Drug‑Drug and Drug‑Nutraceutical Cocrystal/Salt as Alternative Medicine for Combination Therapy: A Crystal Engineering Approach
Abstract
:1. Introduction
2. Case Studies
2.1. Drug-Drug Cocrystal
2.1.1. Aspirin Cocrystals
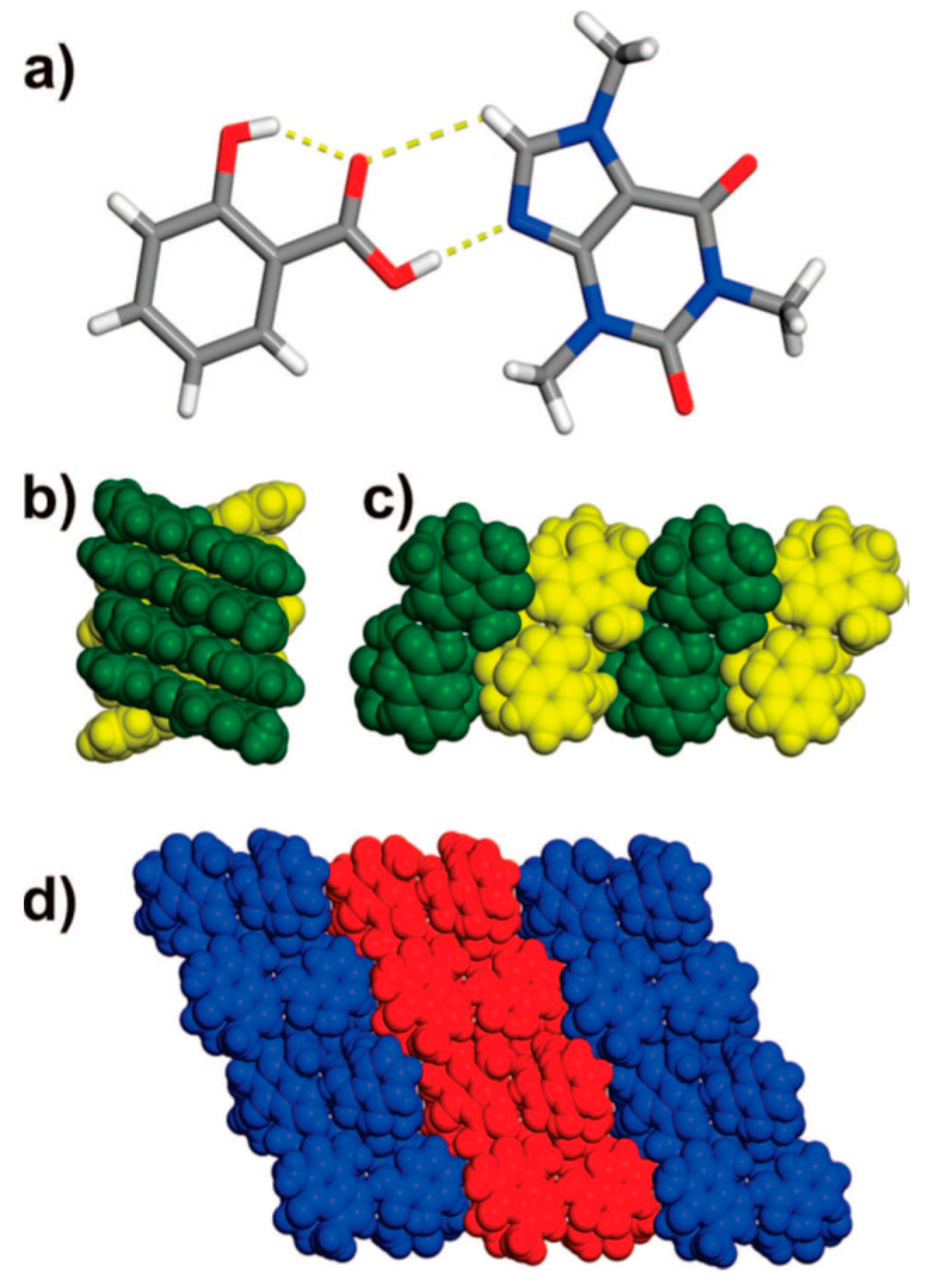
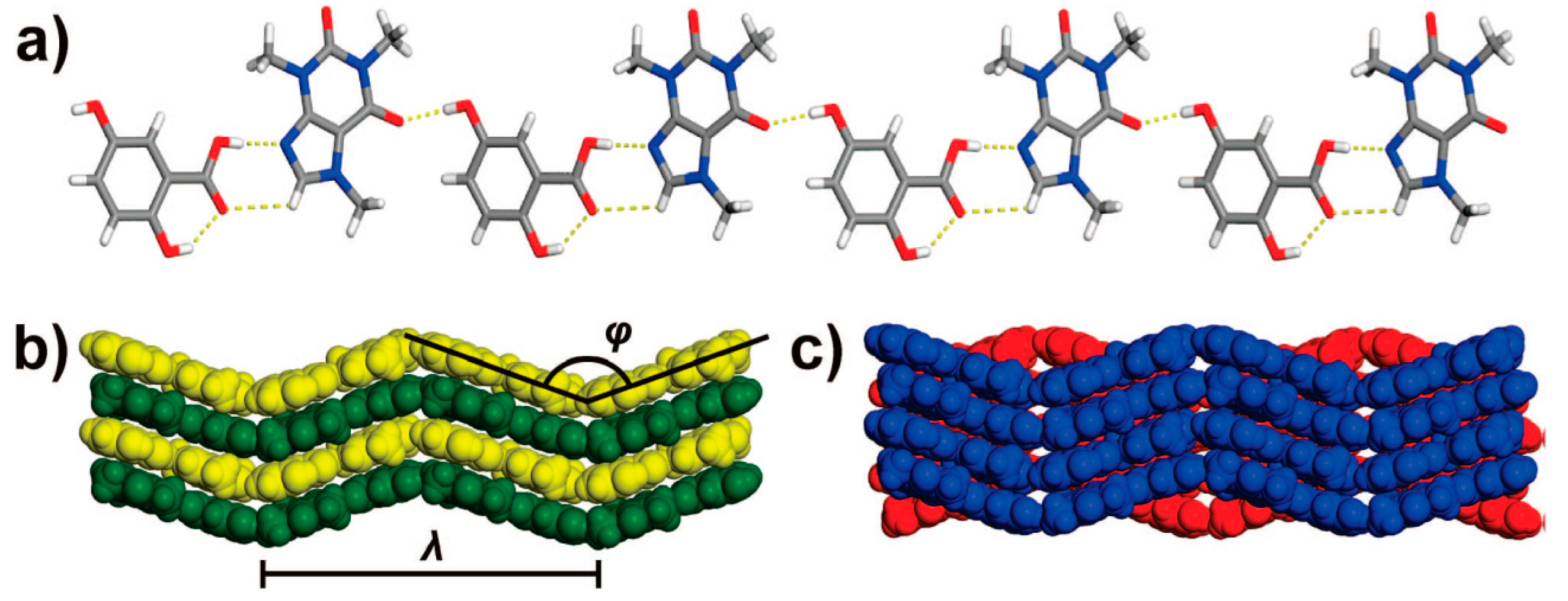
2.1.2. Anti-Tuberculosis Drug Cocrystal
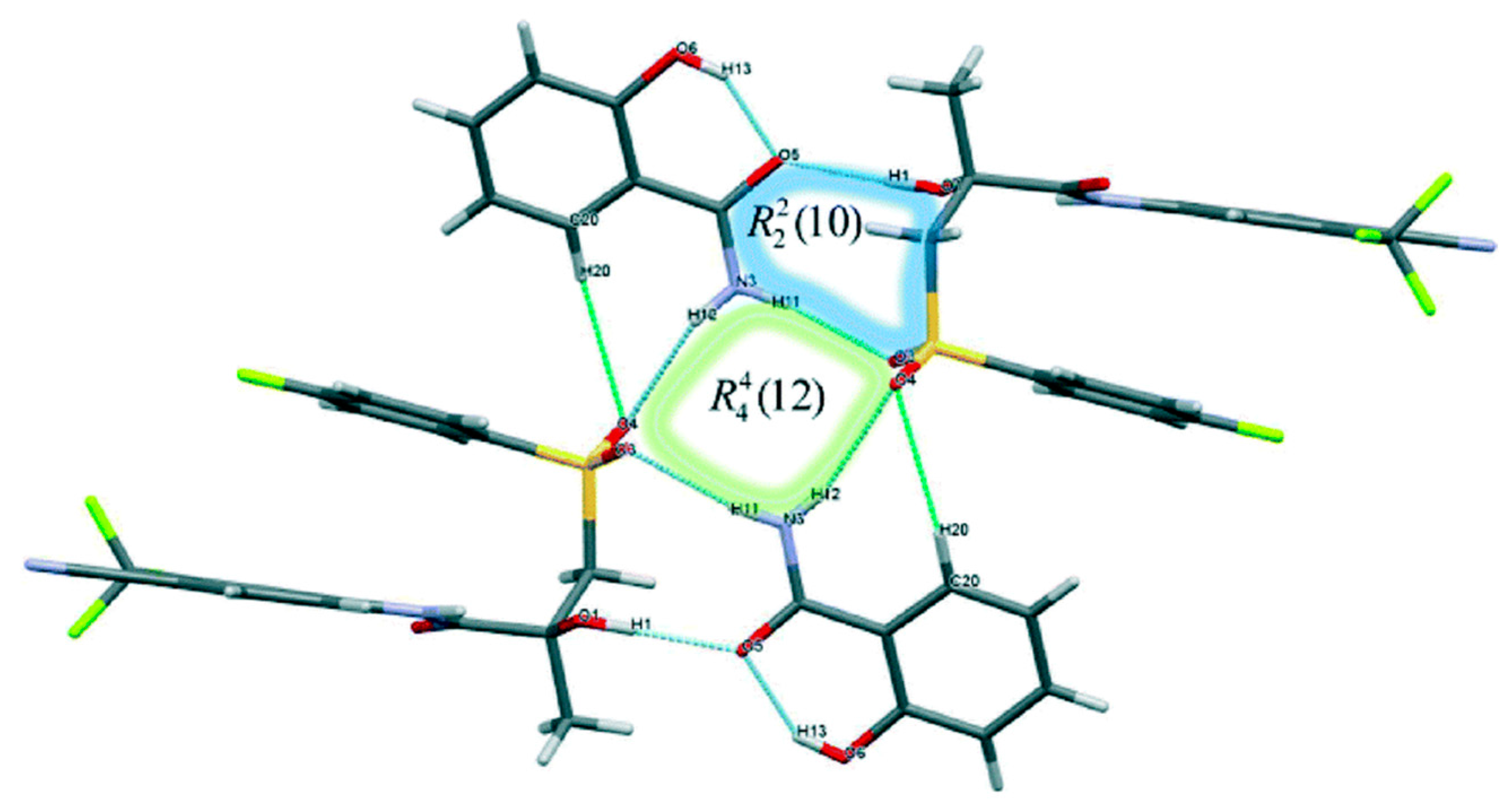
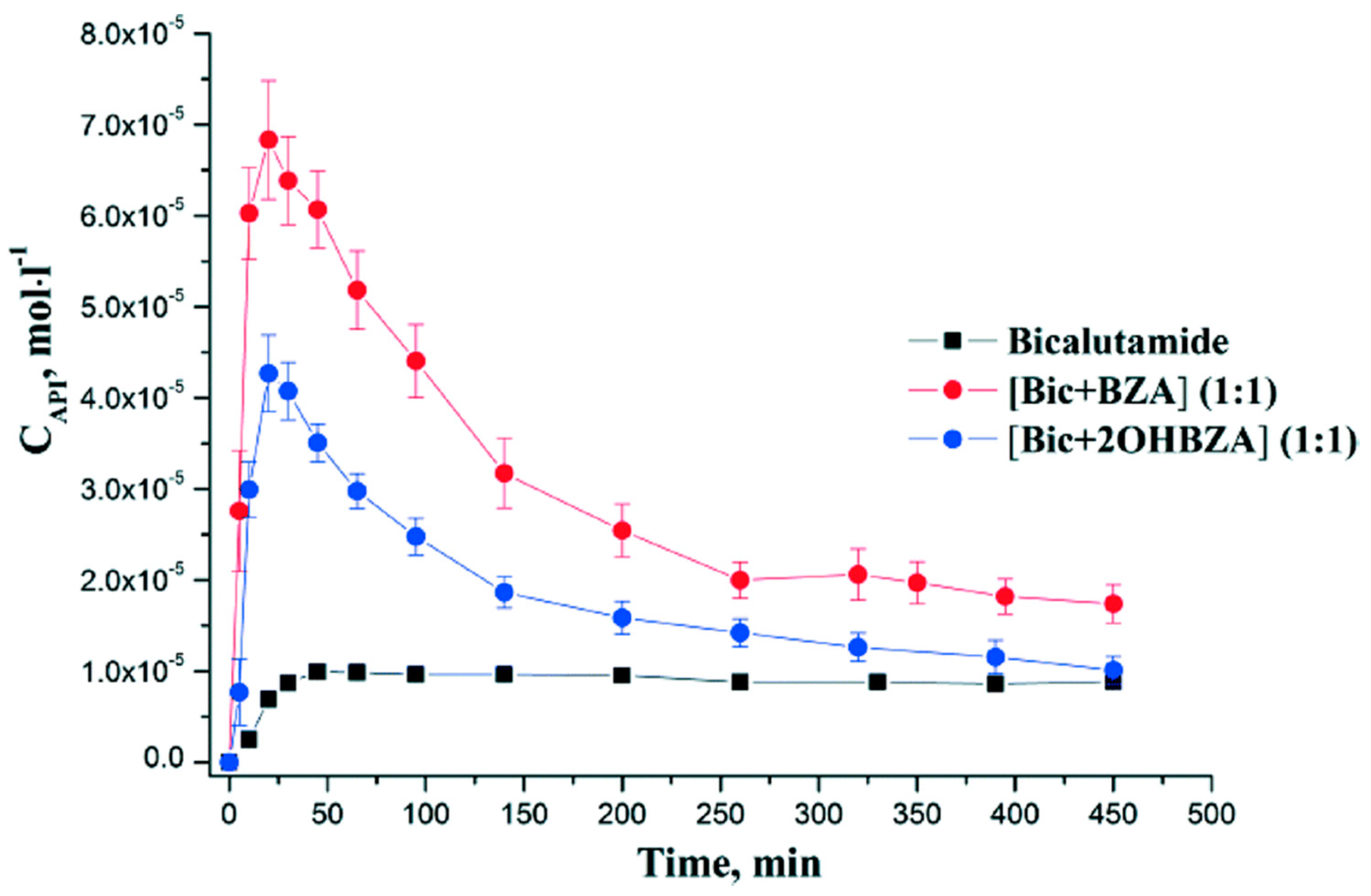
2.1.3. Bicalutamide Cocrystal
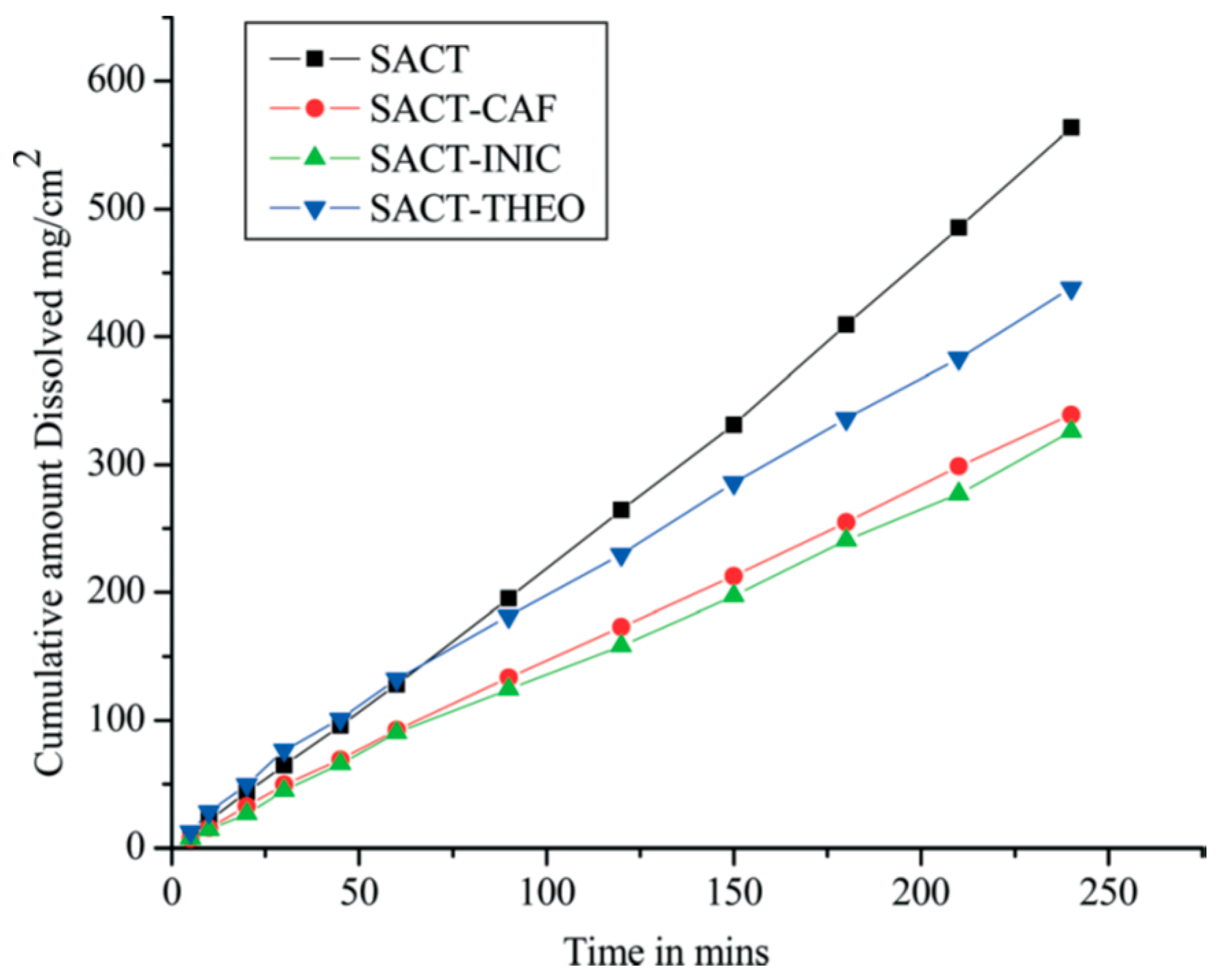
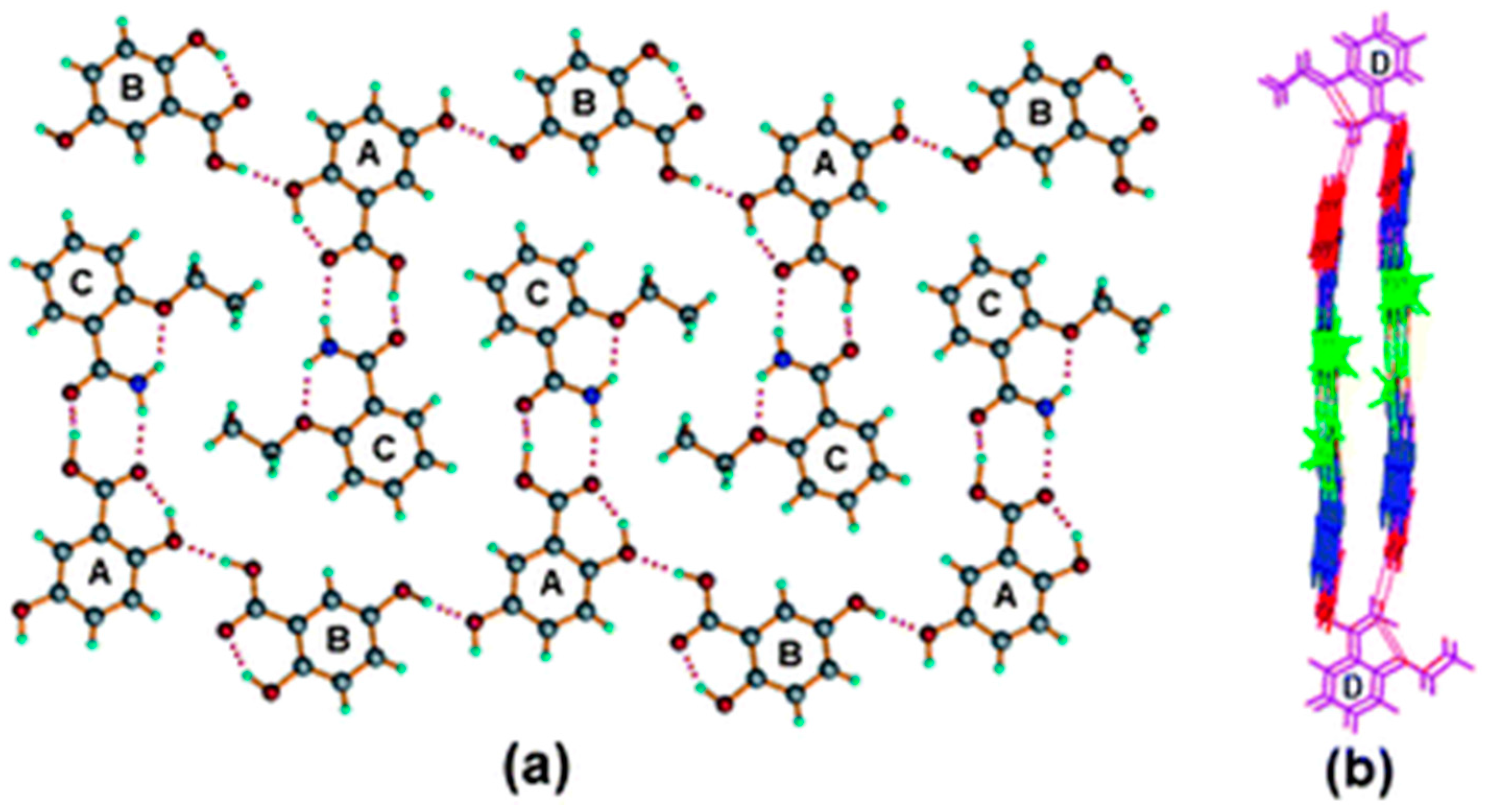
2.1.4. Caffeine Cocrystal System
2.1.5. Carbamazepine Cocrystal System
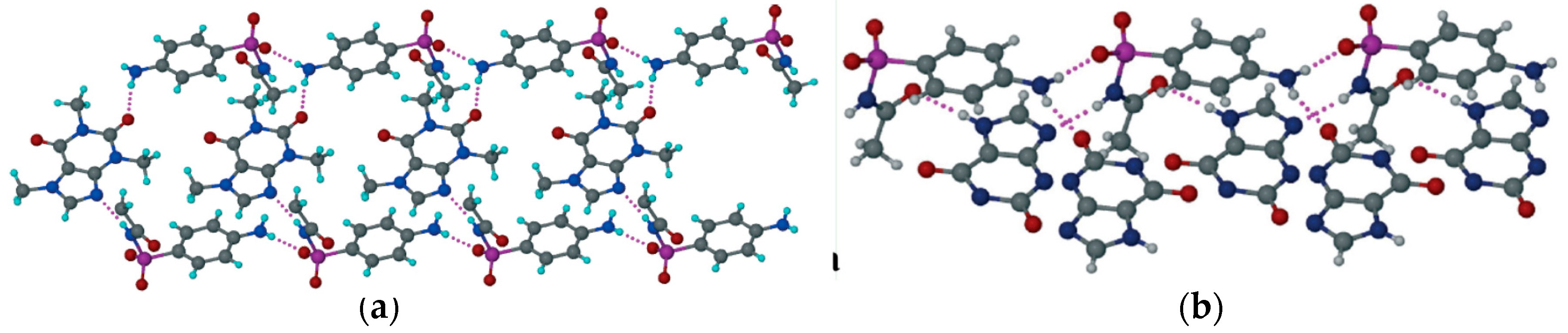
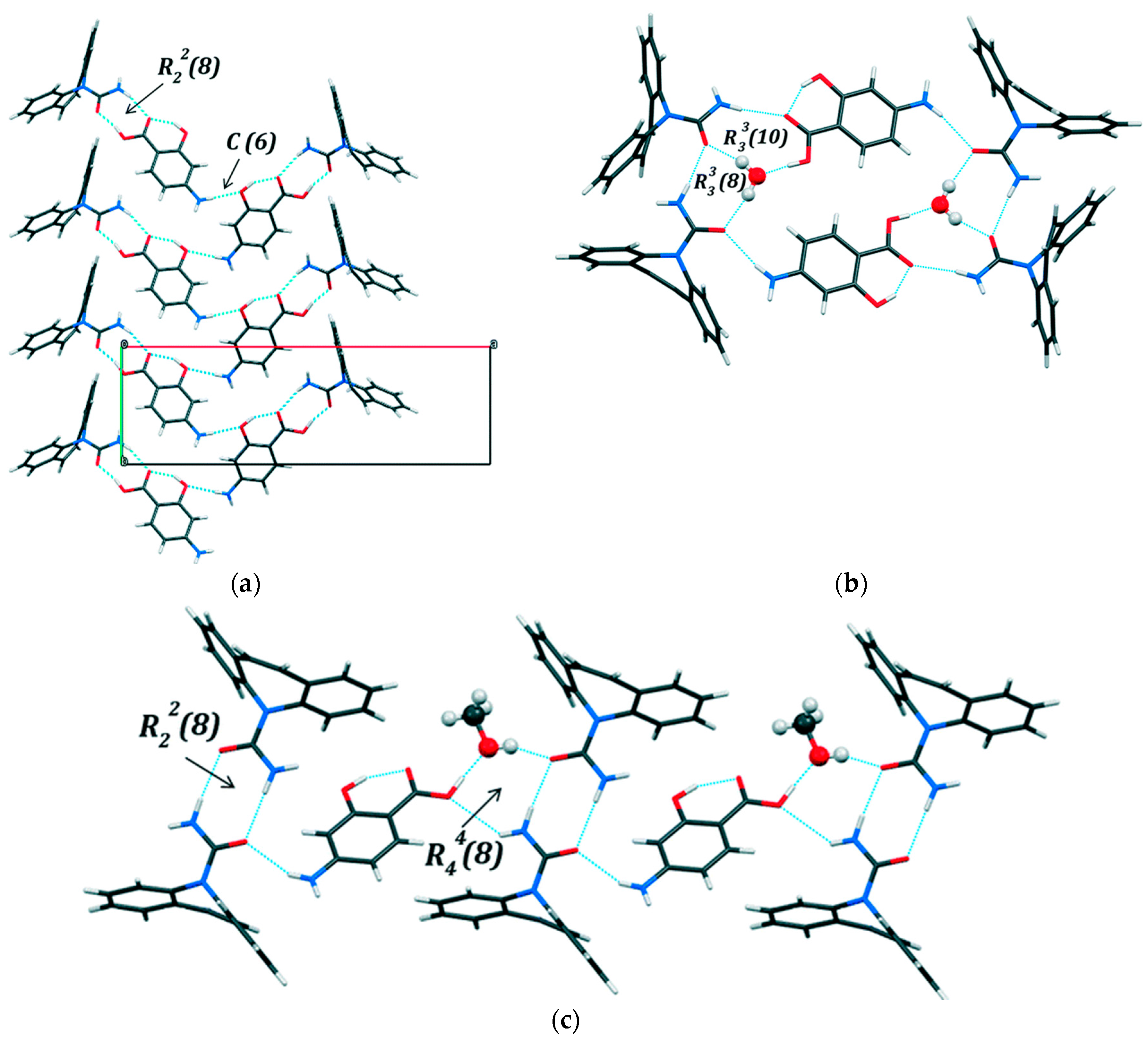
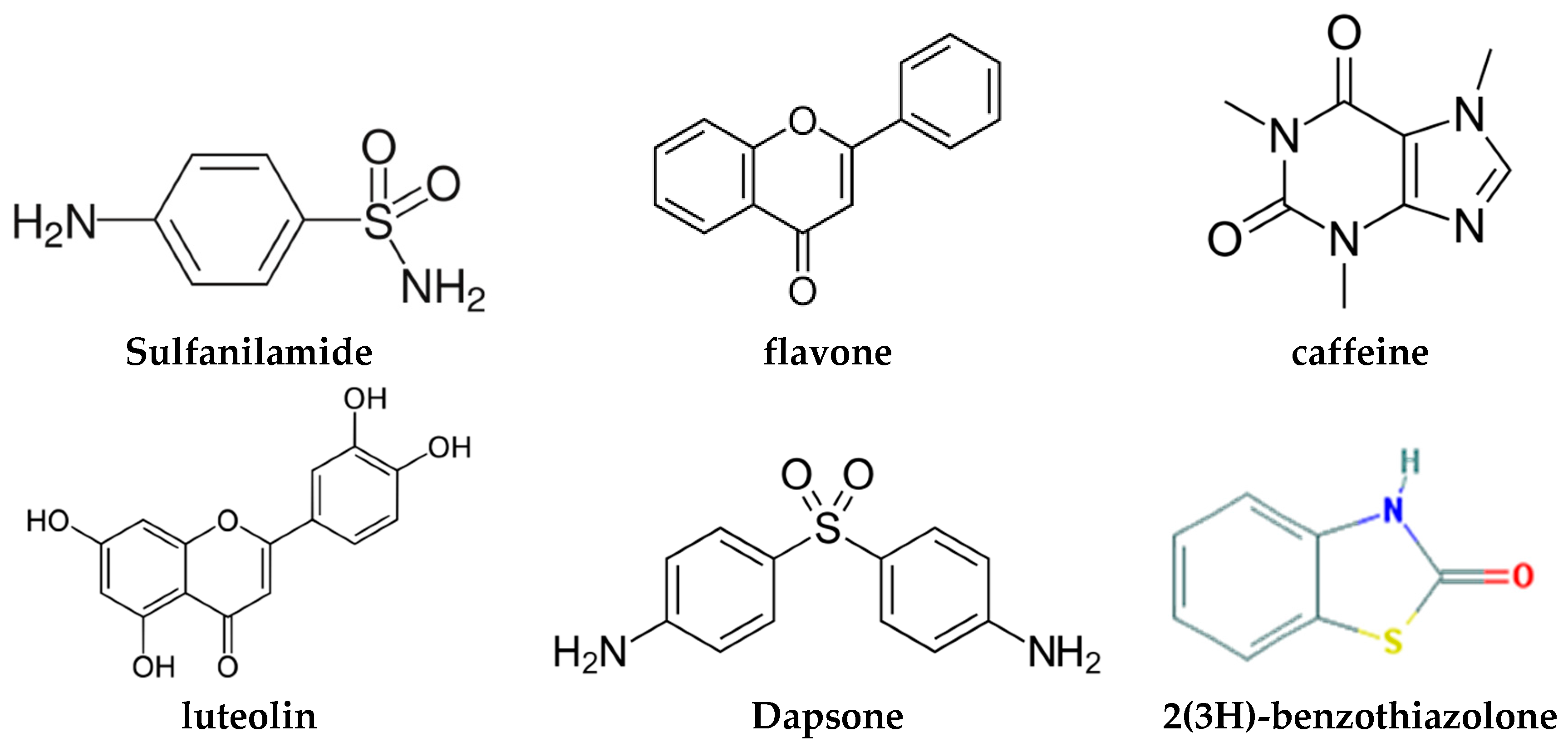
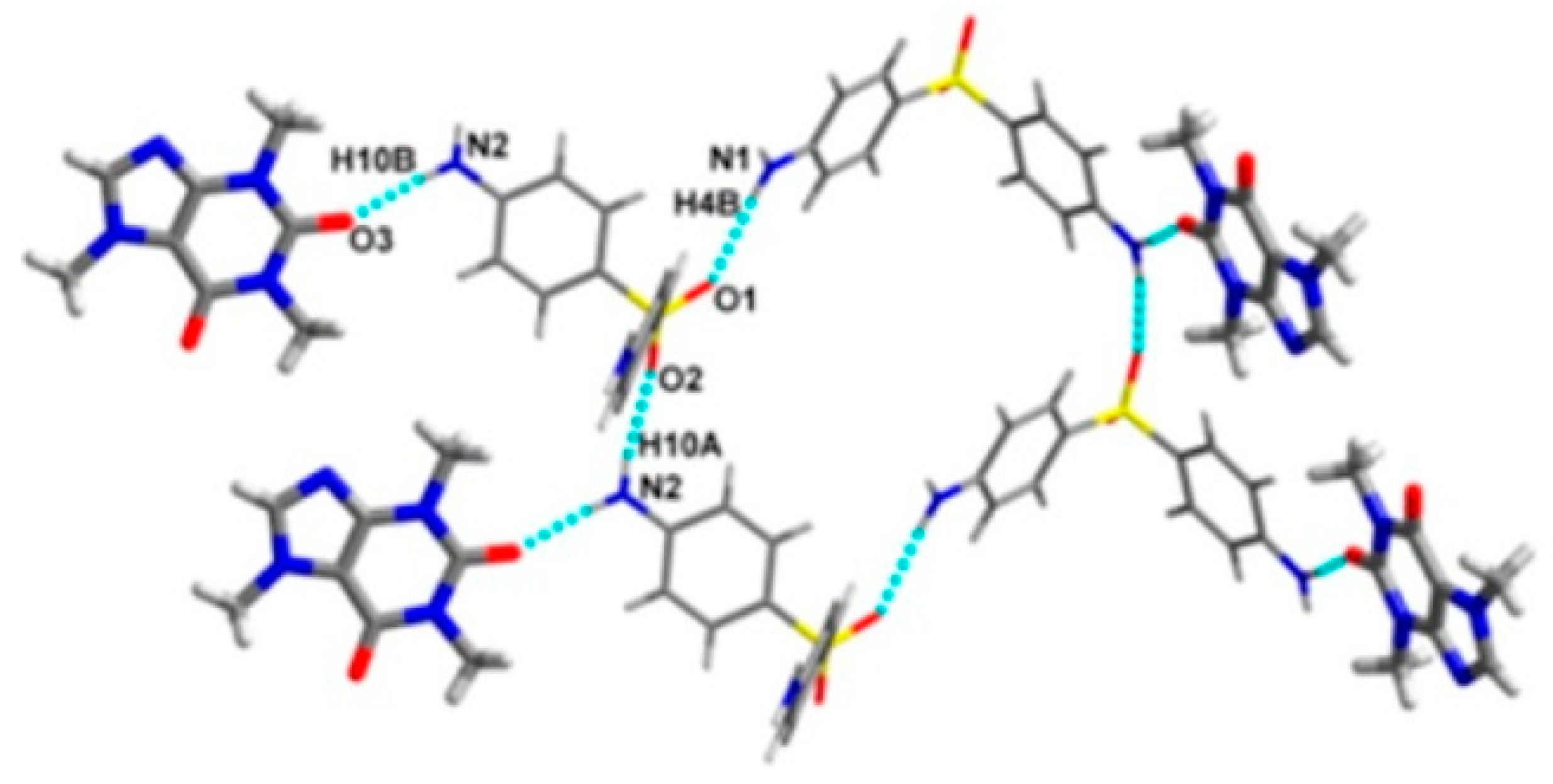
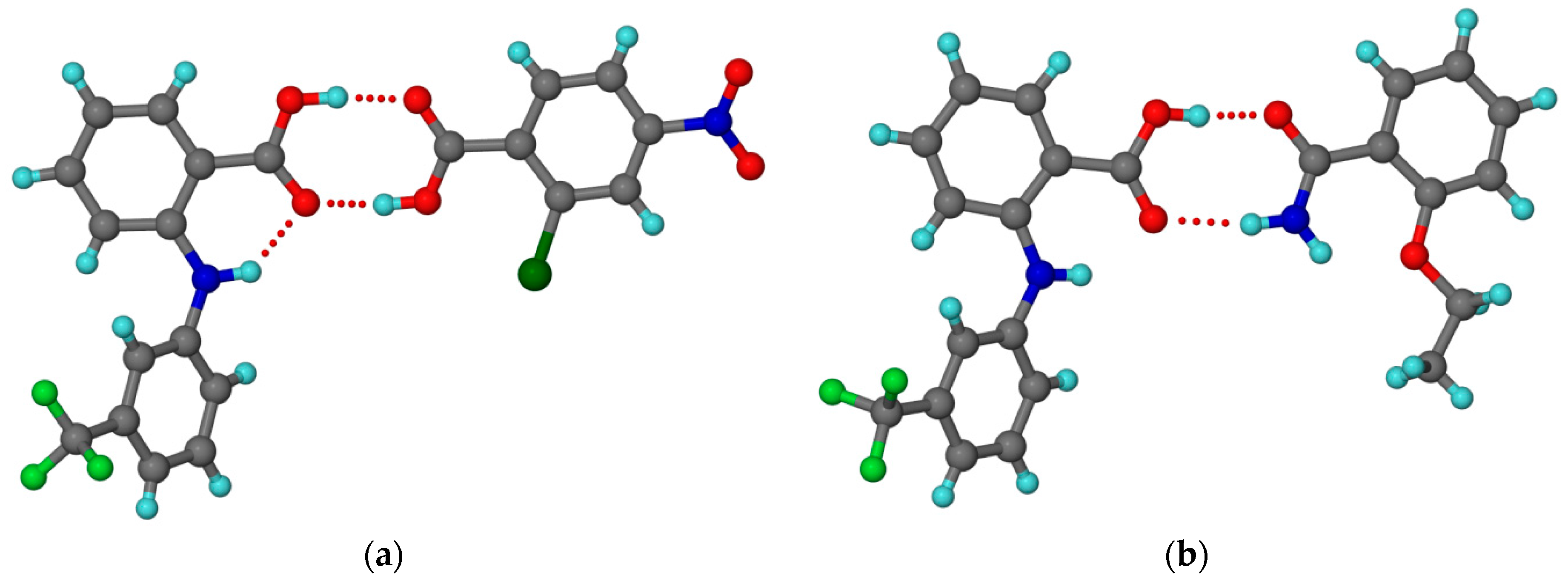
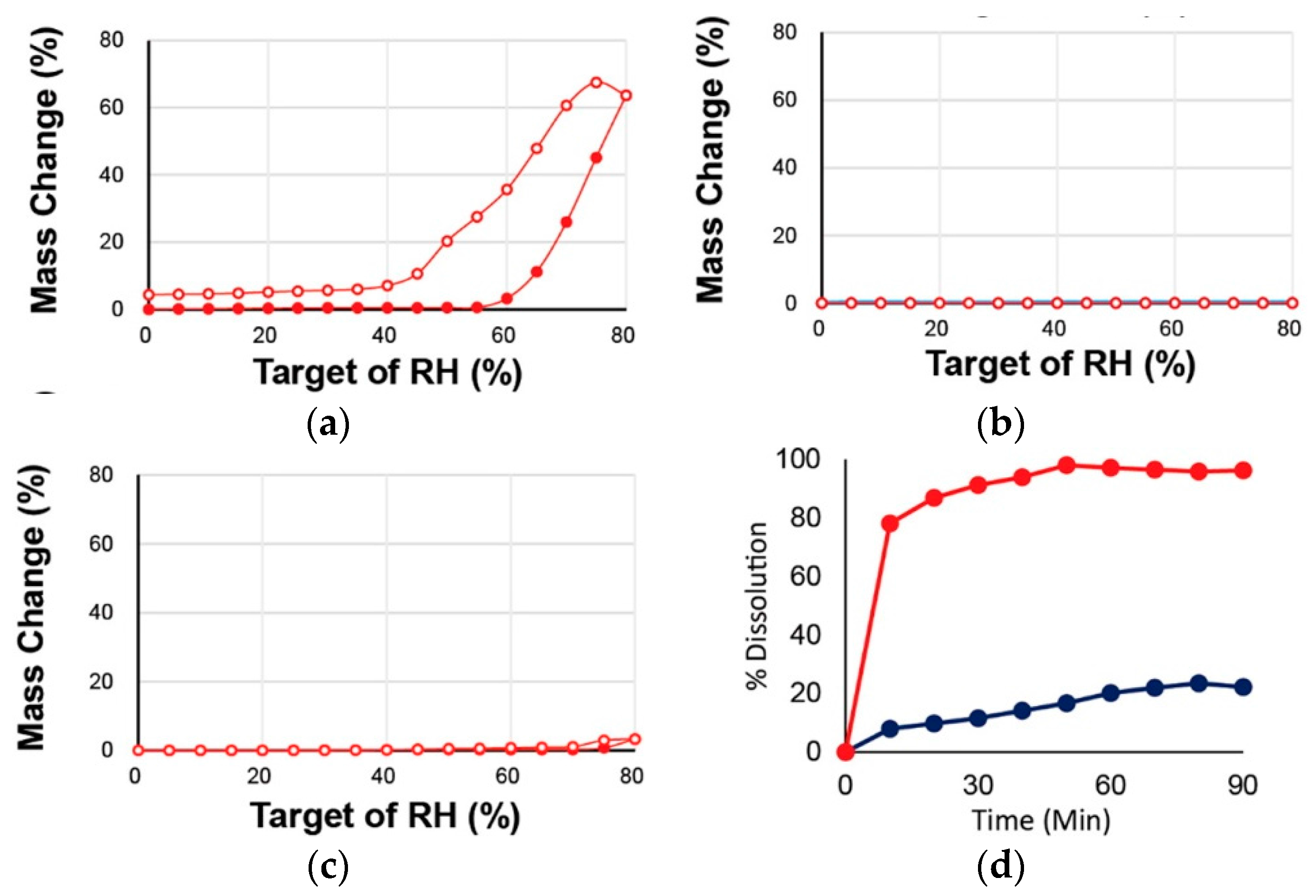
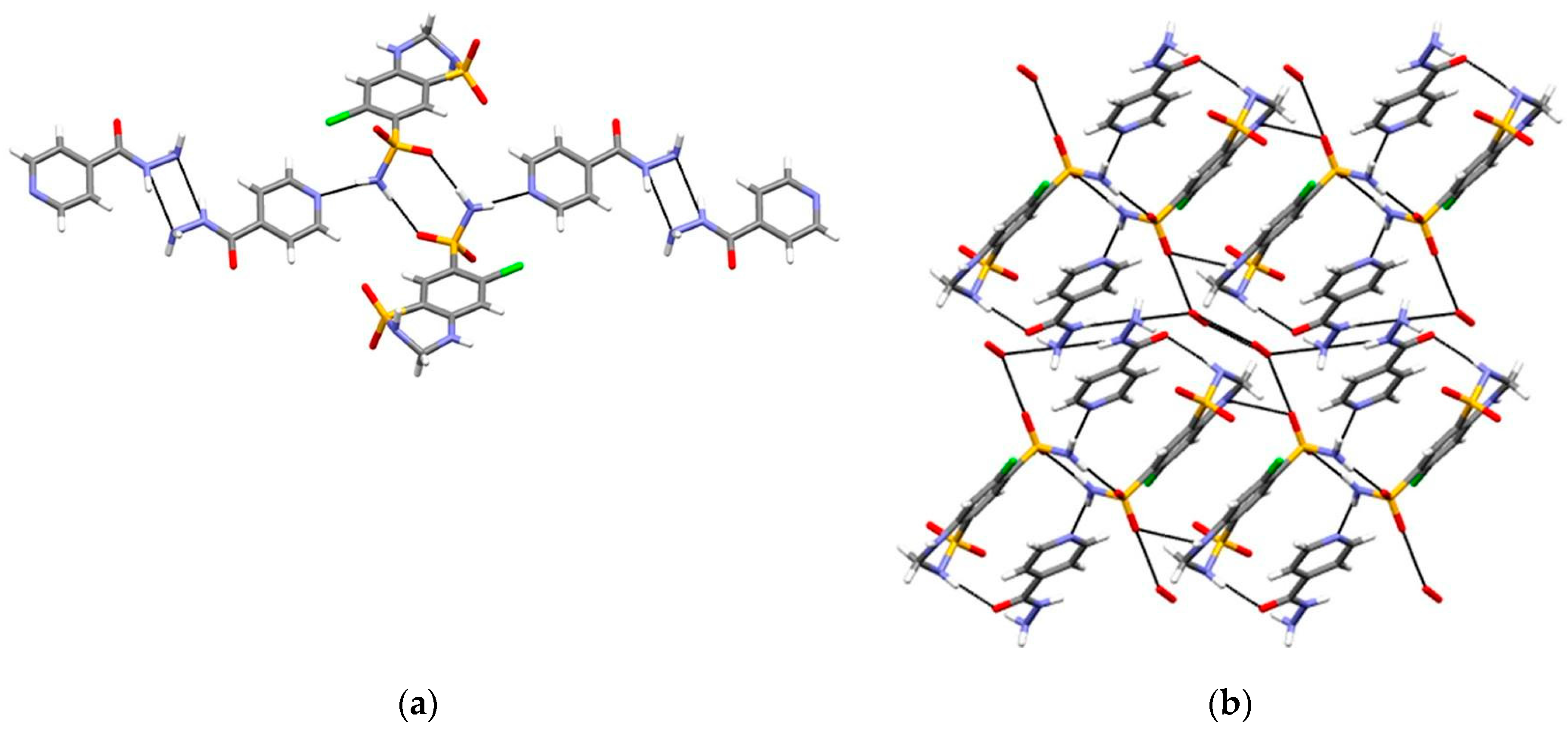
2.1.6. Dapsone-Drug Cocrystals
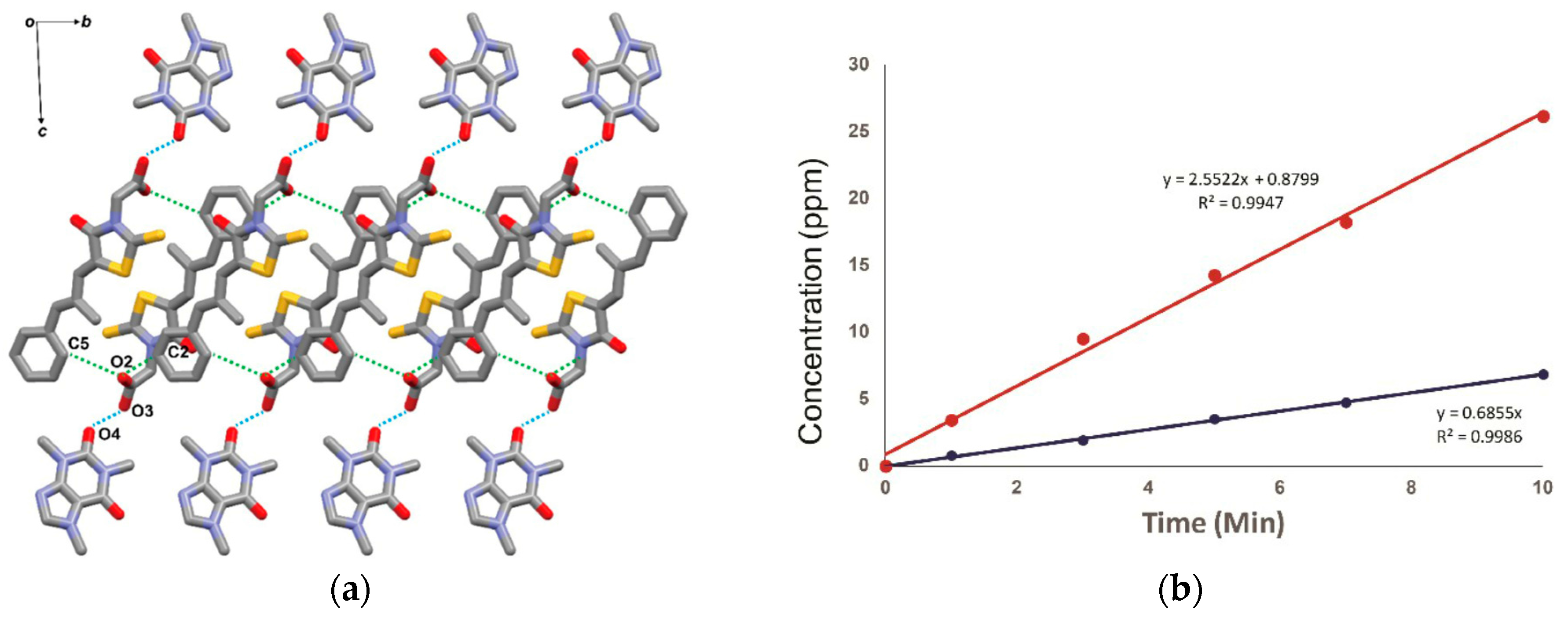
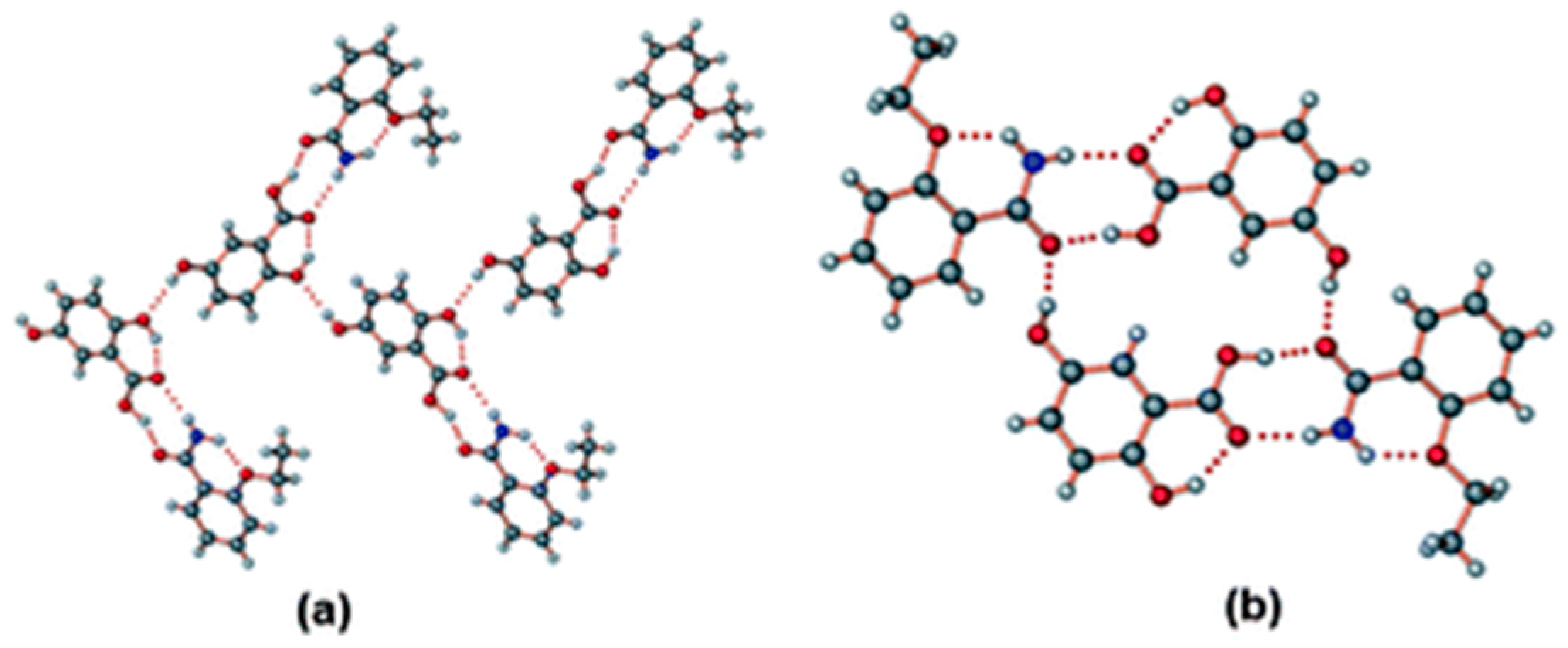
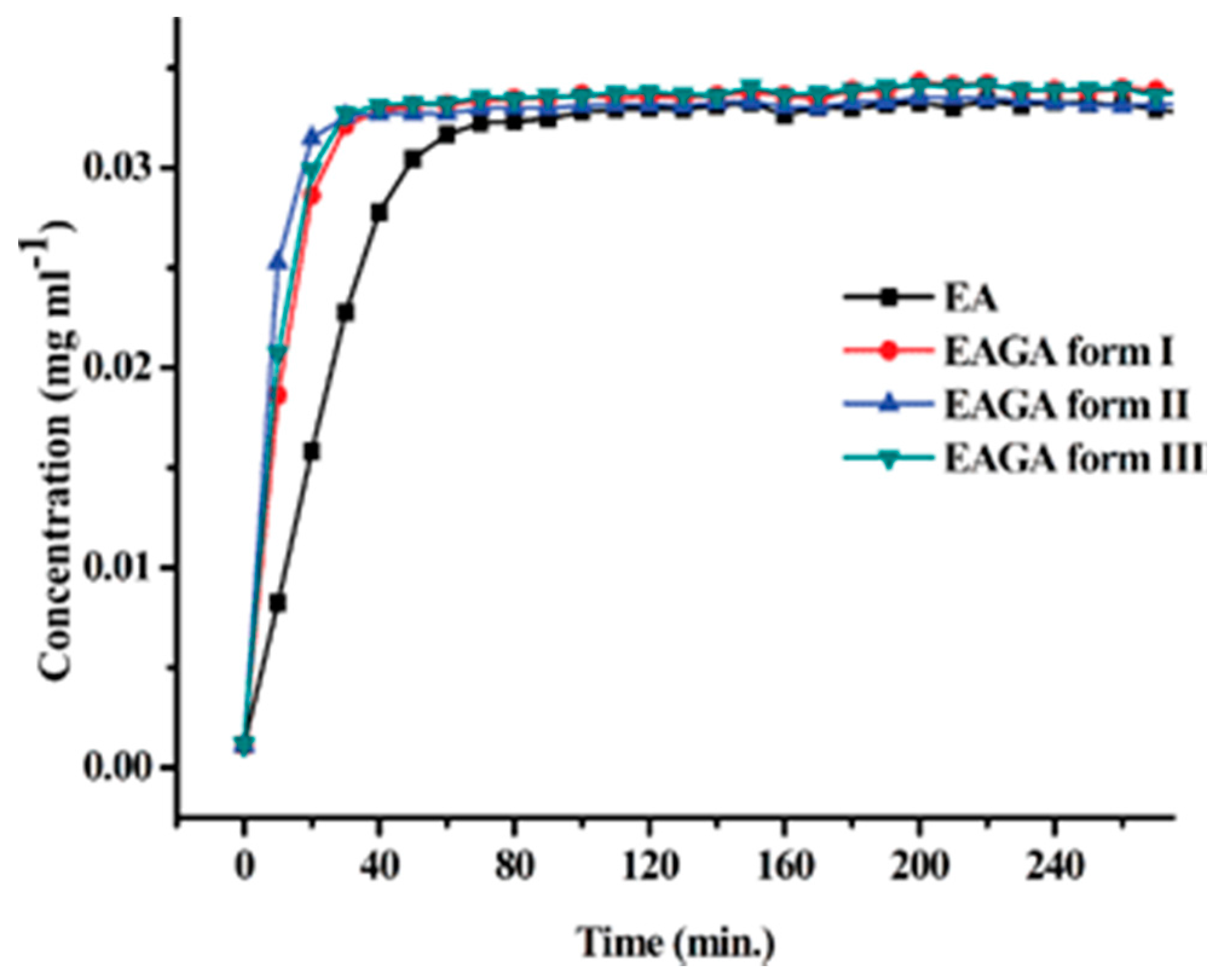
2.1.7. Ethenzamide-Gentisic Acid Cocrystals
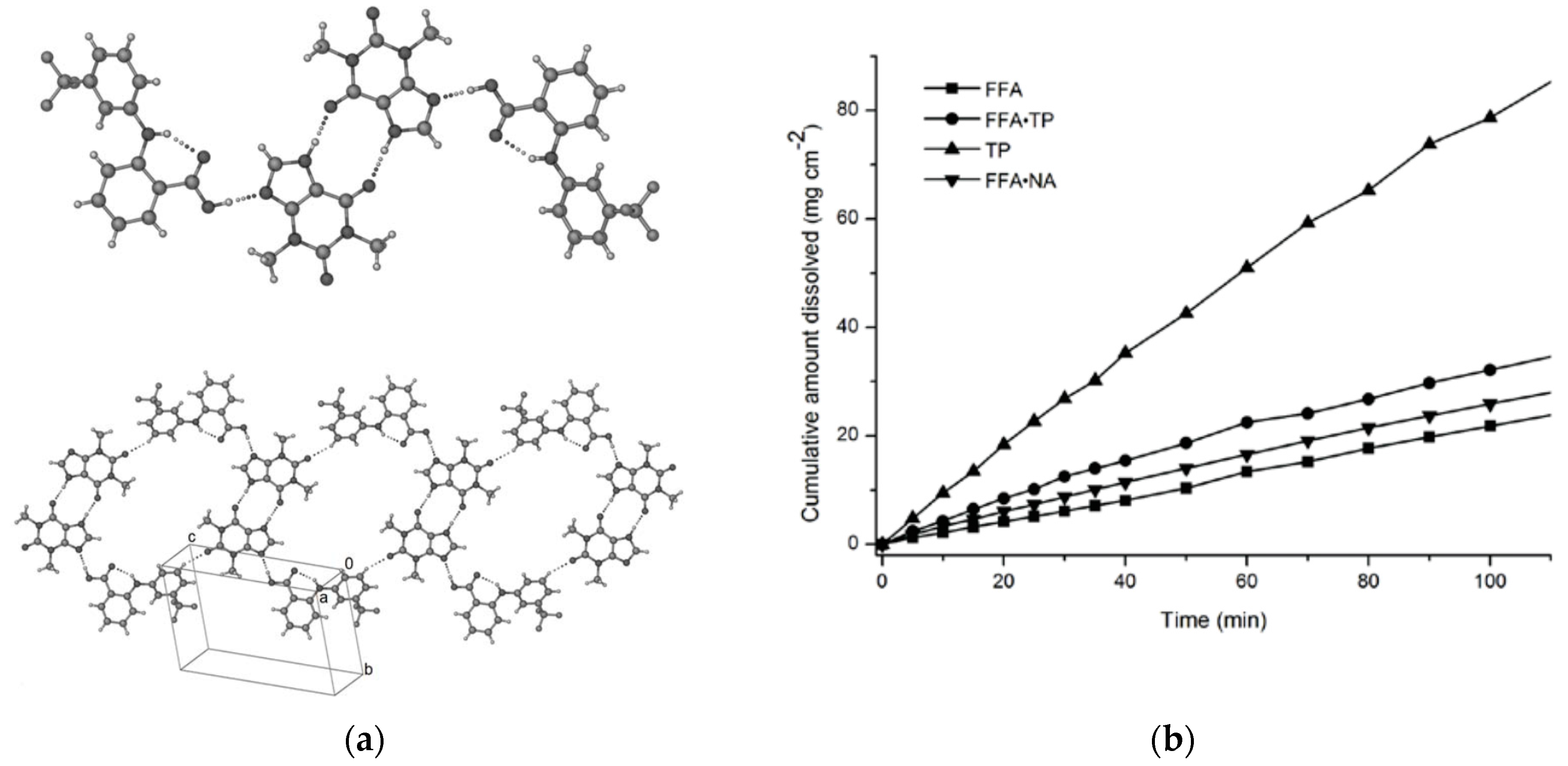
2.1.8. Flufenamic Acid Cocrystals
2.1.9. Furosemide-Caffeine Cocrystals
2.1.10. Gefitinib-Furosemide Salt Hydrate
2.1.11. Gliclazide-Metformin Salt
2.1.12. Hydrochlorothiazide Cocrystal
2.1.13. Lamivudine-Zidovudine Cocrystal
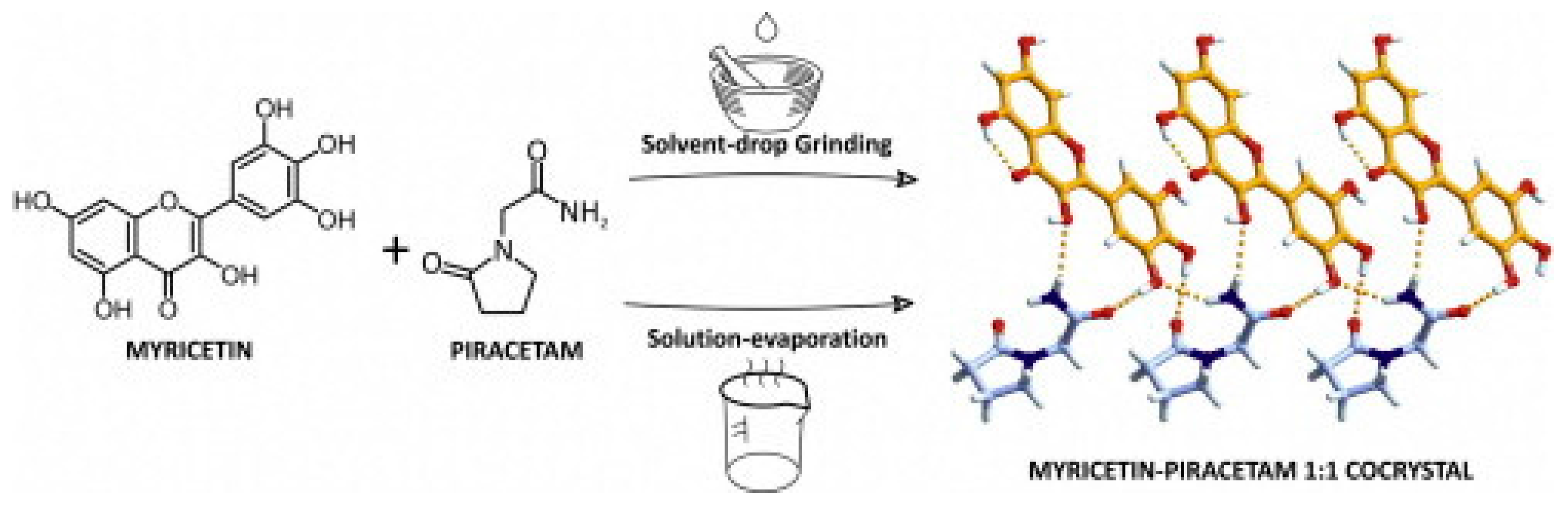
2.1.14. Myricetin–Piracetam Cocrystal
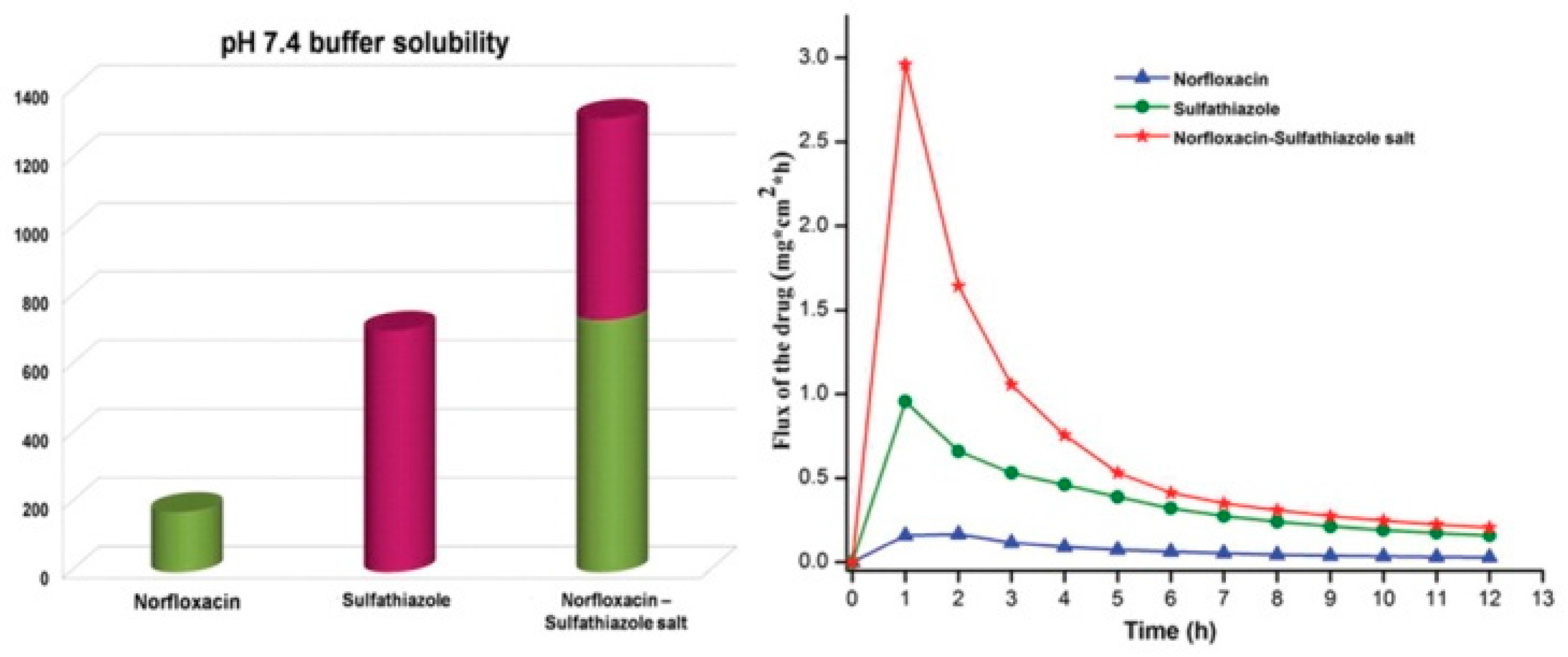
2.1.15. Norfloxacin–Sulfathiazole Salt Hydrate
2.1.16. Oxaprozin-Salbutamol Salt
2.1.17. Paracetamol Cocrystal System
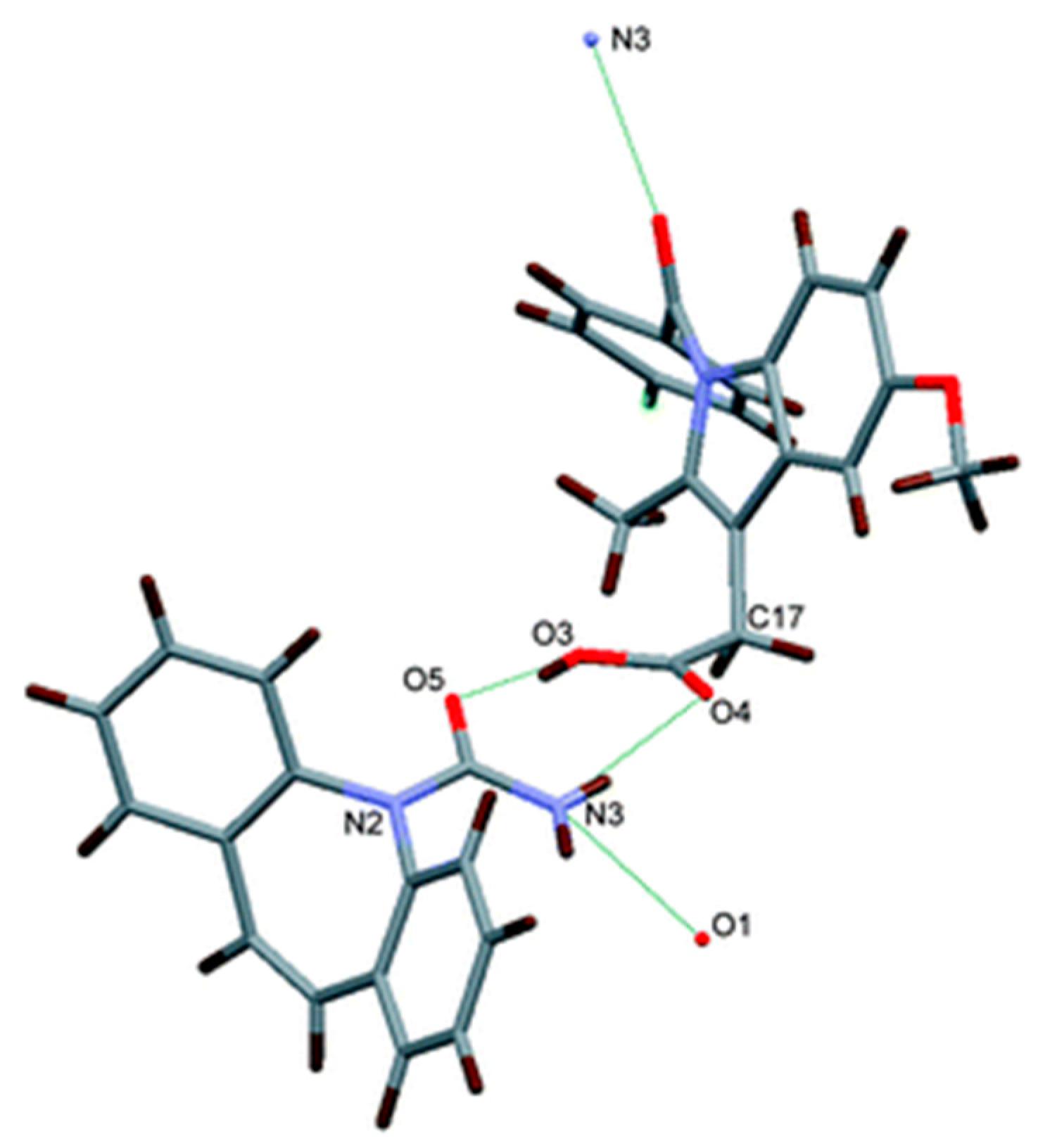
2.1.18. Pyrazinamide-Diflunisal Cocrystal
2.1.19. Pyrimethamine-Drug Cocrystals
2.1.20. Temozolomide Cocrystal System
2.1.21. Theophylline-Acetazolomide Cocrystal
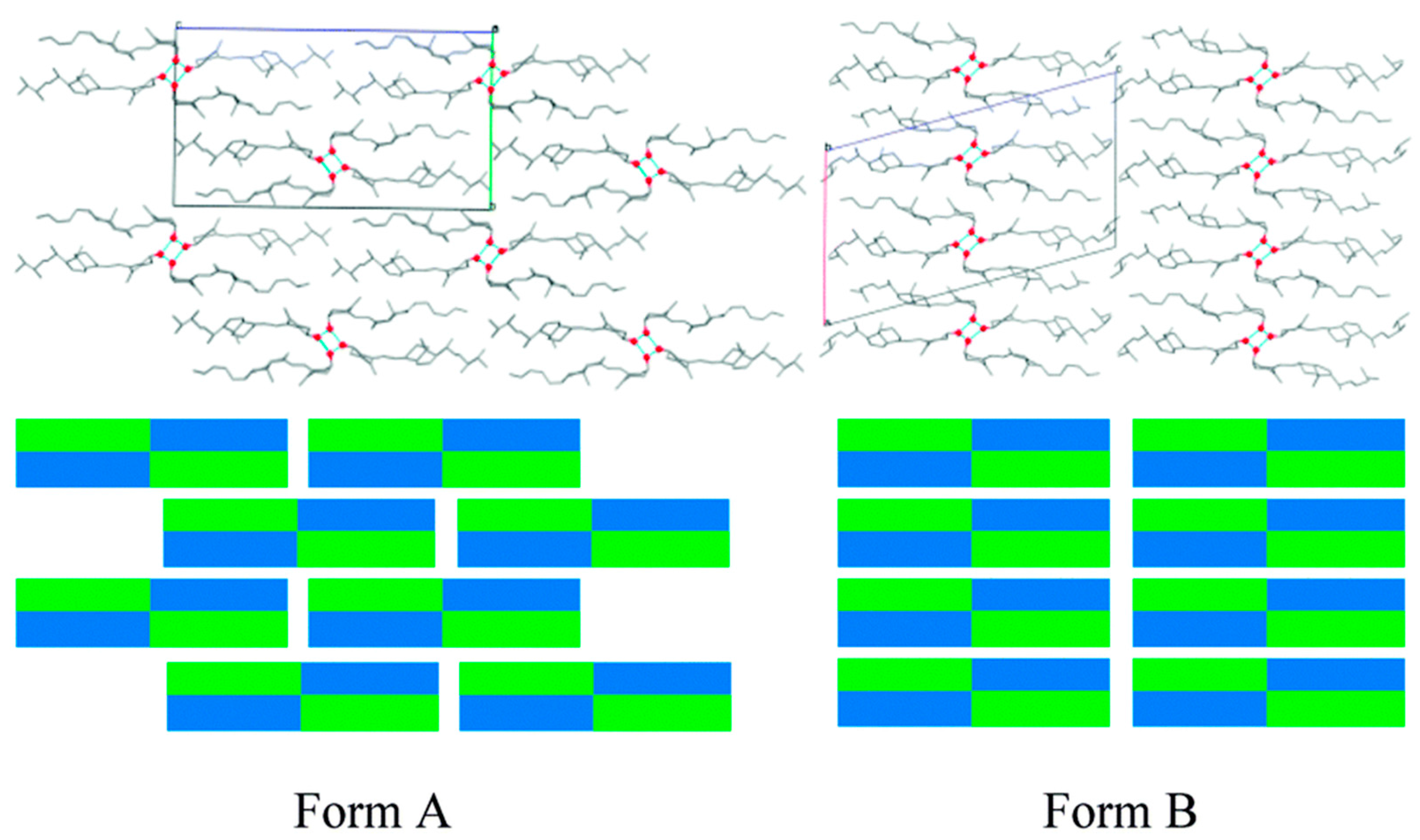
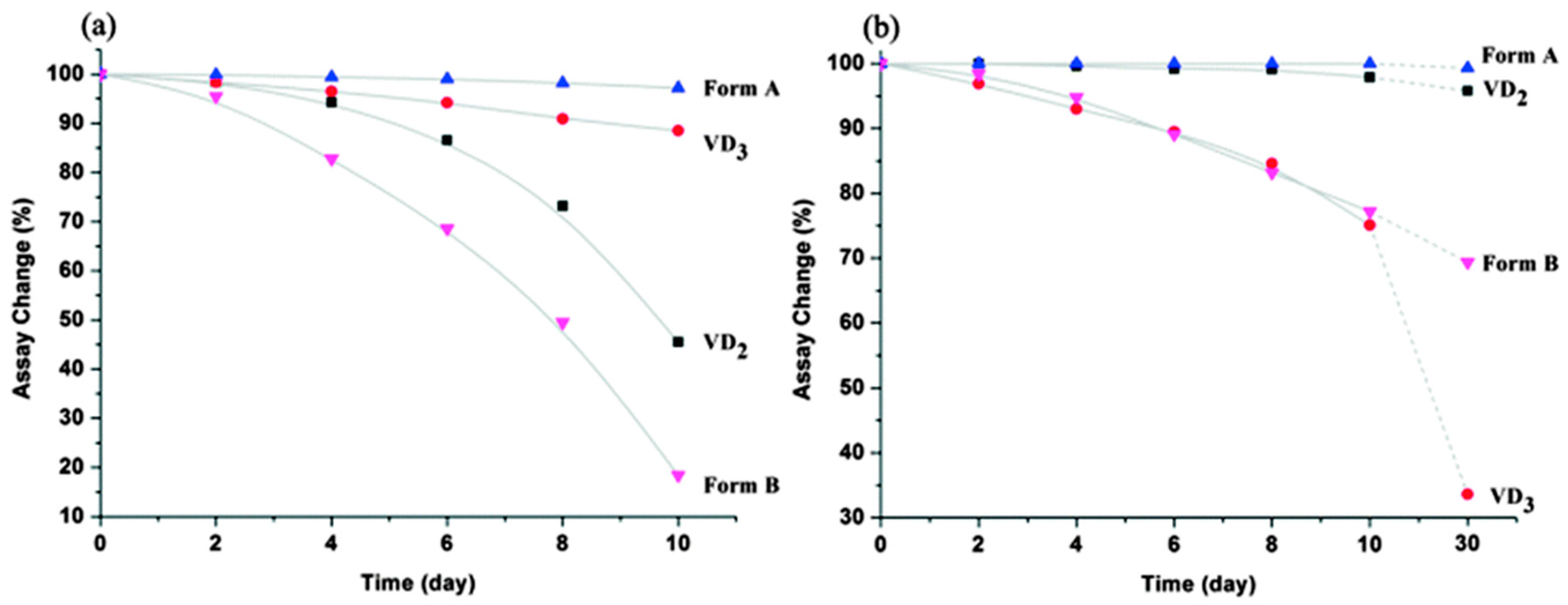
2.1.22. Vitamin D2-Vitamin D3 Cocrystal
2.2. Drug-Nutraceutical Cocrystals
3. Future Prospects
Acknowledgments
Conflicts of Interest
References
- Regulatory Classification of Pharmaceutical Co-Crystals Guidance for Industry. Available online: www.fda.gov/downloads/Drugs/Guidances/UCM516813 (accessed on 2 December 2017).
- Reflection paper on the use of cocrystals of active substances in medicinal products. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/07/WC500189927.pdf (accessed on 2 December 2017).
- Takagi, T.; Ramachandran, C.; Bermejo, M.; Yamashita, S.; Yu, L.X.; Amidon, G.L. A Provisional Biopharmaceutical Classification of the Top 200 Oral Drug Products in the United States, Great Britain, Spain, and Japan. Mol. Pharm. 2006, 3, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, N.; Newman, A. Pharmaceutical Cocrystals and Their Physicochemical Properties. Cryst. Growth Des. 2009, 9, 2950–2967. [Google Scholar] [CrossRef] [PubMed]
- Sarma, B.; Saikia, B. Hydrogen bond synthon competition in the stabilization of theophylline cocrystals. CrystEngComm 2014, 16, 4753–4765. [Google Scholar] [CrossRef]
- Qiao, N.; Li, M.; Schlindwein, W.; Malek, N.; Davies, A.; Trappitt, G. Pharmaceutical cocrystals: An overview. Int. J. Pharm. 2011, 419, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Saikia, B.; Bora, P.; Khatioda, R.; Sarma, B. Hydrogen Bond Synthons in the Interplay of Solubility and Membrane Permeability/Diffusion in Variable Stoichiometry Drug Cocrystals. Cryst. Growth Des. 2015, 15, 5593–5603. [Google Scholar] [CrossRef]
- Saikia, B.; Khatioda, R.; Bora, P.; Sarma, B. Pyridine N-oxides as coformers in the development of drug cocrystals. CrystEngComm 2016, 18, 8454–8464. [Google Scholar] [CrossRef]
- Thakuria, R.; Delori, A.; Jones, W.; Lipert, M.P.; Roy, L.; Rodríguez-Hornedo, N. Pharmaceutical cocrystals and poorly soluble drugs. Int. J. Pharm. 2013, 453, 101–125. [Google Scholar] [CrossRef] [PubMed]
- Thakuria, R.; Sarma, B.; Nangia, A. 7.03—Hydrogen Bonding in Molecular Crystals A2—Atwood, Jerry L. In Comprehensive Supramolecular Chemistry II; Elsevier: Oxford, UK, 2017; pp. 25–48. [Google Scholar]
- Galcera, J.; Molins, E. Effect of the Counterion on the Solubility of Isostructural Pharmaceutical Lamotrigine Salts. Cryst. Growth Des. 2009, 9, 327–334. [Google Scholar] [CrossRef]
- Babu, N.J.; Nangia, A. Solubility Advantage of Amorphous Drugs and Pharmaceutical Cocrystals. Cryst. Growth Des. 2011, 11, 2662–2679. [Google Scholar] [CrossRef]
- Variankaval, N.; Wenslow, R.; Murry, J.; Hartman, R.; Helmy, R.; Kwong, E.; Clas, S.-D.; Dalton, C.; Santos, I. Preparation and Solid-State Characterization of Nonstoichiometric Cocrystals of a Phosphodiesterase-IV Inhibitor and l-Tartaric Acid. Cryst. Growth Des. 2006, 6, 690–700. [Google Scholar] [CrossRef]
- Bethune, S.J.; Huang, N.; Jayasankar, A.; Rodríguez-Hornedo, N. Understanding and Predicting the Effect of Cocrystal Components and pH on Cocrystal Solubility. Cryst. Growth Des. 2009, 9, 3976–3988. [Google Scholar] [CrossRef]
- Bhatt, P.M.; Ravindra, N.V.; Banerjee, R.; Desiraju, G.R. Saccharin as a salt former. Enhanced solubilities of saccharinates of active pharmaceutical ingredients. Chem. Commun. 2005, 1073–1075. [Google Scholar] [CrossRef] [PubMed]
- Cheney, M.L.; Shan, N.; Healey, E.R.; Hanna, M.; Wojtas, L.; Zaworotko, M.J.; Sava, V.; Song, S.; Sanchez-Ramos, J.R. Effects of Crystal Form on Solubility and Pharmacokinetics: A Crystal Engineering Case Study of Lamotrigine. Cryst. Growth Des. 2010, 10, 394–405. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Forbes, S.; Desper, J. Using Cocrystals to Systematically Modulate Aqueous Solubility and Melting Behavior of an Anticancer Drug. J. Am. Chem. Soc. 2009, 131, 17048–17049. [Google Scholar] [CrossRef] [PubMed]
- McNamara, D.P.; Childs, S.L.; Giordano, J.; Iarriccio, A.; Cassidy, J.; Shet, M.S.; Mannion, R.; O’Donnell, E.; Park, A. Use of a Glutaric Acid Cocrystal to Improve Oral Bioavailability of a Low Solubility API. Pharm. Res. 2006, 23, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.D.B.; Bradner, M.W.; Fleischman, S.; Morales, L.A.; Moulton, B.; Rodriguez-Hornedo, N.; Zaworotko, M.J. Crystal engineering of the composition of pharmaceutical phases. Chem. Commun. 2003, 186–187. [Google Scholar] [CrossRef]
- Trask, A.V.; Motherwell, W.D.S.; Jones, W. Pharmaceutical Cocrystallization: Engineering a Remedy for Caffeine Hydration. Cryst. Growth Des. 2005, 5, 1013–1021. [Google Scholar] [CrossRef]
- Trask, A.V.; Motherwell, W.D.S.; Jones, W. Physical stability enhancement of theophylline via cocrystallization. Int. J. Pharm. 2006, 320, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.; Friščić, T.; Fábián, L.; Laity, P.R.; Day, G.M.; Jones, W. Improving Mechanical Properties of Crystalline Solids by Cocrystal Formation: New Compressible Forms of Paracetamol. Adv. Mater. 2009, 21, 3905–3909. [Google Scholar] [CrossRef]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Thayer, A.M. Finding solutions. Chem. Eng. News 2010, 88, 13–18. [Google Scholar]
- Available online: http://apps.who.int/iris/bitstream/10665/43443/1/WHO_TRS_937_eng.pdf (accessed on 2 December 2017).
- Hajduk, P.J.; Greer, J. A decade of fragment-based drug design: Strategic advances and lessons learned. Nat. Rev. Drug Discov. 2007, 6, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.E.; Coyne, A.G.; Hudson, S.A.; Abell, C. Fragment-Based Approaches in Drug Discovery and Chemical Biology. Biochemistry 2012, 51, 4990–5003. [Google Scholar] [CrossRef] [PubMed]
- Chellat, M.F.; Raguž, L.; Riedl, R. Targeting Antibiotic Resistance. Angew. Chem. Int. Ed. 2016, 55, 6600–6626. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Sanderson, P.E.; Zheng, W. Drug combination therapy increases successful drug repositioning. Drug Discov. Today 2016, 21, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Brittain, H.G. Pharmaceutical cocrystals: The coming wave of new drug substances. J. Pharm. Sci. 2013, 102, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Shan, N.; Perry, M.L.; Weyna, D.R.; Zaworotko, M.J. Impact of pharmaceutical cocrystals: The effects on drug pharmacokinetics. Expert Opin. Drug Metab. Toxicol. 2014, 10, 1255–1271. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sarma, B.; Evans, J.M.B.; Myerson, A.S. Pharmaceutical Crystallization. Cryst. Growth Des. 2011, 11, 887–895. [Google Scholar] [CrossRef]
- Sarma, B.; Chen, J.; Hsi, H.-Y.; Myerson, A.S. Solid forms of pharmaceuticals: Polymorphs, salts and cocrystals. Korean J. Chem. Eng. 2011, 28, 315–322. [Google Scholar] [CrossRef]
- Good, D.J.; Rodríguez-Hornedo, N. Solubility Advantage of Pharmaceutical Cocrystals. Cryst. Growth Des. 2009, 9, 2252–2264. [Google Scholar] [CrossRef]
- Almarsson, O.; Zaworotko, M.J. Crystal engineering of the composition of pharmaceutical phases. Do pharmaceutical co-crystals represent a new path to improved medicines? Chem. Commun. 2004, 1889–1896. [Google Scholar] [CrossRef] [PubMed]
- Generally Regarded as Safe Chemicals by the US-FDA. Available online: https://www.fda.gov/Food/IngredientsPackagingLabeling/FoodAdditivesIngredients/ucm091048.htm (accessed on 2 December 2017).
- Lemmerer, A. Covalent assistance to supramolecular synthesis: Modifying the drug functionality of the antituberculosis API isoniazid in situ during co-crystallization with GRAS and API compounds. CrystEngComm 2012, 14, 2465–2478. [Google Scholar] [CrossRef]
- Electronic Code of Federal Regulations. Available online: https://www.ecfr.gov/cgi-bin/text-idx?rgn=div5&node=21:3.0.1.1.13 (accessed on 2 December 2017).
- Everything Added to Food in the United States (EAFUS). Available online: https://www.accessdata.fda.gov/scripts/fcn/fcnnavigation.cfm?rpt=eafuslisting (accessed on 2 December 2017).
- Available online: https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/entresto.pdf (accessed on 2 December 2017).
- Harrison, W.T.A.; Yathirajan, H.S.; Bindya, S.; Anilkumar, H.G. Escitalopram oxalate: Co-existence of oxalate dianions and oxalic acid molecules in the same crystal. Acta Crystallogr. Sect. C 2007, 63, o129–o131. [Google Scholar] [CrossRef]
- Khatioda, R.; Saikia, B.; Das, P.J.; Sarma, B. Solubility and in vitro drug permeation behavior of ethenzamide cocrystals regulated in physiological pH environments. CrystEngComm 2017, 19, 6992–7000. [Google Scholar] [CrossRef]
- Thakuria, R.; Cherukuvada, S.; Nangia, A. Crystal Structures of Pyrogallol, Its Hydrate, and Stable Multiple Z′ Cocrystals with N-Heterocycles Containing Metastable Conformers of Pyrogallol. Cryst. Growth Des. 2012, 12, 3944–3953. [Google Scholar] [CrossRef]
- Maddileti, D.; Thakuria, R.; Cherukuvada, S.; Nangia, A. Blonanserin HCl salt and its monohydrate. CrystEngComm 2012, 14, 2367–2372. [Google Scholar] [CrossRef]
- Kumar, S.S.; Thakuria, R.; Nangia, A. Pharmaceutical cocrystals and a nitrate salt of voriconazole. CrystEngComm 2014, 16, 4722–4731. [Google Scholar] [CrossRef]
- Eddleston, M.D.; Thakuria, R.; Aldous, B.J.; Jones, W. An Investigation of the Causes of Cocrystal Dissociation at High Humidity. J. Pharm. Sci. 2014, 103, 2859–2864. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.; Lindeman, J.A. Chapter 14 Co-Crystals: Commercial Opportunities and Patent Considerations. In Pharmaceutical Salts and Co-Crystals; The Royal Society of Chemistry: London, UK, 2012; pp. 318–329. [Google Scholar]
- Buschmann, H.H.D.; Solà, C.L.; Benet, B.J.; Ceron, B.J.C. Co-Crystals of Duloxetine and Co-Crystal Formers for the Treatment of Pain. Patent EP2,123,626, 25 November 2009. [Google Scholar]
- Heinrich, B.H.; Farran, J.; Tesson, N. Co-Crystals of Tramadol and Paracetamol. Patent WO2,010,069,561, 24 June 2010. [Google Scholar]
- Solá, C.L.; Cerón, B.J.C.; Benet, B.J.; Buschmann, H.H. Co-Crystals of Tramadol and NSAIDs. Patent WO2,010,043,412, 22 April 2010. [Google Scholar]
- Plata, S.C.R.; Tesson, N. Co-Crystals of Tramadol and Coxibs. U.S. Patent 8,598,152, 3 December 2013. [Google Scholar]
- Plata, S.C.R.; Videla, C.S.; Tesson, N.; Trilla, C.M. Co-Crystals of Venlafaxine and Celecoxib. Patent EP2,515,892, 31 October 2012. [Google Scholar]
- Plata, S.C.R.; Tesson, N.; Jiménez, G.C.; Vaiana, L. Crystalline Forms of Sartans Like Telmisartan with Beta Blockers. Patent EP2,649,996, 16 October 2013. [Google Scholar]
- Sowa, C.; Gold, R.E.; Chiodo, T.; Vogel, R. Co-Crystals of Cyprodinil and Dithianon. Patent WO2,013,030,777, 7 March 2013. [Google Scholar]
- Entresto 97 mg/103 mg Film-Coated Tablets. Available online: http://www.medicines.org.uk/emc/medicine/31244 (accessed on 2 December 2017).
- Cafcit. Available online: https://www.drugs.com/pro/cafcit.html (accessed on 3 December 2017).
- Smit, J.P.; Hagen, E.J. Polymorphism in Caffeine Citric Acid Cocrystals. J. Chem. Crystallogr. 2015, 45, 128–133. [Google Scholar] [CrossRef]
- Karki, S.; Friščić, T.; Jones, W.; Motherwell, W.D.S. Screening for Pharmaceutical Cocrystal Hydrates via Neat and Liquid-Assisted Grinding. Mol. Pharm. 2007, 4, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Petruševski, G.; Naumov, P.; Jovanovski, G.; Ng, S.W. Unprecedented sodium–oxygen clusters in the solid-state structure of trisodium hydrogentetravalproate monohydrate: A model for the physiological activity of the anticonvulsant drug Epilim®. Inorg. Chem. Commun. 2008, 11, 81–84. [Google Scholar] [CrossRef]
- Putra, O.D.; Yoshida, T.; Umeda, D.; Higashi, K.; Uekusa, H.; Yonemochi, E. Crystal Structure Determination of Dimenhydrinate after More than 60 Years: Solving Salt–Cocrystal Ambiguity via Solid-State Characterizations and Solubility Study. Cryst. Growth Des. 2016, 16, 5223–5229. [Google Scholar] [CrossRef]
- Desiraju, G.R. Supramolecular Synthons in Crystal Engineering—A New Organic Synthesis. Angew. Chem. Int. Ed. Engl. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Sarma, B.; Bora, P.; Saikia, B. Regulation of π···π Stacking Interactions in Small Molecule Cocrystals and/or Salts for Physiochemical Property Modulation. Cryst. Growth Des. 2018. [Google Scholar] [CrossRef]
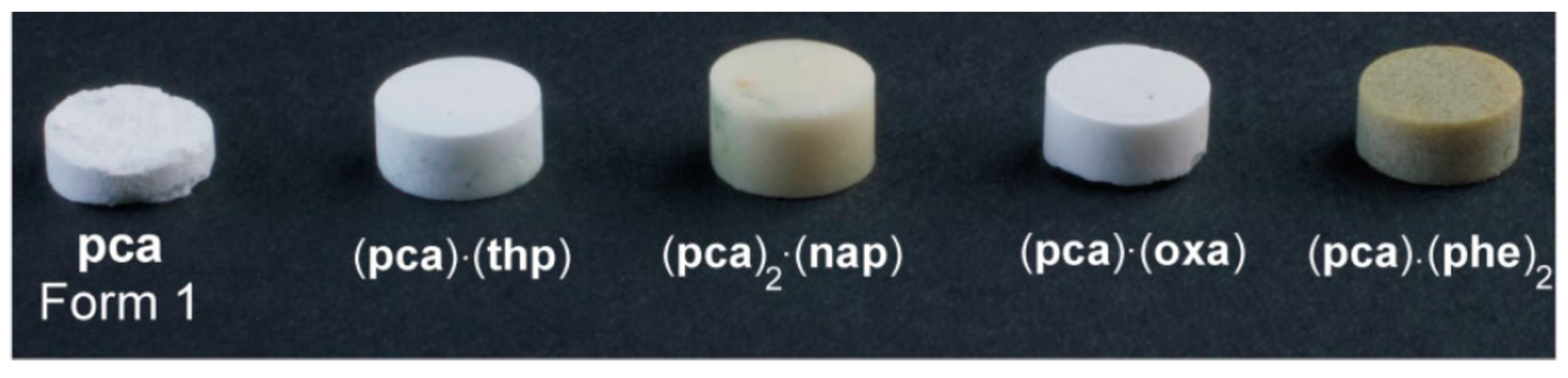
- Bhogala, B.R.; Basavoju, S.; Nangia, A. Three-Component Carboxylic Acid−Bipyridine Lattice Inclusion Host. Supramolecular Synthesis of Ternary Cocrystals. Cryst. Growth Des. 2005, 5, 1683–1686. [Google Scholar] [CrossRef]
- Sarma, B.; Nath, N.K.; Bhogala, B.R.; Nangia, A. Synthon Competition and Cooperation in Molecular Salts of Hydroxybenzoic Acids and Aminopyridines. Cryst. Growth Des. 2009, 9, 1546–1557. [Google Scholar] [CrossRef]
- Bhogala, B.R.; Nangia, A. Cocrystals of 1,3,5-Cyclohexanetricarboxylic Acid with 4,4′-Bipyridine Homologues: Acid···Pyridine Hydrogen Bonding in Neutral and Ionic Complexes. Cryst. Growth Des. 2003, 3, 547–554. [Google Scholar] [CrossRef]
- Childs, S.L.; Stahly, G.P.; Park, A. The Salt–Cocrystal Continuum: The Influence of Crystal Structure on Ionization State. Mol. Pharm. 2007, 4, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Cabeza, A.J. Acid-base crystalline complexes and the pKa rule. CrystEngComm 2012, 14, 6362–6365. [Google Scholar] [CrossRef]
- Sarmah, K.K.; Sarma, A.; Roy, K.; Rao, D.R.; Thakuria, R. Olanzapine Salts and Diversity in Molecular Packing. Cryst. Growth Des. 2016, 16, 1047–1055. [Google Scholar] [CrossRef]
- Thakuria, R.; Nangia, A. Olanzapinium Salts, Isostructural Solvates, and Their Physicochemical Properties. Cryst. Growth Des. 2013, 13, 3672–3680. [Google Scholar] [CrossRef]
- Thakuria, R.; Nangia, A. Highly soluble olanzapinium maleate crystalline salts. CrystEngComm 2011, 13, 1759–1764. [Google Scholar] [CrossRef]
- Sarma, B.; Thakuria, R.; Nath, N.K.; Nangia, A. Crystal structures of mirtazapine molecular salts. CrystEngComm 2011, 13, 3232–3240. [Google Scholar] [CrossRef]
- Laurence, C.; Berthelot, M. Observations on the strength of hydrogen bonding. Perspect. Drug Discov. Des. 2000, 18, 39–60. [Google Scholar] [CrossRef]
- Zegarac, M.; Leksic, E.; Sket, P.; Plavec, J.; Devcic Bogdanovic, M.; Bucar, D.-K.; Dumic, M.; Mestrovic, E. A sildenafil cocrystal based on acetylsalicylic acid exhibits an enhanced intrinsic dissolution rate. CrystEngComm 2014, 16, 32–35. [Google Scholar] [CrossRef]
- Cheney, M.L.; Weyna, D.R.; Shan, N.; Hanna, M.; Wojtas, L.; Zaworotko, M.J. Coformer selection in pharmaceutical cocrystal development: A case study of a meloxicam aspirin cocrystal that exhibits enhanced solubility and pharmacokinetics. J. Pharm. Sci. 2011, 100, 2172–2181. [Google Scholar] [CrossRef] [PubMed]
- Grobelny, P.; Mukherjee, A.; Desiraju, G.R. Drug-drug co-crystals: Temperature-dependent proton mobility in the molecular complex of isoniazid with 4-aminosalicylic acid. CrystEngComm 2011, 13, 4358–4364. [Google Scholar] [CrossRef]
- Liu, F.; Song, Y.; Liu, Y.-N.; Li, Y.-T.; Wu, Z.-Y.; Yan, C.-W. Drug-bridge-drug ternary cocrystallization strategy for anti-tuberculosis drugs combination. Cryst. Growth Des. 2018. [Google Scholar] [CrossRef]
- Surov, A.O.; Solanko, K.A.; Bond, A.D.; Bauer-Brandl, A.; Perlovich, G.L. Cocrystals of the antiandrogenic drug bicalutamide: Screening, crystal structures, formation thermodynamics and lattice energies. CrystEngComm 2016, 18, 4818–4829. [Google Scholar] [CrossRef]
- Bučar, D.-K.; Henry, R.F.; Lou, X.; Duerst, R.W.; MacGillivray, L.R.; Zhang, G.G.Z. Cocrystals of Caffeine and Hydroxybenzoic Acids Composed of Multiple Supramolecular Heterosynthons: Screening via Solution-Mediated Phase Transformation and Structural Characterization. Cryst. Growth Des. 2009, 9, 1932–1943. [Google Scholar] [CrossRef]
- Eddleston, M.D.; Lloyd, G.O.; Jones, W. Cocrystal dissociation and molecular demixing in the solid state. Chem. Commun. 2012, 48, 8075–8077. [Google Scholar] [CrossRef] [PubMed]
- Eddleston, M.D.; Patel, B.; Day, G.M.; Jones, W. Cocrystallization by Freeze-Drying: Preparation of Novel Multicomponent Crystal Forms. Cryst. Growth Des. 2013, 13, 4599–4606. [Google Scholar] [CrossRef]
- Rajesh Goud, N.; Khan, R.A.; Nangia, A. Modulating the solubility of sulfacetamide by means of cocrystals. CrystEngComm 2014, 16, 5859–5869. [Google Scholar] [CrossRef]
- Putra, O.D.; Umeda, D.; Nugraha, Y.P.; Furuishi, T.; Nagase, H.; Fukuzawa, K.; Uekusa, H.; Yonemochi, E. Solubility improvement of epalrestat by layered structure formation via cocrystallization. CrystEngComm 2017, 19, 2614–2622. [Google Scholar] [CrossRef]
- Majumder, M.; Buckton, G.; Rawlinson-Malone, C.; Williams, A.C.; Spillman, M.J.; Shankland, N.; Shankland, K. A carbamazepine-indomethacin (1:1) cocrystal produced by milling. CrystEngComm 2011, 13, 6327–6328. [Google Scholar] [CrossRef]
- Drozd, K.V.; Manin, A.N.; Churakov, A.V.; Perlovich, G.L. Novel drug-drug cocrystals of carbamazepine with para-aminosalicylic acid: Screening, crystal structures and comparative study of carbamazepine cocrystal formation thermodynamics. CrystEngComm 2017, 19, 4273–4286. [Google Scholar] [CrossRef]
- Jiang, L.; Huang, Y.; Zhang, Q.; He, H.; Xu, Y.; Mei, X. Preparation and Solid-State Characterization of Dapsone Drug–Drug Co-Crystals. Cryst. Growth Des. 2014, 14, 4562–4573. [Google Scholar] [CrossRef]
- Aitipamula, S.; Chow, P.S.; Tan, R.B.H. Trimorphs of a pharmaceutical cocrystal involving two active pharmaceutical ingredients: Potential relevance to combination drugs. CrystEngComm 2009, 11, 1823–1827. [Google Scholar] [CrossRef]
- Nechipadappu, S.K.; Tekuri, V.; Trivedi, D.R. Pharmaceutical Co-Crystal of Flufenamic Acid: Synthesis and Characterization of Two Novel Drug-Drug Co-Crystal. J. Pharm. Sci. 2017, 106, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Aitipamula, S.; Wong, A.B.H.; Chow, P.S.; Tan, R.B.H. Cocrystallization with flufenamic acid: Comparison of physicochemical properties of two pharmaceutical cocrystals. CrystEngComm 2014, 16, 5793–5801. [Google Scholar] [CrossRef]
- Surov, A.O.; Voronin, A.P.; Manin, A.N.; Manin, N.G.; Kuzmina, L.G.; Churakov, A.V.; Perlovich, G.L. Pharmaceutical Cocrystals of Diflunisal and Diclofenac with Theophylline. Mol. Pharm. 2014, 11, 3707–3715. [Google Scholar] [CrossRef] [PubMed]
- Goud, N.R.; Gangavaram, S.; Suresh, K.; Pal, S.; Manjunatha, S.G.; Nambiar, S.; Nangia, A. Novel furosemide cocrystals and selection of high solubility drug forms. J. Pharm. Sci. 2012, 101, 664–680. [Google Scholar] [CrossRef] [PubMed]
- Thorat, S.H.; Sahu, S.K.; Patwadkar, M.V.; Badiger, M.V.; Gonnade, R.G. Drug–Drug Molecular Salt Hydrate of an Anticancer Drug Gefitinib and a Loop Diuretic Drug Furosemide: An Alternative for Multidrug Treatment. J. Pharm. Sci. 2015, 104, 4207–4216. [Google Scholar] [CrossRef] [PubMed]
- Putra, O.D.; Furuishi, T.; Yonemochi, E.; Terada, K.; Uekusa, H. Drug–Drug Multicomponent Crystals as an Effective Technique to Overcome Weaknesses in Parent Drugs. Cryst. Growth Des. 2016, 16, 3577–3581. [Google Scholar] [CrossRef]
- Gopi, S.P.; Banik, M.; Desiraju, G.R. New Cocrystals of Hydrochlorothiazide: Optimizing Solubility and Membrane Diffusivity. Cryst. Growth Des. 2017, 17, 308–316. [Google Scholar] [CrossRef]
- Bhatt, P.M.; Azim, Y.; Thakur, T.S.; Desiraju, G.R. Co-Crystals of the Anti-HIV Drugs Lamivudine and Zidovudine. Cryst. Growth Des. 2009, 9, 951–957. [Google Scholar] [CrossRef]
- Sowa, M.; Ślepokura, K.; Matczak-Jon, E. A 1:1 pharmaceutical cocrystal of myricetin in combination with uncommon piracetam conformer: X-ray single crystal analysis and mechanochemical synthesis. J. Mol. Struct. 2014, 1058, 114–121. [Google Scholar] [CrossRef]
- Gopi, S.P.; Ganguly, S.; Desiraju, G.R. A Drug–Drug Salt Hydrate of Norfloxacin and Sulfathiazole: Enhancement of in Vitro Biological Properties via Improved Physicochemical Properties. Mol. Pharm. 2016, 13, 3590–3594. [Google Scholar] [CrossRef] [PubMed]
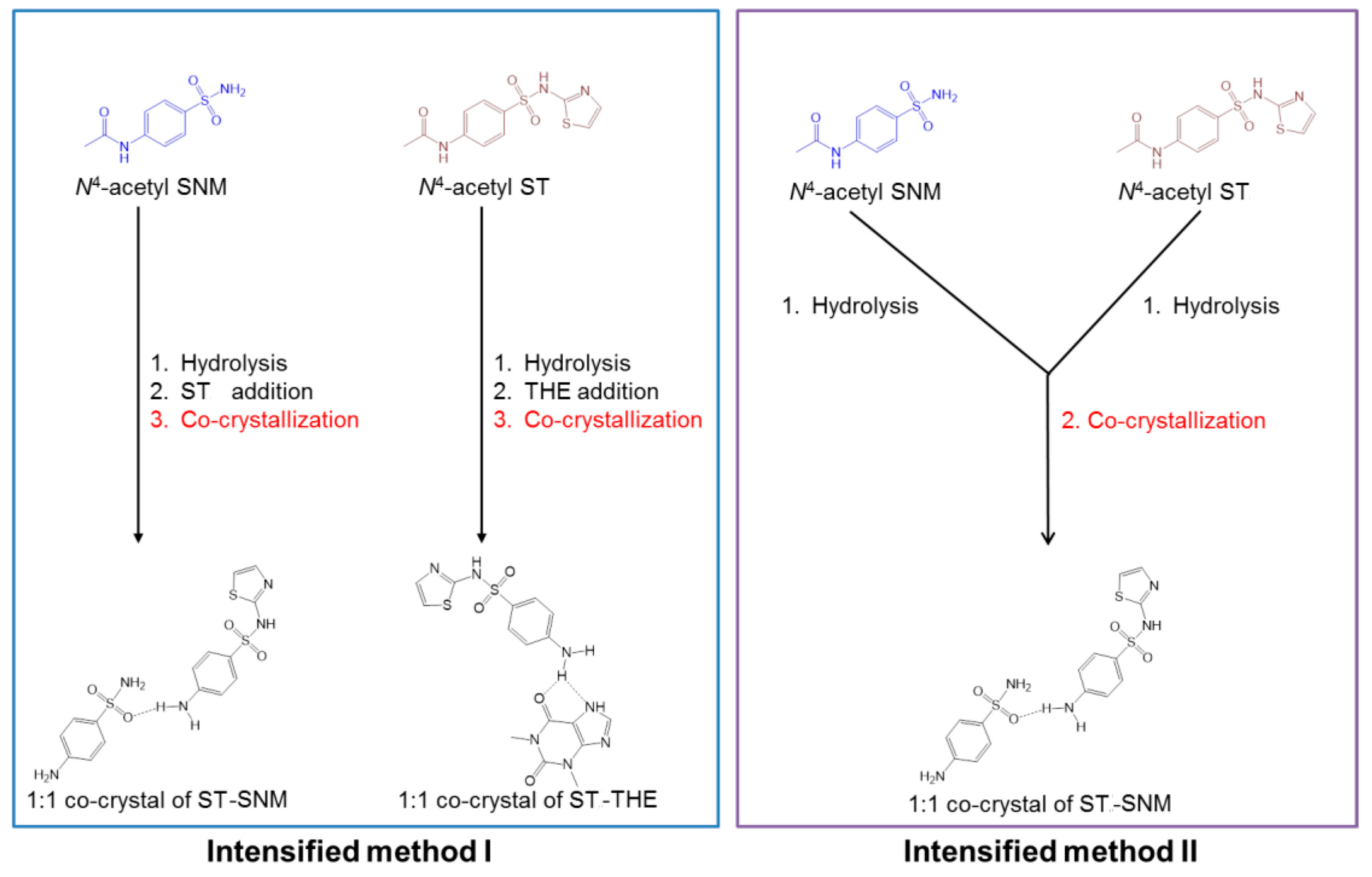
- Yeh, K.L.; Lee, T. Intensified Crystallization Processes for 1:1 Drug-Drug Co-crystals of Sulfathiazole-Theophylline, and Sulfathiazole-Sulfanilamide. Cryst. Growth Des. 2018. [Google Scholar] [CrossRef]
- Aitipamula, S.; Wong, A.B.H.; Chow, P.S.; Tan, R.B.H. Novel solid forms of oxaprozin: Cocrystals and an extended release drug-drug salt of salbutamol. RSC Adv. 2016, 6, 34110–34119. [Google Scholar] [CrossRef]
- Lee, H.L.; Lee, T. Direct co-crystal assembly from synthesis to co-crystallization. CrystEngComm 2015, 17, 9002–9006. [Google Scholar] [CrossRef]
- Évora, A.O.L.; Castro, R.A.E.; Maria, T.M.R.; Rosado, M.T.S.; Ramos Silva, M.; Matos Beja, A.; Canotilho, J.; Eusébio, M.E.S. Pyrazinamide-Diflunisal: A New Dual-Drug Co-Crystal. Cryst. Growth Des. 2011, 11, 4780–4788. [Google Scholar] [CrossRef]
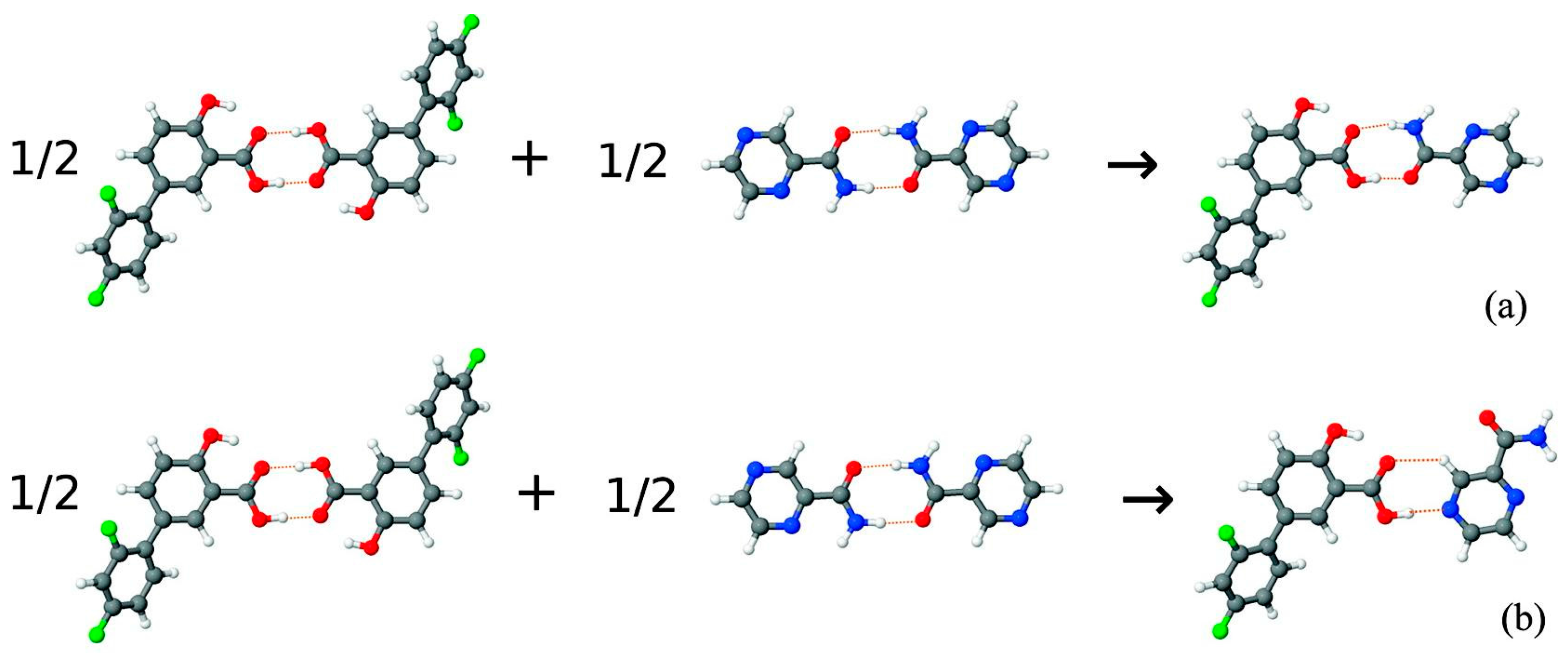
- Delori, A.; Galek, P.T.A.; Pidcock, E.; Patni, M.; Jones, W. Knowledge-based hydrogen bond prediction and the synthesis of salts and cocrystals of the anti-malarial drug pyrimethamine with various drug and GRAS molecules. CrystEngComm 2013, 15, 2916–2928. [Google Scholar] [CrossRef]
- Sanphui, P.; Babu, N.J.; Nangia, A. Temozolomide Cocrystals with Carboxamide Coformers. Cryst. Growth Des. 2013, 13, 2208–2219. [Google Scholar] [CrossRef]
- Kakkar, S.; Bhattacharya, B.; Reddy, C.M.; Ghosh, S. Tuning mechanical behaviour by controlling the structure of a series of theophylline co-crystals. CrystEngComm 2018. [Google Scholar] [CrossRef]
- Wang, J.-R.; Yu, Q.; Dai, W.; Mei, X. Drug-drug co-crystallization presents a new opportunity for the development of stable vitamins. Chem. Commun. 2016, 52, 3572–3575. [Google Scholar] [CrossRef] [PubMed]
- Vishweshwar, P.; McMahon, J.A.; Peterson, M.L.; Hickey, M.B.; Shattock, T.R.; Zaworotko, M.J. Crystal engineering of pharmaceutical co-crystals from polymorphic active pharmaceutical ingredients. Chem. Commun. 2005, 4601–4603. [Google Scholar] [CrossRef] [PubMed]
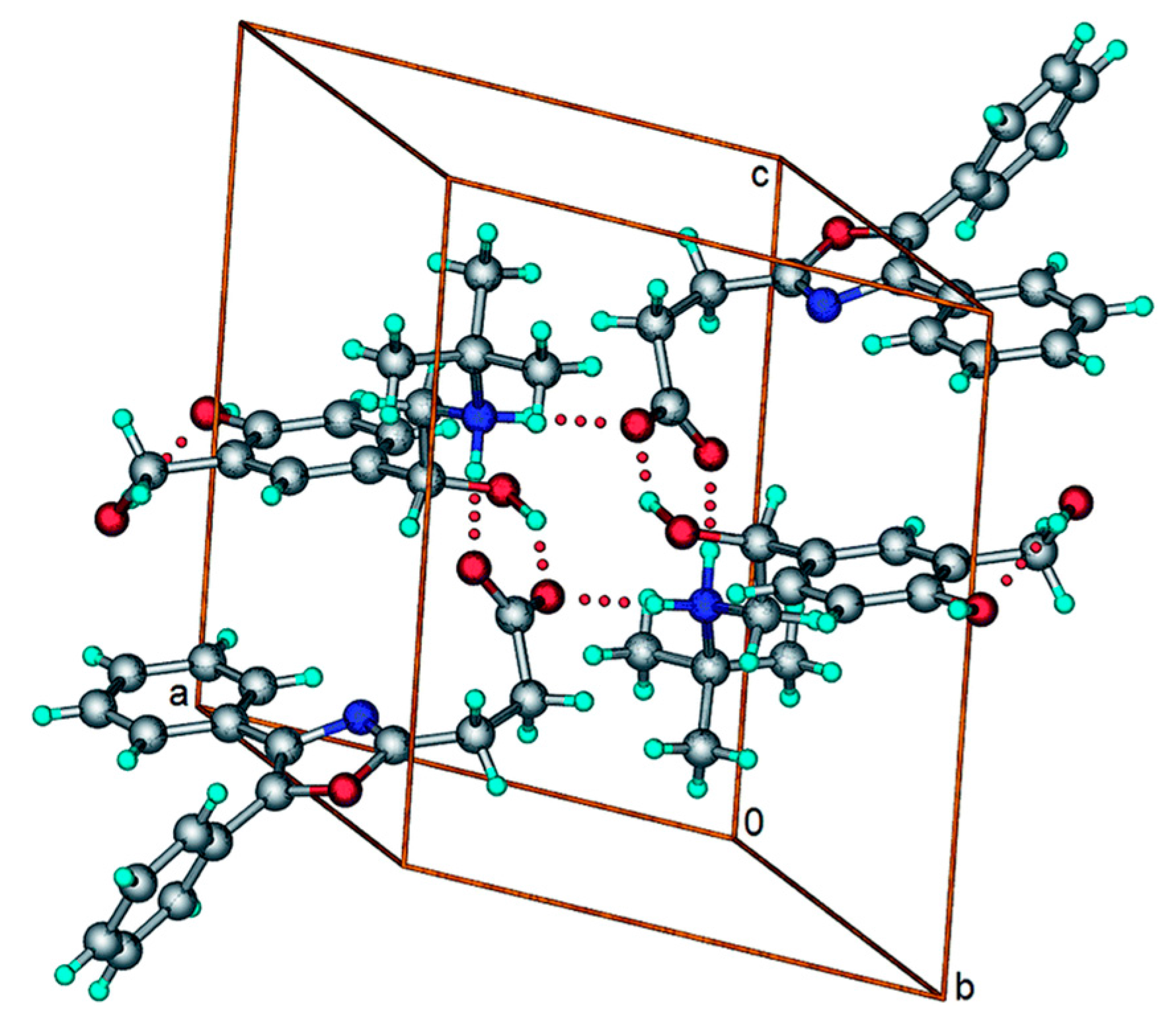
- Kaur, R.; Cavanagh, K.L.; Rodríguez-Hornedo, N.; Matzger, A.J. Multidrug Cocrystal of Anticonvulsants: Influence of Strong Intermolecular Interactions on Physiochemical Properties. Cryst. Growth Des. 2017, 17, 5012–5016. [Google Scholar] [CrossRef]
- Braga, D.; Grepioni, F.; Chelazzi, L.; Campana, M.; Confortini, D.; Viscomi, G.C. The structure-property relationship of four crystal forms of rifaximin. CrystEngComm 2012, 14, 6404–6411. [Google Scholar] [CrossRef]
- Braga, D.; Grepioni, F.; Maini, L.; Capucci, D.; Nanna, S.; Wouters, J.; Aerts, L.; Quere, L. Combining piracetam and lithium salts: Ionic co-crystals and co-drugs? Chem. Commun. 2012, 48, 8219–8221. [Google Scholar] [CrossRef] [PubMed]
- Grifasi, F.; Chierotti, M.R.; Gaglioti, K.; Gobetto, R.; Maini, L.; Braga, D.; Dichiarante, E.; Curzi, M. Using Salt Cocrystals to Improve the Solubility of Niclosamide. Cryst. Growth Des. 2015, 15, 1939–1948. [Google Scholar] [CrossRef]
- Braga, D.; Chelazzi, L.; Grepioni, F.; Dichiarante, E.; Chierotti, M.R.; Gobetto, R. Molecular Salts of Anesthetic Lidocaine with Dicarboxylic Acids: Solid-State Properties and a Combined Structural and Spectroscopic Study. Cryst. Growth Des. 2013, 13, 2564–2572. [Google Scholar] [CrossRef]
- Sun, C.C.; Hou, H. Improving Mechanical Properties of Caffeine and Methyl Gallate Crystals by Cocrystallization. Cryst. Growth Des. 2008, 8, 1575–1579. [Google Scholar] [CrossRef]
- Chow, S.F.; Chen, M.; Shi, L.; Chow, A.H.L.; Sun, C.C. Simultaneously Improving the Mechanical Properties, Dissolution Performance, and Hygroscopicity of Ibuprofen and Flurbiprofen by Cocrystallization with Nicotinamide. Pharm. Res. 2012, 29, 1854–1865. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.C. Cocrystallization for successful drug delivery. Expert Opin. Drug Deliv. 2013, 10, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Chattoraj, S.; Shi, L.; Sun, C.C. Understanding the relationship between crystal structure, plasticity and compaction behaviour of theophylline, methyl gallate, and their 1:1 co-crystal. CrystEngComm 2010, 12, 2466–2472. [Google Scholar] [CrossRef]
- Jayasankar, A.; Reddy, L.S.; Bethune, S.J.; Rodríguez-Hornedo, N. Role of Cocrystal and Solution Chemistry on the Formation and Stability of Cocrystals with Different Stoichiometry. Cryst. Growth Des. 2009, 9, 889–897. [Google Scholar] [CrossRef]
- Rodríguez-Spong, B.; Price, C.P.; Jayasankar, A.; Matzger, A.J.; Rodrı́guez-Hornedo, N.R. General principles of pharmaceutical solid polymorphism: A supramolecular perspective. Adv. Drug Deliv. Rev. 2004, 56, 241–274. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Rutledge, G.C.; Myerson, A.S.; Trout, B.L. Production and Characterization of Carbamazepine Nanocrystals by Electrospraying for Continuous Pharmaceutical Manufacturing. J. Pharm. Sci. 2012, 101, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Matzger, A.J. Influence of Coformer Stoichiometric Ratio on Pharmaceutical Cocrystal Dissolution: Three Cocrystals of Carbamazepine/4-Aminobenzoic Acid. Mol. Pharm. 2016, 13, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Porter Iii, W.W.; Elie, S.C.; Matzger, A.J. Polymorphism in Carbamazepine Cocrystals. Cryst. Growth Des. 2008, 8, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Bučar, D.-K.; Elliott, J.A.; Eddleston, M.D.; Cockcroft, J.K.; Jones, W. Sonocrystallization Yields Monoclinic Paracetamol with Significantly Improved Compaction Behavior. Angew. Chem. 2015, 127, 251–255. [Google Scholar] [CrossRef]
- Bucar, D.-K.; Day, G.M.; Halasz, I.; Zhang, G.G.Z.; Sander, J.R.G.; Reid, D.G.; MacGillivray, L.R.; Duer, M.J.; Jones, W. The curious case of (caffeine)[middle dot](benzoic acid): How heteronuclear seeding allowed the formation of an elusive cocrystal. Chem. Sci. 2013, 4, 4417–4425. [Google Scholar] [CrossRef]
- Bucar, D.-K.; Henry, R.F.; Lou, X.; Borchardt, T.B.; Zhang, G.G.Z. A “hidden” co-crystal of caffeine and adipic acid. Chem. Commun. 2007, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Sander, J.R.G.; Bučar, D.-K.; Henry, R.F.; Baltrusaitis, J.; Zhang, G.G.Z.; Macgillivray, L.R. A Red Zwitterionic Co-Crystal of Acetaminophen and 2,4-Pyridinedicarboxylic Acid. J. Pharm. Sci. 2010, 99, 3676–3683. [Google Scholar] [CrossRef] [PubMed]
- Bučar, D.-K.; Henry, R.F.; Duerst, R.W.; Lou, X.; MacGillivray, L.R.; Zhang, G.G.Z. A 1:1 Cocrystal of Caffeine and 2-Hydroxy-1-Naphthoic Acid Obtained via a Slurry Screening Method. J. Chem. Crystallogr. 2010, 40, 933–939. [Google Scholar] [CrossRef]
- Berry, D.J.; Seaton, C.C.; Clegg, W.; Harrington, R.W.; Coles, S.J.; Horton, P.N.; Hursthouse, M.B.; Storey, R.; Jones, W.; Friščić, T.; et al. Applying Hot-Stage Microscopy to Co-Crystal Screening: A Study of Nicotinamide with Seven Active Pharmaceutical Ingredients. Cryst. Growth Des. 2008, 8, 1697–1712. [Google Scholar] [CrossRef]
- Velaga, S.P.; Basavoju, S.; Boström, D. Norfloxacin saccharinate–saccharin dihydrate cocrystal—A new pharmaceutical cocrystal with an organic counter ion. J. Mol. Struct. 2008, 889, 150–153. [Google Scholar] [CrossRef]
- Hong, C.; Xie, Y.; Yao, Y.; Li, G.; Yuan, X.; Shen, H. A Novel Strategy for Pharmaceutical Cocrystal Generation without Knowledge of Stoichiometric Ratio: Myricetin Cocrystals and a Ternary Phase Diagram. Pharm. Res. 2015, 32, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Basavoju, S.; Boström, D.; Velaga, S.P. Indomethacin–Saccharin Cocrystal: Design, Synthesis and Preliminary Pharmaceutical Characterization. Pharm. Res. 2008, 25, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Weyna, D.R.; Shattock, T.; Vishweshwar, P.; Zaworotko, M.J. Synthesis and Structural Characterization of Cocrystals and Pharmaceutical Cocrystals: Mechanochemistry vs Slow Evaporation from Solution. Cryst. Growth Des. 2009, 9, 1106–1123. [Google Scholar] [CrossRef]
- Basavoju, S.; Boström, D.; Velaga, S.P. Pharmaceutical Cocrystal and Salts of Norfloxacin. Cryst. Growth Des. 2006, 6, 2699–2708. [Google Scholar] [CrossRef]
- Friščić, T.; Jones, W. Benefits of cocrystallisation in pharmaceutical materials science: An update. J. Pharm. Pharmacol. 2010, 62, 1547–1559. [Google Scholar] [CrossRef] [PubMed]
- Kalra, E.K. Nutraceutical-definition and introduction. AAPS PharmSci 2003, 5, 27–28. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.S.; Maguire, A.R.; Lawrence, S.E. Cocrystallization of Nutraceuticals. Cryst. Growth Des. 2015, 15, 984–1009. [Google Scholar] [CrossRef]
- Caira, M.R. Sulfa Drugs as Model Cocrystal Formers. Mol. Pharm. 2007, 4, 310–316. [Google Scholar] [CrossRef] [PubMed]
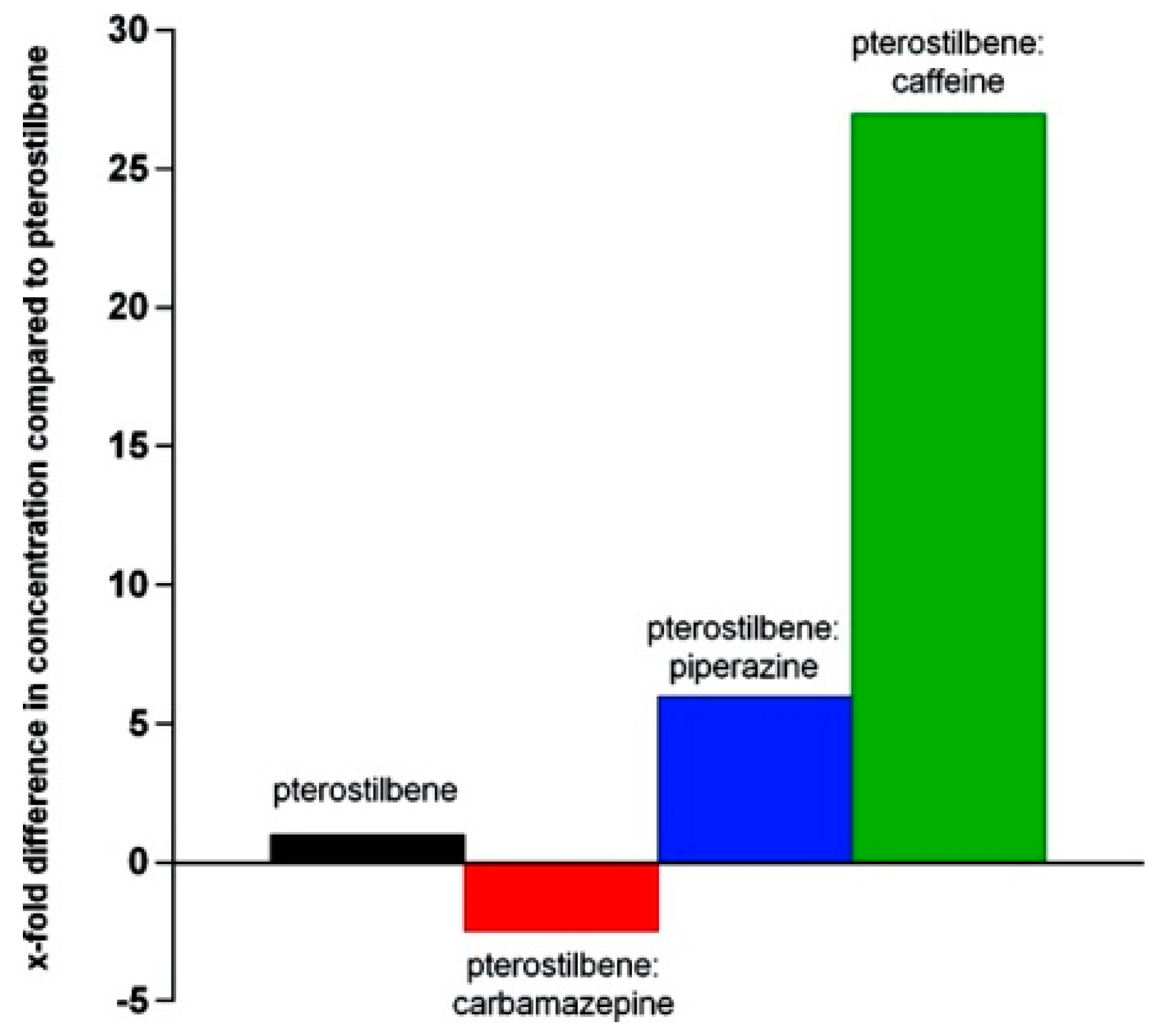
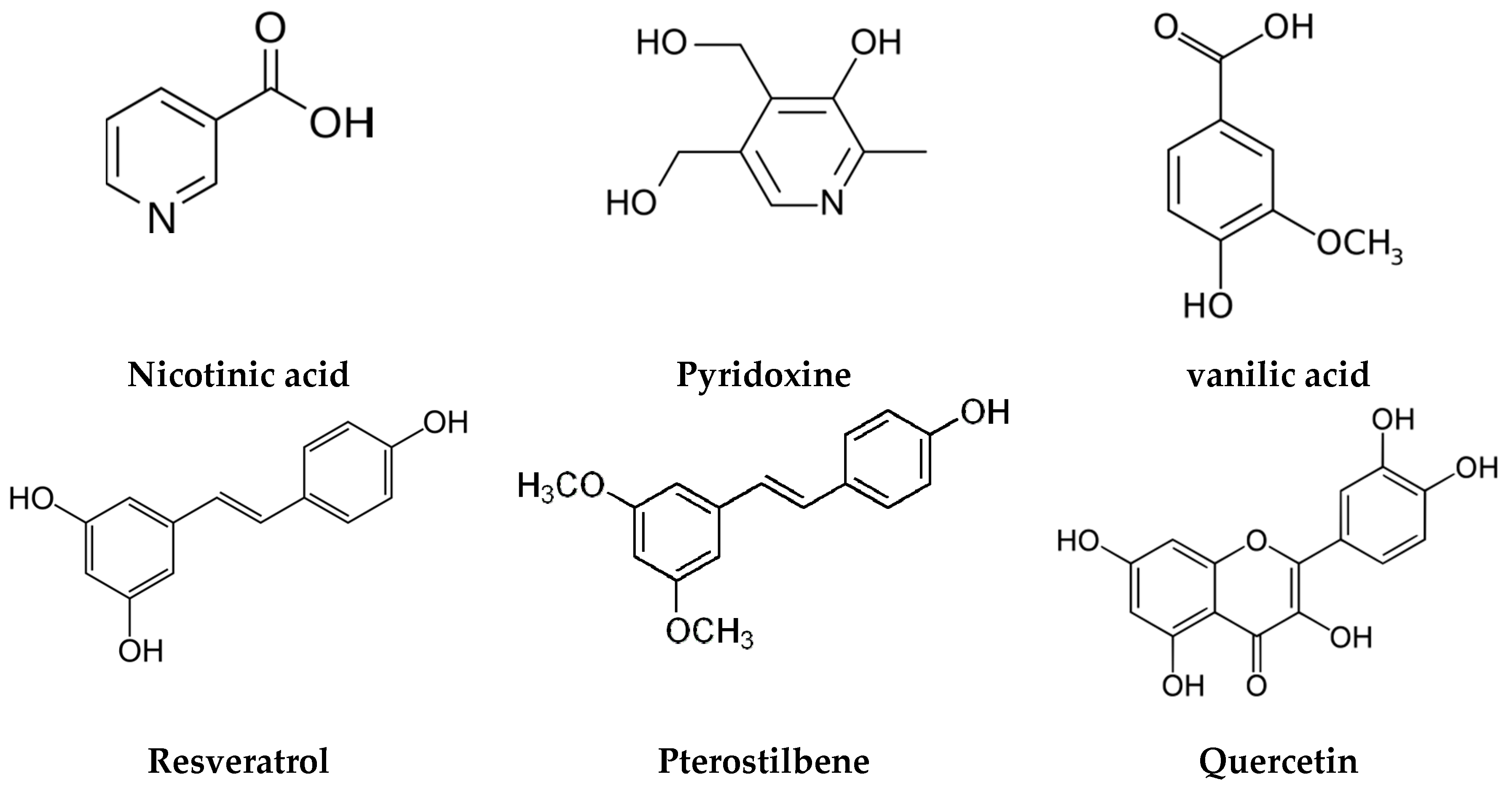
- Bethune, S.J.; Schultheiss, N.; Henck, J.-O. Improving the Poor Aqueous Solubility of Nutraceutical Compound Pterostilbene through Cocrystal Formation. Cryst. Growth Des. 2011, 11, 2817–2823. [Google Scholar] [CrossRef]
- Schultheiss, N.; Bethune, S.; Henck, J.-O. Nutraceutical cocrystals: Utilizing pterostilbene as a cocrystal former. CrystEngComm 2010, 12, 2436–2442. [Google Scholar] [CrossRef]
- Schultheiss, N.; Roe, M.; Boerrigter, S.X.M. Cocrystals of nutraceutical p-coumaric acid with caffeine and theophylline: Polymorphism and solid-state stability explored in detail using their crystal graphs. CrystEngComm 2011, 13, 611–619. [Google Scholar] [CrossRef]
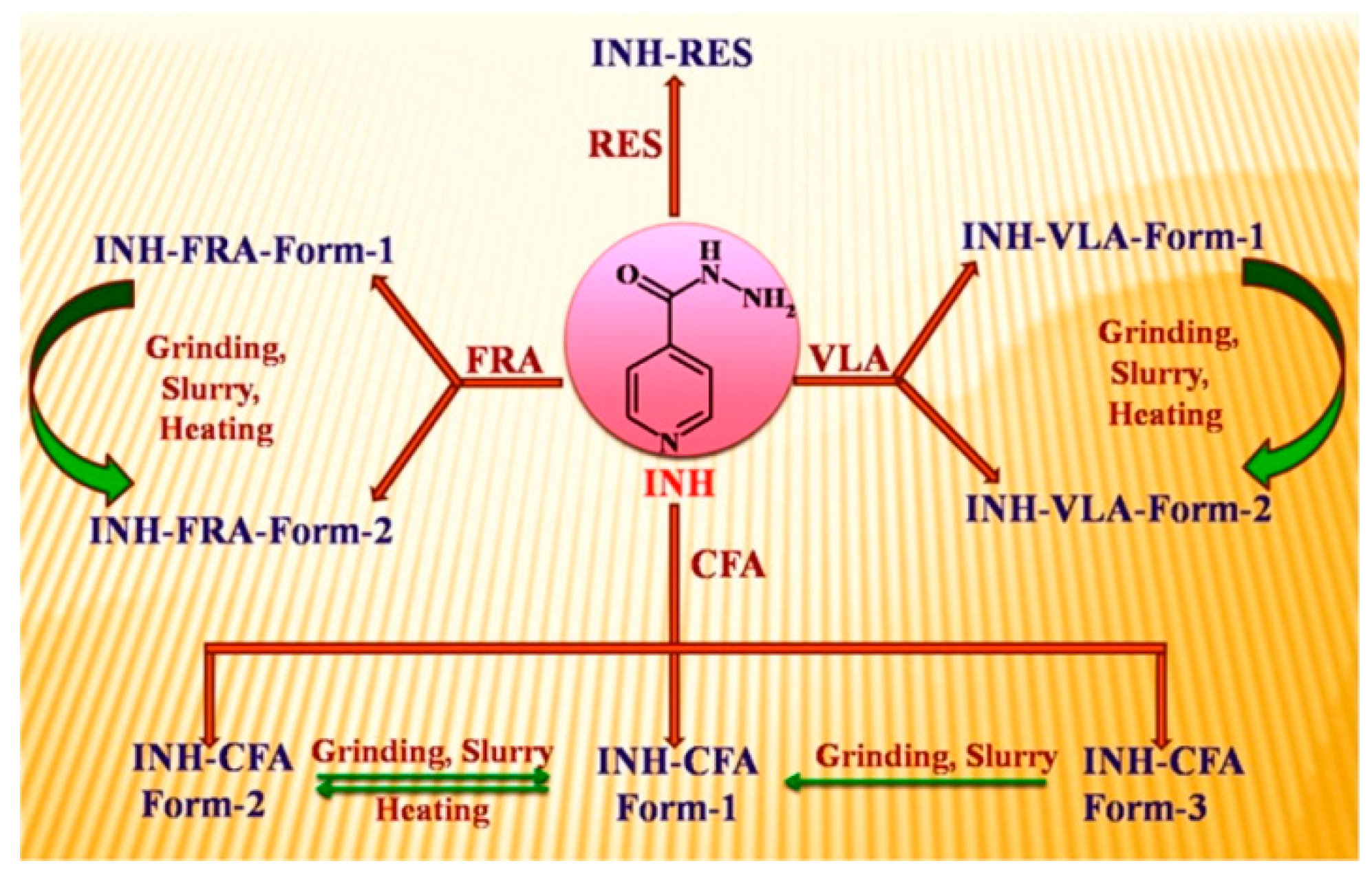
- Swapna, B.; Maddileti, D.; Nangia, A. Cocrystals of the Tuberculosis Drug Isoniazid: Polymorphism, Isostructurality, and Stability. Cryst. Growth Des. 2014, 14, 5991–6005. [Google Scholar] [CrossRef]
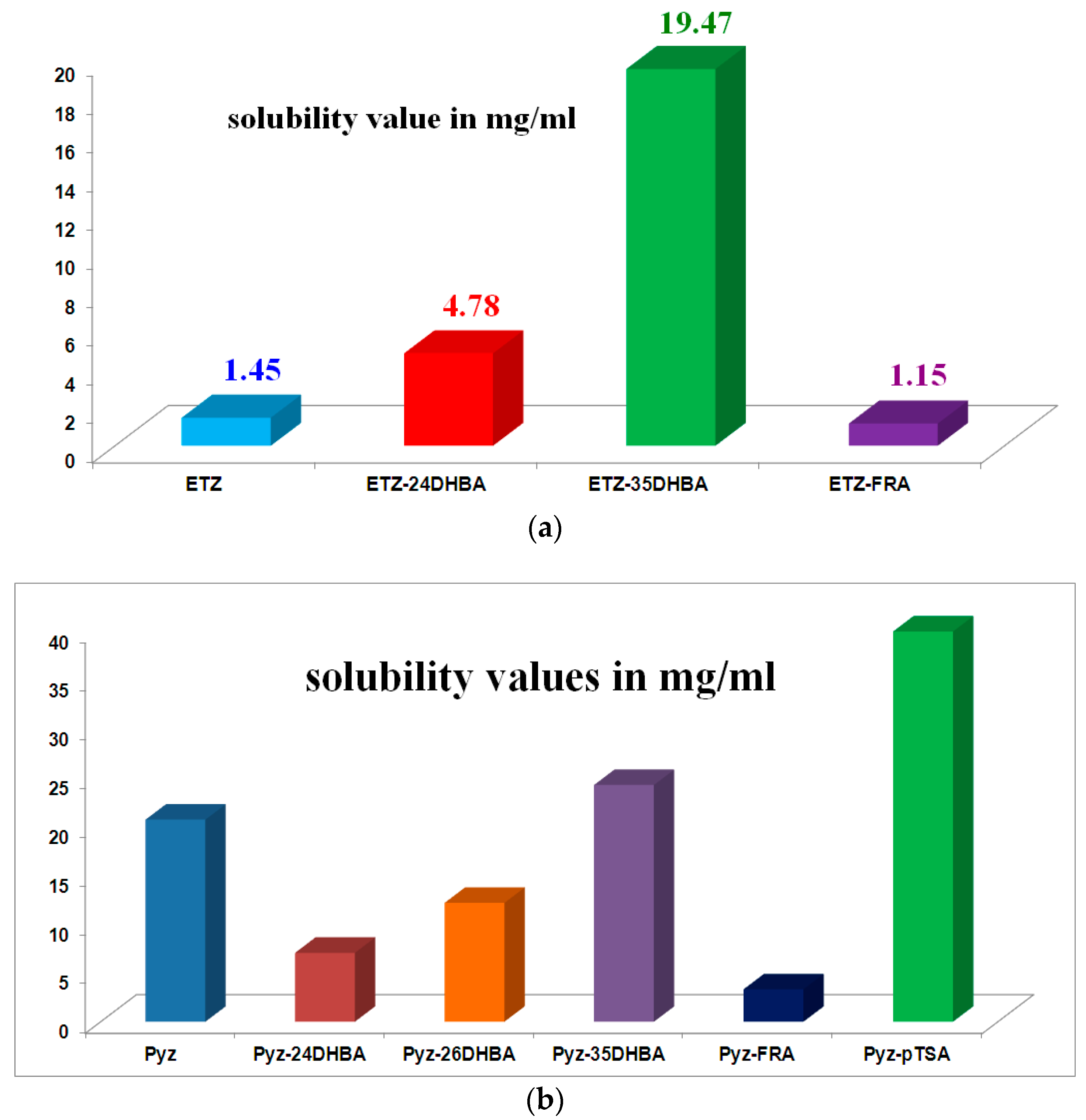
- Sarmah, K.K.; Boro, K.; Arhangelskis, M.; Thakuria, R. Crystal structure landscape of ethenzamide: A physicochemical property study. CrystEngComm 2017, 19, 826–833. [Google Scholar] [CrossRef]
- Sarmah, K.K.; Rajbongshi, T.; Bhowmick, S.; Thakuria, R. First-line antituberculosis drug, pyrazinamide, its pharmaceutically relevant cocrystals and a salt. Acta Crystallogr. Sect. B 2017, 73, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
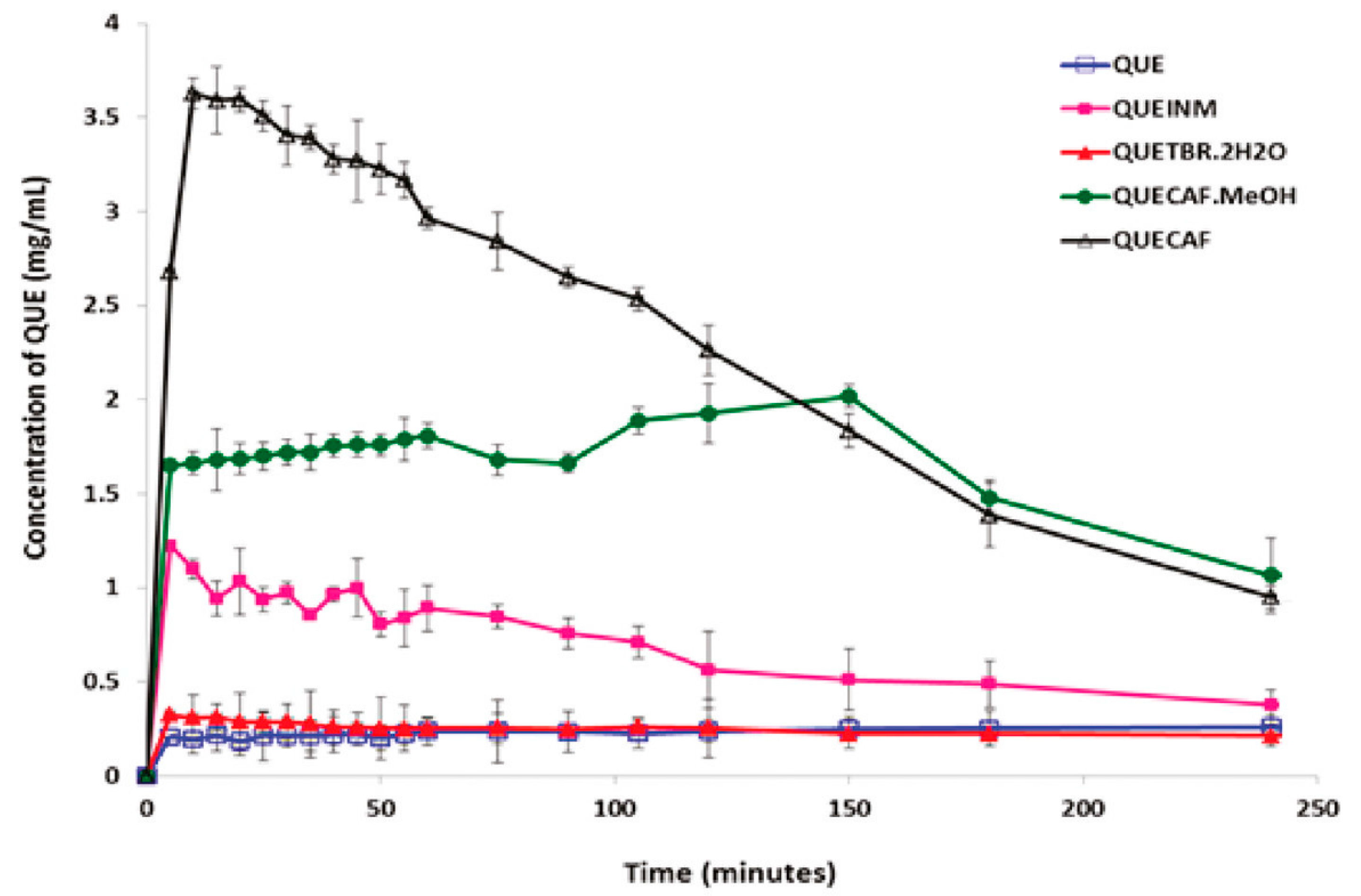
- Smith, A.J.; Kavuru, P.; Wojtas, L.; Zaworotko, M.J.; Shytle, R.D. Cocrystals of Quercetin with Improved Solubility and Oral Bioavailability. Mol. Pharm. 2011, 8, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Simon, F. The trouble with making combination drugs. Nat. Rev. Drug Discov. 2006, 5, 881–882. [Google Scholar] [CrossRef] [PubMed]
- Thipparaboina, R.; Kumar, D.; Chavan, R.B.; Shastri, N.R. Multidrug co-crystals: Towards the development of effective therapeutic hybrids. Drug Discov. Today 2016, 21, 481–490. [Google Scholar] [CrossRef] [PubMed]
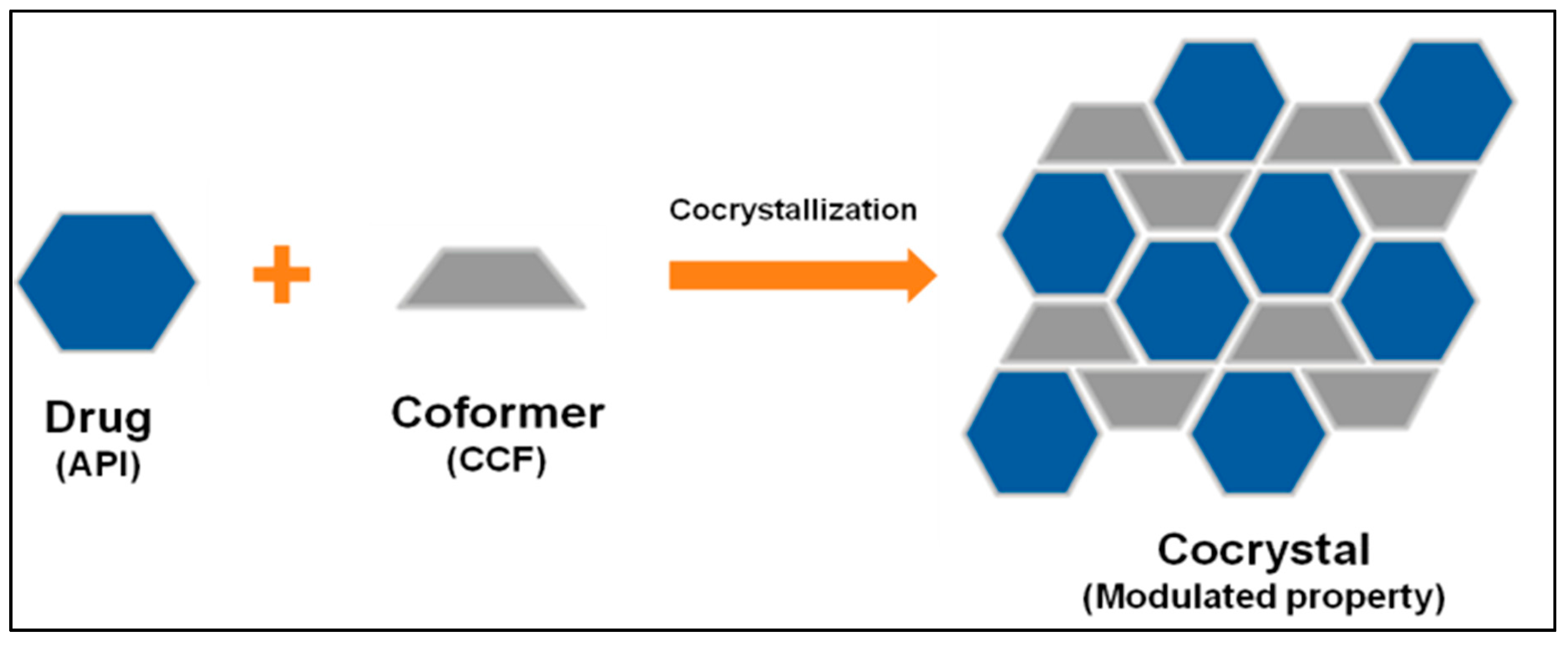
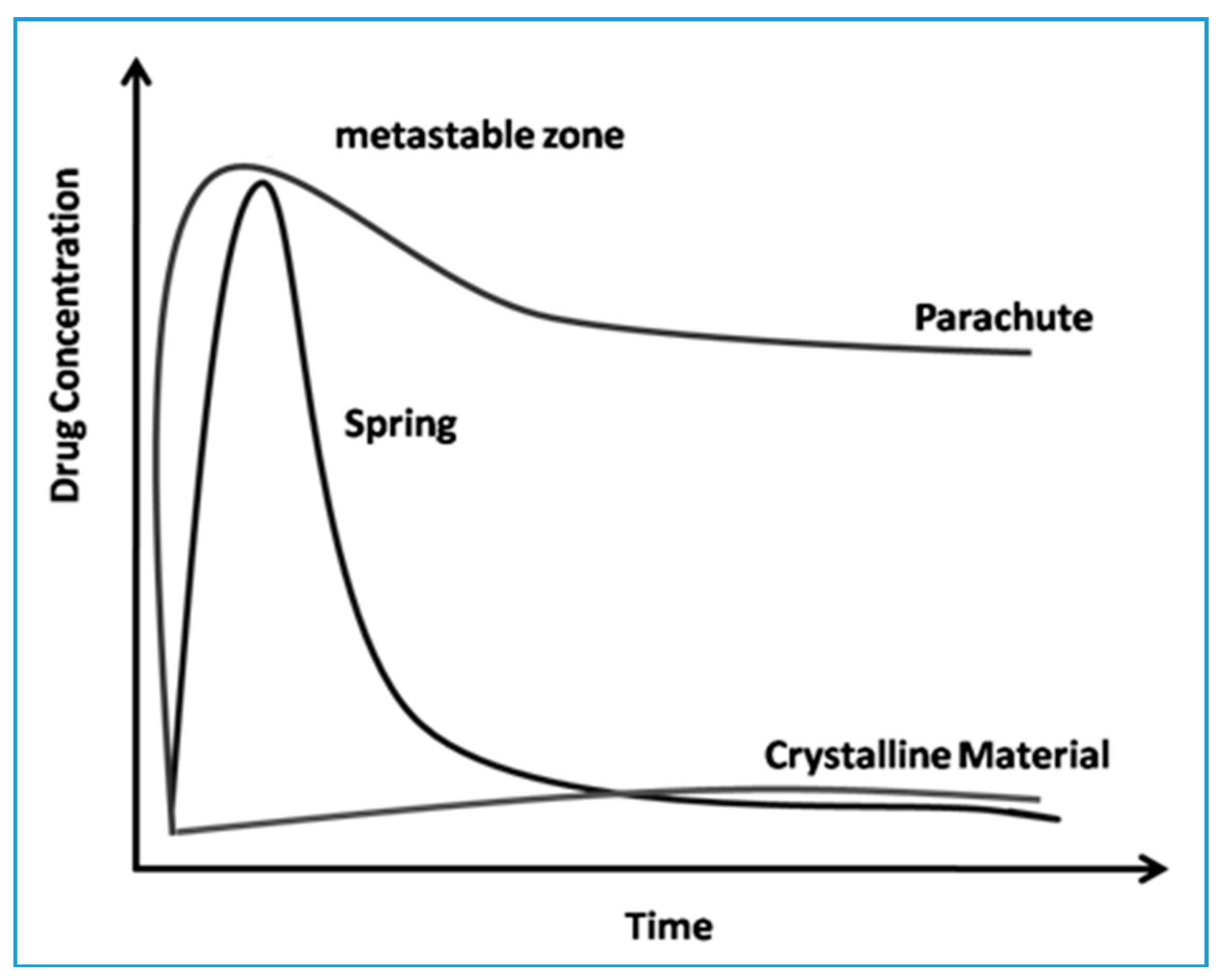
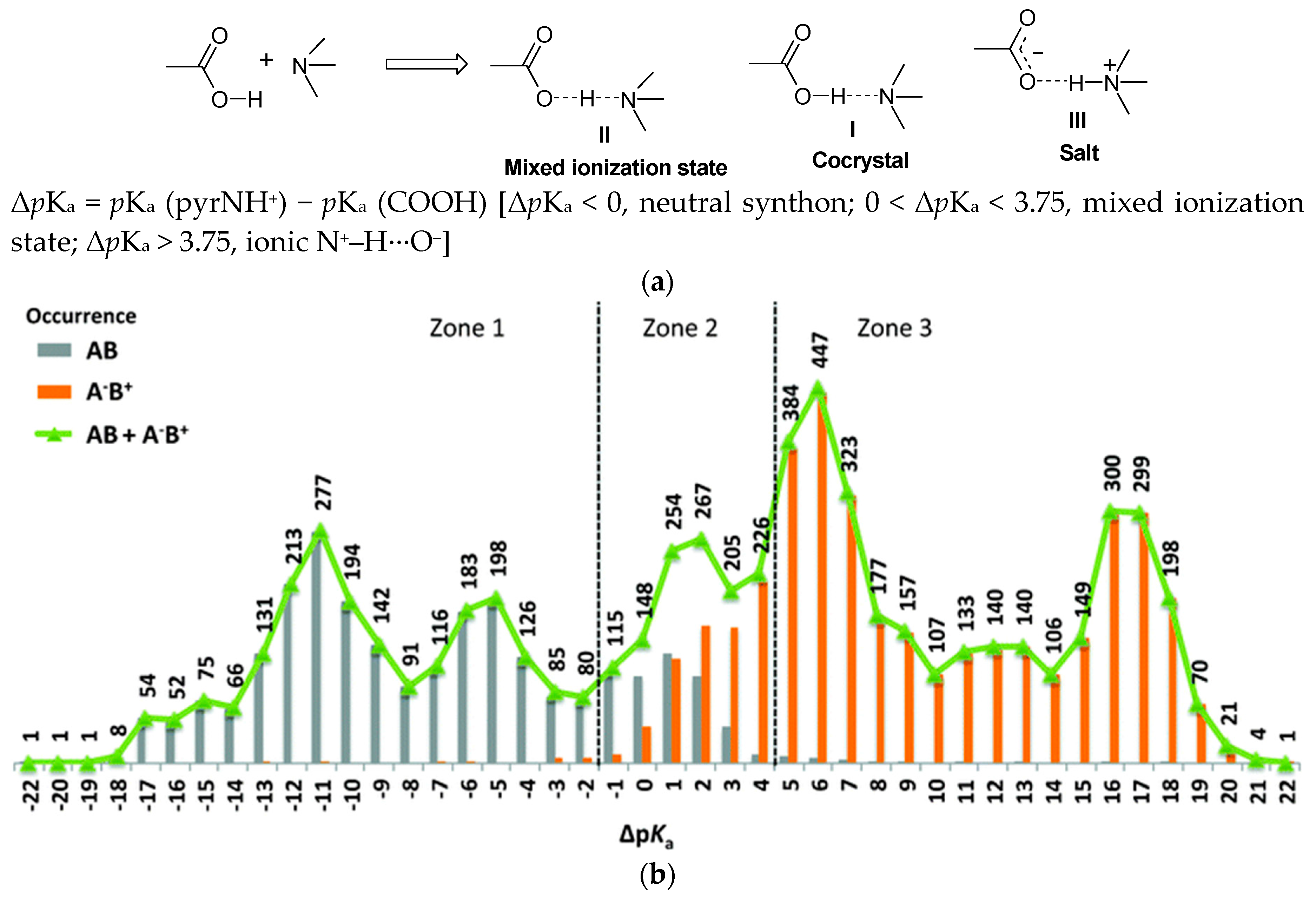
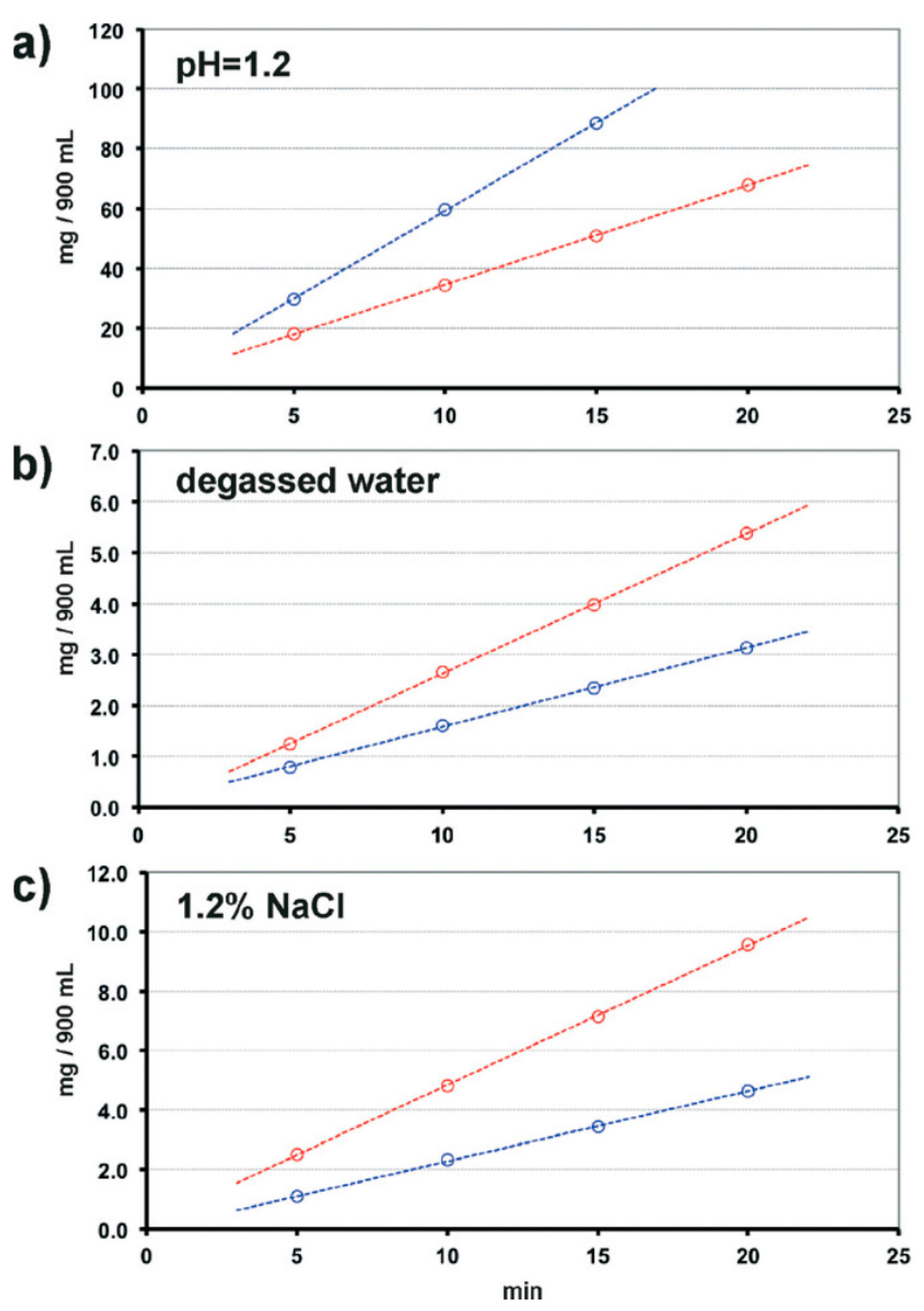

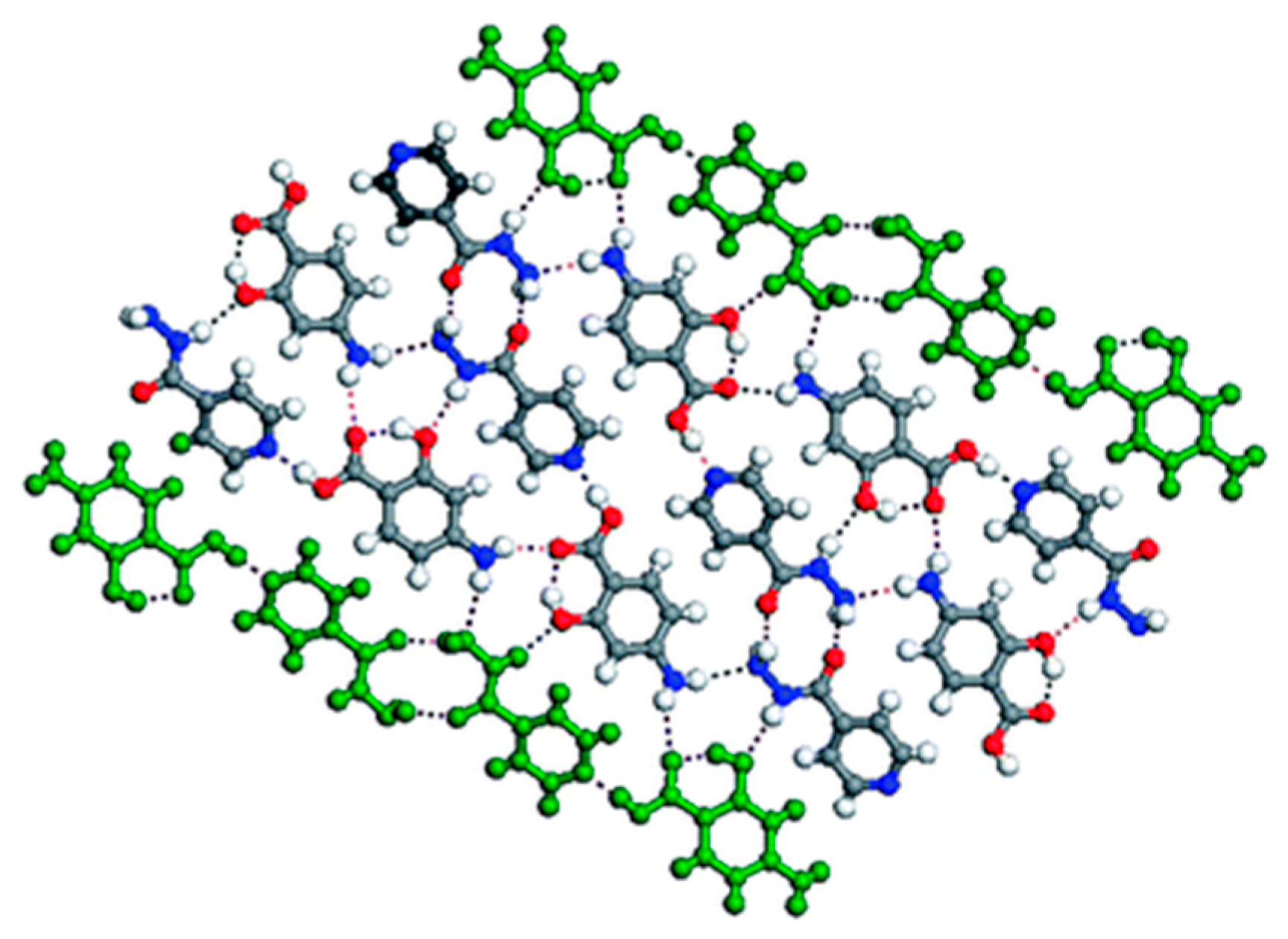
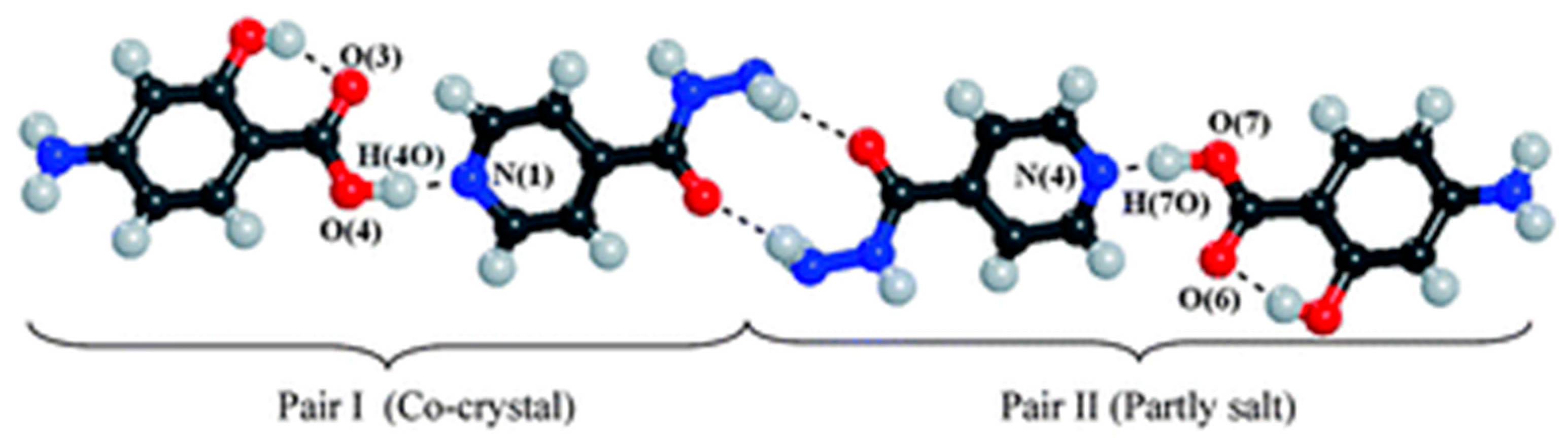
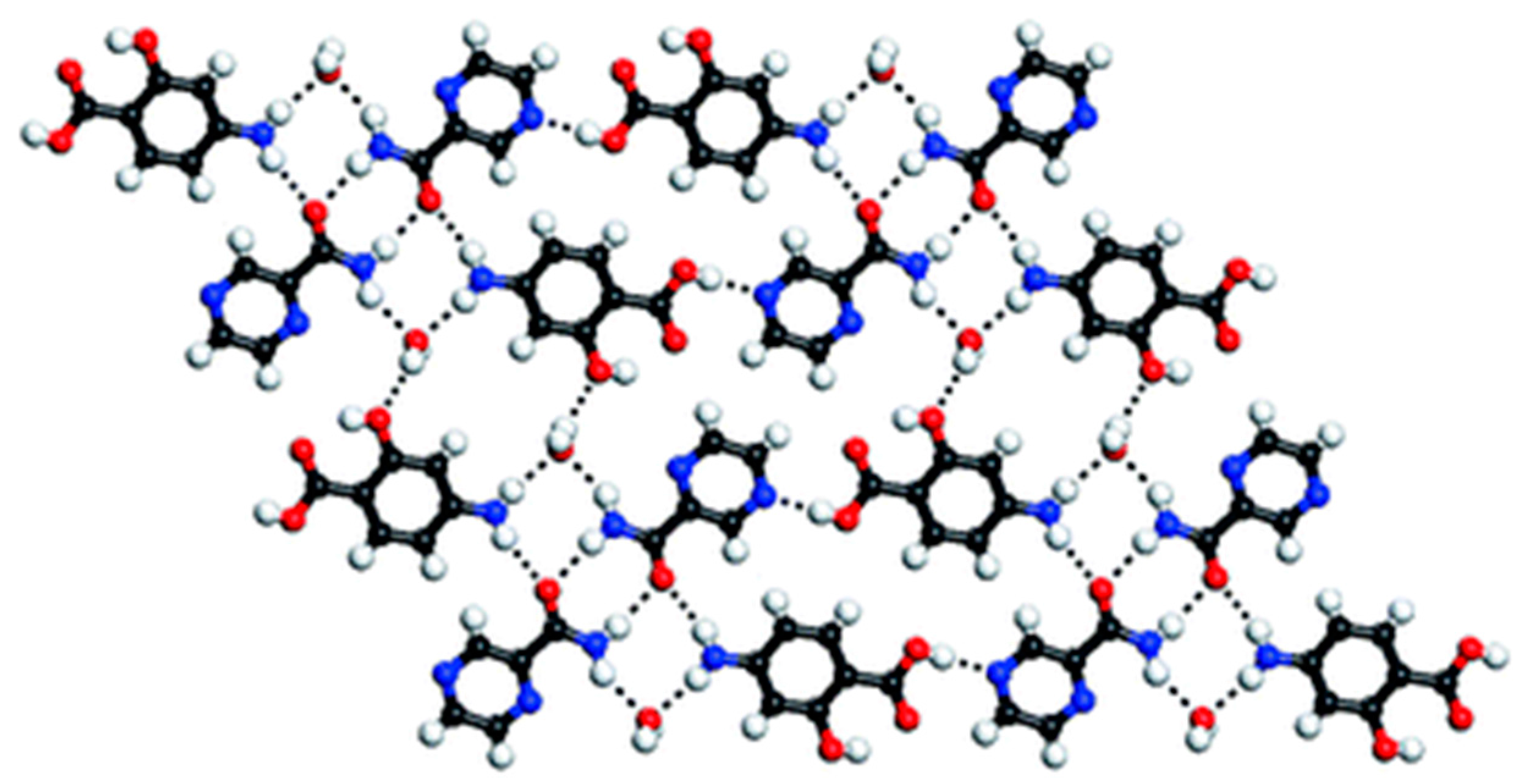
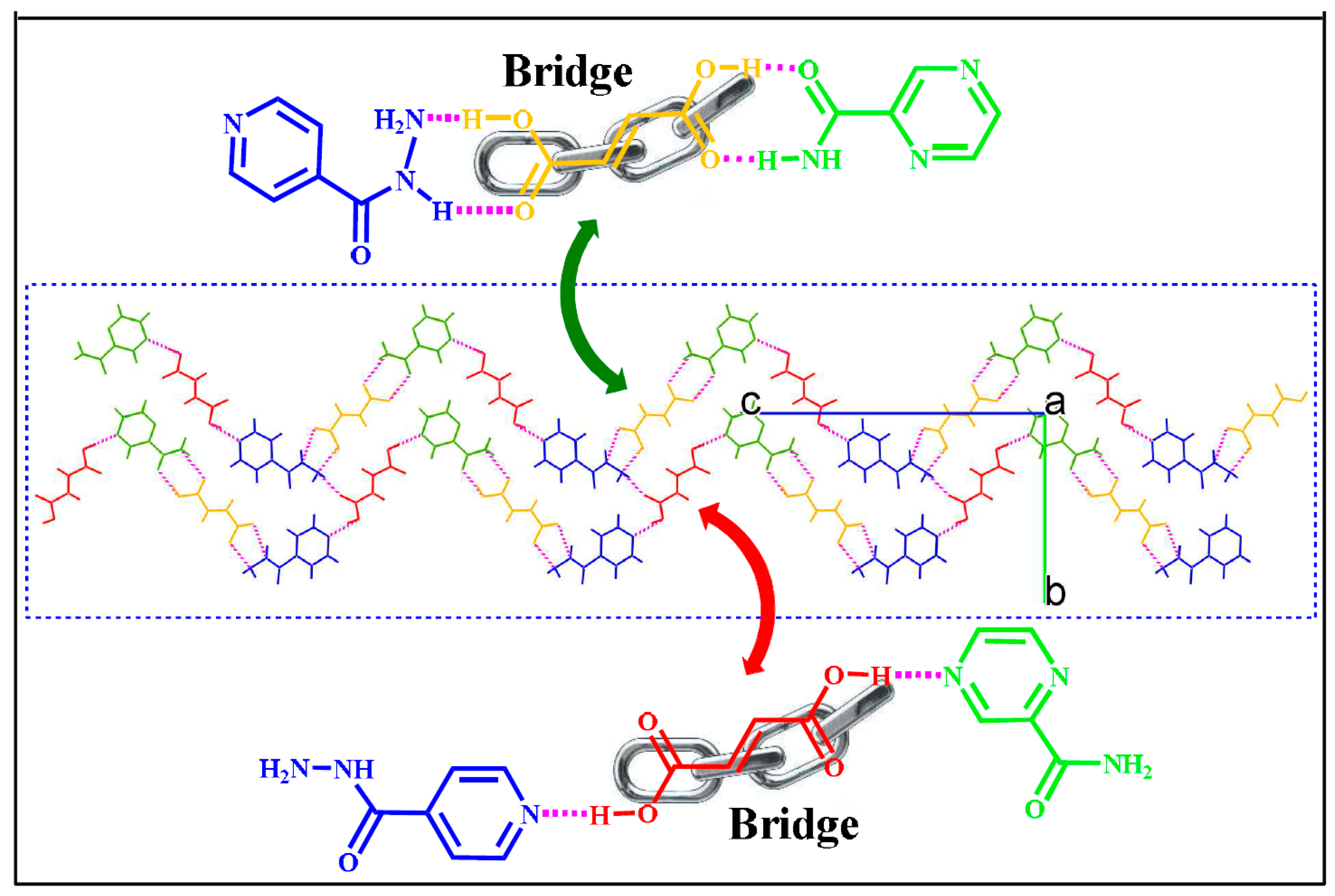

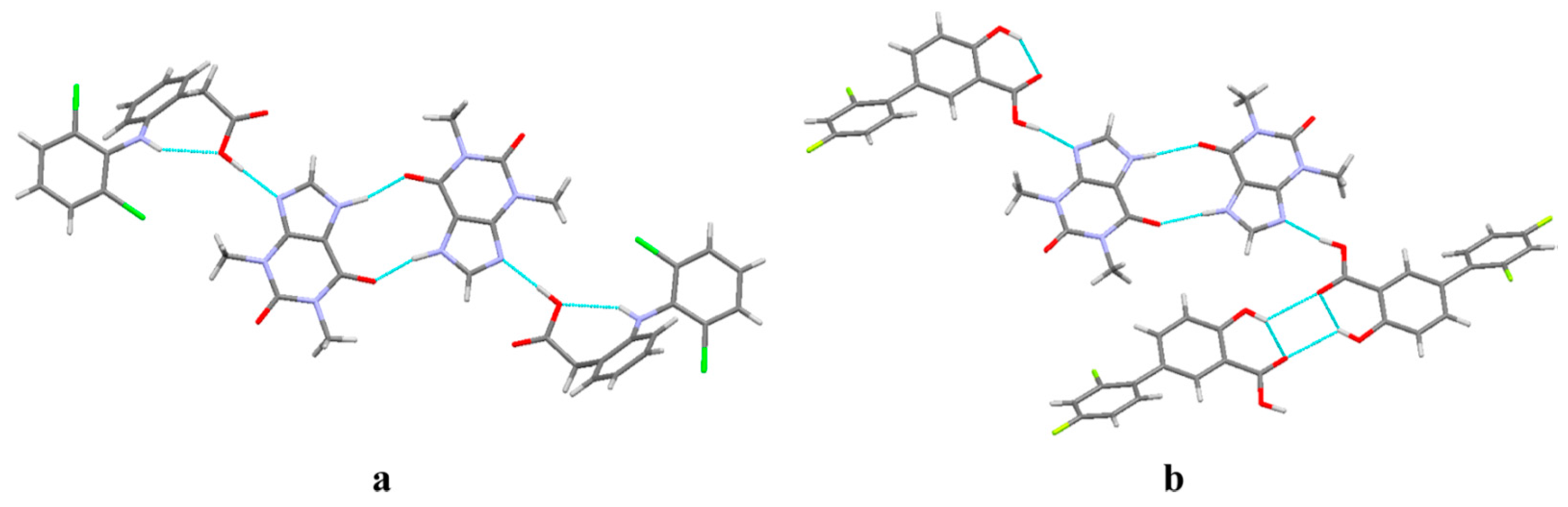
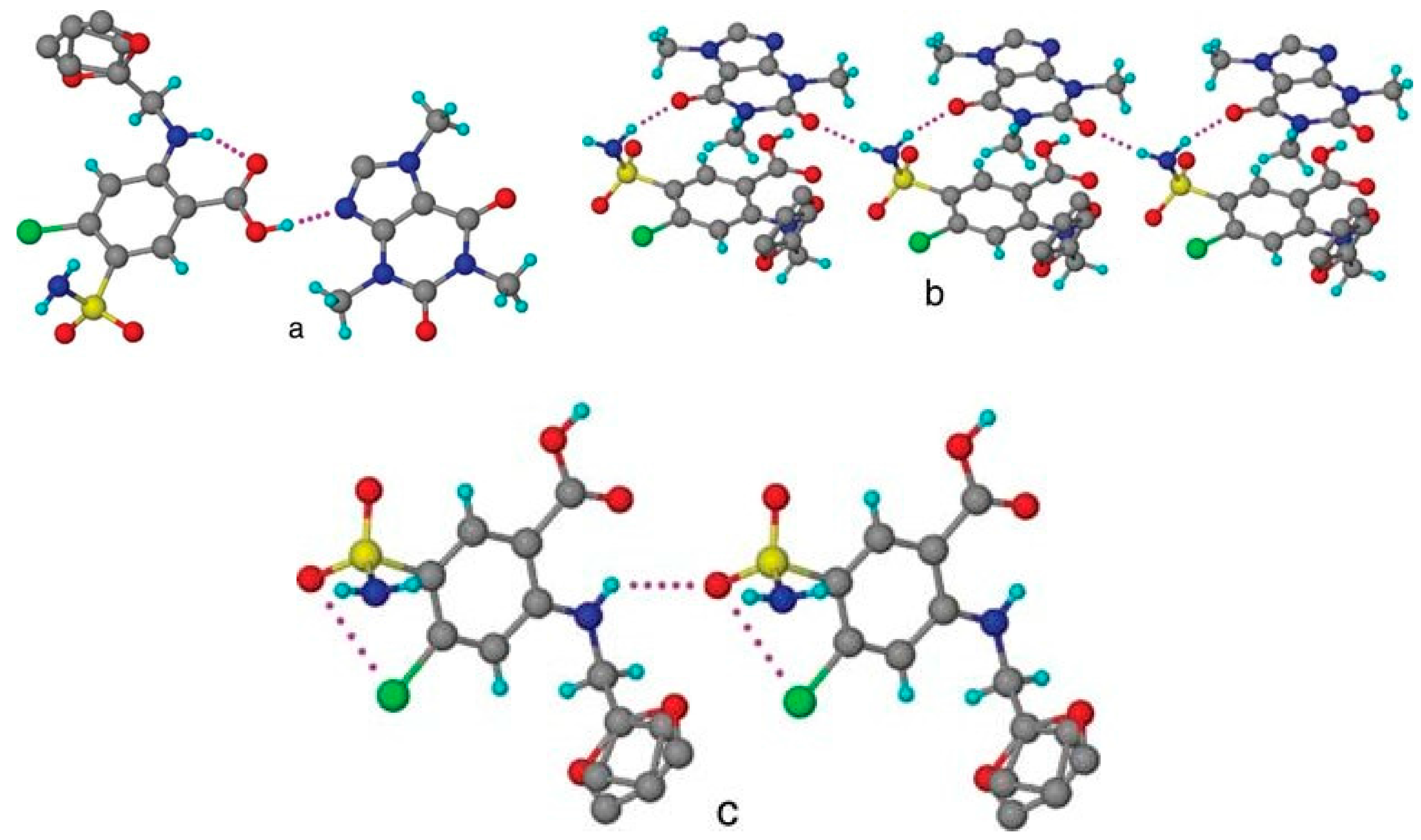

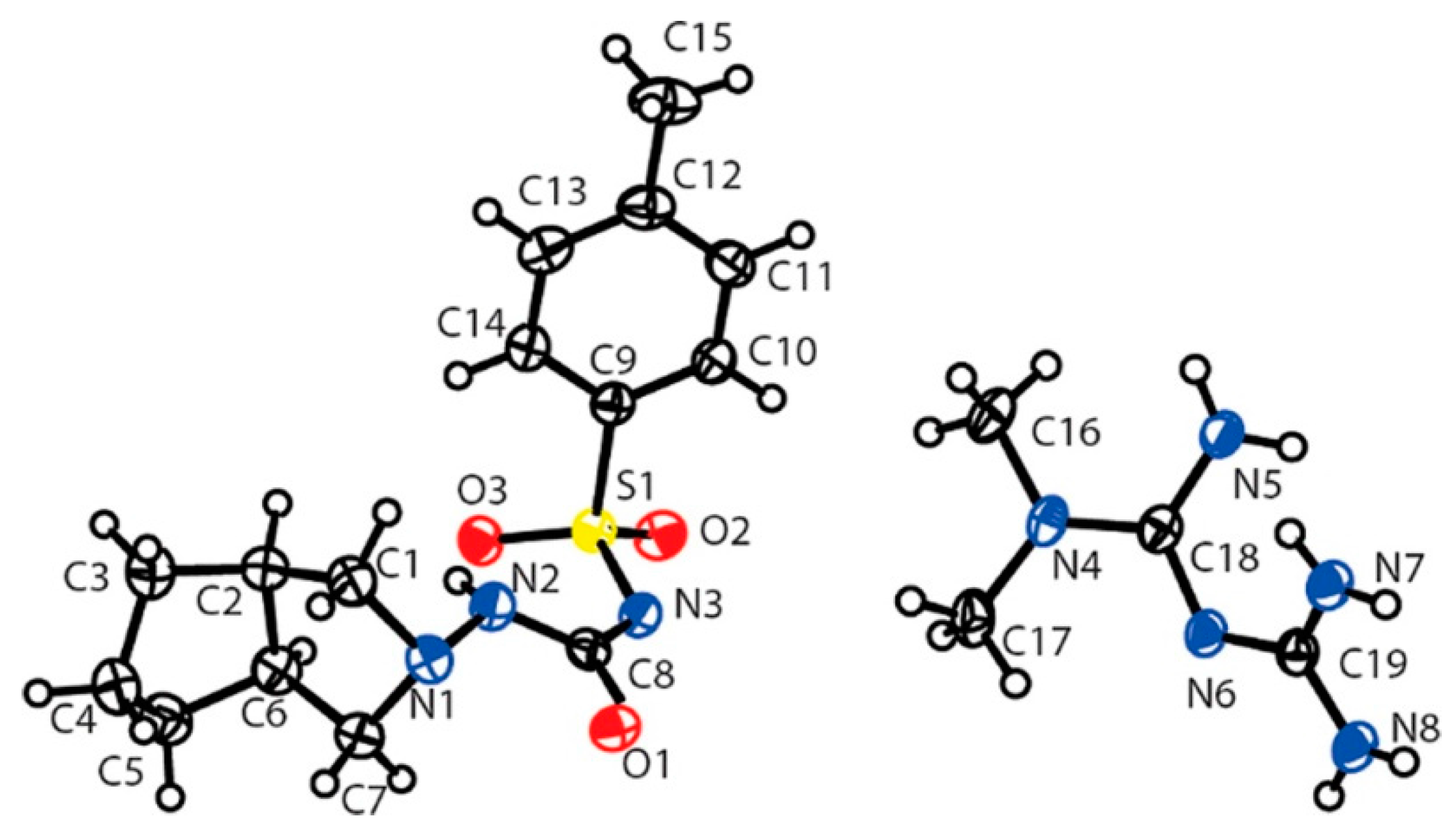
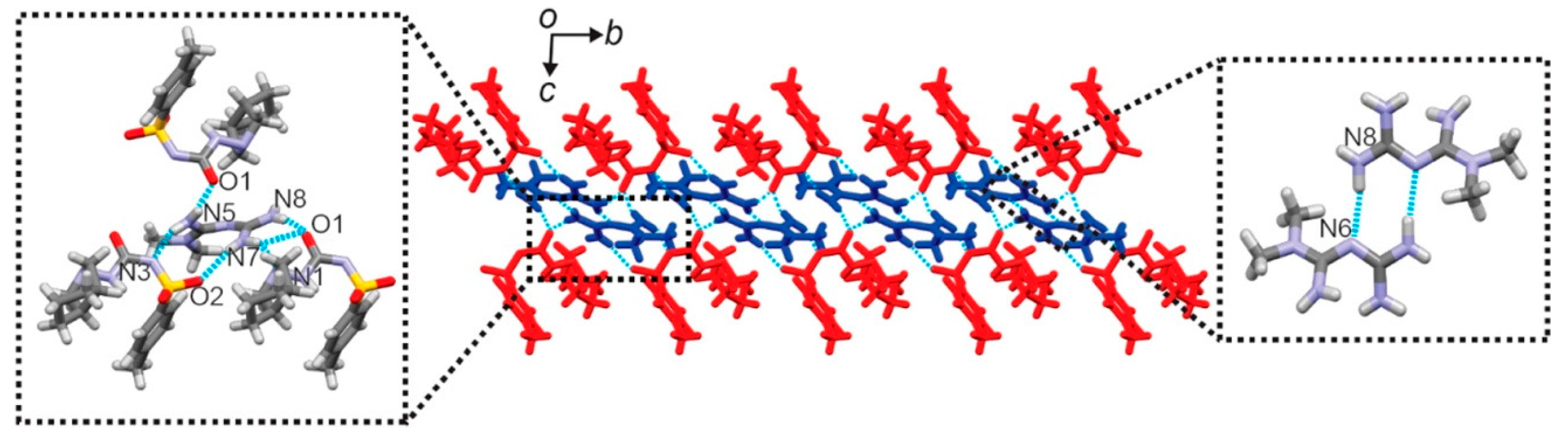


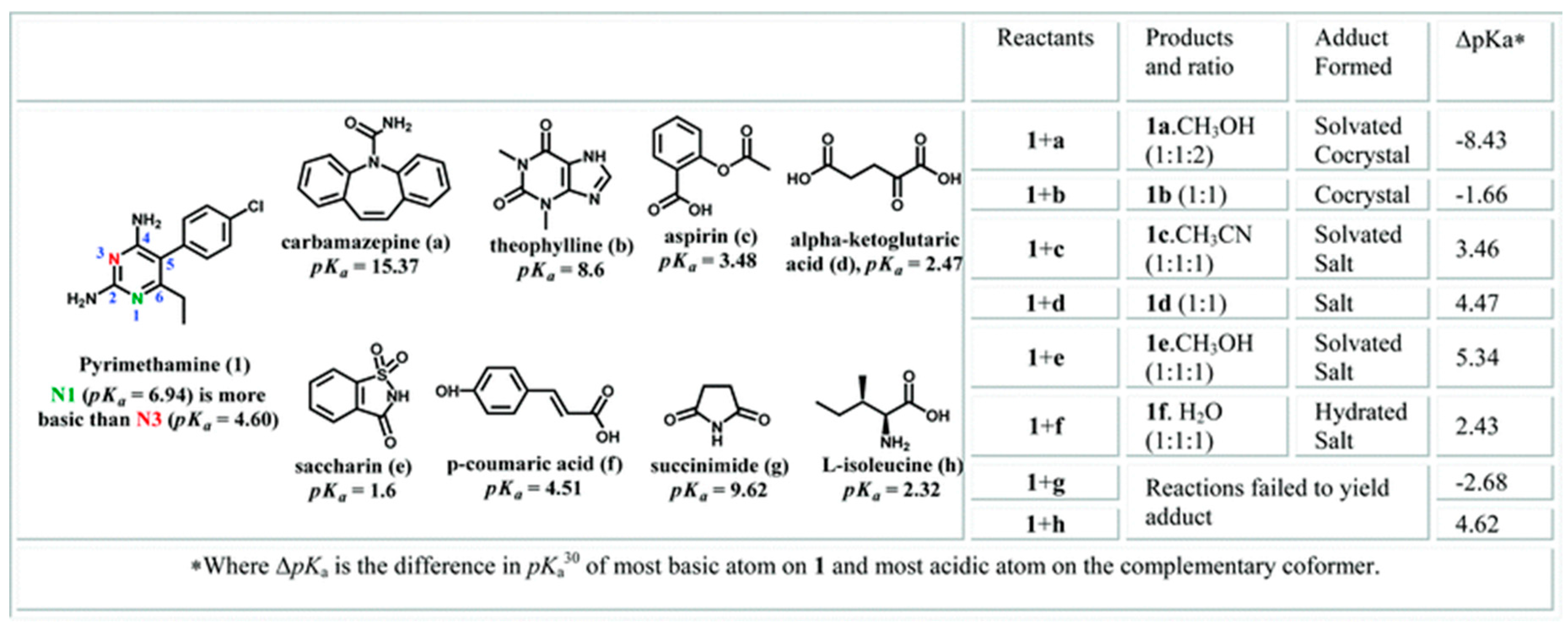
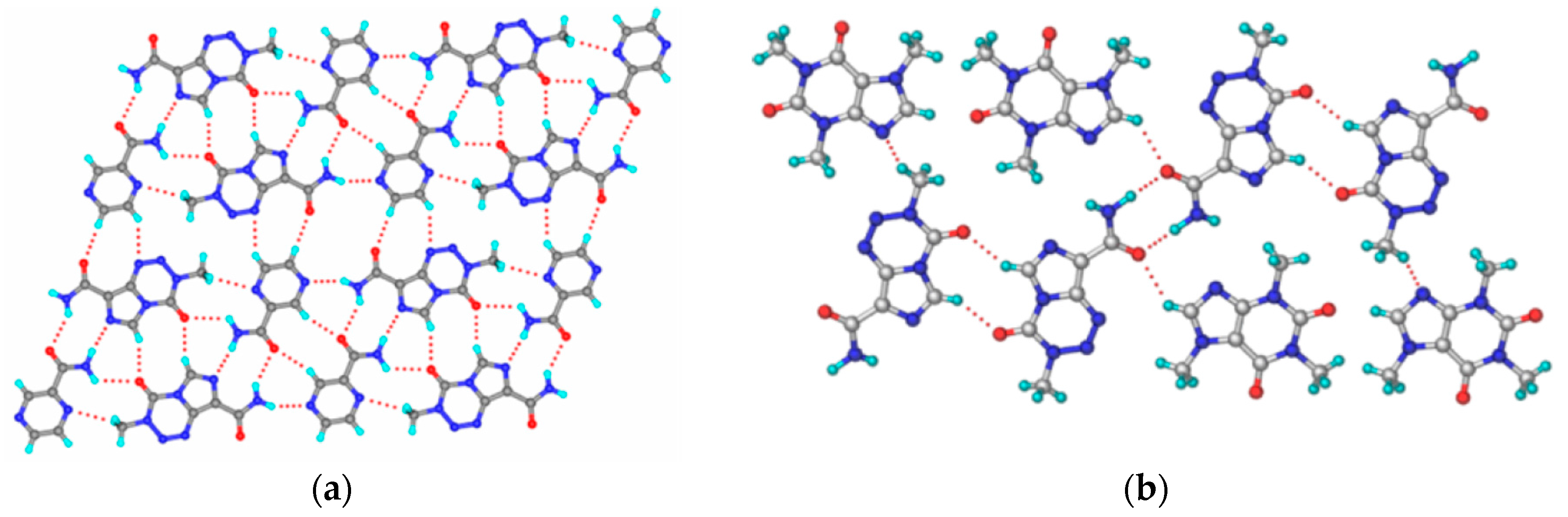
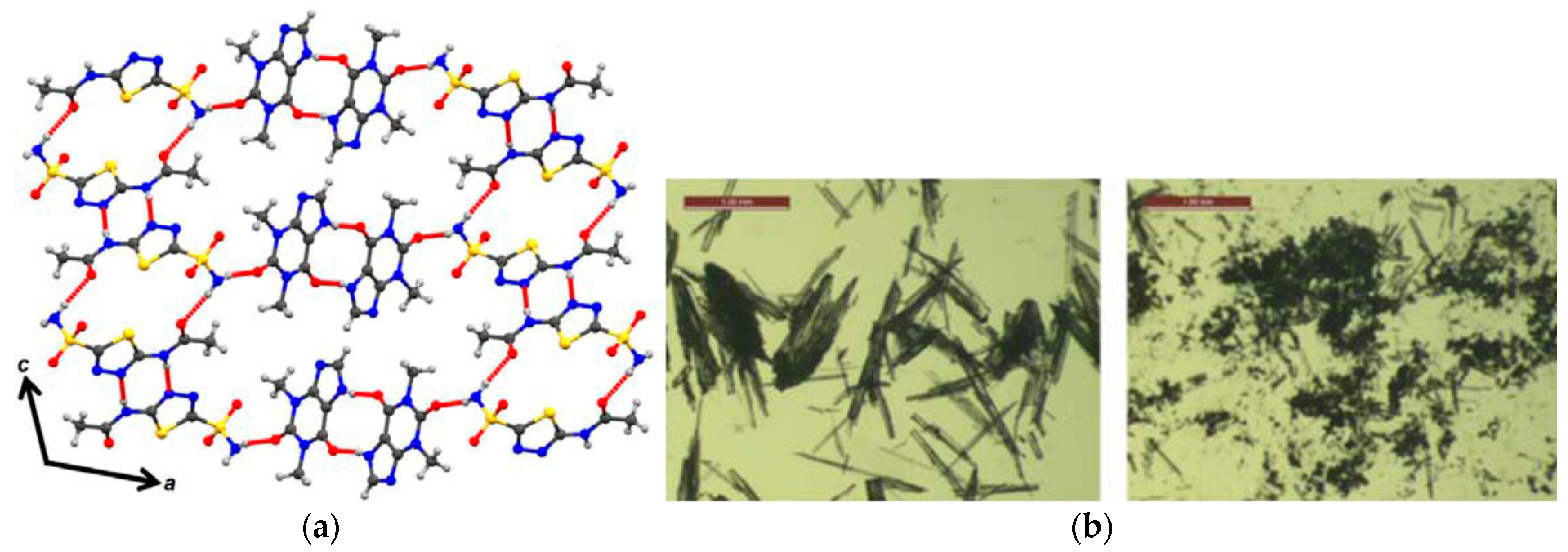
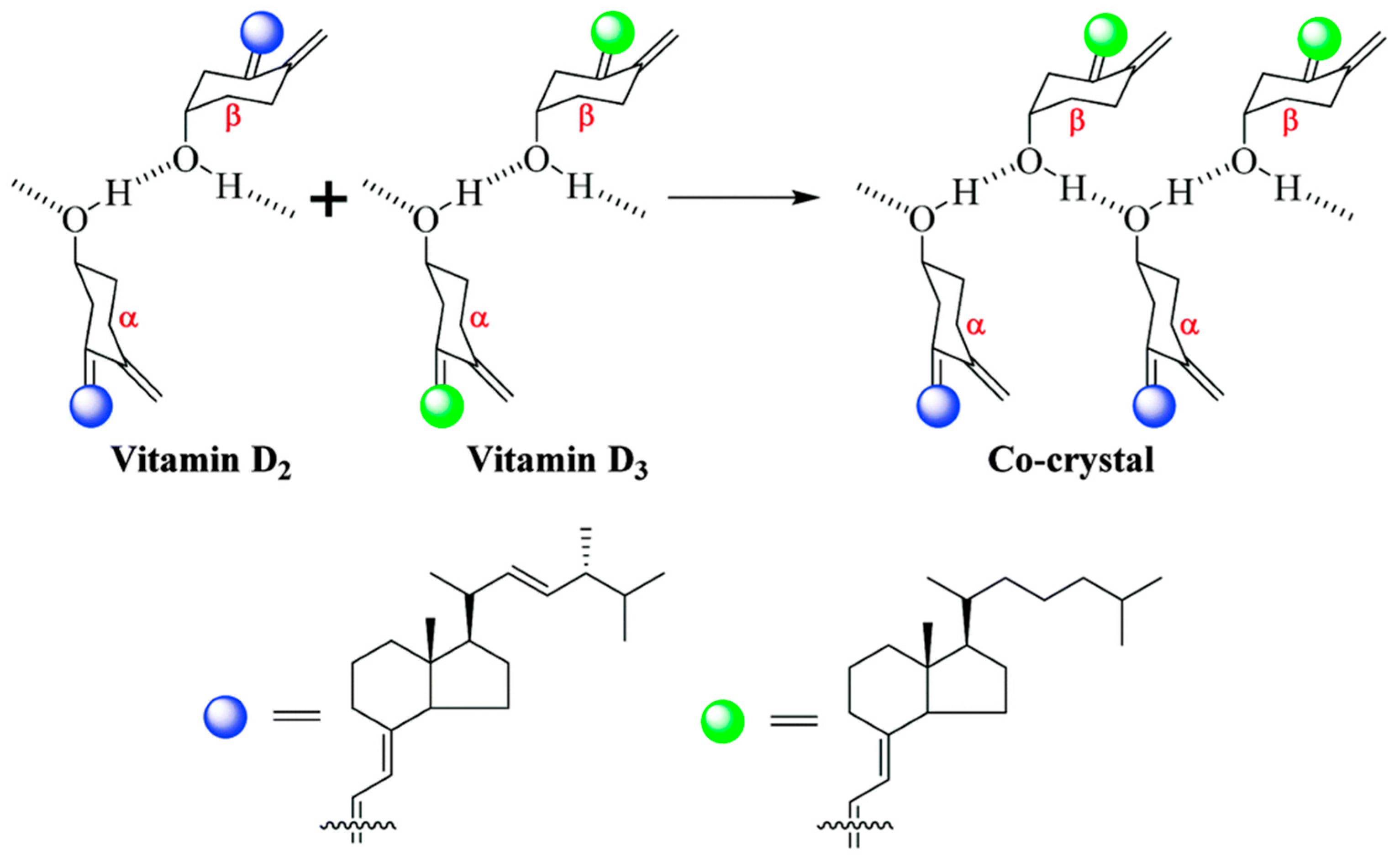
| Polymorph | Solvent of Crystallization |
|---|---|
| Form I | Acetonitrile Formic acid Toluene + ethylacetate |
| Form II | 2-Propanol Toluene + acetonitrile Chloroform + ethylacetate Ethylacetate + n-hexane Ethylacetate + cyclohexane |
| Form III | Acetone Acetonitrile + acetone Methanol + chloroform Chloroform + acetonitrile Toluene + acetonitrile |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thakuria, R.; Sarma, B. Drug‑Drug and Drug‑Nutraceutical Cocrystal/Salt as Alternative Medicine for Combination Therapy: A Crystal Engineering Approach. Crystals 2018, 8, 101. https://doi.org/10.3390/cryst8020101
Thakuria R, Sarma B. Drug‑Drug and Drug‑Nutraceutical Cocrystal/Salt as Alternative Medicine for Combination Therapy: A Crystal Engineering Approach. Crystals. 2018; 8(2):101. https://doi.org/10.3390/cryst8020101
Chicago/Turabian StyleThakuria, Ranjit, and Bipul Sarma. 2018. "Drug‑Drug and Drug‑Nutraceutical Cocrystal/Salt as Alternative Medicine for Combination Therapy: A Crystal Engineering Approach" Crystals 8, no. 2: 101. https://doi.org/10.3390/cryst8020101





