Two Supramolecular Inorganic–Organic Hybrid Crystals Based on Keggin Polyoxometalates and Crown Ethers
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Measurements
2.2. [(3-F-4-MeAnis)([18]crown-6)]2[SMo12O40]•CH3CN (1)
2.3. [(3-IAnis)([18]crown-6)]3[PMo12O40]•4CH3CN (2)
3. Results and Discussion
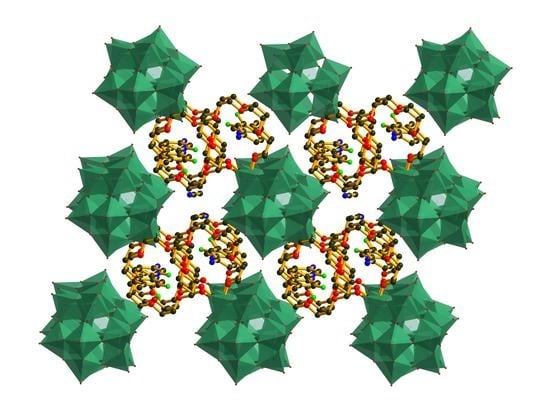
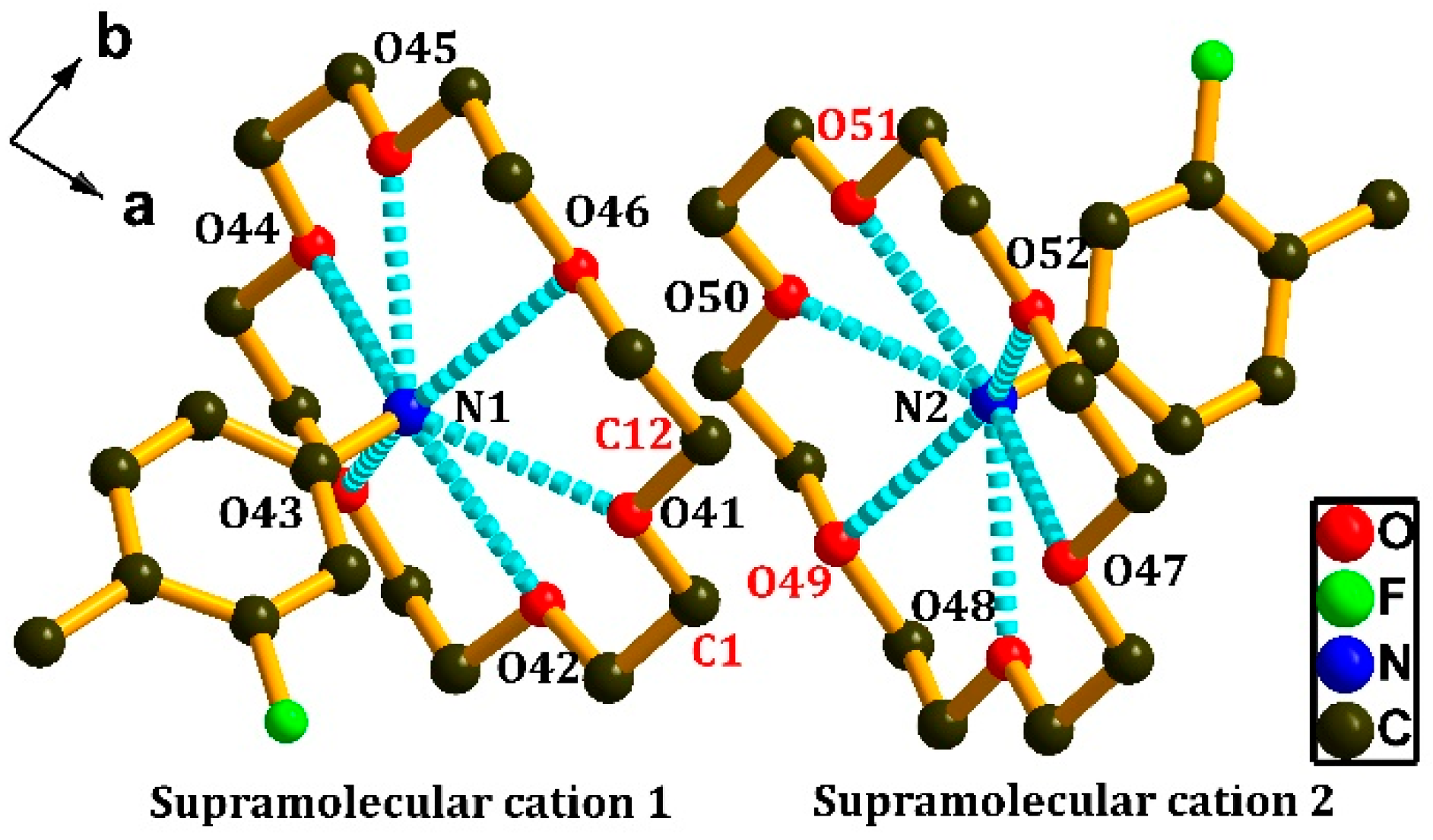
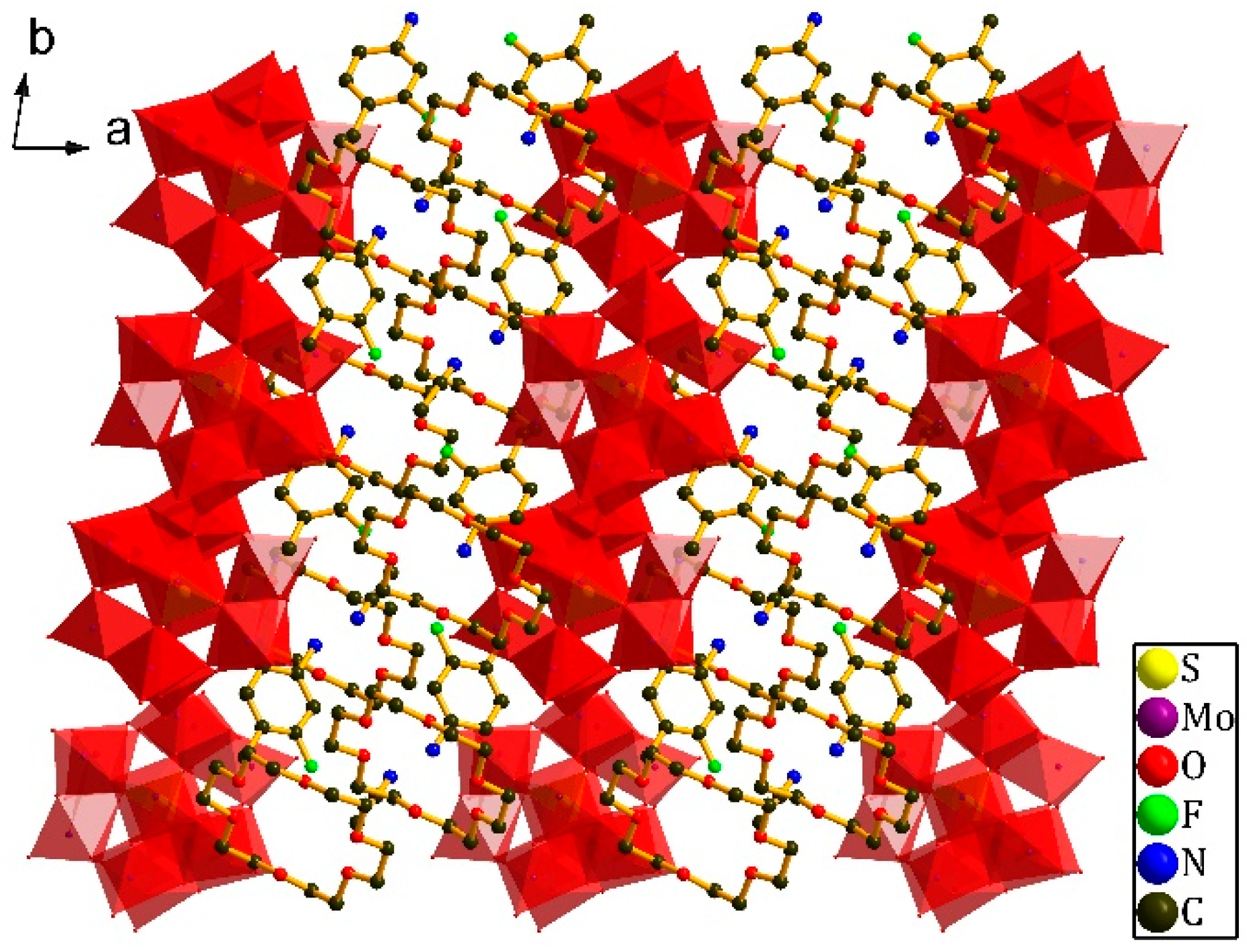
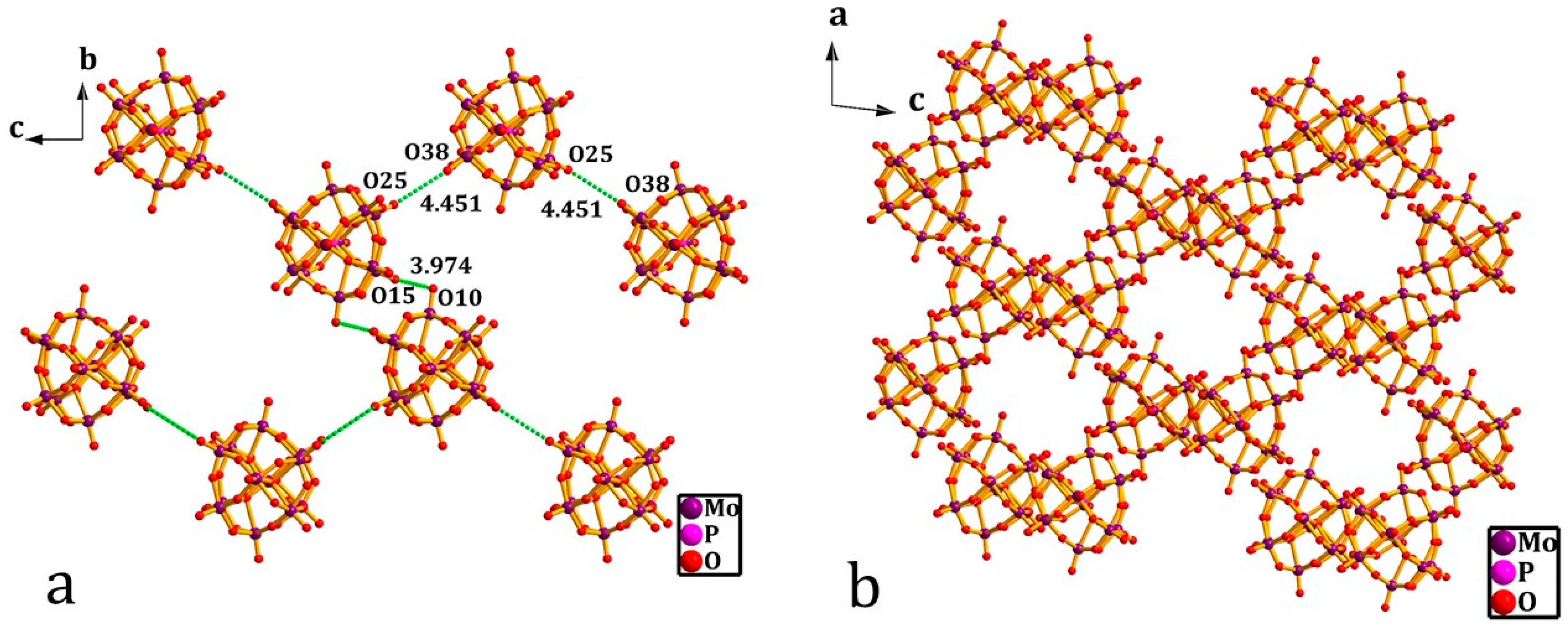
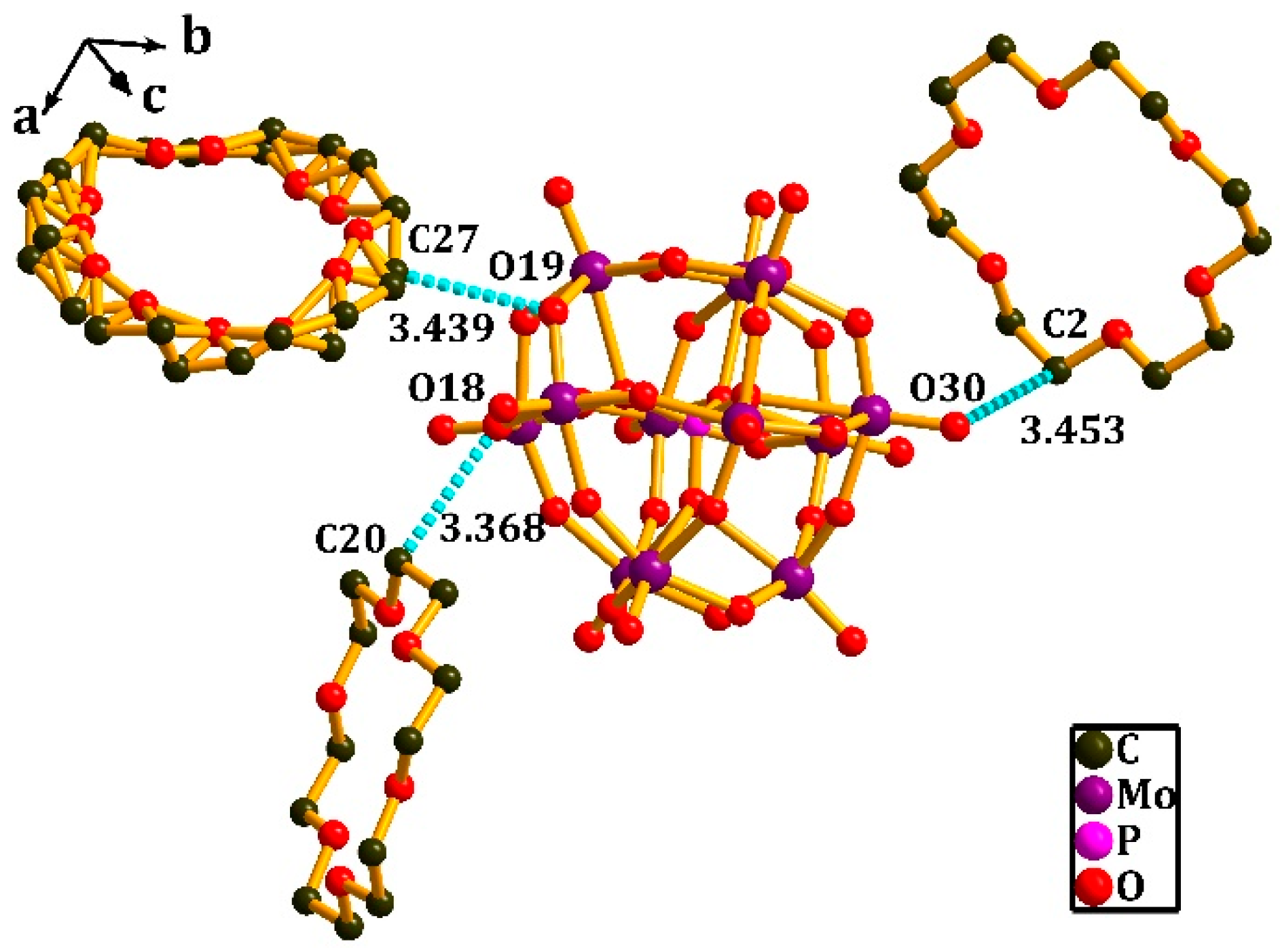
3.1. Crystal Structure of [(3-F-4-MeAnis)([18]crown-6)]2[SMo12O40]•CH3CN (1)
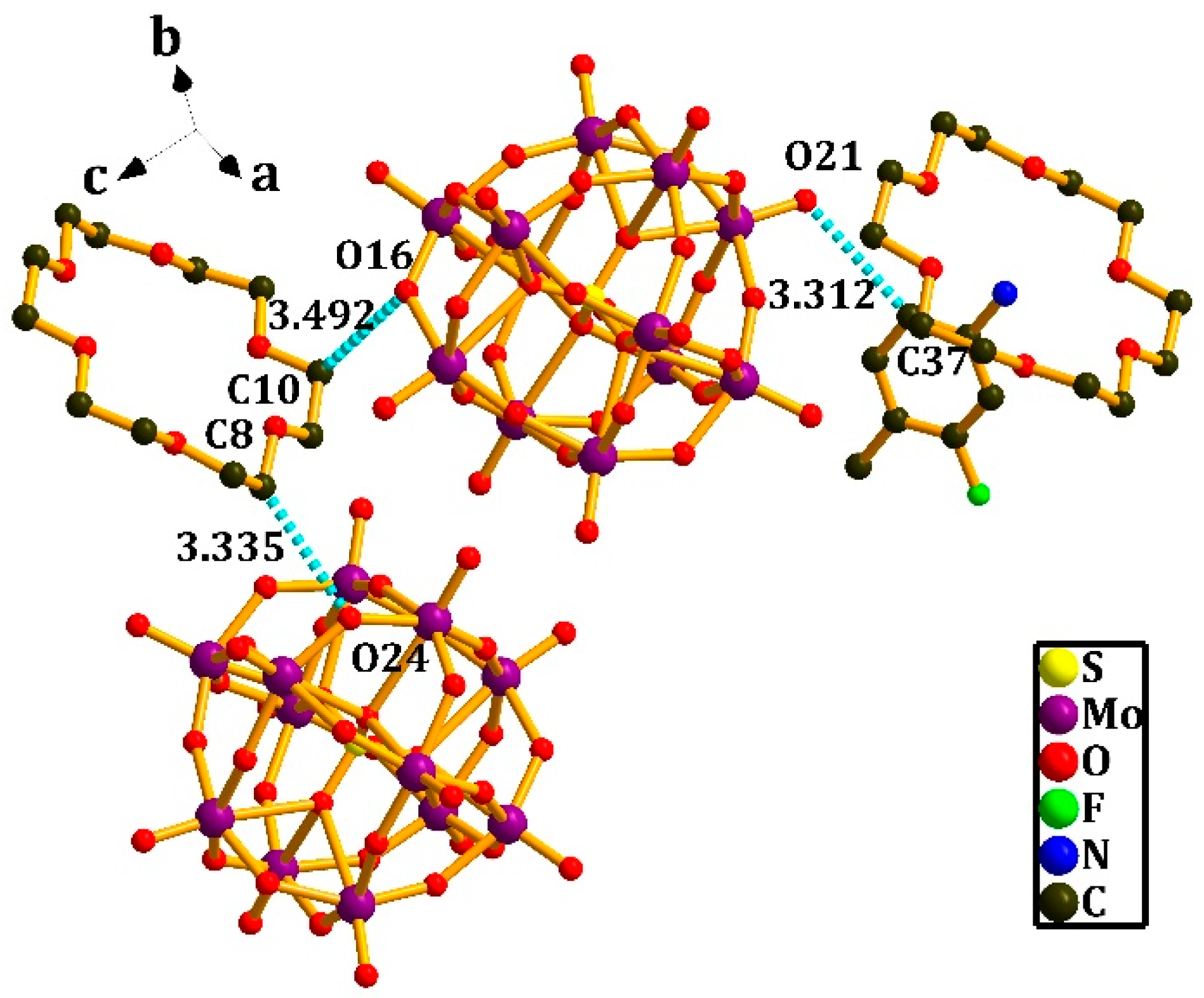
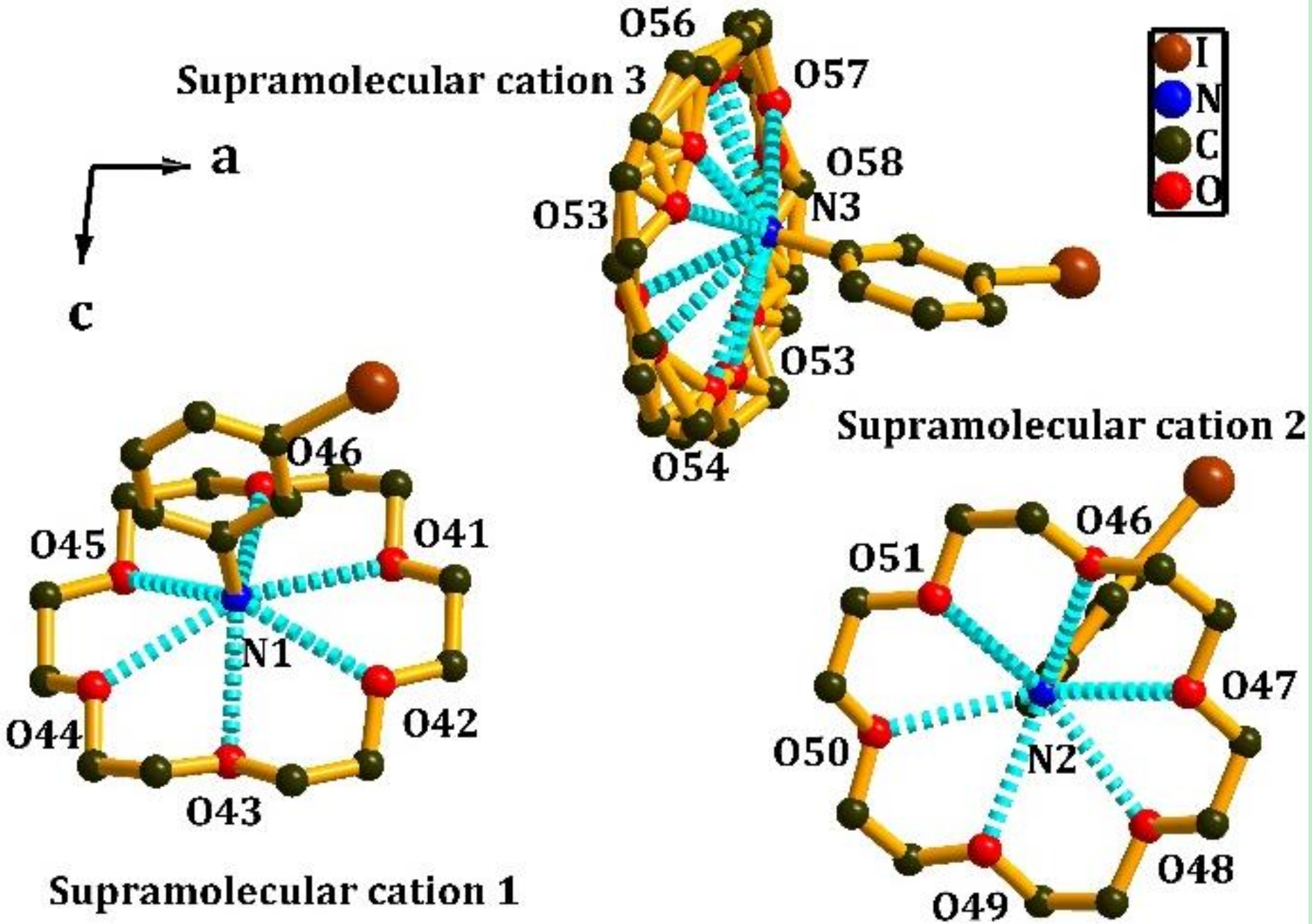
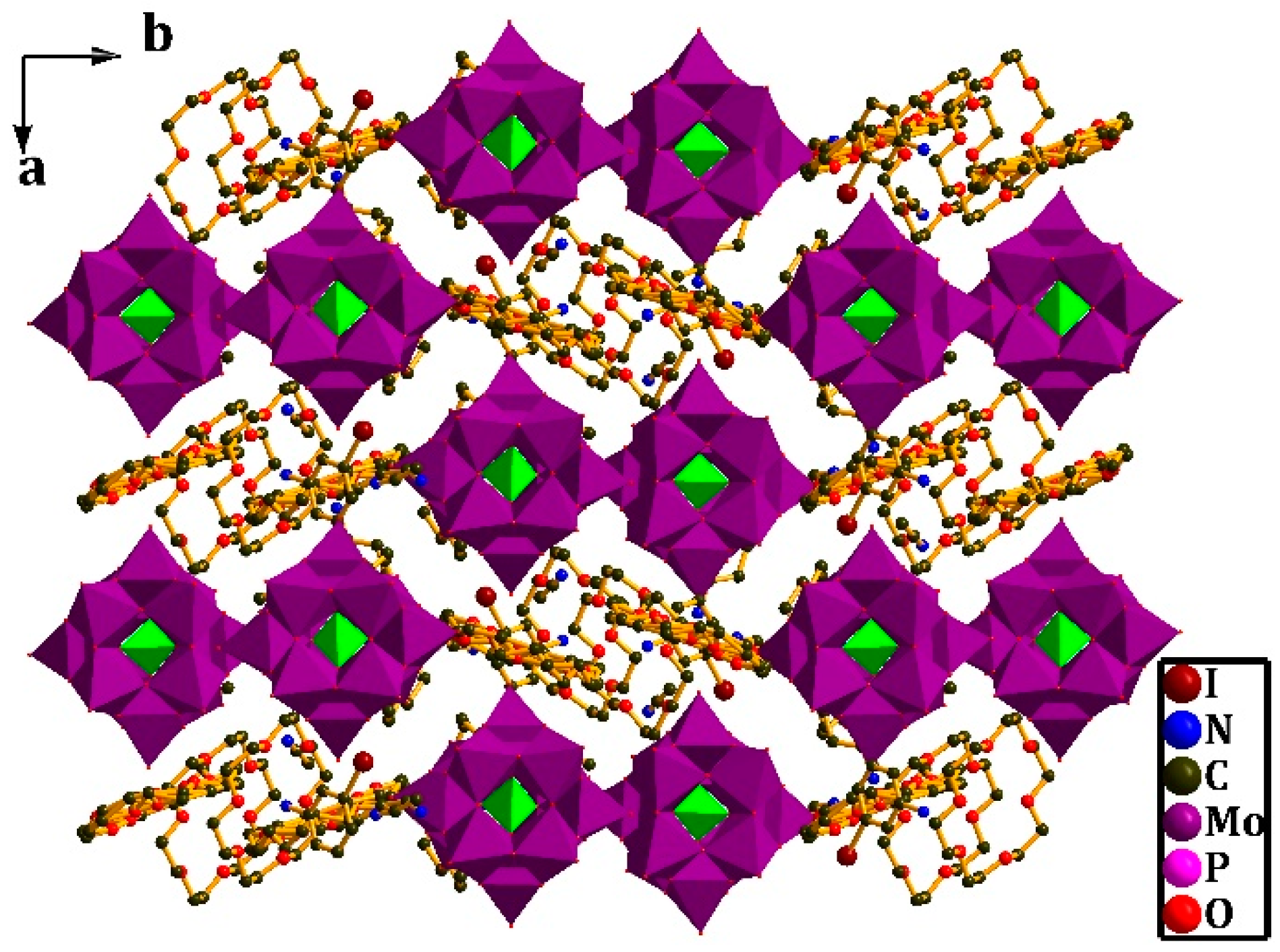
3.2. Crystal Structure of [(4-IAnis)([18]crown-6)]3[PMo12O40]•4CH3CN (2)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lee, B. Developments in inorganic crystal engineering. Chem. Soc. Rev. 2004, 33, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, M.D. Crystal engineering: From structure to function. Science 2002, 295, 2410–2413. [Google Scholar] [PubMed]
- Moulton, B.; Zaworotko, M.J. From Molecules to crystal engineering: Supramolecular isomerism and polymorphism in network solids. Chem. Rev. 2001, 101, 1629–1658. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Li, B.; He, H.; Zhou, W.; Chen, B.; Qian, G. Metal-organic frameworks as platforms for functional materials. Acc. Chem. Res. 2016, 49, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Sliwa, M.; Létard, S.; Malfant, I.; Nierlich, M.; Lacroix, R.G.; Asahi, T.; Masuhara, H.; Yu, P.; Nakatani, K. Design, synthesis, structural and nonlinear optical properties of photochromic crystals: Toward reversible molecular switches. Chem. Mater. 2005, 17, 4727–4735. [Google Scholar] [CrossRef]
- Zhao, M.; Ou, S.; Wu, C.D. Porous metal-organic frameworks for heterogeneous biomimetic catalysis. Acc. Chem. Res. 2014, 47, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.Q.; Shao, D.; Wei, X.Q.; Shen, F.X.; Shi, L.; Kempe, D.; Zhang, Y.Z.; Dunbar, K.R.; Wang, X.Y. Reversible on-off switching of a single-molecular magnet via a crystal chemical transformation. J. Am. Chem. Soc. 2017, 139, 11714–11717. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.N.; Kubo, K.; Noro, S.; Akutagawa, T.; Nakamura, T. Crystal structure and physical properties of a dithiolene complex crystal with adamantine supramolecular rotator. Bull. Chem. Soc. Jpn. 2014, 87, 417–419. [Google Scholar] [CrossRef]
- Akutagawa, T.; Sato, D.; Koshinaka, H.; Aonuma, M.; Noro, S.; Takeda, S.; Nakamura, T. Solid-state molecular rotators of anilinium and adamantylammonium in [Ni(dmit)2]− salts with diverse magnetic properties. Inorg. Chem. 2008, 47, 5951–5962. [Google Scholar] [CrossRef] [PubMed]
- Akutagawa, T.; Koshinaka, H.; Sato, D.; Takeda, S.; Noro, S.; Takahashi, H.; Kumai, R.; Tokura, Y.; Nakamura, T. Ferroelectricity and polarity control in solid-state flip-flop supramolecular rotators. Nat. Mater. 2009, 8, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Aakeröy, C.B.; Seddon, K.R. The hydrogen bond and crystal engineering. Chem. Soc. Rev. 1993, 22, 397–407. [Google Scholar] [CrossRef]
- Desiraju, G.R. Hyrogen bridges in crystal engineering: Interactions without borders. Acc. Chem. Res. 2002, 35, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Aakeröy, C.B.; Fasulo, M.; Schulthesis, N.; Desper, J.; Moore, C. Structural competition between hydrogen bonds and halogen bonds. J. Am. Chem. Soc. 2007, 192, 13772–13773. [Google Scholar] [CrossRef] [PubMed]
- Canac, Y.; Debono, N.; Lepetit, C.; Duhayon, C.; Chauvin, R. Flexible diphosphine ligands with overall charges of 0, +1, and +2: Critical role of the electrostatics in favoring trans over cis coordination. Inorg. Chem. 2011, 50, 10810–10819. [Google Scholar] [CrossRef] [PubMed]
- Akutagawa, T.; Kudo, F.; Tsunashima, R.; Noro, S.; Cronin, L.; Nakamura, T. Hydrogen-bonded assemblies of two-electron reduced mix-valence [XMo12O40] (X = P and Si) with p-phenylenediamines. Inorg. Chem. 2011, 50, 6711–6718. [Google Scholar] [CrossRef] [PubMed]
- Ito, T. Inorganic-organic hybrid surfactant crystals: Structural aspects and functions. Crystals 2016, 6, 24. [Google Scholar] [CrossRef]
- Xu, H.; Li, Z.; Liu, B.; Xue, G.; Hu, H.; Fu, F.; Wang, J. Charge-transfer salts via cocrystallization of the cationic ferrocenyl donor with polyoxometalate acceptors. Cryst. Growth Des. 2010, 10, 1096–1103. [Google Scholar] [CrossRef]
- Ito, T.; Fujimoto, N.; Uchida, S.; Iijima, J.; Naruke, H.; Mizuno, N. Polyoxotungstate-surfactant layered crystal toward conductive inorganic-organic hybrid. Crystals 2012, 2, 362–373. [Google Scholar] [CrossRef]
- Vu, T.; Bond, A.M.; Hockless, D.C.R.; Moubaraki, B.; Murray, K.S.; Lazarev, G.; Wedd, A.G. Electrochemical synthesis and structural and physical characterization of one- and two-electro-reduced forms of [SMo12O402−]. Inorg. Chem. 2001, 40, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Kubo, K.; Noro, S.; Akutagawa, T.; Nakamura, T. Self-assembled structure of inorganic-organic hybrid crystals based on Keggin polyoxometallates [SMo12O402−] and supramolecular cations. Cryst. Growth Des. 2016, 16, 800–807. [Google Scholar] [CrossRef]
- Li, Q.; Lu, Z.; Boas, J.F.; Traore, D.A.K.; Wilce, M.C.J.; Martin, L.L.; Ueda, T.; Bond, A.M. Spontaneous redox synthesis and characterization of the tetrathiafulvalene-vanadium-substituted polyoxometalate charge-transfer material TTF4[SVW11O40]: Comparison with the Mo analogue. Inorg. Chem. 2014, 53, 10996–11006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lu, X.; Sun, J.; Zhao, Y.; Shao, X. Honeycomb supramolecular frameworks of organic-inorganic hybrid cluster composed of cation radical and Keggin-type polyoxometalate. CrystEngComm 2015, 17, 4110–4116. [Google Scholar] [CrossRef]
- Li, Q.; Lu, J.; Boas, J.F.; Traore, D.A.K.; Wilce, M.C.J.; Huang, F.; Martin, L.L.; Ueda, T.; Bond, A.M. Spontaneous redox synthesis of the charge transfer material TTF4[SVMo11O40]. Inorg. Chem. 2012, 51, 12929–12937. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Niu, Y.; Xu, H.; Cao, G.; Liu, B.; Hu, H.; Xue, G. Charge-transfer salts based on Lindqvist and Keggin polyoxoanion acceptors and ferrocenyl cationic donors. New J. Chem. 2012, 36, 1224–1230. [Google Scholar] [CrossRef]
- Veya, P.L.; Kochi, J.K. Structural and spectral characterization of novel charge-transfer salts of polyoxometalates and the cationic ferrocenyl donor. J. Organomet. Chem. 1995, 488, C4–C8. [Google Scholar] [CrossRef]
- Akutagawa, T.; Endo, D.; Noro, S.; Cronin, L.; Nakamura, T. Directing organic-inorganic hybrid molecular-assemblies of polyoxometalate crown-ether complexes with supramolecular cations. Coord. Chem. Rev. 2007, 251, 2547–2561. [Google Scholar] [CrossRef]
- Xiong, J.; Kubo, K.; Noro, S.; Akutagawa, T.; Nakamura, T. Supramolecular cations of (m-halogenated-anilinium)(dibenzo[18]crown-6) in Keggin [SMo12O40]2− polyoxometallates. CrystEngComm 2015, 17, 856–861. [Google Scholar] [CrossRef]
- Endo, D.; Akutagawa, T.; Kubo, K.; Noro, S.; Cronin, L.; Nakamura, T. Molecular motions and hydrogen-bonding networks in (o-aminoanilinium)-(crown ethers)-[PMo12O40]4− crystals. Bull. Chem. Soc. Jpn. 2012, 85, 305–315. [Google Scholar] [CrossRef]
- Akutagawa, T.; Nakamura, T. Supramolecular approach for solid state Brownian rotators. Dalton Trans. 2008, 45, 6335–6345. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Kubo, K.; Noro, S.; Akutagawa, T.; Nakamura, T. Design of crystalline spaces for molecular rotations in crystals. Cryst. Growth Des. 2014, 14, 537–543. [Google Scholar] [CrossRef]
- Nishihara, S.; Akutagawa, T.; Sato, D.; Takeda, S.; Noro, S.; Nakamura, T. Multirotations of (anilinium)([18]crown-6) supramolecular cation structure in magnetic salt of [Ni(dmit)2]−. Chem. Asian J. 2007, 2, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Livage, J.; Launay, J.P.; Fournier, M.; Jeannin, Y. Electron delocalization in mixed-valence molybdenum polyanions. J. Am. Chem. Soc. 1982, 104, 3194–3202. [Google Scholar] [CrossRef]
- Xiong, J.; Lv, S.F.; Peng, J.J.; Li, M.; Li, W.; Yang, S.B. Two Inorganic-organic hybrid crystals based on polyoxometallates and supramolecular cation: Syntheses and crystal structures. Chin. J. Inorg. Chem. 2017, 33, 1649–1655. [Google Scholar]
- Steiner, T. The hydrogen bond in the solid state. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar]


| Crystal | 1 | 2 |
|---|---|---|
| Formula | C40H69F2Mo12N3O52S | C62H105I3Mo12N7O58P |
| Formula weight | 2645.32 | 3439.48 |
| T/K | 173 | 173 |
| Crystal system | Triclinic | Monoclinic |
| Space group | Pī | P21/n |
| a/Å | 14.928(3) | 14.3880(3) |
| b/Å | 15.358(3) | 31.1929(6) |
| c/Å | 17.008(3) | 23.1485(4) |
| α/° | 85.46(3) | 90 |
| β/° | 81.86(3) | 96.685(7) |
| γ/° | 80.65(3) | 90 |
| V/Å3 | 3802.5(13) | 10318.5(4) |
| Z | 2 | 4 |
| dcalc/g cm−3 | 2.310 | 2.214 |
| μ/mm−1 | 2.045 | 19.602 |
| GoF on F2 | 1.223 | 1.141 |
| R1 [I > 2σ(I)] | 0.0482 | 0.0512 |
| wR2 [I > 2σ(I)] | 0.1268 | 0.1305 |
| Supramolecular Cation 1 | Distance | Supramolecular Cation 2 | Distance |
|---|---|---|---|
| N(1)–O(41) | 2.8847 | N(2)–O(47) | 2.9704 |
| N(1)–O(42) | 2.9782 | N(2)–O(48) | 2.8732 |
| N(1)–O(43) | 2.9037 | N(2)–O(49) | 2.9799 |
| N(1)–O(44) | 2.9757 | N(2)–O(50) | 2.8553 |
| N(1)–O(45) | 2.8720 | N(2)–O(51) | 2.9414 |
| N(1)–O(46) | 2.9503 | N(2)–O(52) | 2.8622 |
| Average distance | 2.9274 | Average distance | 2.9137 |
| Supramolecular Cation 1 | Distance | Supramolecular Cation 2 | Distance | Supramolecular Cation 3 | Distance |
|---|---|---|---|---|---|
| N(1)–O(41) | 2.8937 | N(2)–O(47) | 2.9218 | N(3)–O(53) | 2.9131 |
| N(1)–O(42) | 2.9378 | N(2)–O(48) | 2.9127 | N(3)–O(54) | 2.9691 |
| N(1)–O(43) | 2.9267 | N(2)–O(49) | 2.9147 | N(3)–O(55) | 2.9629 |
| N(1)–O(44) | 2.8978 | N(2)–O(50) | 2.9968 | N(3)–O(56) | 2.9171 |
| N(1)–O(45) | 2.8937 | N(2)–O(51) | 2.8158 | N(3)–O(57) | 2.8991 |
| N(1)–O(46) | 2.8537 | N(2)–O(52) | 2.8697 | N(3)–O(58) | 3.0802 |
| Average distance | 2.9004 | Average distance | 2.9053 | Average distance | 2.9569 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, J.; Luo, T.; Zhang, J.; Li, X.-X.; Lv, S.-F.; Peng, J.-J.; Li, M.; Li, W.; Nakamura, T. Two Supramolecular Inorganic–Organic Hybrid Crystals Based on Keggin Polyoxometalates and Crown Ethers. Crystals 2018, 8, 17. https://doi.org/10.3390/cryst8020017
Xiong J, Luo T, Zhang J, Li X-X, Lv S-F, Peng J-J, Li M, Li W, Nakamura T. Two Supramolecular Inorganic–Organic Hybrid Crystals Based on Keggin Polyoxometalates and Crown Ethers. Crystals. 2018; 8(2):17. https://doi.org/10.3390/cryst8020017
Chicago/Turabian StyleXiong, Jun, Teng Luo, Jun Zhang, Xiao-Xia Li, Shao-Fang Lv, Jun-Jun Peng, Ming Li, Wei Li, and Takayoshi Nakamura. 2018. "Two Supramolecular Inorganic–Organic Hybrid Crystals Based on Keggin Polyoxometalates and Crown Ethers" Crystals 8, no. 2: 17. https://doi.org/10.3390/cryst8020017