Rare-Earth Tantalates and Niobates Single Crystals: Promising Scintillators and Laser Materials
Abstract
:1. Introduction
2. Experiments
2.1. Polycrystalline Synthesis
2.2. Single Crystal Growth
2.3. Crystal Structure and Chemical Etching
2.4. Measurements
2.5. Judd–Ofelt Calculation
2.6. Emission Cross-Section Calculation
3. Scintillator Materials
3.1. GdTaO4 and Nd:GdTaO4 Crystals
3.2. LuTaO4
4. Laser Materials
4.1. Er:GdTaO4 Crystal
4.2. Nd:GdTaO4 and Nd:GdYTaO4 Crystals
4.3. Yb:GdTaO4 Crystal
4.4. Tm,Ho:GdYTaO4 and Yb,Ho:GdYTaO4 Crystal
4.5. Yb:GdNbO4 and Yb:YNbO4
4.6. Nd:GdNbO4, Nd:YNbO4, Nd:GdYNbO4, and Nd:GdLaNbO4 Crystals
5. Crystal Field Calculation
5.1. Calculations of Energy Levels
5.2. Crystal Field Analysis
6. Conclusions
- Rare-earth orthotantalates have high density deserve to be studied as scintillators. Large volume and high-quality single crystals are grown and their properties are researched systematically by our group. The scintillation decay time of Nd:GdTaO4 single crystal is faster than GdTaO4 single crystal. LuTaO4 has the highest density 9.8 g/cm3 among the present luminescent material hosts, and the fluorescence lifetime of Nd:LuTaO4 is about 263.2 ns and expected as the most promising heavy scintillator.
- Er:GdTaO4 and Tm,Ho:GdYTaO4 single crystals have good visible and mid-infrared fluorescence properties and can be the potential laser materials. Nd3+, Yb3+-doped othotantalates and orthoniobates have been realized laser output, which prove that they can be used as laser matrix hosts successfully.
- The ab initio self-consistent DV-Xα method has been used in its relativistic model to investigate the crystal-field and spin-orbit parameters of Nd3+ doped in rare earth tantalates. The deviations of the calculated energy levels and experimental energy levels are less than 30 cm−1, which indicates that the energy levels fitting results are satisfactory. Through the calculation of the crystal field parameters it is shown that the crystal field is strong and is beneficial to the widen of the ion absorption band, which indicated that rare-earth orthotantalates will be promising laser crystal materials.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abe, R.; Higashi, M.; Sayama, K.; Abe, Y.; Sugihara, H. Photocatalytic activity of R3MO7 and R2Ti2O7 (R = Y, Gd, La; M = Nb, Ta) for water splitting into H2 and O2. J. Phys. Chem. B 2006, 110, 2219–2226. [Google Scholar] [CrossRef] [PubMed]
- Haugsrud, R.; Norby, T. Proton conduction in rare-earth ortho-niobates and ortho-tantalates. Nat. Mater. 2006, 5, 193–196. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Xie, L. Luminescent properties of Tb3+ activated GdTaO4 with M and M′ type structure under UV-VUV excitation. J. Lumin. 2010, 130, 2089–2092. [Google Scholar] [CrossRef]
- Forbes, T.Z.; Nyman, M.; Rodriguez, M.A.; Navrotskya, A. The energetics of lanthanum tantalate materials. J. Solid State Chem. 2010, 183, 2516–2521. [Google Scholar] [CrossRef]
- Sych, A.M.; Golub, A.M. Niobates and Tantalates of Trivalent Elements. Uspekhi Khimii 1977, 46, 417–444. [Google Scholar]
- Gundobin, N.V.; Petrov, K.I.; Plotkin, S.S. Vibration-Spectra and Structure Ln3MO7-Type Niobates and Tantalates. Zhurnal Neorganicheskoi Khimii 1977, 22, 2973–2977. [Google Scholar]
- Siqueira, K.P.F.; Carmo, A.P.; Bell, M.J.V.; Dias, A. Optical properties of undoped NdTaO4, ErTaO4 and YbTaO4 ceramics. J. Lumin. 2016, 179, 146–153. [Google Scholar] [CrossRef]
- Blasse, G.; Bril, A. Luminescence phenomena in compounds with fergusonite structure. J. Lumin. 1970, 3, 109–131. [Google Scholar] [CrossRef]
- Voloshyna, O.; Neicheva, S.V.; Starzhinskiy, N.G.; Zenyaa, I.M.; Gridina, S.S.; Baumerb, V.N.; Sidletskiya, O.T. Luminescent and scintillation properties of orthotantalates with common formulae RETaO4 (RE = Y, Sc, La, Lu and Gd). Mater. Sci. Eng. B 2013, 178, 1491–1496. [Google Scholar] [CrossRef]
- Kazakova, L.I.; Bykov, I.S.; Dubovsky, A.B. The luminescence of rare-earth tantalate single crystals. J. Lumin. 1997, 72–74, 211–212. [Google Scholar] [CrossRef]
- Brixner, L. On the structural and luminescent properties of the M′ LnTaO4 rare earth tantalates. J. Electrochem. Soc. 1983, 130, 2435–2443. [Google Scholar] [CrossRef]
- Stubičan, V. High-Temperature Transitions in Rare-Earth Niobates and TantaIates. J. Am. Ceram. Soc. 1964, 47, 55–58. [Google Scholar] [CrossRef]
- Siqueira, K.P.F.; Carvalho, G.B.; Dias, A. Influence of the processing conditions and chemical environment on the crystal structures and phonon modes of lanthanide orthotantalates. Dalton Trans. 2011, 40, 9454–9460. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Han, K.; Liu, X.; Gu, M.; Huang, S.; Ni, C.; Qi, Z.; Zhang, G. Luminescent properties of GdTaO4 and GdTaO4:Eu3+ under VUV-UV excitation. Solid State Commun. 2007, 144, 484–487. [Google Scholar] [CrossRef]
- Liu, X.; Chen, S.; Gu, M.; Wu, M.; Qiu, Z.; Liu, B.; Ni, C.; Huang, S. A novel M′-type LuTaO4:Ln(3+) (Ln = Eu, Tb) transparent scintillator films. Opt. Mater. Express 2014, 4, 172–178. [Google Scholar] [CrossRef]
- Yang, H.; Peng, F.; Zhang, Q.; Guo, C.; Shi, C.; Liu, W.; Sun, G.; Zhao, Y.; Zhang, D.; Sun, D.; et al. A promising high-density scintillator of GdTaO4 single crystal. CrystEngComm 2014, 16, 2480–2485. [Google Scholar] [CrossRef]
- Ning, K.; Zhang, Q.; Zhang, D.; Fan, J.; Sun, D.; Wang, X.; Hang, Y. Crystal growth, characterization of NdTaO4: A new promising stoichiometric neodymium laser material. J. Cryst. Growth 2014, 388, 83–86. [Google Scholar] [CrossRef]
- Ofelt, G.S. Intensities of Crystal Spectra of Rare-Earth Ions. J. Chem. Phys. 1962, 37, 511–520. [Google Scholar] [CrossRef]
- Judd, B.R. Optical Absorption Intensities of Rare-Earth Ions. Phys. Rev. 1962, 127, 750–761. [Google Scholar] [CrossRef]
- Nikl, M.; Bruza, P.; Panek, D.; Vrbova, M.; Mihokova, E.; Mares, J.A.; Beitlerova, A.; Kawaguchi, N.; Fukuda, K.; Yoshikawa, A. Scintillation characteristics of LiCaAlF6-based single crystals under X-ray excitation. Appl. Phys. Lett. 2013, 102, 161907. [Google Scholar] [CrossRef]
- Rooh, G.; Kim H, J.; Park, H.; Kim, S. Investigation of scintillation and luminescence properties of cerium doped Rb2LiGdCl6 single crystals. J. Cryst. Growth 2013, 377, 28–31. [Google Scholar] [CrossRef]
- Nikl, M.; Solovieva, N.; Pejchal, J.; Shim, J.B.; Yoshikawa, A.; Fukuda, T.; Vedda, A.; Martini, M.; Yoon, D.H. Very fast YbxY1−xAlO3 single-crystal scintillators. Appl. Phys. Lett. 2004, 84, 882–884. [Google Scholar] [CrossRef]
- Moses, W.W.; Weber, M.J.; Derenzo, S.E.; Perry, D.; Berdahl, P.; Boatner, L.A. Prospects for dense, infrared emitting scintillators. IEEE Trans. Nucl. Sci. 1998, 45, 462–466. [Google Scholar] [CrossRef]
- Lecoq, P.; Dafinei, I.; Auffray, E.; Schneegans, M.; Korzhik, M.V.; Missevitch, O.V.; Pavlenko, V.B.; Fedorov, A.A.; Annenkov, A.N.; Kostylev, V.L.; et al. Lead tungstate (PbWO4) scintillators for LHC EM calorimetry. Nucl. Instrum. Methods Phys. Res. Sect. A 1995, 365, 291–298. [Google Scholar] [CrossRef]
- Almeida Silva, R.; Tirao, G.; Cusatis, C.; Andreeta, J.P. Growth and X-ray characterization of GdxYb1−xTaO4 (0 ≤ x ≤ 1) single crystals with large lattice spacing gradient. J. Cryst. Growth 2005, 277, 308–313. [Google Scholar] [CrossRef]
- Almeida Silva, R.; Tirao, G.; Cusatis, C.; Andreeta, J.P. Growth and structural characterization of M-type GdTaO4 single crystal fiber. J. Cryst. Growth 2005, 274, 512–517. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Q.; Zhou, W.; Gu, C.; Yin, S. Growth and Luminescence of M-Type GdTaO4 and Tb: GdTaO4 Scintillation Single Crystals. IEEE Trans. Nucl. Sci. 2010, 57, 1287–1290. [Google Scholar] [CrossRef]
- Yang, H.; Peng, F.; Zhang, Q.; Shi, C.; Guo, C.; Wei, X.; Sun, D.; Wang, X.; Dou, R.; Xing, X.; et al. Temperature-dependent luminescence of GdTaO4 single crystal. J. Lumin. 2015, 160, 90–94. [Google Scholar] [CrossRef]
- Peng, F.; Liu, W.; Zhang, Q.; Yang, H.; Shi, C.; Mao, R.; Sun, D.; Luo, J.; Sun, G. Crystal growth, optical and scintillation properties of Nd3+ doped GdTaO4 single crystal. J. Cryst. Growth 2014, 406, 31–35. [Google Scholar] [CrossRef]
- Blasse, G.; Dirksen, G.J.; Brixner, L.H.; Crawford, M.K. Luminescence of Materials Based On LuTaO4. J. Alloy. Compd. 1994, 209, 1–6. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Q.; Ding, L.; Sun, D.; Luo, J.; Yin, S. Photoluminescence properties of LuTaO4:RE3+ (RE3+ = Eu3+, Tb3+) with M′-type structure. J. Alloy. Compd. 2009, 474, 226–228. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Q.; Guo, C.; Shi, C.; Peng, F.; Yang, H.; Sun, D.; Luo, J.; Liu, W. The luminescence properties of the high-density phosphor Lu1−xNdxTaO4. J. Lumin. 2014, 155, 165–169. [Google Scholar] [CrossRef]
- Xing, X.; Wang, X.; Zhang, Q.; Sun, G.; Shi, C.; Liu, W.; Sun, D.; Yin, S. High-Temperature Phase Relations in the Lu2O3-Ta2O5 System. J. Am. Ceram. Soc. 2016, 99, 1042–1046. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Q.; Zhou, P.; Ning, K.; Gao, J.; He, Y.; Luo, J.; Sun, D.; Yin, S. Czochralski growth and optical investigations of Er3+:GdTaO4 laser crystal. In Proceedings of the SPIE 8206, Pacific Rim Laser Damage 2011: Optical Materials for High Power Lasers, Shanghai, China, 12 January 2012. [Google Scholar]
- Chen, Y.; Peng, F.; Zhang, Q.; Liu, W.; Dou, R.; Ding, S.; Luo, J.; Sun, D.; Sun, G.; Wang, X. Growth, structure and spectroscopic properties of 1 at.% Er3+: GdTaO4 laser crystal. J. Lumin. 2017, 192, 555–561. [Google Scholar] [CrossRef]
- Lupei, V.; Georgescu, S.; Florea, V. On the dynamics of population inversion for 3 μm Er3+ lasers. IEEE J. Quantum Electron. 1993, 29, 426–434. [Google Scholar] [CrossRef]
- Su, J.; Yang, C.; Li, Q.; Zhang, Q.; Luo, J. Optical spectroscopy of Er:YSGG laser crystal. J. Lumin. 2010, 130, 1546–1550. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, G.; Wu, F.; Xu, W.; Zong, Y.; Wang, X.; Zhao, Z.; Zhou, G.; Xu, J. Growth and spectral properties of Er:Gd2SiO5 crystal. J. Cryst. Growth 2008, 310, 156–159. [Google Scholar] [CrossRef]
- Ding, Y.; Zhao, G.; Xu, X. Crystal growth and spectroscopic properties of erbium doped Lu2SiO5. J. Cryst. Growth 2010, 312, 2103–2106. [Google Scholar] [CrossRef]
- Peng, F.; Yang, H.; Zhang, Q.; Luo, J.; Liu, W.; Sun, D.; Dou, R.; Sun, G. Spectroscopic properties and laser performance at 1066 nm of a new laser crystal Nd:GdTaO4. Appl. Phys. B 2015, 118, 549–554. [Google Scholar] [CrossRef]
- Peng, F.; Yang, H.; Zhang, Q.; Luo, J.; Sun, D.; Liu, W.; Sun, G.; Dou, R.; Wang, X.; Xing, X. Growth, thermal properties, and LD-pumped 1066 nm laser performance of Nd3+ doped Gd/YTaO4 mixed single crystal. Opt. Mater. Express 2015, 5, 2536–2544. [Google Scholar] [CrossRef]
- Tempus, M.; Luthy, W.; Weber, H.P.; Ostroumov, V.G.; Shcherbakov, I.A. 2.79 μm YSGG:Cr:Er Laser pumped at 790 nm. IEEE J. Quantum Electron. 1994, 30, 2608–2611. [Google Scholar] [CrossRef]
- Vodopyanov, K.L.; Ganikhanov, F.; Maffetone, J.P.; Zwieback, I.; Ruderman, W. ZnGeP2 optical parametric oscillator with 3.8–12.4 μm tunability. Opt. Lett. 2000, 25, 841–843. [Google Scholar] [CrossRef] [PubMed]
- Diening, A.; Kuck, S. Spectroscopy and diode-pumped laser oscillation of Yb3+, Ho3+-doped yttrium scandium gallium garnet. J. Appl. Phys. 2000, 87, 4063–4068. [Google Scholar] [CrossRef]
- Dou, R.; Zhang, Q.; Liu, W.; Luo, J.; Wang, X.; Ding, S.; Sun, D. Growth, structure, chemical etching, and spectroscopic properties of a 2.9 μm Tm,Ho:GdYTaO4 laser crystal. Opt. Mater. 2015, 48, 80–85. [Google Scholar] [CrossRef]
- Dou, R.; Zhang, Q.; Sun, D.; Luo, J.; Yang, H.; Liu, W.; Sun, G. Growth, thermal, and spectroscopic properties of a 2.911 μm Yb,Ho:GdYTaO4 laser crystal. CrystEngComm 2014, 16, 11007–11012. [Google Scholar] [CrossRef]
- Chen, D.W.; Fincher, C.L.; Rose, T.S.; Vernon, F.L.; Fields, R.A. Diode-pumped 1-W continuous-wave Er:YAG 3 μm laser. Opt. Lett. 1999, 24, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Deloach, L.D.; Payne, S.A.; Smith, L.K.; Kway, W.L.; Krupke, W.F. Laser And Spectroscopic Properties of Sr5(PO4)(3)F:Yb. J. Opt. Soc. Am. B 1994, 11, 269–276. [Google Scholar] [CrossRef]
- Boulon, G.; Collombet, A.; Brenier, A.; Cohen-Adad, M.-T.; Yoshikawa, A.; Lebbou, K.; Lee, J.H.; Fukuda, T. Structural and spectroscopic characterization of nominal Yb3+:Ca8La2(PO4)(6)O2 oxyapatite single crystal fibers grown by the micro-pulling-down method. Adv. Funct. Mater. 2001, 11, 263–270. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, Q.; Luo, J.; Liu, W.; Lu, W.; Xu, J.; Sun, G.; Sun, D. Crystal growth, structure, defects, mechanical and spectral properties of Nd0.01:Gd0.89La0.1NbO4 mixed crystal. Appl. Phys. A 2017, 123, 636. [Google Scholar] [CrossRef]
- Ding, S.; Peng, F.; Zhang, Q.; Luo, J.; Liu, W.; Sun, D.; Dou, R.; Sun, G. Structure, spectroscopic properties and laser performance of Nd:YNbO4 at 1066 nm. Opt. Mater. 2016, 62, 7–11. [Google Scholar] [CrossRef]
- Ding, S.; Peng, F.; Zhang, Q.; Luo, J.; Liu, W.; Sun, D.; Dou, R.; Gao, J.; Sun, G.; Cheng, M. Crystal growth, spectral properties, and continuous wave laser operation of Nd:GdNbO4. J. Alloy. Compd. 2017, 693, 339–343. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, Q.; Peng, F.; Liu, W.; Luo, J.; Dou, R.; Sun, G.; Wang, X.; Sun, D. Crystal growth, spectral properties and continuous wave laser operation of new mixed Nd:GdYNbO4 laser crystal. J. Alloy. Compd. 2017, 698, 59–63. [Google Scholar] [CrossRef]
- Rosen, A.; Ellis, D.E. Relativistic Molecular Calculations in Dirac-Slater Model. J. Chem. Phys. 1975, 62, 3039–3049. [Google Scholar] [CrossRef]
- Van Pieterson, L.; Reid, M.F.; Wegh, R.T.; Soverna, S.; Meijerink, A. 4fn → 4fn−15d transitions of the light lanthanides: Experiment and theory. Phys. Rev. B 2002, 65, 045113. [Google Scholar] [CrossRef]
- Reid, M.F.; Duan, C.-K.; Zhou, H. Crystal-field parameters from ab initio calculations. J. Alloy. Compd. 2009, 488, 591–594. [Google Scholar] [CrossRef]
- Molina, P.; Loro, H.; Álvarez-García, S.; Bausá, L.E.; Rodriguez, E.M.; Guillot-Noël, O.; Goldner, P.; Bettinelli, M.; Ghigna, P.; Solé, J.G. Site location and crystal field of Nd3+ ions in congruent strontium barium niobate. Phys. Rev. B 2009, 80, 054111. [Google Scholar] [CrossRef]
- Loro, H.; Vasquez, D.; Camarillo, G.E.; del Castillo, H.; Camarillo, I.; Muñoz, G.; Flores, J.C.; Hernández, A.J.; Murrieta, S.H. Experimental study and crystal-field modeling of Nd3+ 4f3 energy levels in Bi4Ge3O12 and Bi4Si3O12. Phys. Rev. B 2007, 75, 125405. [Google Scholar] [CrossRef]
- Dagama, A.A.S.; Desa, G.F.; Porcher, P.; Caro, P. Energy-Levels of Nd3+ in LiYF4. J. Chem. Phys. 1981, 75, 2583–2587. [Google Scholar] [CrossRef]
- Duan, C.-K.; Tanner, P.A.; Makhov, V.N.; Kirm, M. Vacuum ultraviolet spectra and crystal field analysis of YAlO3 doped with Nd3+ and Er3+. Phys. Rev. B 2007, 75, 195130. [Google Scholar] [CrossRef]
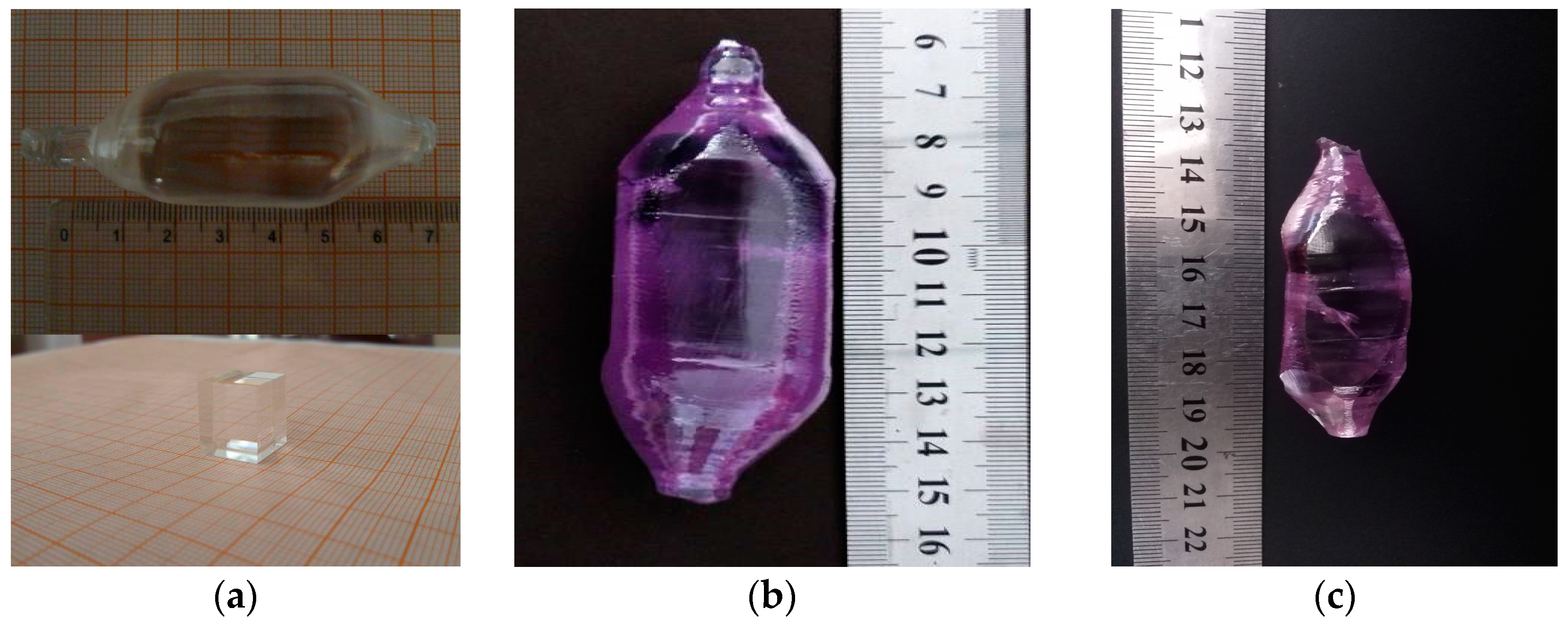

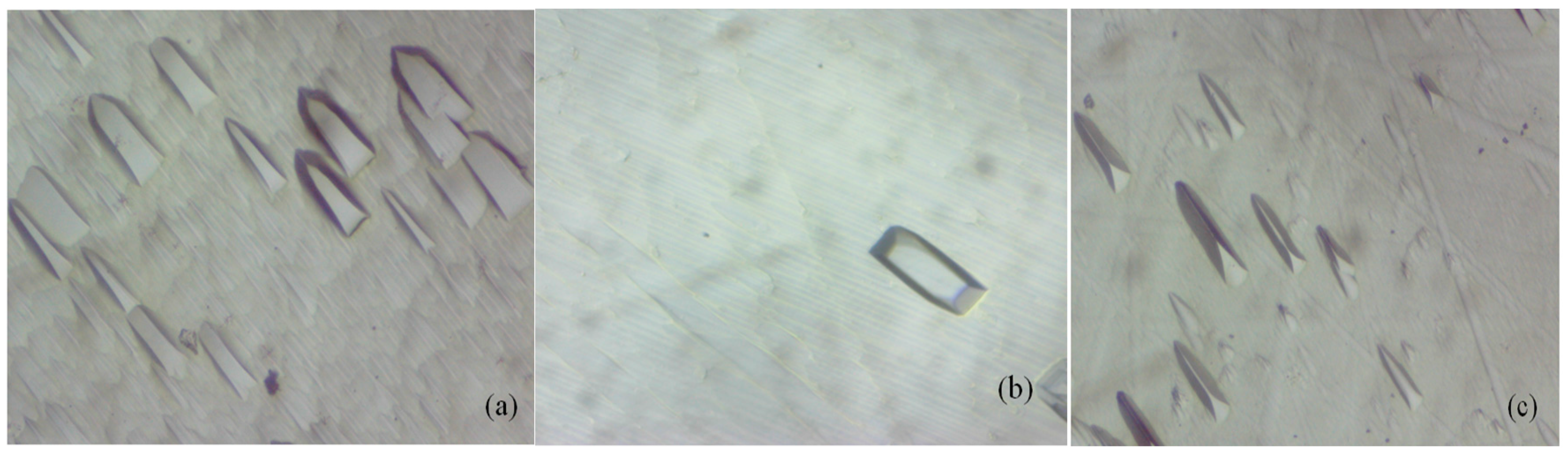
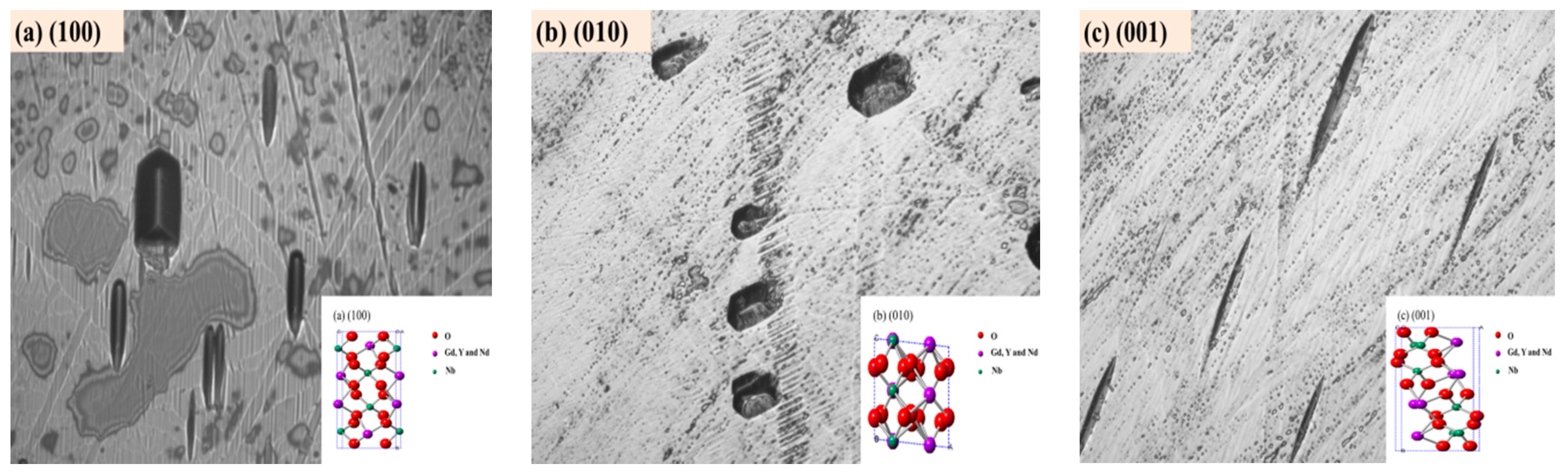
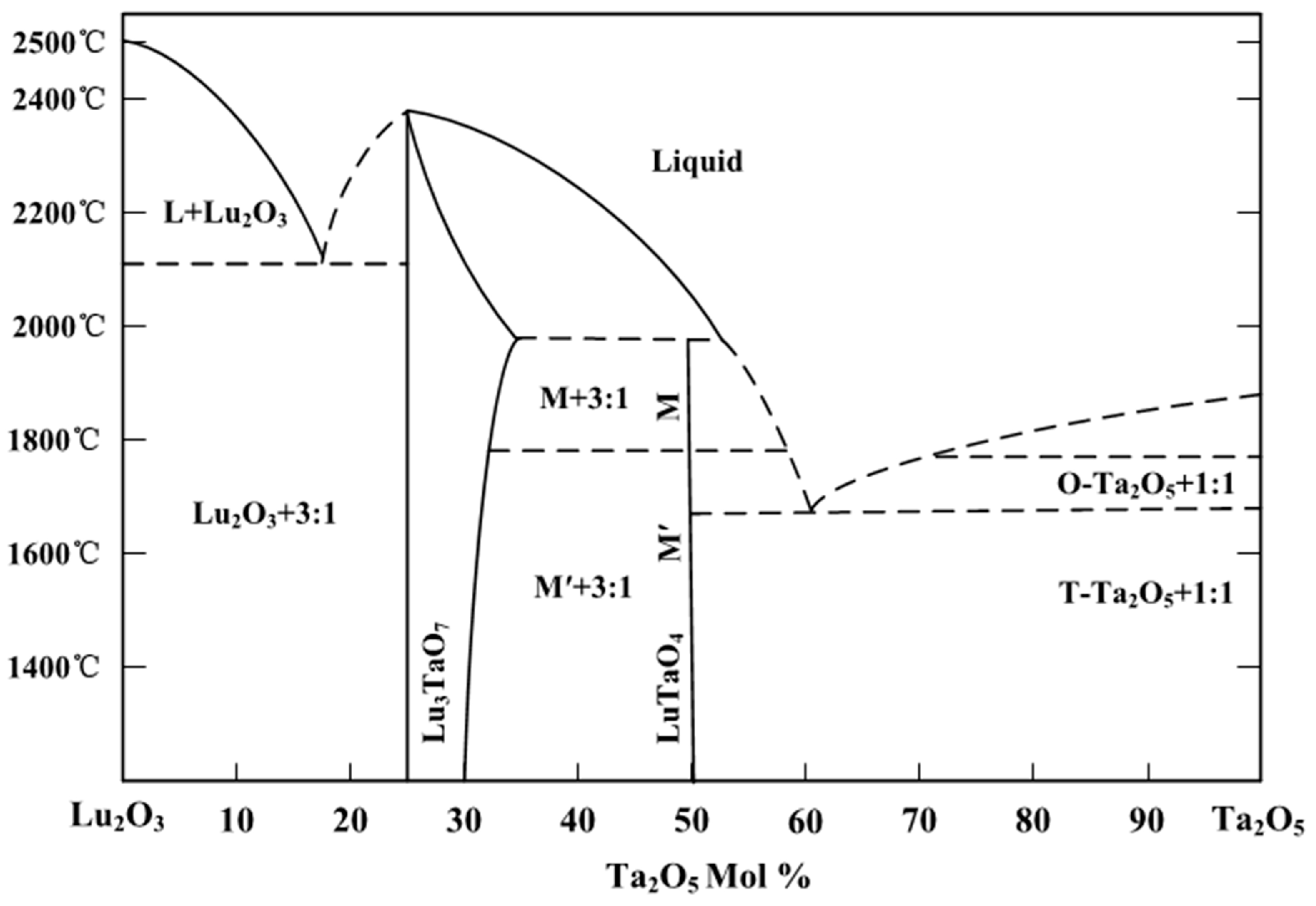

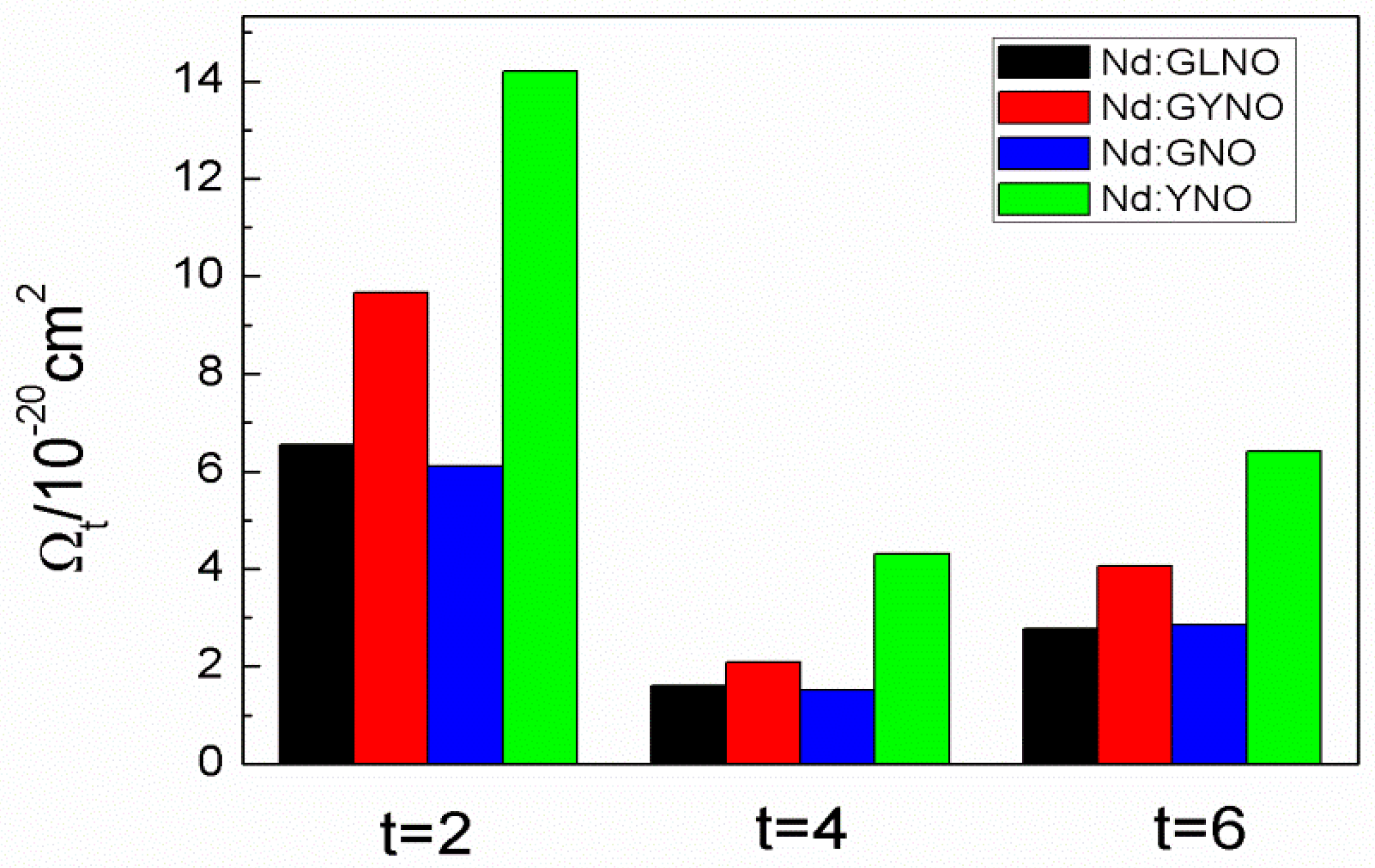
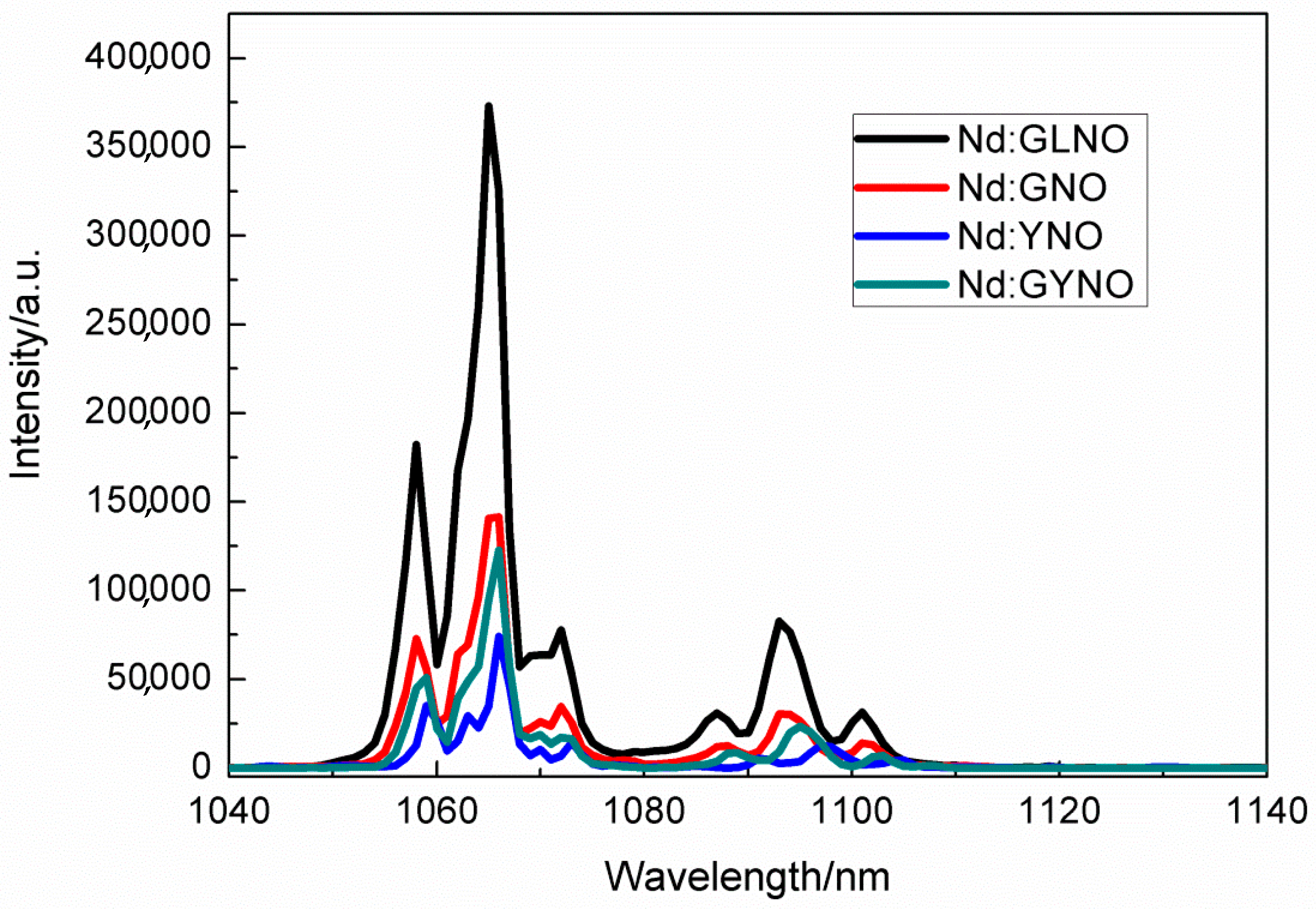
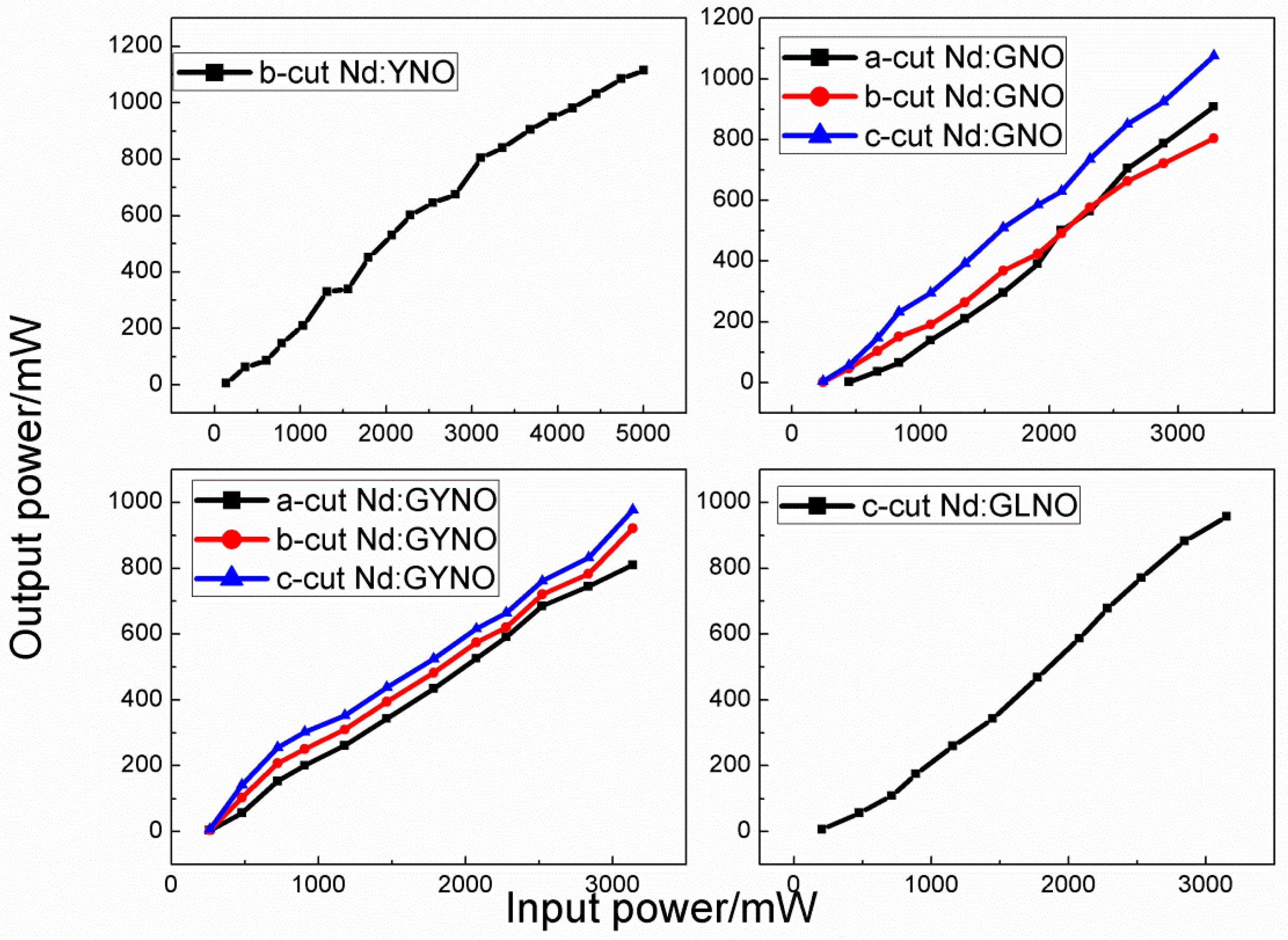
| Crystal | a (Å) | b (Å) | c (Å) | β (°) | ρ (Å3) | V (g cm3) | Rp% | Rwp% |
|---|---|---|---|---|---|---|---|---|
| GdTaO4 | 5.41 | 11.05 | 5.07 | 95.58 | 8.83 | 302.56 | - | - |
| Nd:GdTaO4 | 5.40 | 11.06 | 5.08 | 95.61 | 8.89 | 302.09 | 4.3 | 6.24 |
| Nd:GdYTaO4 | 5.38 | 11.04 | 5.08 | 95.58 | 8.42 | 300.25 | 9.9 | 7.34 |
| Er:GdTaO4 | 5.41 | 11.06 | 5.09 | 95.61 | 8.79 | 302.70 | 5.20 | 7.20 |
| Tm,Ho:GdYTaO4 | 5.39 | 11.03 | 5.08 | 95.61 | 8.595 | 300.43 | 8.91 | 6.22 |
| Yb,Ho:GdYTaO4 | 5.39 | 11.04 | 5.08 | 95.60 | 8.602 | 300.68 | 2.92 | 1.11 |
| Yb:GdNbO4 | 5.36 | 11.07 | 5.10 | 94.57 | 7.140 | 301.459 | 4.58 | 6.26 |
| Nd:GdNbO4 | 5.38 | 11.09 | 5.11 | 94.56 | 6.875 | 303.410 | 4.14 | 5.44 |
| Nd:YNbO4 | 7.041 | 10.952 | 5.301 | 94.56 | 5.568 | 299.640 | 6.70 | 8.75 |
| Nd:GdYNbO4 | 5.350 | 11.050 | 5.090 | 94.56 | 6.525 | 300.312 | 4.04 | 5.28 |
| Nd:GdLaNbO4 | 5.381 | 11.112 | 5.111 | 94.56 | 6.791 | 304.694 | 5.86 | 4.20 |
| Crystals | Ω2/10−20 cm2 | Ω4/10−20 cm2 | Ω6/10−20 cm2 | Ω4/Ω6 |
|---|---|---|---|---|
| 30 at% Er:YSGG [37] | 0.23 | 0.86 | 0.37 | 2.32 |
| 0.6 at% Er:Gd2SiO5 [38] | 6.168 | 1.878 | 1.255 | 1.50 |
| 0.5 at% Er:Lu2SiO5 [39] | 4.451 | 1.614 | 1.158 | 1.39 |
| 30 at% Er:GdTaO4 [34] | 0.98 | 1.17 | 4.67 | 0.25 |
| 1 at% Er:GdTaO4 [35] | 7.553 | 1.999 | 1.404 | 1.42 |
| Crystals | FWHM (@808 nm) | σα (10–20 cm2) (@808 nm) | σem (10−20 cm2) (@1.06 μm) | τem (μs) |
|---|---|---|---|---|
| Nd:GdYTaO4 | 6~12 | 6.9 | 22 | 182 |
| Nd:GdTaO4 | 6 | 5.1 | 39 | 178 |
| Nd:YAG | 1.5 | 8.3 | 34 | 240 |
| Nd:YVO4 | 4.4 | 40 | 156 | 99 |
| Crystals | α/cm−1 (3H6→3H4) | α/cm−1 (2F7/2→2F5/2) | ||||
|---|---|---|---|---|---|---|
| a | b | c | a | b | c | |
| Tm,Ho:GdYTaO4 | 2.75 | 2.79 | 3.88 | – | – | – |
| Yb,Ho:GdYTaO4 | – | – | – | 5.08 | 5.60 | 9.67 |
| Crystals | 5I6/μs | 5I7/ms | 5I7/5I6 |
|---|---|---|---|
| Tm,Ho:GdYTaO4 | 131 | 4.09 | 31.2 |
| Yb,Ho:GdYTaO4 | 419 | 7.3 | 17.4 |
| Yb,Ho:YSGG | 585 | 10.2 | 17.4 |
| Tm,Ho:LuAG | 250 | 7.5 | 30.0 |
| Tm,Ho:YAG | 40 | 11.4 | 285 |
| Crystal | σabs/10−20 cm2 | σem/10−20 cm2 |
|---|---|---|
| Yb:YNbO4 | 0.73 (933 nm) | 1.81 (1005 nm) |
| 1.85 (955 nm) | 1.11 (1021 nm) | |
| 0.86 (974 nm) | 0.57 (1030 nm) | |
| 0.44 (1003 nm) | ||
| Yb:GdNbO4 | 0.87 (936 nm) | 0.446 (1003) |
| 0.97 (955 nm) | 0.487 (1018) | |
| 0.85 (975 nm) | 0.466 (1030) | |
| 0.34 (1000 nm) | ||
| Yb:Y2O3 | 1.16 (978 nm) | 0.95 (1032 nm) |
| Yb:YAG | 0.86 (935 nm) | 2.50 (1030 nm) |
| Yb:Lu2O3 | 1.23 (978 nm) | 0.95 (1034.5 nm) |
| Yb:GSO | 0.69 (925 nm) | 0.66 (1030 nm) 0.38 (1048 nm) 0.41 (1088 nm) |
| Yb:FAP | 10.0 (905 nm) | 5.9 (942 nm) |
| Crystals | β9/2 | β11/2 | β13/2 | β15/2 | τrad |
|---|---|---|---|---|---|
| 1 at% Nd:GLNO | 31.22 | 55.10 | 13.04 | 0.64 | 176 |
| 1 at% Nd:GYNO | 33.04 | 53.88 | 12.47 | 0.61 | 156 |
| 2 at% Nd:GNO | 31.53 | 54.90 | 12.95 | 0.62 | 178 |
| 1 at% Nd:YNO | 34.39 | 52.98 | 12.05 | 0.58 | 152 |
| Parameters | Nd3+: GdTaO4 | Nd3+: LuTaO4 |
|---|---|---|
| −1891 | 778 | |
| −299 | −321 | |
| 702 | −682 | |
| 601 + 267i | −1017 + 795i | |
| −302 + 149i | 105 + 648i | |
| −696 | −421 | |
| 248 + 620i | −302 − 118i | |
| 579 − 12i | −651 − 848i | |
| 77 − 168i | 359 + 741i | |
| ξ | 998 | 902 |
| 2S+1LJ | Nd3+ Energy Levels of GdTaO4 | Nd3+ Energy Levels of LuTaO4 | ||||
|---|---|---|---|---|---|---|
| E (calc) | E (exp) | ΔE (cm−1) | E (calc) | E (exp) | ΔE (cm−1) | |
| 4I9/2 | −4.81 | 0 | 4.81 | −21.81 | 0 | 21.81 |
| 122.09 | 117 | −5.09 | 105.21 | 115 | 9.79 | |
| 242.10 | 238 | −4.10 | 162.71 | 151 | −11.71 | |
| 372.17 | 367 | −5.17 | 289.78 | 275 | −14.78 | |
| 489.45 | 498 | 8.55 | 640.44 | 631 | −9.44 | |
| 4I11/2 | 2319.31 | – | – | 1978.34 | 1983 | 4.66 |
| 2369.08 | – | – | 1992.01 | 2005 | 12.99 | |
| 2450.84 | – | – | 2058.63 | 2039 | −19.63 | |
| 2516.72 | – | – | 2123.43 | 2123 | −0.43 | |
| 2592.42 | – | – | 2292.47 | 2304 | 11.53 | |
| 2656.79 | – | – | 2354.40 | 2361 | 6.60 | |
| 4I13/2 | 4619.13 | 4632 | 12.87 | 3924.45 | 3921 | −3.45 |
| 4672.95 | 4668 | −4.95 | 3933.55 | 3939 | 5.45 | |
| 4735.59 | 4745 | 9.41 | 3991.05 | 3980 | −11.05 | |
| 4829.13 | 4842 | 12.87 | 4055.60 | 4057 | 1.40 | |
| 4873.80 | – | – | 4250.42 | 4260 | 9.58 | |
| 4936.67 | 4944 | 7.33 | 4297.58 | 4310 | 12.42 | |
| 5044.43 | 5036 | −8.43 | 4383.78 | 4354 | −29.78 | |
| 4I15/2 | 6874.39 | 6872 | −2.39 | 5830.42 | – | – |
| 7000.32 | 7013 | 12.68 | 5880.84 | – | – | |
| 7071.00 | 7084 | 13.00 | 5929.69 | – | – | |
| 7134.39 | 7148 | 13.61 | 5989.77 | – | – | |
| 7226.49 | 7239 | 12.51 | 6349.72 | – | – | |
| 7322.92 | 7324 | 1.08 | 6405.49 | – | – | |
| 7462.48 | 7470 | 7.52 | 6552.21 | – | – | |
| 7621.33 | – | – | 6630.59 | – | – | |
| 4F3/2 | 11,617.98 | 11,615 | −2.98 | 11,368.59 | 11,365 | −3.59 |
| 11,878.56 | 11,877 | −1.56 | 11,451.70 | 11,450 | −1.70 | |
| 4F5/2 + 2H9/2(2) | 12,755.05 | 12,731 | −24.05 | 12,336.64 | 12,341 | 4.36 |
| 12,874.09 | 12,862 | −12.09 | 12,390.72 | 12,409 | 18.28 | |
| 12,907.30 | 12,898 | −9.30 | 12,510.25 | 12,509 | −1.25 | |
| 12,954.06 | 12,935 | −19.06 | 12,551.60 | 12,550 | −1.60 | |
| 13,026.22 | 13,014 | −12.22 | 12,599.59 | 12,590 | −9.59 | |
| 13,185.56 | 13,171 | −14.56 | 12,695.51 | 12,677 | −18.51 | |
| 4F7/2 + 4S3/2 | 13,926.81 | 13,936 | 9.19 | 13,349.10 | 13,321 | −28.10 |
| 14,094.82 | 14,082 | −12.82 | 13,400.94 | 13,382 | −18.94 | |
| 14,151.69 | 14,163 | 11.31 | 13,487.04 | 13,497 | 9.96 | |
| 14,184.81 | – | – | 13,499.67 | 13,517 | 17.33 | |
| 13,514.64 | 13,532 | 17.36 | ||||
| 13,611.03 | 13,6.23 | 11.97 | ||||
| 4F9/2 | 15,451.31 | 15,464 | 12.69 | 14,583.44 | 14,573 | −10.44 |
| 15,623.02 | 15,601 | −22.02 | 14,795.59 | 14,767 | −28.59 | |
| 15,675.85 | 15,664 | −11.85 | 14,868.52 | 14,873 | 4.48 | |
| 15,740.08 | – | – | 15,842.02 | 15,844 | 1.98 | |
| 15,814.32 | 15,825 | 10.68 | 15,929.31 | 15,927 | −2.31 | |
| 2H11/2(2) | 16,824.27 | 16,833 | 8.73 | 16,846.25 | 16,844 | −2.25 |
| 16,881.07 | 16,896 | 14.93 | 16,919.53 | 16,922 | 2.47 | |
| 16,911.49 | – | – | 17,058.50 | 17,036 | −22.50 | |
| 16,947.09 | 16,965 | 17.91 | 17,168.31 | 17,194 | 25.69 | |
| 4G5/2 + 2G7/2 | 17,629.64 | 17,646 | 16.36 | 17,214.23 | 17,221 | 6.77 |
| 17,898.56 | 17,884 | −14.56 | 17,236.09 | 17,256 | 19.91 | |
| 17,959.88 | 17,940 | −19.88 | 17,261.02 | – | – | |
| 17,288.86 | – | – | ||||
| 17,455.47 | 17,459 | 3.53 | ||||
| 4G7/2 | 19,681.74 | 19,697 | 15.26 | 18,707.70 | 18,711 | 3.30 |
| 19,834.97 | 19,825 | −9.97 | 18,811.84 | 18,811 | −0.84 | |
| 18,914.48 | 18,924 | 9.52 | ||||
| 18,949.08 | – | – | ||||
| 2K13/2 + 4G9/2 | 20,511.95 | 20,502 | −9.95 | 19,135.78 | 19,150 | 14.22 |
| 20,680.22 | 20,687 | 6.78 | 19,292.55 | 19,307 | 14.45 | |
| 19,378.41 | 19,379 | 0.59 | ||||
| 2G9/2(1) + 4G11/2 + 2D3/2(1) + 2K15/2 | 21,672.12 | 21,678 | 5.88 | 20,780.79 | 20,790 | 9.21 |
| 22,014.24 | – | – | 20,844.56 | 20,843 | −1.56 | |
| 22,240.88 | 22,230 | −10.88 | 20,977.02 | 20,973 | −4.02 | |
| 22,847.41 | 22,841 | −6.41 | 21,022.94 | 21,017 | −5.94 | |
| 21,098.53 | 21,088 | −10.53 | ||||
| 21,183.83 | 21,194 | 10.17 | ||||
| 21,519.96 | 21,523 | 3.04 | ||||
| 21,604.87 | 21,606 | 1.13 | ||||
| 21,762.06 | 21,774 | 11.94 | ||||
| 2P1/2 | 23,973.77 | 23,960 | −13.77 | 23,085.11 | 23,095 | 9.89 |
| 2D(1)5/2 | 23,597.10 | 23,585 | −12.10 | |||
| 23,698.37 | 23,685 | −13.37 | ||||
| 23,811.15 | 23,787 | −24.15 | ||||
| 2P3/2 | 25,952.60 | 25,965 | 12.40 | |||
| 26,074.57 | 26,074 | −0.57 | ||||
| 4D3/2 | 27,638.97 | 27,628 | −10.97 | 27,535.98 | 27,539 | 3.02 |
| 27,648.17 | 27,636 | −12.17 | ||||
| 4D5/2 | 27,840.16 | 27,848 | 7.84 | |||
| 28,029.62 | 28,027 | −2.62 | ||||
| 2I11/2 + 4D1/2 | 29,682.98 | 29,676 | −6.98 | 28,221.24 | 28,218 | −3.24 |
| 28,328.95 | 28,324 | −4.95 | ||||
| 2I13/2 + 2L17/2 | 31,640.01 | 31,650 | 9.99 | 28,928.96 | 28,917 | −11.96 |
| … | – | – | 29,682.43 | 29,677 | −5.43 | |
| 4D7/2 + 2D(2)3/2 | 30,182.43 | 30,177 | −5.43 | |||
| 32,893.13 | 32,895 | 1.87 | ||||
| 2H(1)11/2 | 33,790.34 | 33,806 | 15.66 | |||
| 2F(2)5/2 | 35,808.85 | 35,822 | 13.15 | 34,382.58 | – | – |
| … | – | – | … | – | – | |
| Parameters | Nd3+:GdTaO4 | Nd3+:LuTaO4 | Nd3+: YAlO3 [60] |
|---|---|---|---|
| Eavg | 25,167 | 24,141 | 24,119 |
| F2 | 73,018 | 73,290 | 70,925 |
| F4 | 52,789 | 60,030 | 50,794 |
| F6 | 35,757 | 44,220 | 35,424 |
| ξ | 1034 | 883 | 875 |
| α | 21.34 | 11.22 | 23 |
| β | −593 | −436 | −691 |
| γ | 1445 | −1694 | 1690 |
| T2 | [298] | [298] | [458] |
| T3 | [35] | [35] | [38] |
| T4 | [59] | [59] | [75] |
| T6 | [−285] | [−285] | [−290] |
| T7 | [332] | [332] | [237] |
| T8 | [305] | [305] | [496] |
| M | 2.11 | 2.11 | 1.90 |
| P | 192 | 192 | 206 |
| −1560 | 591 | −154 | |
| −415 | −153 − 70i | 578 | |
| 626 | −753 | −541 | |
| 422+315i | −1428 + 611i | 967 + 24i | |
| 244 + 18i | 516 + 898i | −309+608i | |
| −613 | −522 | −671 | |
| −301 + 807i | −147 − 43i | 512 − 18i | |
| −100 − 103i | 172 − 820i | 1611 + 0i | |
| −279 − 107i | 533 + 864i | 0 + 132i | |
| σ | 12.66 | 14.6 | 15.50 |
| Nν | 2959 | 2927 | 2545 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, R.; Zhang, Q.; Gao, J.; Chen, Y.; Ding, S.; Peng, F.; Liu, W.; Sun, D. Rare-Earth Tantalates and Niobates Single Crystals: Promising Scintillators and Laser Materials. Crystals 2018, 8, 55. https://doi.org/10.3390/cryst8020055
Dou R, Zhang Q, Gao J, Chen Y, Ding S, Peng F, Liu W, Sun D. Rare-Earth Tantalates and Niobates Single Crystals: Promising Scintillators and Laser Materials. Crystals. 2018; 8(2):55. https://doi.org/10.3390/cryst8020055
Chicago/Turabian StyleDou, Renqin, Qingli Zhang, Jinyun Gao, Yuanzhi Chen, Shoujun Ding, Fang Peng, Wenpeng Liu, and Dunlu Sun. 2018. "Rare-Earth Tantalates and Niobates Single Crystals: Promising Scintillators and Laser Materials" Crystals 8, no. 2: 55. https://doi.org/10.3390/cryst8020055




