Synthesis, Crystal Structure, Luminescence, Electrochemical and Antimicrobial Properties of Bis(salamo)-Based Co(II) Complex
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
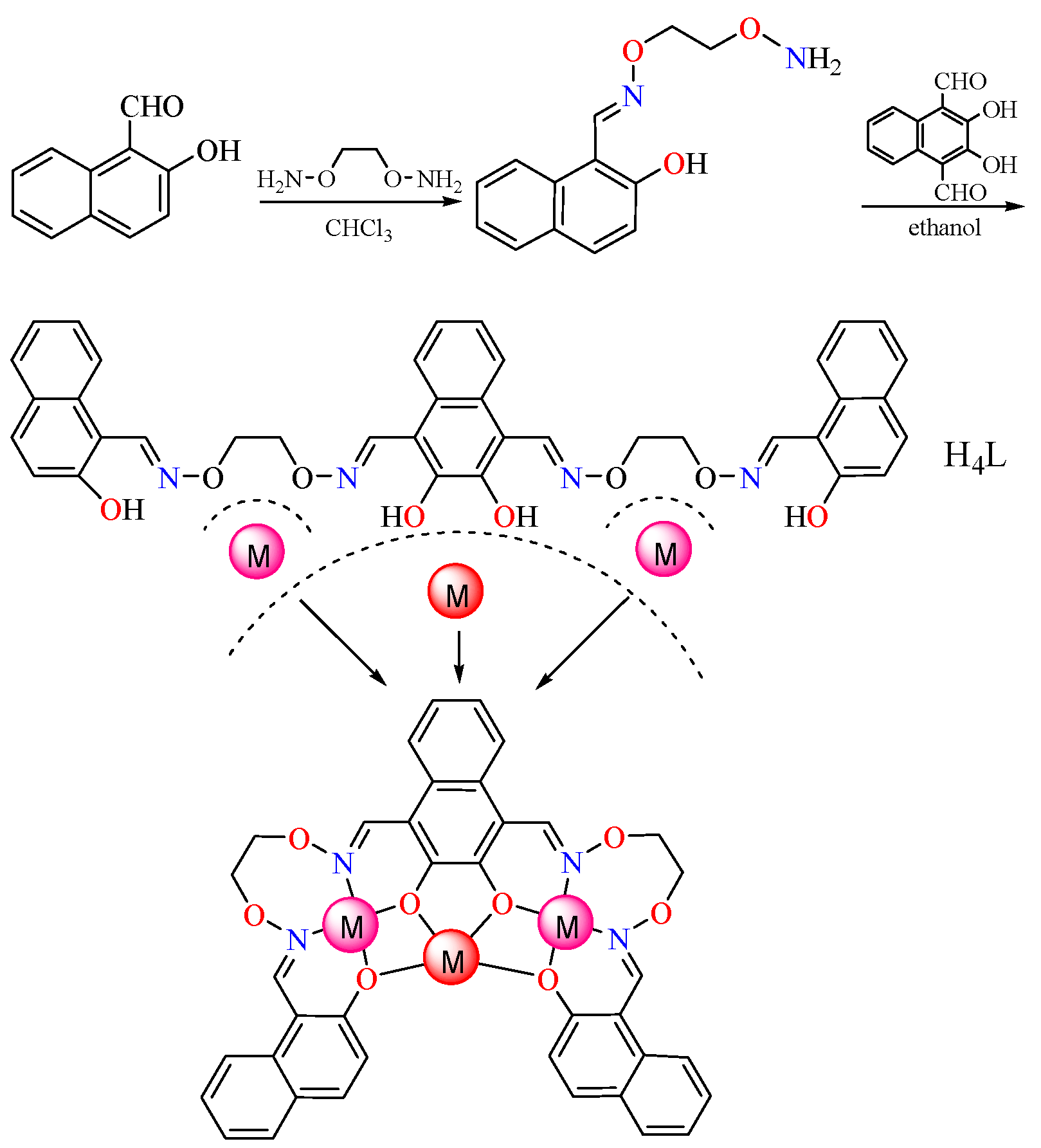
2.2. Synthesis of H4L
2.3. Synthesis of the Co(II) Complex
2.4. Crystal Structure Determination of the Co(II) Complex
3. Results and Discussion
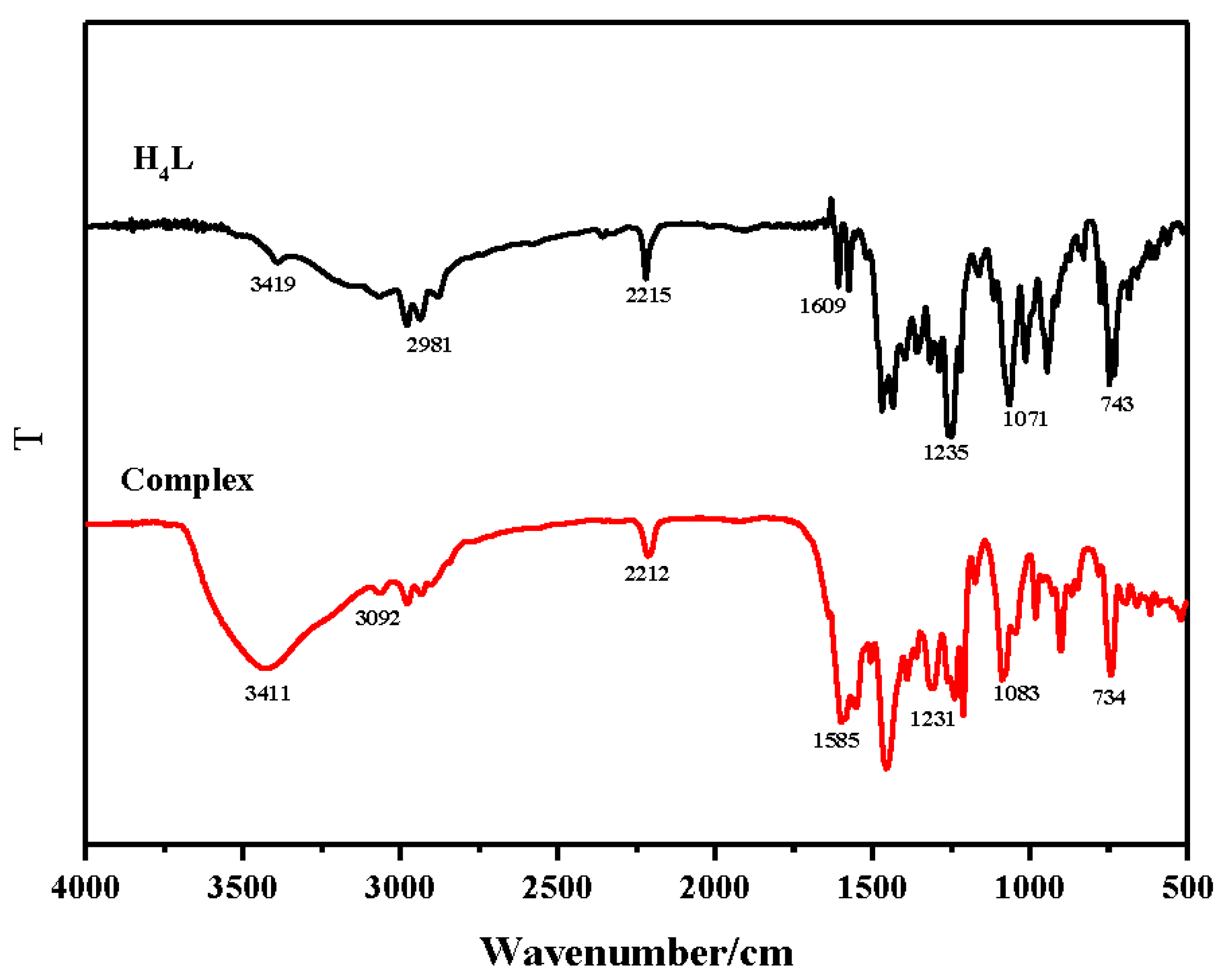
3.1. IR Spectra
3.2. UV-Vis Titration
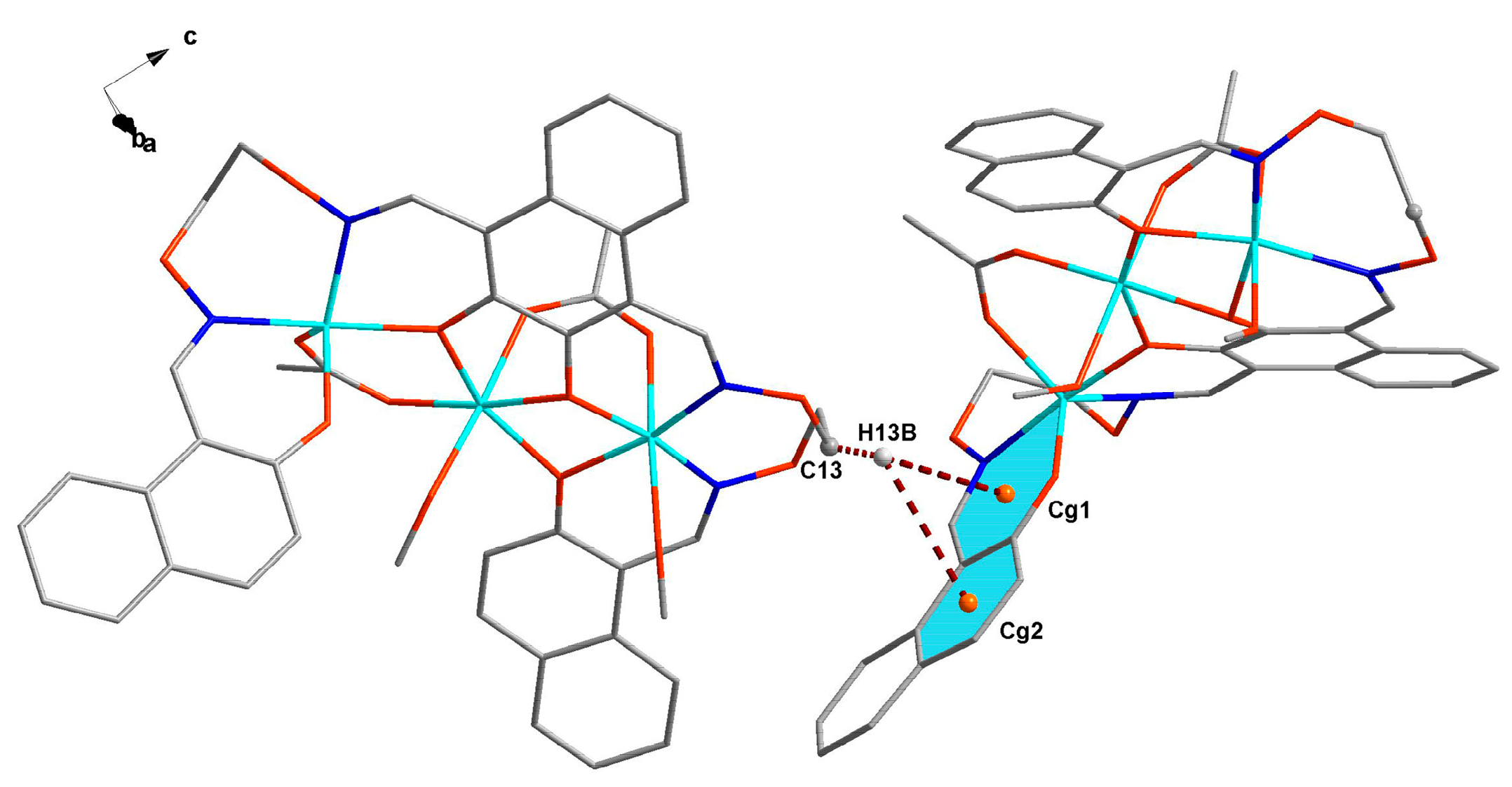
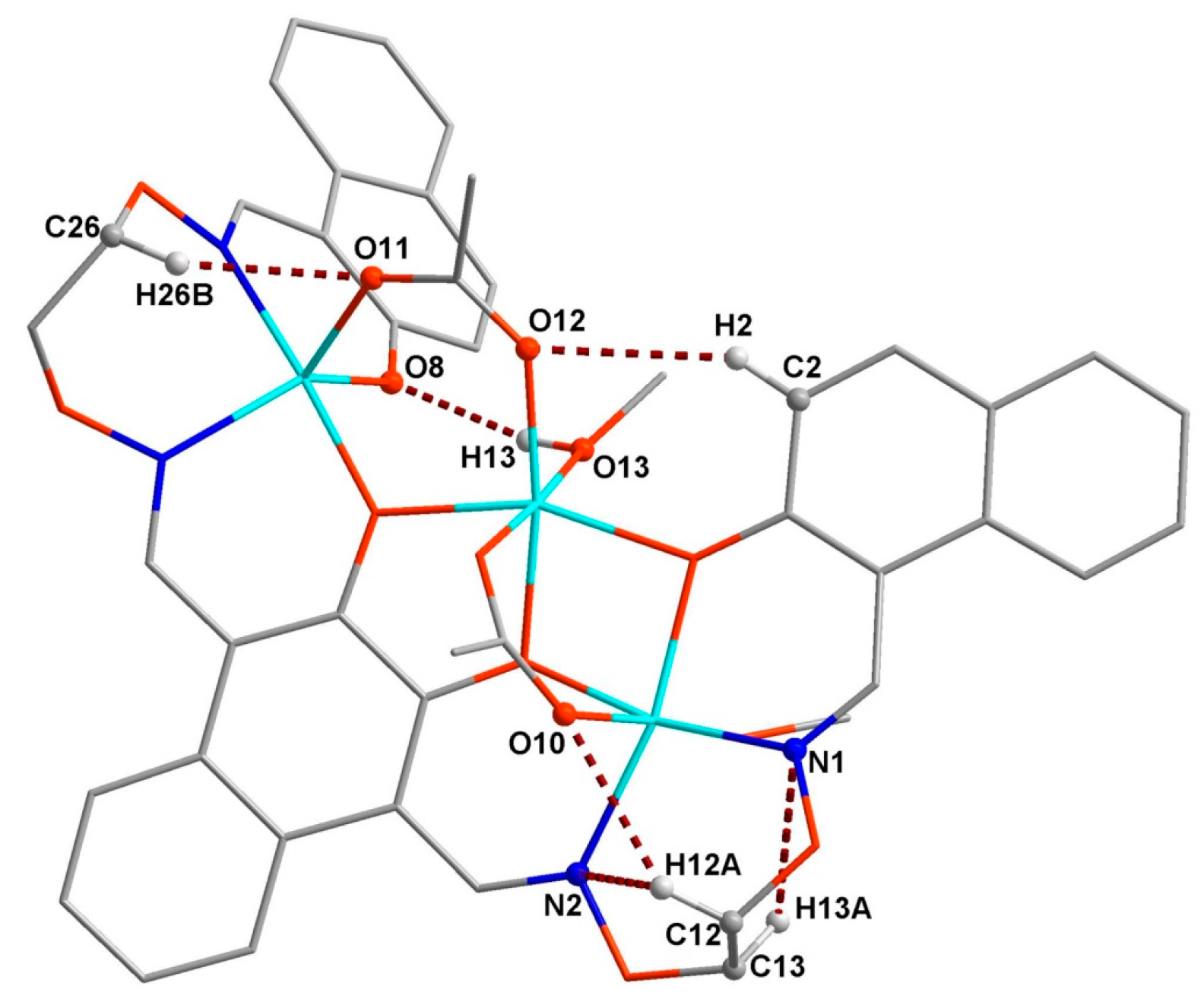
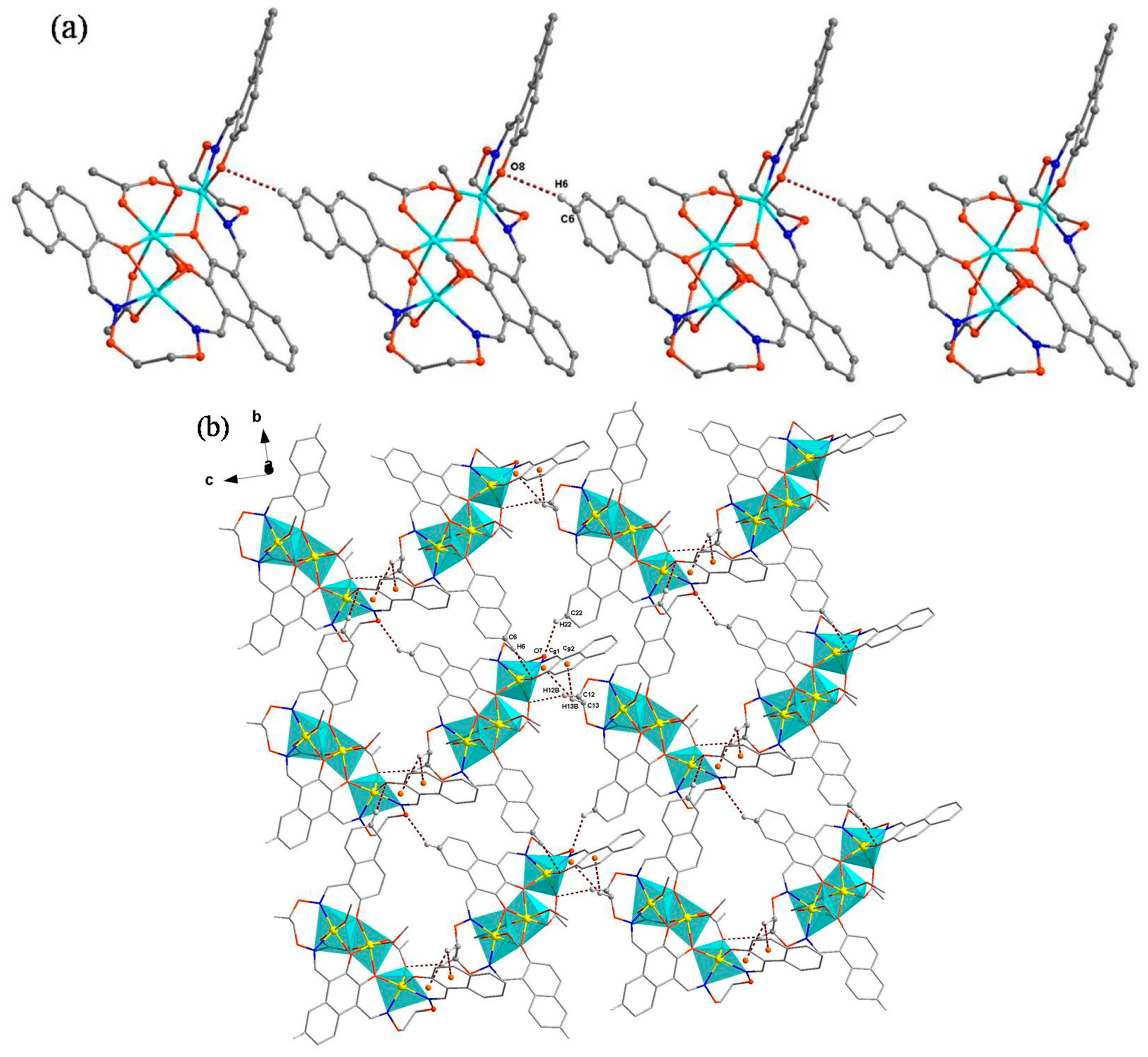
3.3. Crystal Structure
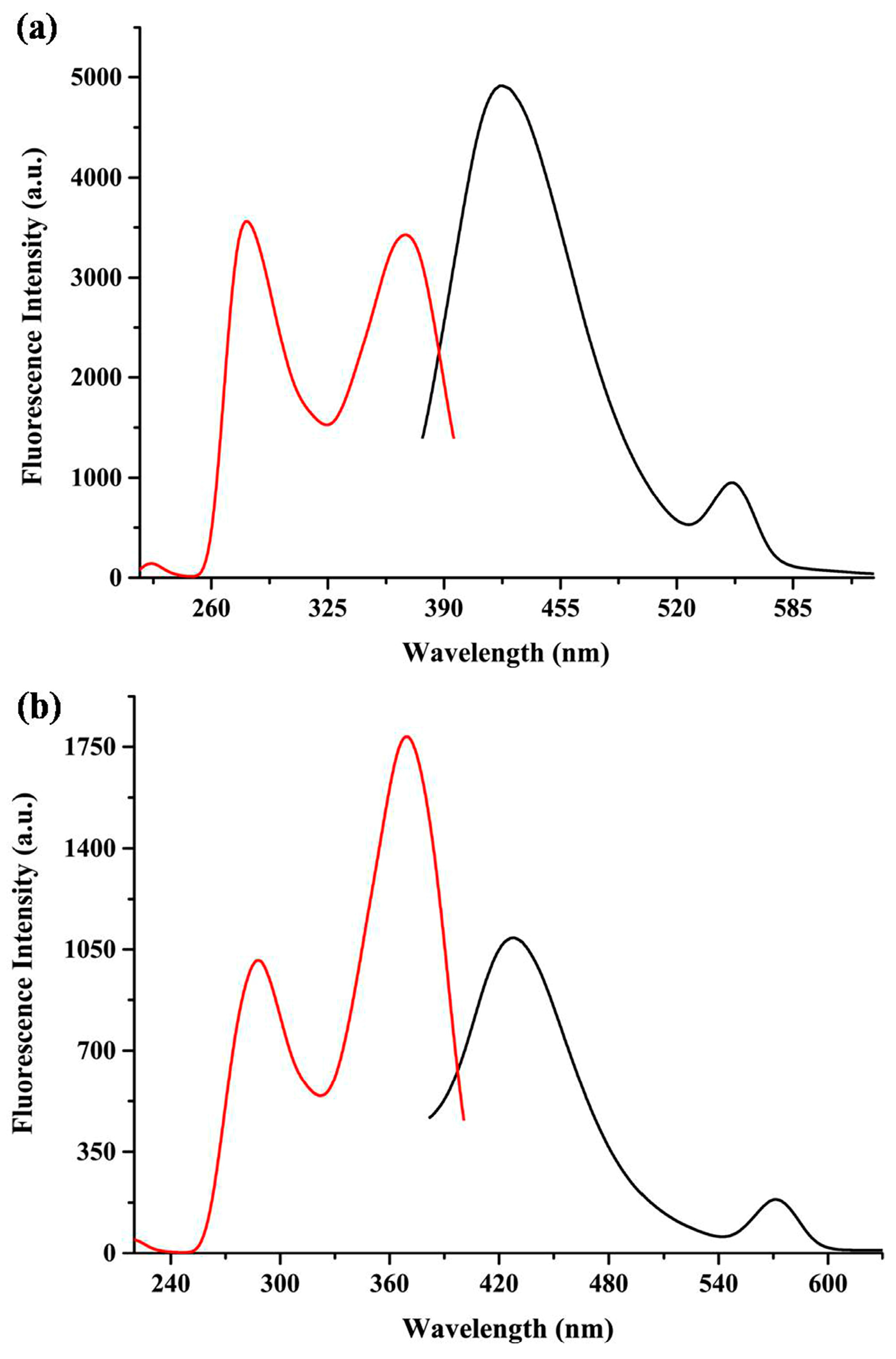
3.4. Fluorescence Properties
3.5. Antimicobial Activities
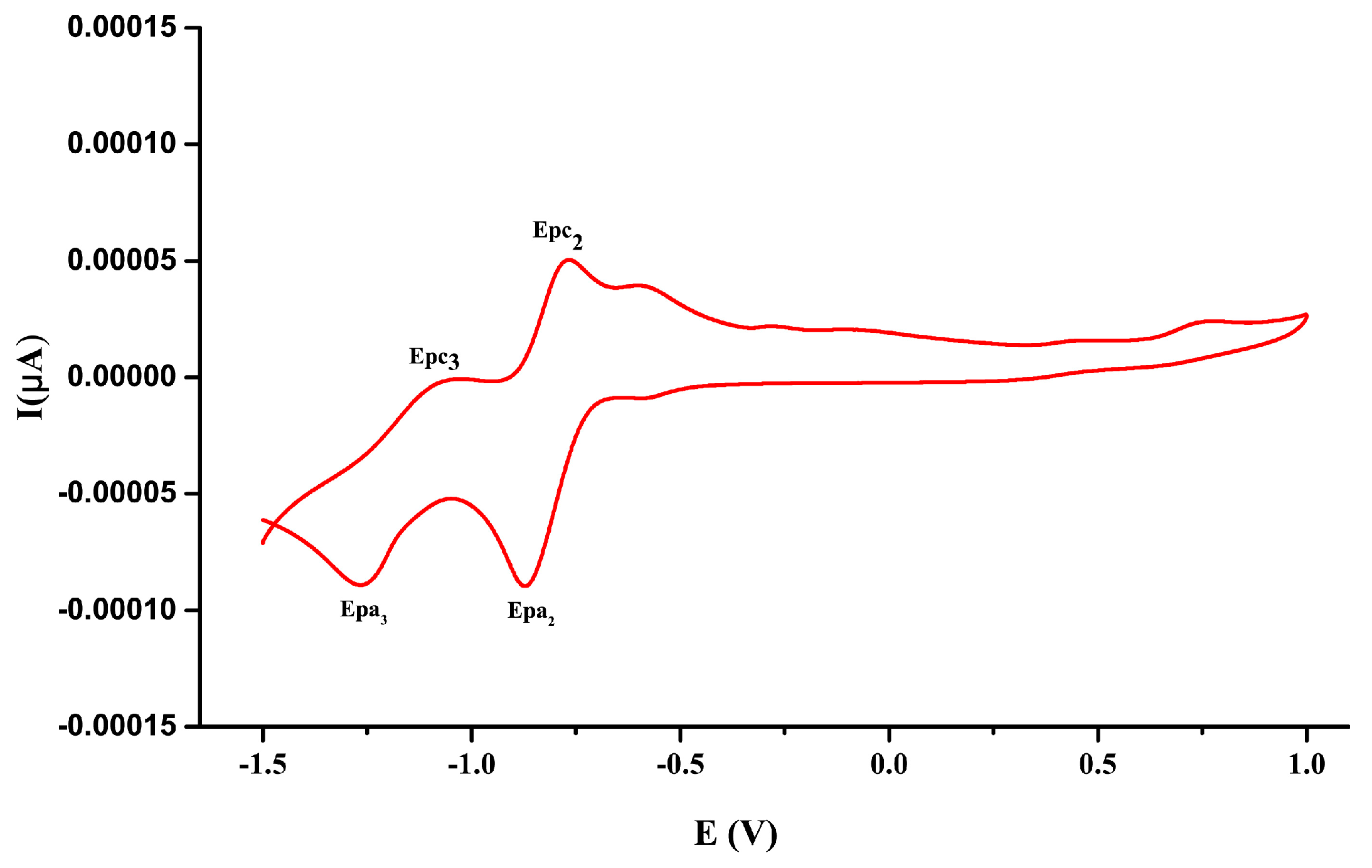
3.6. Electrochemistry Studies
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, Y.A.; Wang, C.Y.; Zhang, M.; Song, X.Q. Structures and magnetic properties of cyclic heterometallictetranuclear clusters. Polyhedron 2017, 127, 278–286. [Google Scholar] [CrossRef]
- Dong, W.K.; Lan, P.F.; Zhou, W.M.; Zhang, Y. Salamo-type trinuclear and tetranuclear cobalt(II) complexes based on a new asymmetry salamo-type ligand: Syntheses, crystal structures and fluorescence properties. J. Coord. Chem. 2016, 65, 1272–1283. [Google Scholar] [CrossRef]
- Dong, X.Y.; Akogun, S.F.; Zhou, W.M.; Dong, W.K. Tetranuclear Zn(II) complex based on an asymmetrical Salamo-type chelating ligand: Synthesis, structural characterization, and fluorescence property. J. Chin. Chem. Soc. 2017, 64, 412–419. [Google Scholar] [CrossRef]
- Wu, H.L.; Pan, G.L.; Bai, Y.C.; Wang, H.; Kong, J.; Shi, F.R.; Zhang, Y.H.; Wang, X.L. Synthesis, structure, antioxidation, and DNA-binding studies of a binuclear ytterbium(III) complex with bis(N-salicylidene)-3-oxapentane-1,5-diamine. Res. Chem. Intermed. 2015, 41, 3375–3388. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, L.C.; Ma, J.C.; Zhang, Y.; Zhang, J.; Dong, W.K. Syntheses, structures and spectral properties of mononuclear CuII and dimeric ZnII complexes based on an asymmetric Salamo-type N2O2 ligand. Z. Anorg. Allg. Chem. 2015, 641, 2520–2524. [Google Scholar] [CrossRef]
- Tao, C.H.; Ma, J.C.; Zhu, L.C.; Zhang, Y.; Dong, W.K. Heterobimetallic 3d–4f Zn(II)–Ln(III) (Ln = Sm, Eu, Tb and Dy) complexes with a N2O4 bisoxime chelate ligand and a simple auxiliary ligand Py: Syntheses, structures and luminescence properties. Polyhedron 2017, 128, 38–45. [Google Scholar] [CrossRef]
- Dong, Y.J.; Dong, X.Y.; Dong, W.K.; Zhang, Y.; Zhang, L.S. Three asymmetric salamo-type copper(II) and cobalt(II) complexes: Syntheses, structures, fluorescent properties. Polyhedron 2017, 123, 305–315. [Google Scholar] [CrossRef]
- Chai, L.Q.; Zhang, J.Y.; Chen, L.C.; Li, Y.X.; Tang, L.J. Synthesis, crystal structure, spectroscopic properties and DFT calculations of a new Schiff base-type zinc(II) complex. Res Chem. Intermed. 2016, 42, 3473–3488. [Google Scholar] [CrossRef]
- Song, X.Q.; Peng, Y.J.; Chen, G.Q.; Wang, X.R.; Liu, P.P.; Xu, W.Y. Substituted group-directed assembly of Zn(II) coordination complexes based on two new structural related pyrazolone based salen ligands: Syntheses, structures and fluorescence properties. Inorg. Chim. Acta 2015, 427, 13–21. [Google Scholar] [CrossRef]
- Dong, Y.J.; Ma, J.C.; Zhu, L.C.; Dong, W.K.; Zhang, Y. Four 3d–4f heteromultinuclear zinc(II)–lanthanide(III) complexes constructed from a distinct hexadentate N2O2-type ligand: Syntheses, structures and photophysical properties. J. Coord. Chem. 2017, 70, 103–115. [Google Scholar] [CrossRef]
- Wang, L.; Ma, J.C.; Dong, W.K.; Zhu, L.C.; Zhang, Y. A novel Self–assembled nickel(II)–cerium(III) heterotetranuclear dimer constructed from N2O2-type bisoxime and terephthalic acid: Synthesis, structure and photophysical properties. Z. Anorg. Allg. Chem. 2016, 642, 834–839. [Google Scholar] [CrossRef]
- Wu, H.L.; Wang, C.P.; Wang, F.; Peng, H.P.; Zhang, H.; Bai, Y.C. A new manganese(III) complex from bis(5-methylsalicylaldehyde)-3-oxapentane-1,5-diamine: Synthesis, characterization, antioxidant activity and luminescence. J. Chin. Chem. Soc. 2015, 62, 1028–1034. [Google Scholar] [CrossRef]
- Chai, L.Q.; Huang, J.J.; Zhang, H.S. An unexpected cobalt (III) complex containing a schiff base ligand: Synthesis, crystal structure, spectroscopic behavior, electrochemical property and SOD-like activity. Spectrochim. Acta Part A 2014, 131, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.K.; Li, X.L.; Wang, L.; Zhang, Y.; Ding, Y.J. A new application of salamo-type bisoximes: As a relay–sensor for Zn2+/Cu2+ and its novel complexes for successive sensing of H+/OH−. Sens. Actuators B 2016, 229, 370–378. [Google Scholar] [CrossRef]
- Song, X.Q.; Liu, P.P.; Xiao, Z.R.; Li, X.; Liu, Y.A. Four polynuclear complexes based on a versatile salicylamide salen-like ligand: Synthesis, structural variations and magnetic properties. Inorg. Chim. Acta 2015, 438, 232–244. [Google Scholar] [CrossRef]
- Zheng, S.S.; Dong, W.K.; Zhang, Y.; Chen, L.; Ding, Y.J. Four Salamo-type 3d–4f hetero-bimetallic [ZnIILnIII] complexes: Syntheses, crystal structures, and luminescent and magnetic properties. New J. Chem. 2017, 41, 4966–4973. [Google Scholar] [CrossRef]
- Wang, B.J.; Dong, W.K.; Zhang, Y.; Akogun, S.F. A novel relay-sensor for highly sensitive and selective detection of Zn2+/Pic− and fluorescence on/off switch response of H+/OH−. Sens. Actuators B 2017, 247, 254–264. [Google Scholar] [CrossRef]
- Dong, W.K.; Zhang, J.; Zhang, Y.; Li, N. Novel multinuclear transition metal(II) complexes based on an asymmetric salamo-type ligand: Syntheses, structure characterizations and fluorescent properties. Inorg. Chim. Acta 2016, 444, 95–102. [Google Scholar] [CrossRef]
- Chai, L.Q.; Li, Y.X.; Chen, L.C.; Zhang, J.Y.; Huang, J.J. Synthesis, X-ray structure, spectroscopic, electrochemical properties and DFT calculation of a bridged dinuclear copper(II) complex. Inorg. Chim. Acta 2016, 444, 193–201. [Google Scholar] [CrossRef]
- Chai, L.Q.; Zhang, K.Y.; Tang, L.J.; Zhang, J.Y.; Zhang, H.S. Two mono- and dinuclear ni(II) complexes constructed from quinazoline-type ligands: Synthesis, X-ray structures, spectroscopic, electrochemical, thermal, and antimicrobial studies. Polyhedron 2017, 130, 100–107. [Google Scholar] [CrossRef]
- Chai, L.Q.; Tang, L.J.; Chen, L.C.; Huang, J.J. Structural, spectral, electrochemical and DFT studies of two mononuclear manganese(II) and zinc(II) complexes. Polyhedron 2017, 122, 228–240. [Google Scholar] [CrossRef]
- Chai, L.Q.; Huang, J.J.; Zhang, J.Y.; Li, Y.X. Two 1-D and 2-D cobalt(II) complexes: Synthesis, crystal structures, spectroscopic and electrochemical properties. J. Coord. Chem. 2015, 68, 1224–1237. [Google Scholar] [CrossRef]
- Dong, Y.J.; Li, X.L.; Zhang, Y.; Dong, W.K. A highly selective visual and fluorescent sensor for Pb2+ and Zn2+ and crystal structure of Cu2+ complex based-on a novel single-armed salamo-type bisoxime. Supramol. Chem. 2017, 29, 518–527. [Google Scholar] [CrossRef]
- Pu, L.M.; Akogun, S.F.; Li, X.L.; Long, H.T.; Dong, W.K.; Zhang, Y. A salamo-type fluorescent sensor for selective detection of Zn2+/Cu2+ and its novel Cd2+ complex with triangular prism geometry. Polyhedron 2017, 134, 356–364. [Google Scholar] [CrossRef]
- Dong, W.K.; Akogun, S.F.; Zhang, Y.; Sun, Y.X.; Dong, X.Y. A reversible “turn-on” fluorescent sensor for selective detection of Zn2+. Sens. Actuators B 2017, 238, 723–734. [Google Scholar] [CrossRef]
- Liu, P.P.; Wang, C.Y.; Zhang, M.; Song, X.Q. Pentanuclear sandwich-type ZnII-LnIII clusters based on a new salen-like salicylamide ligand: Structure, near-infrared emission and magnetic properties. Polyhedron 2017, 129, 133–140. [Google Scholar] [CrossRef]
- Chen, L.; Dong, W.K.; Zhang, H.; Zhang, Y.; Sun, Y.X. Structural variation and luminescence properties of tri- and dinuclear CuII and ZnII complexes constructed from a naphthalenediol-based bis(Salamo)-type ligand. Cryst. Growth Des. 2017, 17, 3636–3648. [Google Scholar] [CrossRef]
- Liu, P.P.; Sheng, L.; Song, X.Q.; Xu, W.Y.; Liu, Y.A. Synthesis, structure and magnetic properties of a new one dimensional manganese coordination polymer constructed by a new asymmetrical ligand. Inorg. Chim. Acta 2015, 434, 252–257. [Google Scholar] [CrossRef]
- Dong, W.K.; Ma, J.C.; Zhu, L.C.; Sun, Y.X.; Zhang, Y. A series of heteromultinuclear zinc(II)-lanthanide(III) complexes based on 3-MeOsalamo: Syntheses, structural characterizations, and luminescent properties. Cryst. Growth Des. 2016, 16, 6903–6914. [Google Scholar] [CrossRef]
- Dong, W.K.; Zhang, F.; Li, N.; Xu, L.; Zhang, Y.; Zhang, J.; Zhu, L.C. Trinuclear cobalt(II) and zinc(II) salamo-type complexes: Syntheses, crystal structures, and fluorescent properties. Z. Anorg. Allg. Chem. 2016, 642, 532–538. [Google Scholar] [CrossRef]
- Dong, W.K.; Sun, Y.X.; Zhao, C.Y.; Dong, X.Y.; Xu, L. Synthesis, structure and properties of supramolecular MnII, CoII, NiII and ZnII complexes containing salen-type bisoxime ligands. Polyhedron 2010, 29, 2087–2097. [Google Scholar] [CrossRef]
- Dong, W.K.; Du, W.; Zhang, X.Y.; Li, G.; Dong, X.Y. Synthesis, crystal structure and spectral properties of a supramolecular trinuclear nickel(II) complex with 5-methoxy-4′-bromo-2,2′-[ethylenedioxybis (nitrilomethylidyne)]diphenol. Spectrochim. Acta Part A 2014, 132, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.K.; Zhu, L.C.; Dong, Y.J.; Ma, J.C.; Zhang, Y. Mono, di and heptanuclear metal(II) complexes based on symmetric and asymmetric tetradentate salamo-type ligands: Syntheses, structures and spectroscopic properties. Polyhedron 2016, 117, 148–154. [Google Scholar] [CrossRef]
- Dong, W.K.; Zhang, X.Y.; Sun, Y.X.; Dong, X.Y.; Li, G.; Wang, J. A 2D supramolecular copper(II) complex with an asymmetric salamo-type ligand: Synthsis, crystal structure, and fluorescent property. Synth. React. Inorg. Met.-Org. Nano-Met Chem. 2015, 45, 956–962. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, L. Synthesis and crystal structure of supramolecular copper(II) complex based on N2O2 coordination sphere. Asian J. Chem. 2015, 4, 1424–1426. [Google Scholar] [CrossRef]
- Sun, Y.X.; Sun, W.Y. Zinc(II)- and cadmium(II)-organic frameworks with 1-imidazole-containing and 1-imidazole-carboxylate Liands. Cryst. Eng. Commun. 2015, 17, 4045–4063. [Google Scholar] [CrossRef]
- Dong, W.K.; Wang, G.; Gong, S.S.; Tong, J.F.; Sun, Y.X.; Gao, X.H. Synthesis, structural characterization and substituent effects of two copper(II) complexes with benzaldehyde ortho-oxime ligands. Transit. Met. Chem. 2012, 37, 271–277. [Google Scholar] [CrossRef]
- Wu, H.L.; Bai, Y.C.; Zhang, Y.H.; Pan, G.L.; Kong, J.; Shi, F.; Wang, X.L. Two lanthanide(III) complexes based on the schiff base N,N-Bis(salicylidene)-1,5-diamino-3-oxapentane: Synthesis, characterization, DNA-binding properties, and antioxidation. Z. Anorg. Allg. Chem. 2014, 640, 2062–2071. [Google Scholar] [CrossRef]
- Chen, C.Y.; Zhang, J.W.; Zhang, Y.H.; Yang, Z.H.; Wu, H.L.; Pana, G.L.; Bai, Y.C. Gadolinium(III) and dysprosium(III) complexes with a schiff base bis(N-salicylidene)-3-oxapentane-1,5-diamine: Synthesis, characterization, antioxidation, and DNA-binding studies. J. Coord. Chem. 2015, 68, 1054–1071. [Google Scholar] [CrossRef]
- Wu, H.L.; Bai, Y.; Yuan, J.K.; Wang, H.; Pan, G.L.; Fan, X.Y.; Kong, J. A zinc(II) complex with tris(2-(N-methyl)benzimidazlylmethyl)amine and salicylate: Synthesis, crystal structure, and DNA-binding. J. Coord. Chem. 2012, 65, 2839–2851. [Google Scholar] [CrossRef]
- Wu, H.L.; Pan, G.L.; Wang, H.; Wang, X.L.; Bai, Y.C.; Zhang, Y.H. Study on synthesis, crystal structure, antioxidant and DNA-binding of mono-, di- and poly-nuclear lanthanides complexes with bis(N-salicylidene)-3-oxapentane-1,5-diamine. J. Photochem. Photobiol. B Biol. 2014, 135, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.L.; Bai, Y.C.; Zhang, Y.H.; Li, Z.; Wu, M.C.; Chen, C.Y.; Zhang, J.W. Synthesis, crystal structure, antioxidation and DNA-binding properties of a dinuclear copper(II) complex with bis(N-salicylidene)-3-oxapentane-1,5-diamine. J. Coord. Chem. 2014, 67, 3054–3066. [Google Scholar] [CrossRef]
- Dong, W.K.; Zhu, L.C.; Ma, J.C.; Sun, Y.X.; Zhang, Y. Two novel mono-and heptanuclear ni(II) complexes constructed from new unsymmetric and symmetric salamo-type bisoximes–synthetic, structural and spectral studies. Inorg. Chim. Acta 2016, 453, 402–408. [Google Scholar] [CrossRef]
- Dong, W.K.; Ma, J.C.; Zhu, L.C.; Zhang, Y.; Li, X.L. Four new nickel(II) complexes based on an asymmetric salamo-type ligand: Synthesis, structure, solvent effect and electrochemical property. Inorg. Chim. Acta 2016, 445, 140–148. [Google Scholar] [CrossRef]
- Dong, W.K.; Ma, J.C.; Dong, Y.J.; Zhu, L.C.; Zhang, Y. Di-and tetranuclear heterometallic 3d–4f cobalt(II)-lanthanide(III) complexes derived from a hexadentate bisoxime: Syntheses, structures and magnetic properties. Polyhedron 2016, 115, 228–235. [Google Scholar] [CrossRef]
- Dong, W.K.; Ma, J.C.; Zhu, L.C.; Zhang, Y. Nine self-assembled nickel(II)-lanthanide(III) heterometallic complexes constructed from a salamo-type bisoxime and bearing a N- or O-donor auxiliary ligand: Syntheses, structures and magnetic properties. New J. Chem. 2016, 40, 6998–7010. [Google Scholar] [CrossRef]
- Akine, S.; Taniguchi, T.; Nabeshima, T. Novel synthetic approach to trinuclear 3d-4f complexes: Specific exchange of the central metal of a trinuclear Zinc(II) complex of a tetraoxime ligand with a lanthanide(III) ion. Angew. Chem. Int. Ed. 2002, 41, 4670–4673. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.X.; Gao, X.H. Synthesis, characterization, and crystal structure of a new CuII complex with salen-type ligand. Synth. React. Inorg. Met-Org. Nano-Met. Chem. 2011, 41, 973–978. [Google Scholar] [CrossRef]
- Hao, J.; Li, L.H.; Zhang, J.T.; Akogun, S.F.; Wang, L.; Dong, W.K. Four homo- and hetero-bismetallic 3d/3d-2s complexes constructed from a naphthalenediol-based acyclic bis(salamo)-type tetraoxime ligand. Polyhedron 2017, 134, 1–10. [Google Scholar] [CrossRef]
- Zhao, L.; Dong, X.T.; Chen, Q.; Zhao, J.X.; Wang, L. Synthesis, crystal structure and spectral properties of a 2D supramolecular copper(II) complex with 1-(4-{[(E)-3-ethoxyl-2-hydroxybenzylidene] amino} phenyl) ethanone oxime. Synth. React. Inorg. Met-Org. Nano-Met. Chem. 2013, 43, 1241–1246. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, L. An infinite 2D supramolecular cobalt(II) complex based on an asymmetric salamo-type ligand: Synthesis, crystal structure, and spectral properties. Synth. React. Inorg. Met-Org. Nano-Met. Chem. 2016, 46, 1095–1101. [Google Scholar] [CrossRef]
- Sun, Y.X.; Wang, L.; Dong, X.Y.; Ren, Z.L.; Meng, W.S. Synthesis, characterization, and crystal structure of a supramolecular CoII complex containing salen-type bisoxime. Synth. React. Inorg. Met-Org. Nano-Met. Chem. 2013, 43, 599–603. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, L. Synthesis, structure and spectroscopic properties of the trinuclear cobalt(II) and nickel(II) complexes based on 2-hydroxynaphthaldehyde and bis(aminooxy) alkane. Spectrochim. Acta Part A 2015, 135, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.K.; Li, G.; Li, X.; Yang, C.J.; Zhao, M.M.; Dong, X.Y. An asymmetrical salamo-type chelating ligand with its cobalt(II) complex: Syntheses, characterizations and crystal structures. Chin. J. Inorg. Chem. 2014, 30, 1911–1919. [Google Scholar]
- Dong, X.Y.; Gao, L.; Wang, F.; Zhang, Y.; Dong, W.K. Tri- and Mono-Nuclear Zinc(II) Complexes Based on Half- and Mono-Salamo Chelating Ligands. Crystals 2017, 7, 267. [Google Scholar] [CrossRef]
- Dong, W.K.; Zhang, J.T.; Dong, Y.J.; Zhang, Y.; Wang, Z.K. Construction of mononuclear copper(II) and trinuclear cobalt(II) complexes based on asymmetric Salamo-Type ligands. Z. Anorg. Allg. Chem. 2016, 642, 189–196. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS-97, Program for the Solution and the Refinement of Crystal Structures; University of Gottingen: Gottingen, Germany, 1997. [Google Scholar]
- Chai, L.Q.; Wang, G.; Sun, Y.X.; Dong, W.K.; Zhao, L.; Gao, X.H. Synthesis, crystal structure, and fluorescence of an unexpected dialkoxo-bridged dinuclear copper(II) complex with bis(salen)-type tetraoxime. J. Coord. Chem. 2012, 65, 1621–1631. [Google Scholar] [CrossRef]
- Dong, W.K.; He, X.N.; Yan, H.B.; Lu, Z.W.; Chen, X.; Zhao, C.Y.; Tang, X.L. Synthesis, structural characterization and solvent effect of copper(II) complexes with a variational multidentate salen-type ligand with bisoxime groups. Polyhedron 2009, 28, 1419–1428. [Google Scholar] [CrossRef]
- Li, G.; Hao, J.; Liu, L.Z.; Zhou, W.M.; Dong, W.K. Syntheses, crystal structures and thermal behaviors of two supramolecular salamo-type cobalt(II) and zinc(II) complexes. Crystals 2017, 7, 217. [Google Scholar]
- Jia, H.R.; Li, J.; Sun, Y.X.; Guo, J.Q.; Yu, B.; Wen, N.; Xu, L. Two supramolecular cobalt(II) complexes: Syntheses, crystal structures, spectroscopic behaviors, and counter anion effects. Crystals 2017, 7, 247. [Google Scholar]
- Dong, W.K.; Li, G.; Wang, Z.K.; Dong, X.Y. A novel trinuclear cobalt(II) complex derived from an asymmetric Salamo-type N2O3 bisoxime chelate ligand: Synthesis, structure and optical properties. Spectrochim. Acta Part A 2014, 133, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Dong, W.K.; Zhang, Y.; Akogun, S.F.; Xu, L. Syntheses, structures and catecholase activities of homo- and hetero-trinuclear cobalt (II) complexes constructed from an acyclic naphthalenediol-based bis (Salamo)-type ligand. Appl. Organomet. Chem. 2017, e3818. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.L. Patterns in hydrogen bonding: Functionality and graph set analysis in crystals. Angew. Chem. Int. Ed. Engl. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Chai, L.Q.; Liu, G.; Zhang, Y.L.; Huang, J.J.; Tong, J.F. Synthesis, crystal structure, fluorescence, electrochemical property, and SOD-like activity of an unexpected nickel(II) complex with a quinazoline-type ligand. J. Coord. Chem. 2013, 66, 3926–3938. [Google Scholar] [CrossRef]
- Song, X.Q.; Cheng, G.Q.; Liu, Y.A. Enhanced Tb(III) luminescence by d1° transition metal coordination. Inorg. Chim. Acta 2016, 450, 386–394. [Google Scholar] [CrossRef]
- Hao, J.; Liu, L.Z.; Dong, W.K.; Zhang, J.; Zhang, Y. Three multinuclear Co(II), Zn(II) and Cd(II) complexes based on a single-armed salamo-type bisoxime: Syntheses, structural characterizations and fluorescent properties. J. Coord. Chem. 2017, 70, 1936–1952. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, W.K.; Zhang, Y.; Akogun, S.F. Naphthalenediol-based bis(Salamo)-type homo- and heterotrinuclear cobalt(II) complexes: Syntheses, structures and magnetic properties. Polyhedron 2017, 133, 279–293. [Google Scholar] [CrossRef]
- Dong, W.K.; Zheng, S.S.; Zhang, J.T.; Zhang, Y.; Sun, Y.X. Luminescent properties of heterotrinuclear 3d–4f complexes constructed from a naphthalenediol-based acyclic bis(salamo)-type ligand. Spectrochim. Acta Part A 2017, 184, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.X.; Zhao, Y.Y.; Li, C.Y.; Yu, B.; Guo, J.Q.; Li, J. Supramolecular Cobalt(II) and Copper(II) complexes with Schiff base ligand: Syntheses, characterizations and crystal structures. Chin. J. Inorg. Chem. 2016, 32, 913–920. [Google Scholar]
- Dong, W.K.; Sun, Y.X.; Zhang, Y.P.; Li, L.; He, X.N.; Tang, X.L. Synthesis, crystal structure, and properties of supramolecular CuII, ZnII, and CdII complexes with Salen-type bisoxime ligands. Inorg. Chim. Acta 2009, 362, 117–124. [Google Scholar] [CrossRef]
- Sun, Y.X.; Yang, C.J.; Zhao, Y.Y.; Guo, J.Q. Two Cu(II) complexes with Schiff base ligands: Syntheses, crystal structures, spectroscopic properties. Chin. J. Inorg. Chem. 2016, 32, 327–335. [Google Scholar]
- Kruszynski, R.; Sierański, T. Can stacking interactions exist beyond the commonly accepted limits? Cryst. Growth Des. 2016, 16, 587–595. [Google Scholar] [CrossRef]
- Dong, W.K.; Li, X.; Yang, C.J.; Zhao, M.M.; Li, G.; Dong, X.Y. Syntheses and crystal structures of 5-Methoxy-6′-hydroxy-2,2′[ethylenedioxybis(nitrilomethylidyne)]diphenol and its tetranuclear zinc(II) complex. Chin. J. Inorg. Chem. 2014, 30, 710–716. [Google Scholar]
- Dong, W.K.; Duan, J.G.; Guan, Y.H.; Shi, J.Y.; Zhao, C.Y. Synthesis, crystal structure and spectroscopic behaviors of Co(II) and Cu(II) complexes with Salen-type bisoxime ligands. Inorg. Chim. Acta 2009, 362, 1129–1134. [Google Scholar] [CrossRef]
- Song, X.Q.; Liu, P.P.; Liu, Y.A.; Zhou, J.J.; Wang, X.L. Two dodecanuclear heterometallic [Zn6Ln6] clusters constructed by a multidentate salicylamide salen-like ligand: Synthesis, structure, luminescence and magnetic properties. Dalton Trans. 2016, 45, 8154–8163. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.Q.; Mao, K.H.; Zhang, J.Y.; Zhang, K.Y.; Zhang, H.S. Synthesis, X-ray crystal structure, spectroscopic, electrochemical and antimicrobial studies of a new dinuclear cobalt(III) complex. Inorg. Chim. Acta 2017, 457, 34–40. [Google Scholar] [CrossRef]




| Formula | C45H46Co3N4O15 |
| Formula weight | 1059.65 |
| Temperature (K) | 293(2) |
| Wavelength (Å) | 0.71073 |
| Crystal system | Monoclinic |
| Space group | P 1 n 1 |
| a (Å) | 8.9831(3) |
| b (Å) | 13.4177(4) |
| c (Å) | 21.4815(7) |
| α (°) | 90.00 |
| β (°) | 90.561(3) |
| γ (°) | 90.00 |
| V (Å3) | 2589.10(15) |
| Z, Dc (g cm−3) | 2, 1.359 |
| μ (mm−1) | 1.015 |
| θ Range (°) | 3.336–24.999 |
| F(000) | 1090 |
| Crystal size (mm) | 0.14 × 0.11 × 0.04 |
| –10 ≤ h ≤ 10 | |
| Index ranges | –15 ≤ k ≤ 15 |
| –18 ≤ l ≤ 25 | |
| Reflections collected/unique | 8775/5819 [Rint = 0.0350] |
| Completeness to θ = 24.999 | 99.7% |
| GOF | 0.982 |
| Data/restraints/parameters | 5819/21/615 |
| Final R1, wR2 indices | 0.0362/0.0750 |
| R1, wR2 indices (all data) | 0.0405/0.0773 |
| Largest differences peak and hole (e Å−3) | 0.412/−0.339 |
| Bond | Lengths | Bond | Lengths |
|---|---|---|---|
| Co1–O1 | 2.075(4) | Co2–O5 | 2.107(4) |
| Co1–O4 | 2.029(4) | Co2–O9 | 2.086(4) |
| Co1–O10 | 2.070(4) | Co2–O12 | 2.018(4) |
| Co1–O15 | 2.143(4) | Co2–O13 | 2.142(5) |
| Co1–N1 | 2.068(4) | Co3–O5 | 2.022(4) |
| Co1–N2 | 2.155(4) | Co3–O8 | 1.950(4) |
| Co2–O1 | 2.123(4) | Co3–O11 | 1.997(4) |
| Co2–O4 | 2.066(4) | Co3–N3 | 2.042(4) |
| Bond | Angles | Bond | Angles |
| O1–Co1–O4 | 83.33(15) | O1–Co1–N1 | 85.63(16) |
| O9–Co2–O13 | 175.45(16) | O5–Co3–O11 | 94.83(16) |
| O1–Co1–O10 | 91.26(15) | O1–Co1–N2 | 165.68(16) |
| O12–Co2–O13 | 90.19(18) | O5–Co3–N3 | 84.87(17) |
| O1–Co1–O15 | 92.68(15) | O4–Co1–O10 | 89.68(15) |
| O5–Co3–O8 | 91.62(15) | O5–Co3–N4 | 174.98(16 |
| O4–Co1–O15 | 90.20(15) | O10–Co1–O15 | 176.02(16) |
| O8–Co3–O11 | 109.57(17) | O11–Co3–N3 | 123.21(18) |
| O4–Co1–N1 | 168.95(16) | O10–Co1–N1 | 91.04(17) |
| O8–Co3–N3 | 127.22(17) | O11–Co3–N4 | 90.18(17) |
| O4–Co1–N2 | 82.36(15) | O10–Co1–N2 | 89.24(16) |
| O8–Co3–N4 | 86.48(17) | N3–Co3–N4 | 92.58(17) |
| O15–Co1–N1 | 89.84(17) | O1–Co2–O4 | 81.28(15) |
| Co1–O1–Co2 | 93.70(16) | O1–Co2–O5 | 158.08(15) |
| O15–Co1–N2 | 86.80(16) | N2–O3–C13 | 110.1(4) |
| Co1–O1–C1 | 129.6(4) | O1–Co2–O9 | 89.69(15) |
| N1–Co1–N2 | 108.68(16) | Co1–O4–Co2 | 96.82(17) |
| Co2–O1–C1 | 136.5(4) | O1–Co2–O12 | 105.68(16) |
| Co1–O4–C16 | 128.3(4) | Co2–O5–C17 | 111.6(3) |
| O1–Co2–O13 | 94.82(16) | O4–Co2–O12 | 172.71(17) |
| Co2–O4–C16 | 113.2(3) | Co3–O5–C17 | 128.5(3) |
| O4–Co2–O5 | 76.85(15) | O4–Co2–O13 | 91.40(16) |
| Co2–O5–Co3 | 119.50(18) | O5–Co2–O9 | 88.35(15) |
| O4–Co2–O9 | 88.62(15) | O5–Co2–O12 | 96.12(15) |
| Co3–O8–C29 | 133.6(3) | Co2–O12–C40 | 134.1(4) |
| O5–Co2–O13 | 87.22(15) | Co2–O13–C42 | 128.4(4) |
| Co2–O9–C38 | 129.2(3) | Co2–O13–C1A | 124.7(17) |
| O9–Co2–O12 | 89.24(17) | Co1–O15–C44 | 135.1(4) |
| Co1–O10–C38 | 128.7(4) | Co1–N1–O2 | 124.9(3) |
| Co3–O11–C40 | 136.1(4) | Co1–N1–C11 | 126.7(4) |
| Co1–N2–O3 | 126.3(3) | Co3–N3–O6 | 118.7(3) |
| Co1–N2–C14 | 126.4(4) | Co3–N3–C24 | 126.5(4) |
| Co1–O15–C44 | 135.5(4) | Co1–O15–H15 | 114(2) |
| Co3–N4–O7 | 123.2(3) | Co2–O13–H13 | 116(3) |
| Co3–N4–C27 | 127.6(4) | Co1–O15–H15 | 115(2) |
| D–X | X···A | D···A | D–X···A | Symmetry Codes | |
|---|---|---|---|---|---|
| C6–H6–O8 | 0.9300 | 2.5900 | 3.382(9) | 144.00 | (x, 1 + y, z) |
| C12–H12A–O10 | 0.9700 | 2.4500 | 3.292(7) | 145.00 | |
| C12–H12A–N2 | 0.9700 | 2.4700 | 2.871(7) | 105.00 | |
| C12–H12B–O11 | 0.9700 | 2.4700 | 3.400(8) | 160.00 | (1/2 + x, 1 − y, 1/2 + z) |
| C13–H13A–N1 | 0.9700 | 2.5500 | 2.919(7) | 102.00 | |
| C22–H22–O7 | 0.9300 | 2.5300 | 3.213(8) | 130.00 | (1/2 + x, −y, 1/2 + z) |
| C26–H26B–O11 | 0.9700 | 2.4200 | 3.262(8) | 145.00 | |
| O13–H13–O8 | 0.87(2) | 1.94(3) | 2.723(6) | 149(5) | |
| O14–H14A–O9 | 0.8200 | 1.9100 | 2.715(6) | 168.00 | |
| O15–H15–O14 | 0.86(3) | 1.80(3) | 2.637(7) | 164(3) | (1 + x, y, z) |
| C13–H13B–Cg1 | 2.87 | ||||
| C13–H13B–Cg2 | 2.99 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Hao, J.; Zhai, L.-X.; Zhang, Y.; Dong, W.-K. Synthesis, Crystal Structure, Luminescence, Electrochemical and Antimicrobial Properties of Bis(salamo)-Based Co(II) Complex. Crystals 2017, 7, 277. https://doi.org/10.3390/cryst7090277
Wang L, Hao J, Zhai L-X, Zhang Y, Dong W-K. Synthesis, Crystal Structure, Luminescence, Electrochemical and Antimicrobial Properties of Bis(salamo)-Based Co(II) Complex. Crystals. 2017; 7(9):277. https://doi.org/10.3390/cryst7090277
Chicago/Turabian StyleWang, Li, Jing Hao, Li-Xiang Zhai, Yang Zhang, and Wen-Kui Dong. 2017. "Synthesis, Crystal Structure, Luminescence, Electrochemical and Antimicrobial Properties of Bis(salamo)-Based Co(II) Complex" Crystals 7, no. 9: 277. https://doi.org/10.3390/cryst7090277






