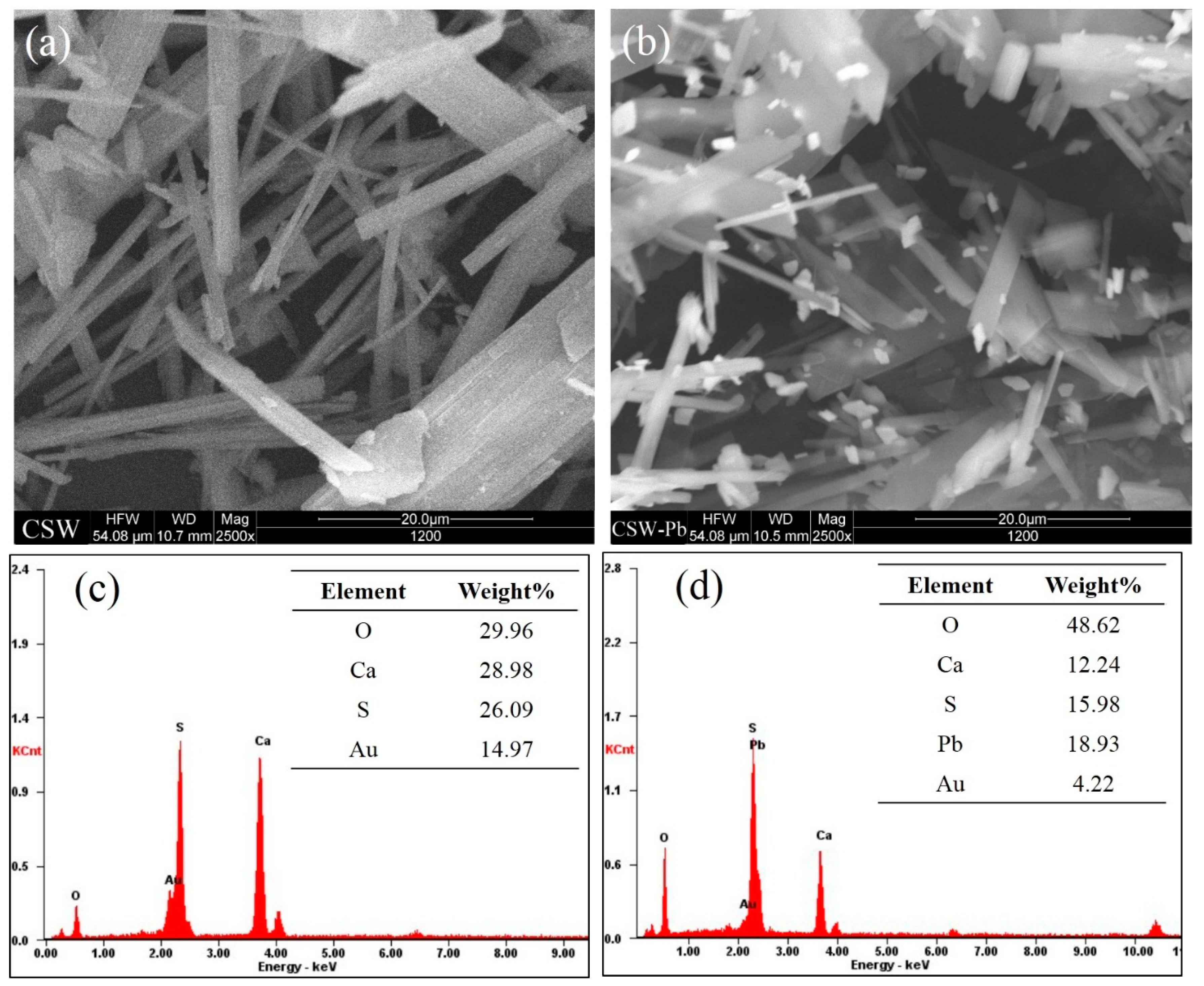
3.5.3. Post Adsorption Characteristics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zheng, D.; Lu, H.; Sun, X.; Liu, X.; Han, W.; Wang, L. Reaction mechanism of reductive decomposition of fgd gypsum with anthracite. Thermochim. Acta 2013, 559, 23–31. [Google Scholar] [CrossRef]
- Nan, J.; Chen, X.; Wang, X.; Lashari, M.S.; Wang, Y.; Guo, Z.; Du, Z. Effects of applying flue gas desulfurization gypsum and humic acid on soil physicochemical properties and rapeseed yield of a saline-sodic cropland in the eastern coastal area of china. J. Soils Sediments 2015, 16, 38–50. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Calabrese, D.; Valenti, G.L.; Montagnaro, F. Flue gas desulfurization gypsum and coal fly ash as basic components of prefabricated building materials. Waste Manag. 2013, 33, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Chandara, C.; Azizli, K.A.; Ahmad, Z.A.; Sakai, E. Use of waste gypsum to replace natural gypsum as set retarders in portland cement. Waste Manag. 2009, 29, 1675–1679. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.H.; Yang, L.; Fu, H.L.; Kong, B.; Li, T.Y.; Yang, L.C. Alpha-calcium sulfate hemihydrate preparation from fgd gypsum in recycling mixed salt solutions. Chem. Eng. J. 2011, 174, 296–303. [Google Scholar] [CrossRef]
- Pan, Z.Y.; Lou, Y.; Yang, G.Y.; Ni, X.; Chen, M.C.; Xu, H.Z.; Miao, X.G.; Liu, J.L.; Hu, C.F.; Huang, Q. Preparation of calcium sulfate dihydrate and calcium sulfate hemihydrate with controllable crystal morphology by using ethanol additive. Ceram. Int. 2013, 39, 5495–5502. [Google Scholar] [CrossRef]
- Hou, S.C.; Xiang, L. Influence of activity of CaSO4·2H2O on hydrothermal formation of CaSO4·0.5H2O whiskers. J. Nanomater. 2013, 2013, 2–7. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Q.; Wang, T. Mechanical, damping, and thermal properties of calcium sulfate whisker-filled castor oil-based polyurethane/epoxy ipn composites. J. Reinf. Plast. Compos. 2011, 30, 509–515. [Google Scholar] [CrossRef]
- Zhao, W.; Wu, Y.; Xu, J.; Gao, C. Effect of ethylene glycol on hydrothermal formation of calcium sulfate hemihydrate whiskers with high aspect ratios. RSC Adv. 2015, 5, 50544–50548. [Google Scholar] [CrossRef]
- Wang, J.; Yang, K.; Lu, S. Preparation and characteristic of novel silicone rubber composites based on organophilic calcium sulfate whisker. High Perform. Polym. 2011, 23, 141–150. [Google Scholar] [CrossRef]
- Yuan, W.; Cui, J.; Cai, Y.; Xu, S. A novel surface modification for calcium sulfate whisker used for reinforcement of poly(vinyl chloride). J. Polym. Res. 2015, 22, 173. [Google Scholar] [CrossRef]
- Han, Q.; Luo, K.B.; Li, H.P.; Xiang, L. Influence of disodium hydrogen phosphate dodecahydrate on hydrothermal formation of hemihydrate calcium sulfate whiskers. Particuology 2014, 17, 131–135. [Google Scholar] [CrossRef]
- Xu, A.-Y.; Li, H.-P.; Luo, K.-B.; Xiang, L. Formation of calcium sulfate whiskers from caco3-bearing desulfurization gypsum. Res. Chem. Intermed. 2011, 37, 449–455. [Google Scholar] [CrossRef]
- Eberl, J.J.; Thelen, E.; Heller, H.L. Calcium Sulfate Whisker Fibers and the Method for the Manufacture Thereof. U.S. Patent 3,822,340, 2 July 1974. [Google Scholar]
- Eberl, J.J.; Thelen, E.; Heller, H.L. Method for the Manufacture of Coated Calcium Sulfate Whisker Fibers. U.S. Patent 3,961,105, 1 June 1976. [Google Scholar]
- Jiang, G.; Wang, H.; Chen, Q.; Zhang, X.; Wu, Z.; Guan, B. Preparation of alpha-calcium sulfate hemihydrate from fgd gypsum in chloride-free ca(no3)(2) solution under mild conditions. Fuel 2016, 174, 235–241. [Google Scholar] [CrossRef]
- Mao, X.; Song, X.; Lu, G.; Sun, Y.; Xu, Y.; Yu, J. Control of crystal morphology and size of calcium sulfate whiskers in aqueous hcl solutions by additives: Experimental and molecular dynamics simulation studies. Ind. Eng. Chem. Res. 2015, 54, 4781–4787. [Google Scholar] [CrossRef]
- Wang, J.; Tang, L.; Wu, D.; Guo, X.; Hao, W. Application of modified calcium sulfate whisker in methyl vinyl silicone rubber composites. Polym. Polym. Compos. 2012, 20, 453–461. [Google Scholar]
- Bradl, H.B. Adsorption of heavy metal ions on soils and soils constituents. J. Colloid Interface Sci. 2004, 277, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sabela, M.I.; Kunene, K.; Kanchi, S.; Xhakaza, N.M.; Bathinapatla, A.; Mdluli, P.; Sharma, D.; Bisetty, K. Removal of copper (ii) from wastewater using green vegetable waste derived activated carbon: An approach to equilibrium and kinetic study. Arab. J. Chem. 2016, in press. [Google Scholar] [CrossRef]
- Cross, J.B.; Hays, J.M.; Schmidt, R.; Katdare, V.Y. Removal of Heavy Metals from Hydrocarbon Gases. U.S. Patent 7,901,486, 8 March 2011. [Google Scholar]
- Samad, A.; Mai, F.; Katsumata, H.; Suzuki, T.; Kaneco, S. Photocatalytic oxidation and simultaneous removal of arsenite with cuo/zno photocatalyst. J. Photochem. Photobiol. A Chem. 2016, 325, 97–103. [Google Scholar] [CrossRef]
- Esalah, J.O.; Weber, M.E.; Vera, J.H. Removal of lead, cadmium and zinc from aqueous solutions by precipitation with sodium di-(n-octyl) phosphinate. Can. J. Chem. Eng. 2000, 78, 948–954. [Google Scholar] [CrossRef]
- Mishra, P.C.; Patel, R.K. Removal of lead and zinc ions from water by low cost adsorbents. J. Hazard. Mater. 2009, 168, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Sun, Y.; Wu, J.; Wu, B.; Creamer, A.E.; Gao, B. Graphene oxide as filter media to remove levofloxacin and lead from aqueous solution. Chemosphere 2015, 150, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Dakiky, M.; Khamis, M.; Manassra, A.; Mer’eb, M. Selective adsorption of chromium(vi) in industrial wastewater using low-cost abundantly available adsorbents. Adv. Environ. Res. 2002, 6, 533–540. [Google Scholar] [CrossRef]
- Goel, J.; Kadirvelu, K.; Rajagopal, C.; Garg, V.K. Removal of lead(ii) by adsorption using treated granular activated carbon: Batch and column studies. J. Hazard. Mater. 2005, 125, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Günay, A.; Arslankaya, E.; Tosun, İ. Lead removal from aqueous solution by natural and pretreated clinoptilolite: Adsorption equilibrium and kinetics. J. Hazard. Mater. 2007, 146, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Agarwal, S.; Saleh, T.A. Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal. J. Hazard. Mater. 2011, 185, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G. Absolute electronegativity and hardness: Application to inorganic chemistry. J. Org. Chem. 1988, 27, 734–740. [Google Scholar] [CrossRef]
- Gomez-Serrano, V.; Macias-Garcia, A.; Espinosa-Mansilla, A.; Valenzuela-Calahorro, C. Adsorption of mercury, cadmium and lead from aqueous solution on heat-treated and sulphurized activated carbon. Water Res. 1998, 32, 1–4. [Google Scholar] [CrossRef]
- Mao, X.; Song, X.; Lu, G.; Sun, Y.; Xu, Y.; Yu, J. Effects of metal ions on crystal morphology and size of calcium sulfate whiskers in aqueous hcl solutions. Ind. Eng. Chem. Res. 2014, 53, 17625–17635. [Google Scholar] [CrossRef]
- Rabizadeh, T.; Stawski, T.M.; Morgan, D.J.; Peacock, C.L.; Benning, L.G. The effects of inorganic additives on the nucleation and growth kinetics of calcium sulfate dihydrate crystals. Cryst. Growth Des. 2017, 17, 582–589. [Google Scholar] [CrossRef]
- Wu, X.Q.; Wang, K.M.; Xiong, Z.Y.; Ye, X.L. Solubility of alpha-calcium sulfate hemihydrate in ca-mg-k chloride salt solution at (353.0 to 371.0) k. J. Chem. Eng. Data 2013, 58, 48–54. [Google Scholar] [CrossRef]
- Yang, L.; Guan, B.; Wu, Z.; Ma, X. Solubility and phase transitions of calcium sulfate in kcl solutions between 85 and 100 °C. Ind. Eng. Chem. Res. 2009, 48, 7773–7779. [Google Scholar] [CrossRef]
- Hamdona, S.K.; Hadad, U.A.A. Crystallization of calcium sulfate dihydrate in the presence of some metal ions. J. Cryst. Growth 2007, 299, 146–151. [Google Scholar] [CrossRef]
- Li, F.; Liu, J.; Yang, G.; Pan, Z.; Ni, X.; Xu, H.; Huang, Q. Effect of ph and succinic acid on the morphology of α-calcium sulfate hemihydrate synthesized by a salt solution method. J. Cryst. Growth 2013, 374, 31–36. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Q.; Wang, Y.; Shi, P.; Jiang, M. Surface modification of calcium sulfate whisker prepared from flue gas desulfurization gypsum. Appl. Surf. Sci. 2016, 360, 263–269. [Google Scholar] [CrossRef]
- Guan, B.H.; Yang, L.C.; Wu, Z.B.; Shen, Z.X.; Ma, X.F.; Ye, Q.Q. Preparation of alpha-calcium sulfate hemihydrate from fgd gypsum in k, mg-containing concentrated cacl(2) solution under mild conditions. Fuel 2009, 88, 1286–1293. [Google Scholar] [CrossRef]
- Fu, H.L.; Jiang, G.M.; Wang, H.; Wu, Z.; Guan, B.H. Solution-mediated transformation kinetics of calcium sulfate dihydrate to alpha-calcium sulfate hemihydrate in cacl2 solutions at elevated temperature. Ind. Eng. Chem. Res. 2013, 52, 17134–17139. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, M.; Ke, X.; Tan, R.; Chen, Y.; Hou, H. Synthesis of alpha hemihydrate particles with lithium and carboxylates via the hydrothermal method. Powder Technol. 2017, 317, 293–300. [Google Scholar] [CrossRef]
- Ling, Y.B.; Demopoulos, G.P. Solubility of calcium sulfate hydrates in (0 to 3.5) mol-kg(-1) sulfuric acid solutions at 100 °C. J. Chem. Eng. Data 2004, 49, 1263–1268. [Google Scholar] [CrossRef]
- El-Ashtoukhy, E.S.Z.; Amin, N.K.; Abdelwahab, O. Removal of lead (ii) and copper (ii) from aqueous solution using pomegranate peel as a new adsorbent. Desalination 2008, 223, 162–173. [Google Scholar] [CrossRef]
- Styles, P.M.; Chanda, M.; Rempel, G.L. Sorption of arsenic anions onto poly(ethylene mercaptoacetimide). React. Funct. Polym. 1996, 31, 89–102. [Google Scholar] [CrossRef]
- Ren, X.; Shao, D.; Yang, S.; Hu, J.; Sheng, G.; Tan, X.; Wang, X. Comparative study of pb(ii) sorption on xc-72 carbon and multi-walled carbon nanotubes from aqueous solutions. Chem. Eng. J. 2011, 170, 170–177. [Google Scholar] [CrossRef]
- Fu, R.; Yang, Y.; Xu, Z.; Zhang, X.; Guo, X.; Bi, D. The removal of chromium (vi) and lead (ii) from groundwater using sepiolite-supported nanoscale zero-valent iron (s-nzvi). Chemosphere 2015, 138, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Amarasinghe, B.M.W.P.K.; Williams, R.A. Tea waste as a low cost adsorbent for the removal of cu and pb from wastewater. Chem. Eng. J. 2007, 132, 299–309. [Google Scholar] [CrossRef]
- Ho, Y.S.; Mckay, G. The sorption of lead(ii) ions on peat. Water Res. 1999, 33, 578–584. [Google Scholar] [CrossRef]
- Rao, M.M.; Rao, G.P.C.; Seshaiah, K.; Choudary, N.V.; Wang, M.C. Activated carbon from ceiba pentandra hulls, an agricultural waste, as an adsorbent in the removal of lead and zinc from aqueous solutions. Waste Manag. 2008, 28, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Heidari, A.; Younesi, H.; Mehraban, Z. Removal of ni(ii), cd(ii), and pb(ii) from a ternary aqueous solution by amino functionalized mesoporous and nano mesoporous silica. Chem. Eng. J. 2009, 153, 70–79. [Google Scholar] [CrossRef]
- Sharma, A.; Bhattacharyya, K.G. Adsorption of chromium (vi) on azadirachta indica (neem) leaf powder. Adsorption 2005, 10, 327–338. [Google Scholar] [CrossRef]
- Wu, C.H. Studies of the equilibrium and thermodynamics of the adsorption of Cu(2+) onto as-produced and modified carbon nanotubes. J. Colloids Interface Sci. 2007, 311, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S. Effect of ph on lead removal from water using tree fern as the sorbent. Bioresour. Technol. 2005, 96, 1292–1296. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Singh, B.; Angove, M. Competitive adsorption behavior of heavy metals on kaolinite. J. Colloids Interface Sci. 2005, 290, 28. [Google Scholar] [CrossRef] [PubMed]
- Caccin, M.; Giorgi, M.; Giacobbo, F.; Ros, M.D.; Besozzi, L.; Mariani, M. Removal of lead (ii) from aqueous solutions by adsorption onto activated carbons prepared from coconut shell. Desalination Water Treat. 2016, 57, 1–19. [Google Scholar] [CrossRef]
- Yan, T.; Luo, X.; Lin, X.; Yang, J. Preparation, characterization and adsorption properties for lead (ii) of alkali-activated porous leather particles. Colloids Surf. A Physicochem. Eng. Asp. 2016, 512, 7–16. [Google Scholar] [CrossRef]
- Allison, J.D.; Brown, D.S.; Novo-Gradac, K.J. Minteqa2/prodefa2, a Geochemical Assessment Model for Environmental Systems: Version 3.0 User’s Manual; Environmental Research Laboratory, Office of Research and Development, US Environmental Protection Agency: Washington, DC, USA, 1991.
- Bernardo, M.; Mendes, S.; Lapa, N.; Gonçalves, M.; Mendes, B.; Pinto, F.; Lopes, H.; Fonseca, I. Removal of lead (Pb2+) from aqueous medium by using chars from co-pyrolysis. J. Colloid Interface Sci. 2013, 409, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.K.; Mandal, T.K. Anion water in gypsum (CaSO4 2H2O) and hemihydrate (CaSO4 0.5 H2O). Cement. Concrete Res. 2002, 32, 313–316. [Google Scholar] [CrossRef]
- Kong, B.; Guan, B.; Yates, M.Z.; Wu, Z. Control of alpha-calcium sulfate hemihydrate morphology using reverse microemulsions. Langmuir 2012, 28, 14137–14142. [Google Scholar] [CrossRef] [PubMed]
- Bosbach, D.; Junta-Rosso, J.L.; Becker, U.; Hochella, M.F. Gypsum growth in the presence of background electrolytes studied by scanning force microscopy. Geochim. Cosmochim. Acta 1996, 60, 3295–3304. [Google Scholar] [CrossRef]
- Martynowicz, E.; Witkamp, G.J.; vanRosmalen, G.M. The effect of aluminium fluoride on the formation of calcium sulfate hydrates. Hydrometallurgy 1996, 41, 171–186. [Google Scholar] [CrossRef]
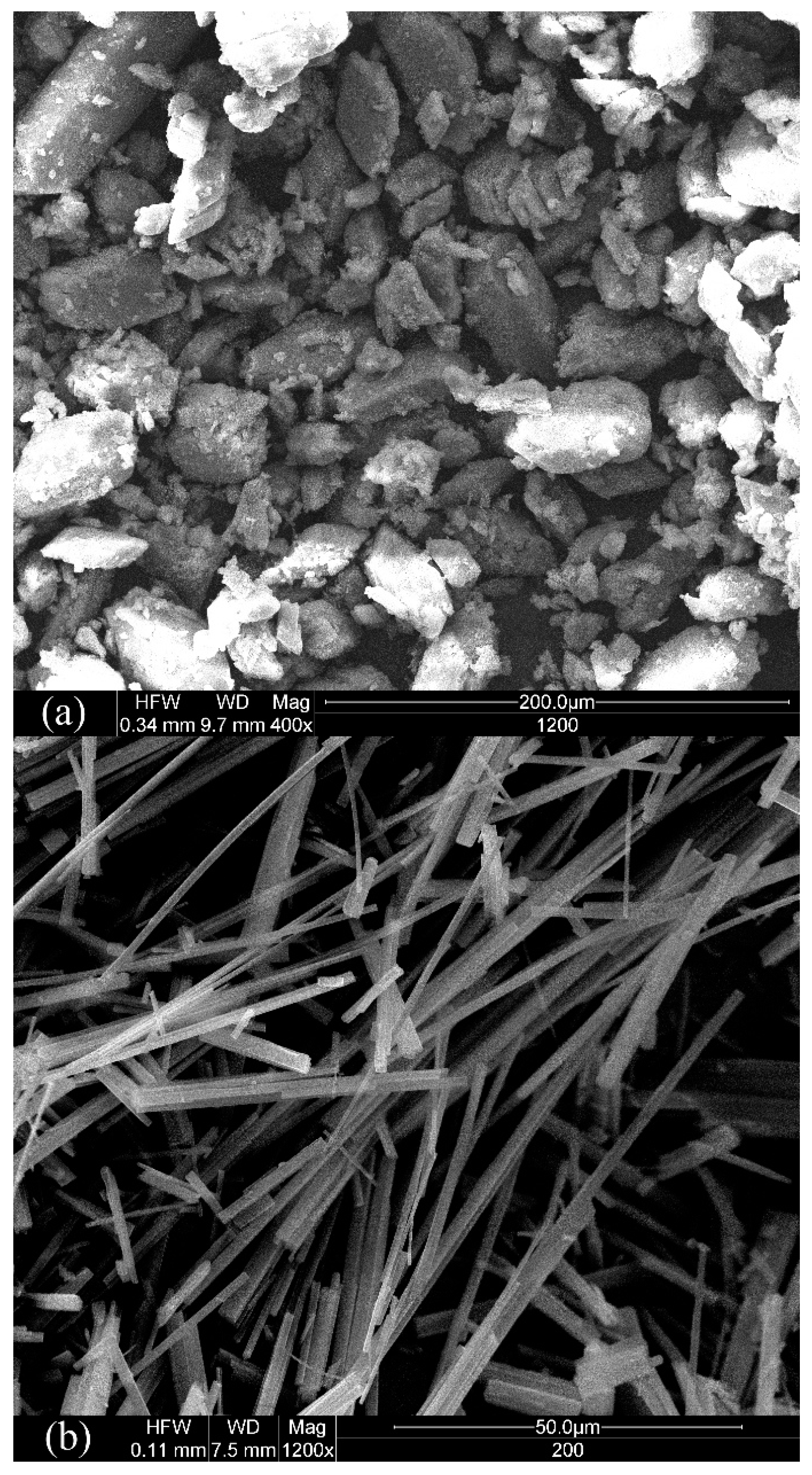
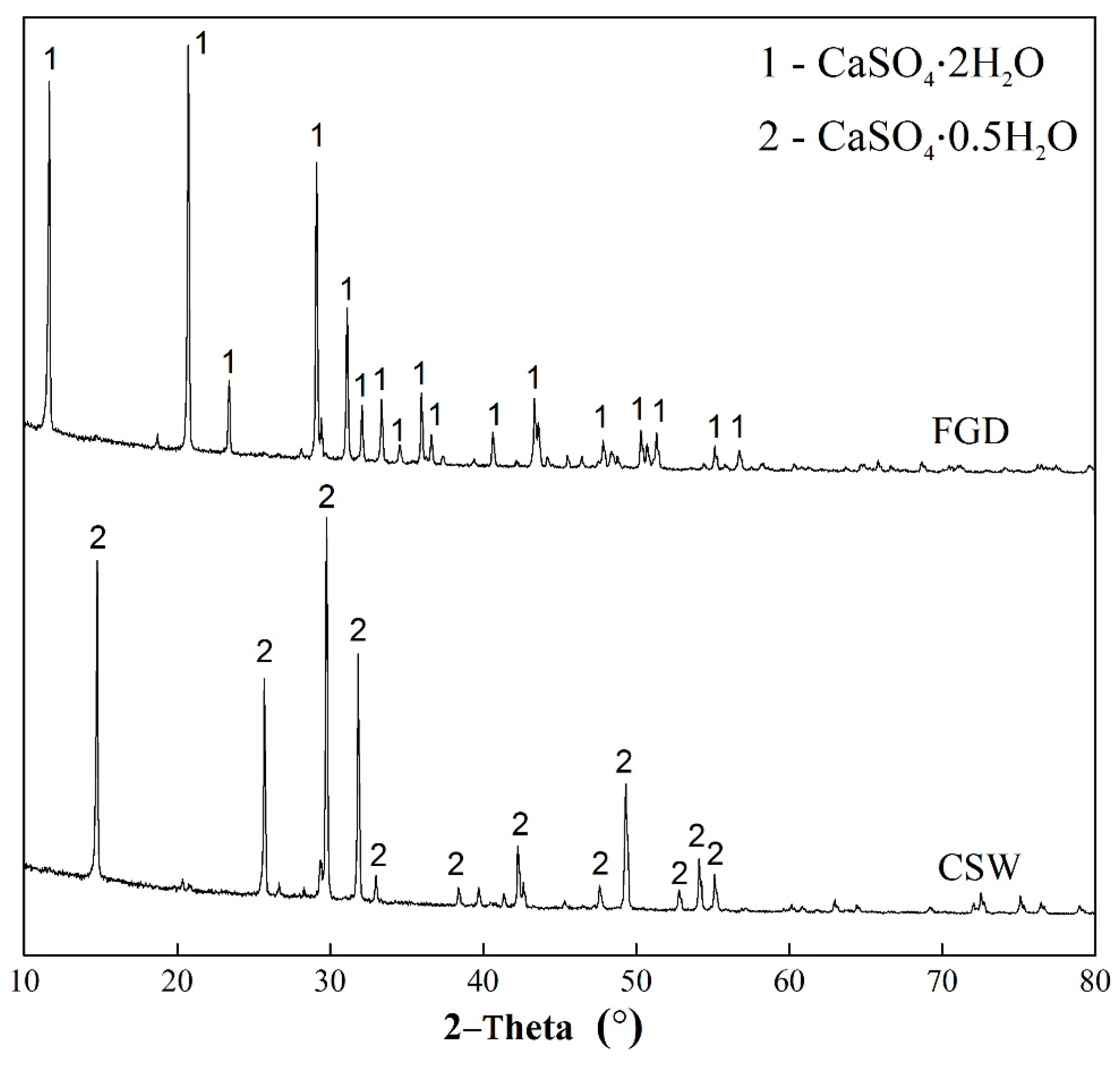
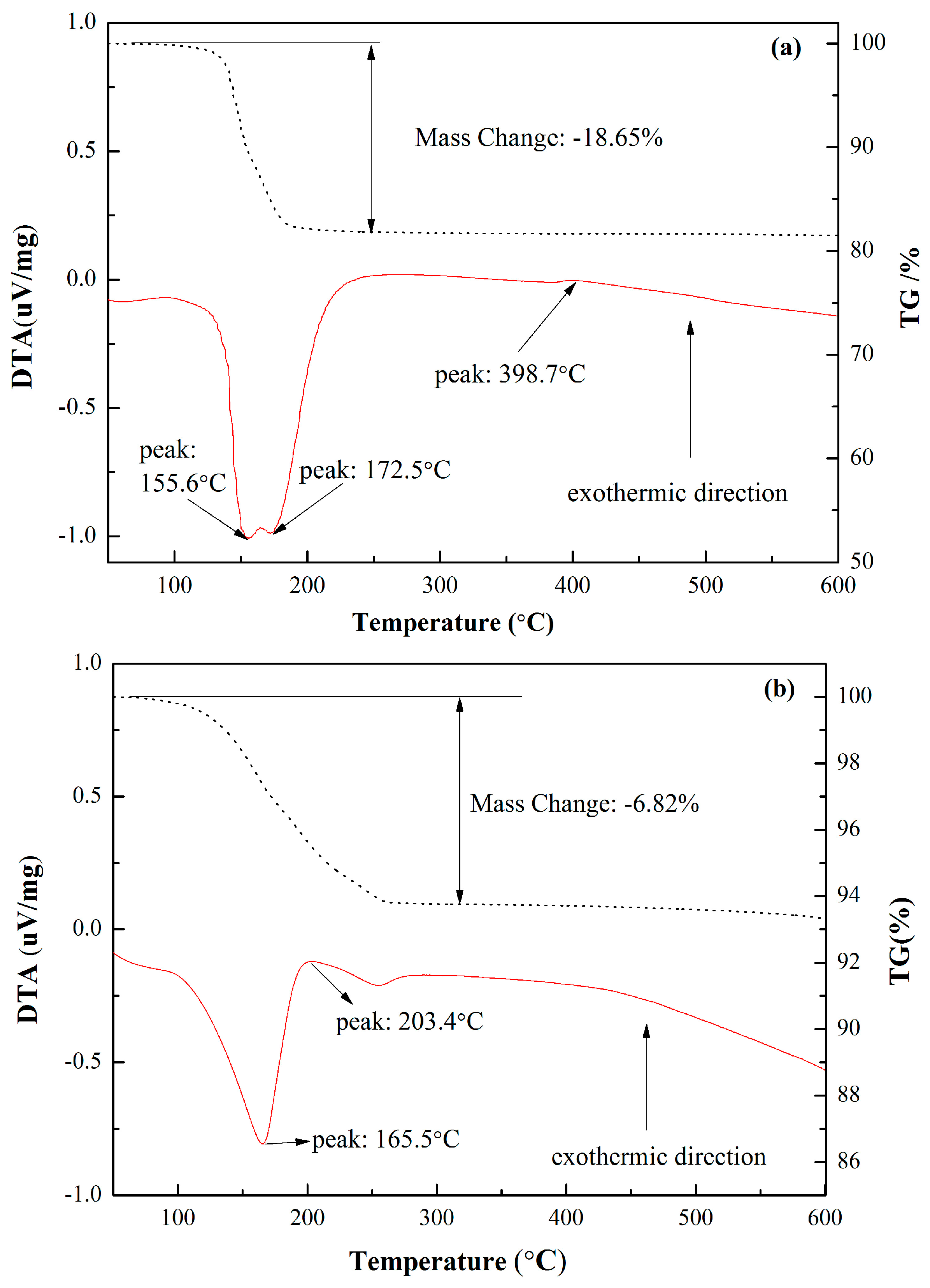

| Parameters | Value | |
|---|---|---|
| FGD Gypsum | CSHW | |
| Bulk density (g mL−1) | 1.06 | 0.26 |
| True density (g mL−1) | 3.19 | 2.78 |
| Particle median diameter (μm) | 38.28 | 37.54 |
| Materials | CaO | SO3 | SiO2 | Al2O3 | Fe2O3 | MgO | Crystal Water (%) |
|---|---|---|---|---|---|---|---|
| FGD gypsum | 44.15 | 52.89 | 1.05 | 0.46 | 0.27 | 0.36 | 18.65 |
| CSHW | 43.43 | 53.12 | 1.36 | 0.61 | 0.25 | 0.39 | 6.82 |
| Isotherm | Parameter | Temperature | |
|---|---|---|---|
| 298 K | 318 K | ||
| Langmuir | (mg g−1) | 9.461 | 9.681 |
| (L mg−1) | 152.59 | 526.12 | |
| 0.991 | 0.996 | ||
| Freundlich | (mg g−1) | 4.814 | 2.718 |
| 2.7334 | 2.8305 | ||
| 0.828 | 0.829 | ||
| Temkin | (KJ mol−1) | 4.025 | 5.328 |
| 3.455 × 10−3 | 3.495 × 10−4 | ||
| 0.972 | 0.981 | ||
| C0 (mg L−1) | k (min−1) | Qe a (mg g−1) | Qe b (mg g−1) | R2 |
|---|---|---|---|---|
| 100 | 0.00419 | 50.50 | 48.18 | 0.999 |
| 80 | 0.00174 | 43.10 | 38.06 | 0.995 |
| 60 | 0.00183 | 31.45 | 27.30 | 0.996 |
| 30 | 0.0104 | 14.12 | 13.91 | 0.996 |
| 10 | 1.122 | 0.43 | 0.42 | 0.998 |
| pH Value | H+ Changes (mmol L−1) | Removal Efficiency (%) | |
|---|---|---|---|
| Initial Value | Equilibrium Value | ||
| 2.51 | 2.89 | −1.802 | 25.32 |
| 2.99 | 3.15 | −0.315 | 40.26 |
| 3.30 | 3.45 | −0.146 | 56.73 |
| 3.98 | 4.11 | −0.0271 | 66.73 |
| 4.48 | 4.60 | −7.99 × 10−3 | 81.76 |
| 5.00 | 5.12 | −2.41 × 10−3 | 87.12 |
| 5.42 a | 5.62 | −1.40 × 10−3 | 88.50 |
| 5.98 | 5.99 | −2.38 × 10−5 | 90.30 |
| 6.59 | 6.38 | 1.60 × 10−4 | 86.27 |
| 7.02 | 6.39 | 3.12 × 10−4 | 76.30 |
| 7.62 | 6.46 | 3.23 × 10−4 | 67.61 |
| 8.08 | 6.65 | 2.26 × 10−4 | 67.44 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wang, L.; Wang, Y.; Tan, R.; Ke, X.; Zhou, X.; Geng, J.; Hou, H.; Zhou, M. Calcium Sulfate Hemihydrate Whiskers Obtained from Flue Gas Desulfurization Gypsum and Used for the Adsorption Removal of Lead. Crystals 2017, 7, 270. https://doi.org/10.3390/cryst7090270
Wang X, Wang L, Wang Y, Tan R, Ke X, Zhou X, Geng J, Hou H, Zhou M. Calcium Sulfate Hemihydrate Whiskers Obtained from Flue Gas Desulfurization Gypsum and Used for the Adsorption Removal of Lead. Crystals. 2017; 7(9):270. https://doi.org/10.3390/cryst7090270
Chicago/Turabian StyleWang, Xiaoshu, Lei Wang, Yan Wang, Ruiqi Tan, Xing Ke, Xian Zhou, Junjun Geng, Haobo Hou, and Min Zhou. 2017. "Calcium Sulfate Hemihydrate Whiskers Obtained from Flue Gas Desulfurization Gypsum and Used for the Adsorption Removal of Lead" Crystals 7, no. 9: 270. https://doi.org/10.3390/cryst7090270