Effect of Sorbitol Templates on the Preferential Crystallographic Growth of Isotactic Polypropylene Wax
Abstract
:1. Introduction
2. Materials and Methods
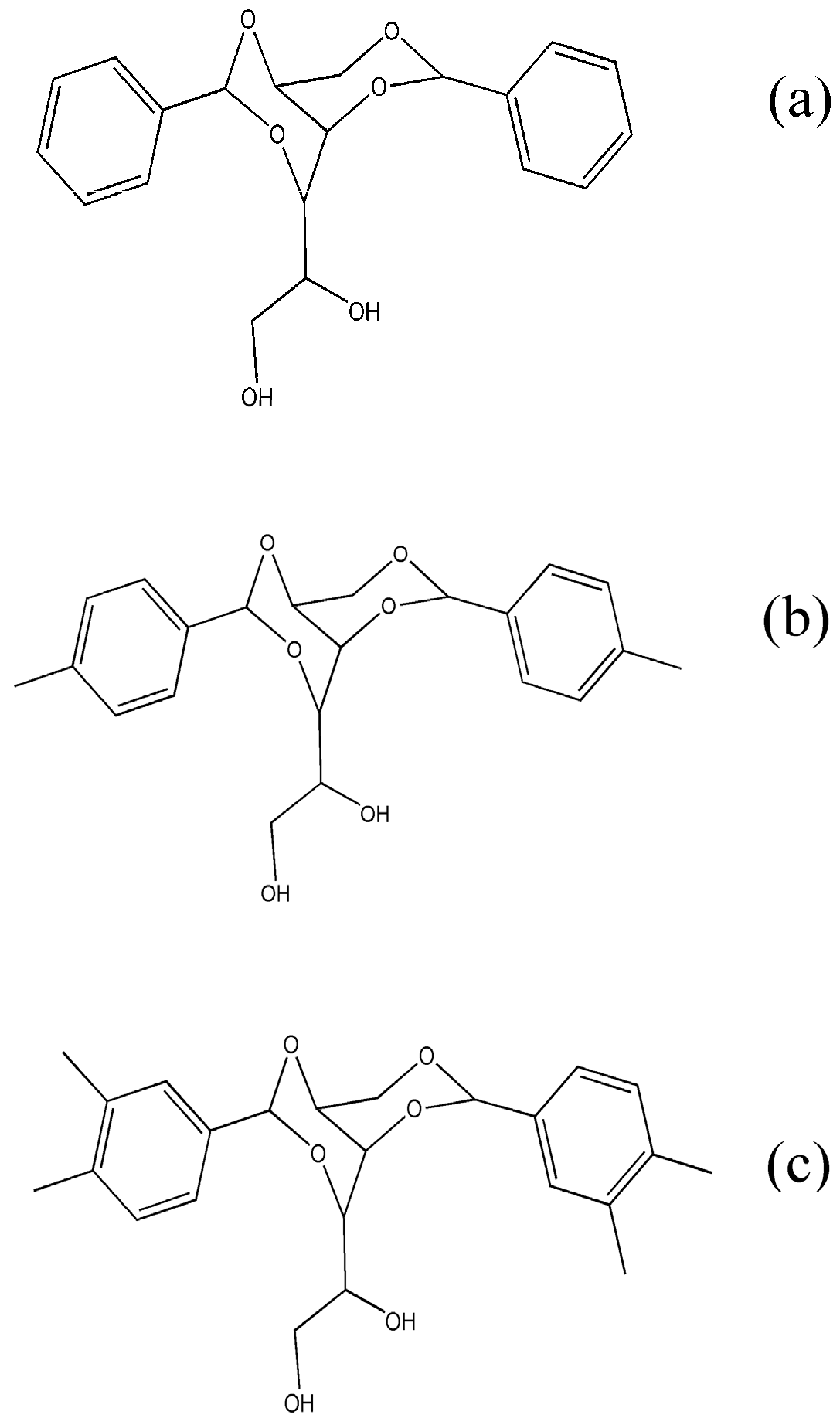
2.1. Materials and Sample Preparations
2.2. Differential Scanning Calorimetry
2.3. Polarized Optical Light Microscopy
2.4. Real-Time Wide Angle X-ray Scattering (WAXS)
3. Results and Discussion
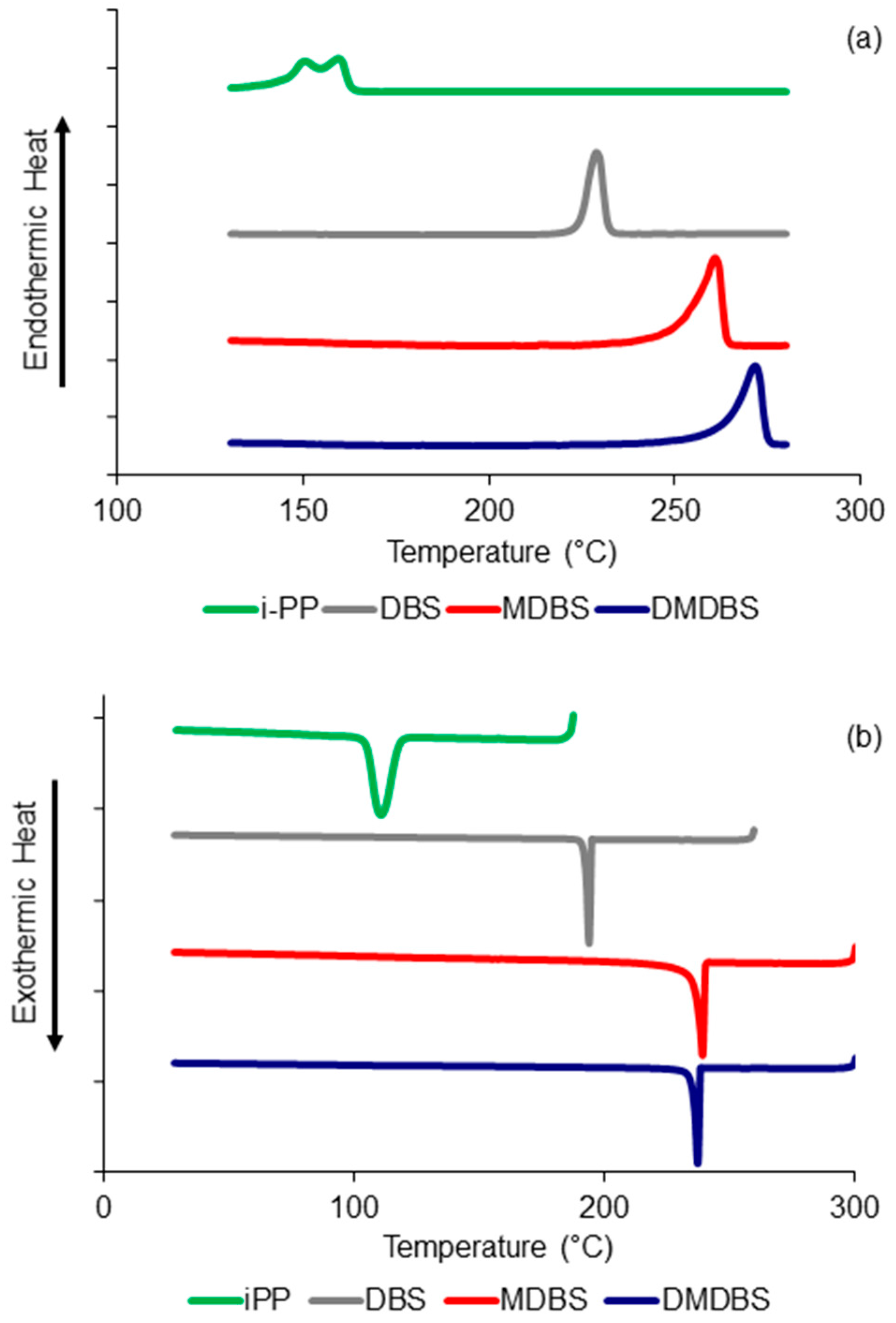
3.1. Thermal Behavior of iPP Wax and Nucleating Agents
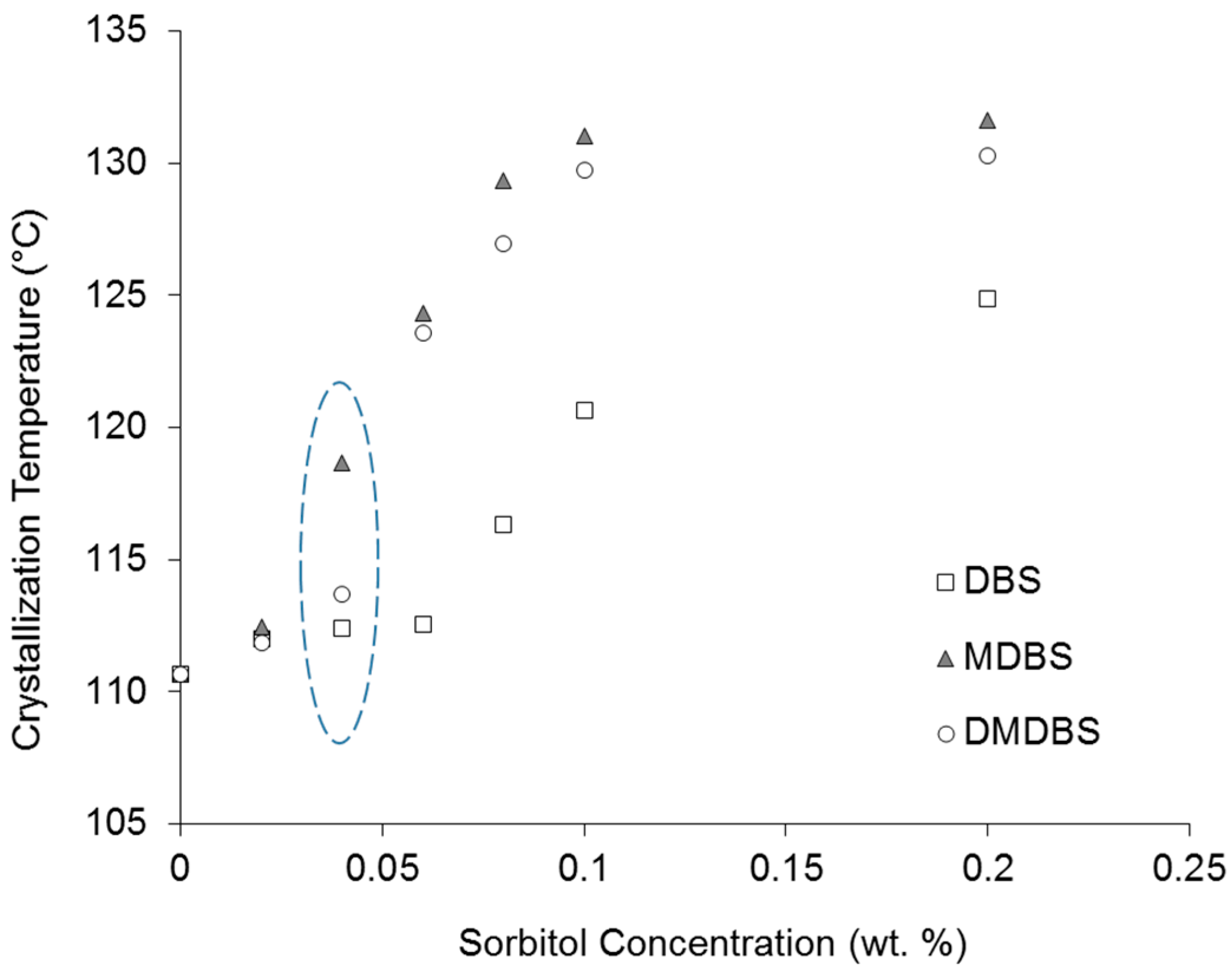
3.2. Sorbitol Nucleating Effect on iPP Wax
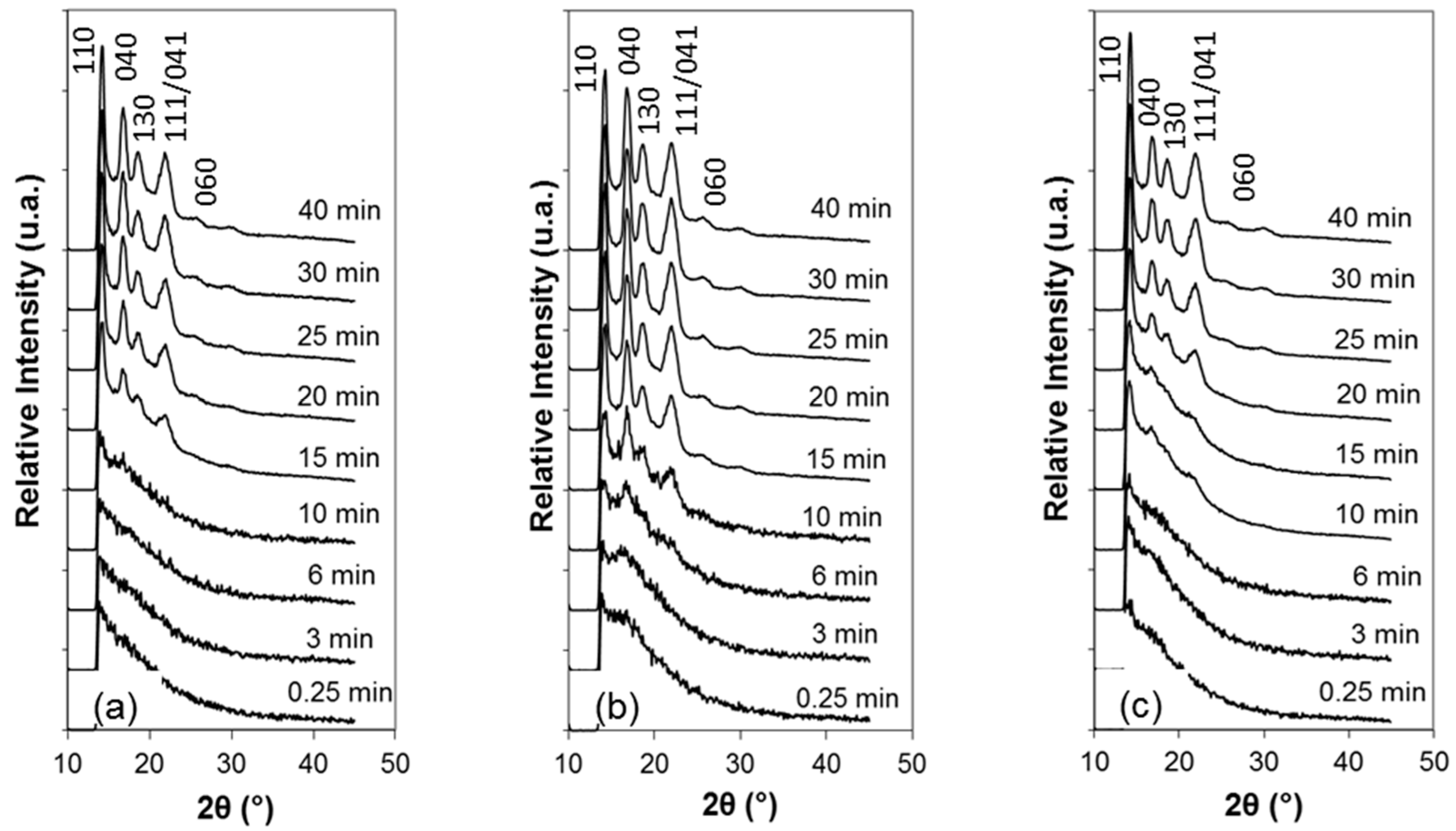
3.3. Unit Cells and Lamellar Structures during Isothermal Crystallization
3.4. Macrostructure Morphology during Isothermal Crystallization
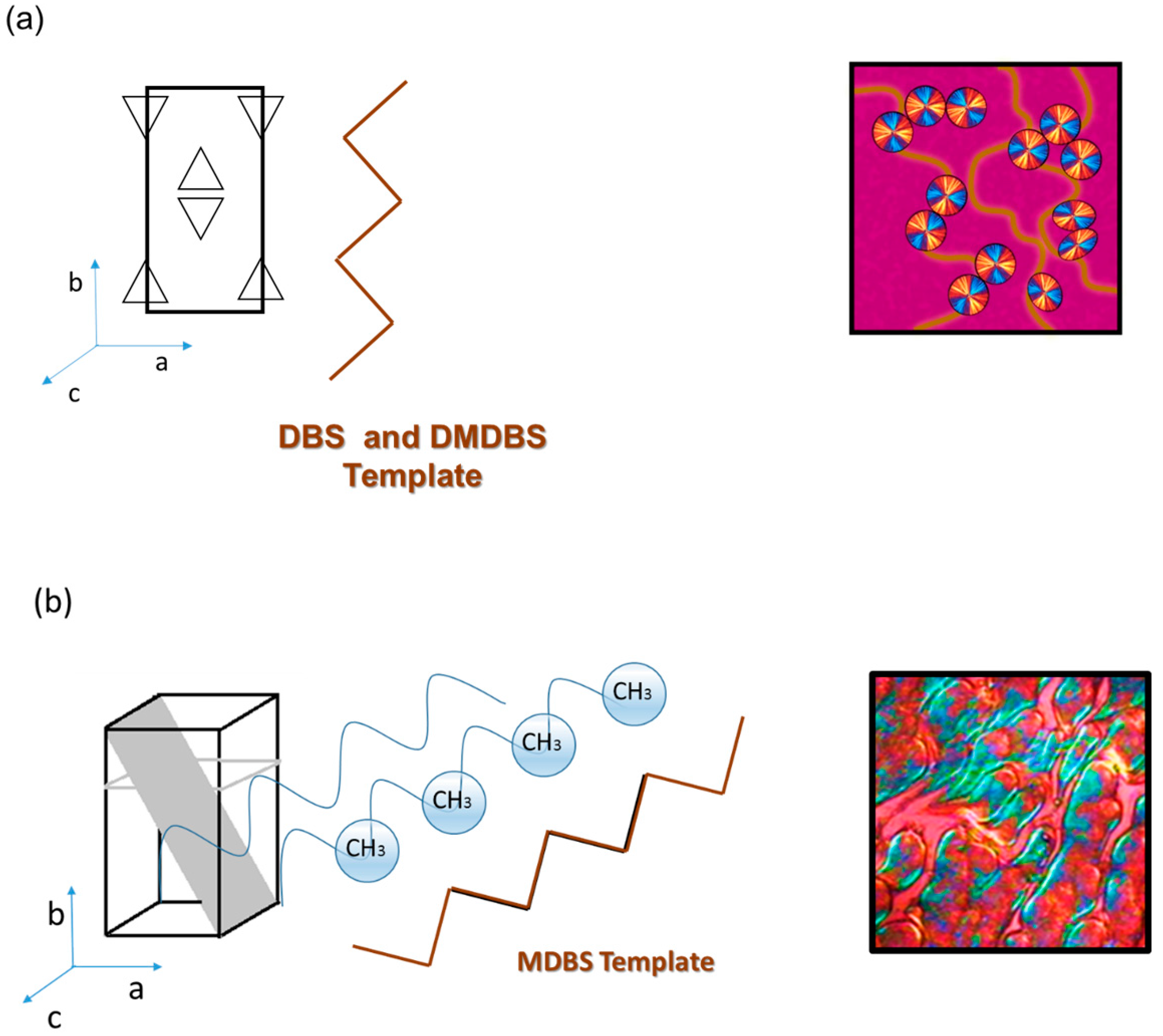
3.5. Interfacial Crystallization of the iPP Wax–Sorbitol Compounds
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Smith, T.L.; Masilamani, D.; Bui, L.K.; Brambilla, R.; Khanna, Y.P.; Garbriel, K.A. Acetals as nucleating agents for polypropylene. J. Appl. Polym. Sci. 1994, 52, 591–596. [Google Scholar] [CrossRef]
- Bernland, K.; Goossens, J.G.P.; Smith, P.; Tervoort, T.A. On clarification of haze in polypropylene. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 865–874. [Google Scholar] [CrossRef]
- Fairgrieve, S. Nucleating Agents, 1st ed.; Rapra Technology, Rapra Review Report 187, SPF Polymer Consultants; iSmithers Rapra Publishing: Shawbury, UK, 2005; Volume 16, p. 6. [Google Scholar]
- Lin, K.-Y.; Xanthos, M.; Sirkar, K.K. Novel polypropylene-based microporous membranes via spherulitic deformation. J. Membr. Sci. 2009, 330, 267–278. [Google Scholar] [CrossRef]
- Folkes, M.J. Interfacial crystallization of polypropylene in composites. In Polypropylene: Structure, Blends, and Composites, 1st ed.; Karger-Kocsis, J., Ed.; Chapman and Hall: London, UK, 1995; Volume 3, p. 340. [Google Scholar]
- Wang, X.; Ouyang, J.; Su, J.; When, Z. Investigating the role of oriented nucleus in polymer shish-kebab crystal growth via phase-field method. J. Chem. Phys. 2004, 140, 114102. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Wang, Z.; Yang, L.; Zhao, J.; Niu, Y.; Hsiao, B. Deformation-induced highly oriented and stable mesomorphic phase in quenched isotactic polypropylene. Polymer 2007, 48, 6934–6947. [Google Scholar] [CrossRef]
- Bruckner, S.; Meille, S.V.; Petraccone, V.; Pirozzi, B. Polymorphism in isotactic polypropylene. Prog. Polym. Sci. 1991, 16, 361–404. [Google Scholar] [CrossRef]
- Fischer, C.; Drummer, D. Crystallization and Mechanical Properties of Polypropylene under Processing-Relevant Cooling Conditions with Respect to Isothermal Holding Time. Int. J. Polym. Sci. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Turner-Jones, A.; Aizlewood, J.M.; Beckett, D.R. Crystalline forms of isotactic polypropylene. Makromol. Chem. 1964, 75, 134–158. [Google Scholar] [CrossRef]
- Varga, J. β-Modification of isotactic polypropylene: Preparation, structure, processing, properties, and application. J. Macromol. Sci. B 2002, 41, 1121–1171. [Google Scholar] [CrossRef]
- Sowinski, P.; Piorkowska, E.; Boyer, S.; Haudin, J. Nucleation of crystallization of isotactic polypropylene in the gamma form under high pressure in nonisothermal conditions. Eur. Polym. J. 2016, 85, 564–574. [Google Scholar] [CrossRef]
- Ma, Z.; Shao, C.G.; Wang, X.; Zhao, B.J.; Li, X.Y.; An, H.N.; Yan, T.Z.; Li, Z.M.; Li, L.B. Critical stress for drawing-induced alpha crystal-mesophase transition in isotactic polypropylene. Polymer 2009, 50, 2706–2715. [Google Scholar] [CrossRef]
- Looijmans, S.; Menyhard, A.; Peters, G.; Alfonso, G.; Cavallo, D. Anomalous temperature dependence of isotactic polypropylene α-on-β cross-nucleation kinetics. Cryst. Growth Des. 2017, 17, 4936–4943. [Google Scholar] [CrossRef]
- Dou, Q.; Xue, J. Effect of an In-Situ Nucleating Agent on the Polymorphs and Mechanical Properties of Isotactic Polypropylene. J. Macromol. Sci. B 2015, 54, 947–961. [Google Scholar] [CrossRef]
- Suzuki, M.; Hanabusa, K. Polymer organogelators that make supramolecular organogels through physical cross-linking and self-assembly. Chem. Soc. Rev. 2010, 39, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Balzano, L.; Rastogi, S.; Gerrit, W.M. Flow Induced Crystallization in Isotactic Polypropylene−1,3:2,4-Bis(3,4-dimethylbenzylidene)sorbitol Blends: Implications on Morphology of Shear and Phase Separation. Macromolecules 2008, 41, 399–408. [Google Scholar] [CrossRef]
- Okesola, B.; Vieira, V.; Cornwell, D.; Whitelaw, N.; Smith, D. 1,3:2,4-Dibenzylidene-D-sorbitol (DBS) and its derivatives—Efficient, versatile and industrially-relevant low-molecular-weight gelators with over 100 years of history and a bright future. Soft Matter 2015, 11, 4768–84787. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, X.; Liu, Y.; Li, L.; Bo, Z.; Zhou, J.; Huo, H. Structure difference of sorbitol derivatives influence the crystallization and performance of P3OT/PCBM organic photovoltaic solar cells. Org. Electron. 2017, 46, 158–165. [Google Scholar] [CrossRef]
- Lai, W.; Tseng, S.; Chao, Y. Effect of Hydrophobicity of Monomers on the Structures and Properties of 1,3:2,4-Dibenzylidene-d-sorbitol Organogels and Polymers Prepared by Templating the Gels. Langmuir 2011, 27, 12630–12635. [Google Scholar] [CrossRef] [PubMed]
- Lipp, J.; Shuster, M.; Terry, A.E.; Cohen, Y. Fibril formation of 1,3:2,4-Di(3,4-dimethylbenzylidene) sorbitol in polymer melts. Polym. Eng. Sci. 2008, 48, 705–710. [Google Scholar] [CrossRef]
- Shepard, T.A.; Delsorbo, C.R.; Louth, R.M.; Walborn, J.L.; Norman, D.A.; Harvey, N.G.; Spontak, R.J. Self-organization and polyolefin nucleation efficacy of 1,3:2,4-di-p-methylbenzylidene sorbitol. J. Polym. Sci. Part B Polym. Phys. 1997, 35, 2617–2628. [Google Scholar] [CrossRef]
- Smith, T.; Masilamani, D.; Bui, L.; Khanna, Y.; Bray, R.; Hammond, W.; Curran, S.; Belles, J.; Binder-Castelli, S. The Mechanism of Action of Sugar Acetals as Nucleating Agents for Polypropylene. Macromolecules 1994, 27, 3147–3155. [Google Scholar] [CrossRef]
- Lin, M.C.; Chen, H.L.; Lin, W.F.; Huang, P.S.; Tsai, J.C. Crystallization of Isotactic Polypropylene under the Spatial Confinement Templated by Block Copolymer Microdomains. J. Phys. Chem. B 2012, 116, 12357–12371. [Google Scholar] [CrossRef] [PubMed]
- Sukhanova, T.; Lednický, F.; Urban, J.; Baklagina, Y.; Mikhailov, G.; Kudryavtsev, V. Morphology of melt crystallized polypropylene in the presence of polyimide fibres. J. Mater. Sci. 1995, 30, 2201–2214. [Google Scholar] [CrossRef]
- Abdou, J.; Braggin, G.; Luo, Y.; Stevenson, A.; Chun, D.; Zhang, S. Graphene-Induced Oriented Interfacial Microstructures in Single Fiber Polymer Composites. Appl. Mater. Interfaces 2015, 7, 13620–13626. [Google Scholar] [CrossRef] [PubMed]
- Ponçot, M.; Martin, J.; Chaudemanche, S.; Ferry, O.; Schenk, T.; Bourson, P. Complementarities of high energy WAXS and Raman spectroscopy measurements to study the crystalline phase orientation in polypropylene blends during tensile test. Polymer 2015, 80, 27–37. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. II Transformation-Time Relations for Random Distribution of Nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Yi, Q.-F.; Wen, X.-J.; Niu, H.; Dong, J.-Y. How does a polymerized compounding affect the nucleation effect of a sorbitol derivative nucleating agent in isotactic polypropylene melt crystallization? J. Appl. Polym. Sci. 2013, 127, 888–903. [Google Scholar] [CrossRef]
- Katsuno, S.; Yoshinaga, M.; Kitade, S.; Sanada, Y.; Akiba, I.; Sakurai, K.; Masunaga, H. Crystallization kinetics of polypropylene containing a sorbitol nucleating agent. Polym. J. 2013, 45, 87–93. [Google Scholar] [CrossRef]
- Mi, D.; La, R.; Chen, W.; Zhang, J. Different kinds of transcrystallinity developed from glass fiber/isotactic polypropylene/β-nucleation agents composite by microinjection molding. Polym. Adv. Technol. 2016, 27, 1220–1227. [Google Scholar] [CrossRef]
- Amitay-Sadovsky, E.; Cohen, S.R.; Wagner, H.D. Nanoscale Shear and Indentation Measurements in Transcrystalline R-Isotactic Polypropylene. Macromolecules 2001, 34, 1252–1257. [Google Scholar] [CrossRef]

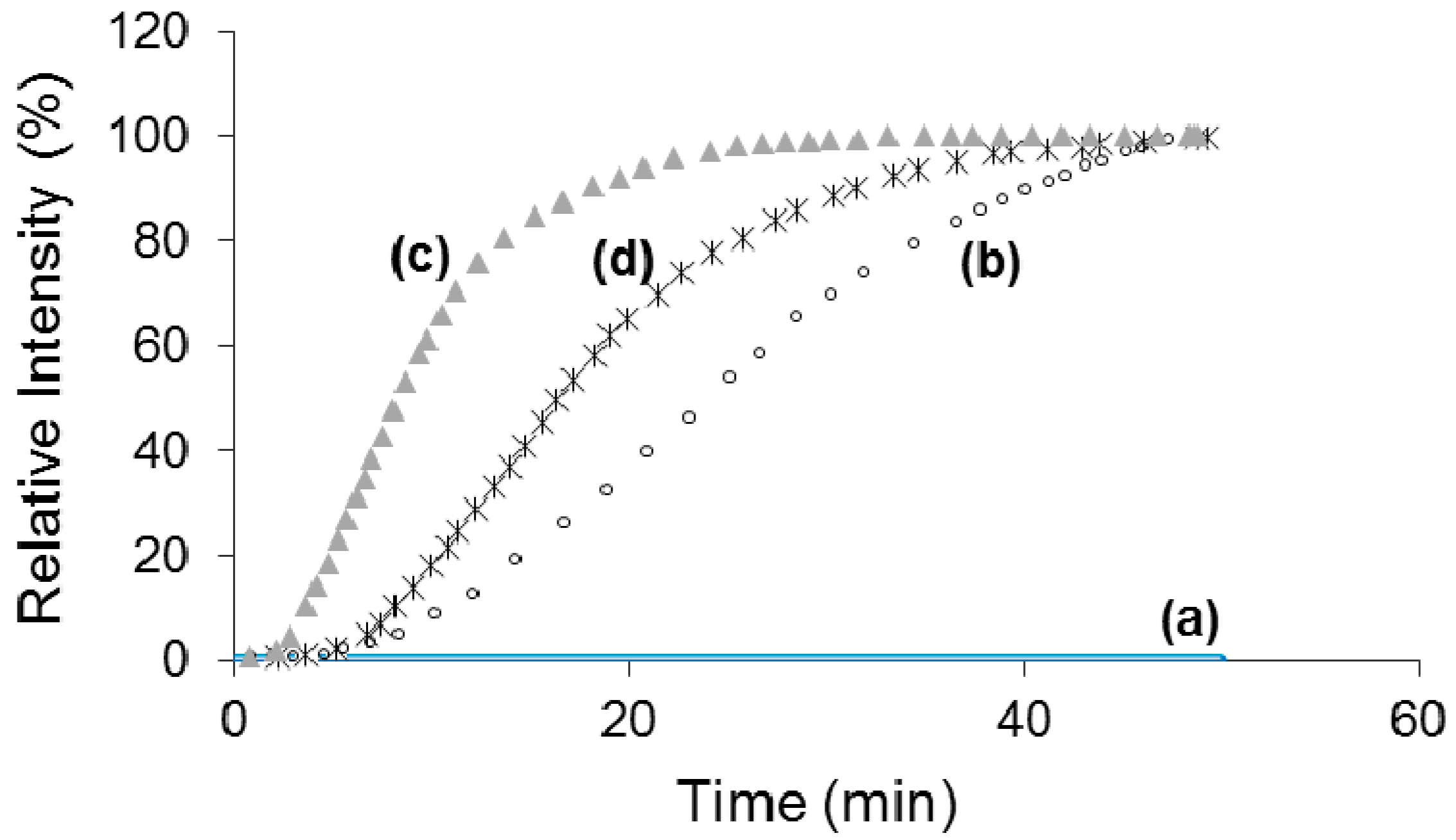
| Sorbitol Template | Avrami Index (n) | Crystal Geometry |
|---|---|---|
| DBS | 3.11 | Spherulitic |
| MDBS | 1.95 | Disk |
| DMDBS | 2.96 | Spherulitic |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quiñones-Jurado, Z.V.; Ávila-Orta, C.A.; Castillo-Reyes, B.E.; Mata-Padilla, J.M.; Hsiao, B.S.; Medellín-Rodríguez, F.J.; Waldo-Mendoza, M.A. Effect of Sorbitol Templates on the Preferential Crystallographic Growth of Isotactic Polypropylene Wax. Crystals 2018, 8, 59. https://doi.org/10.3390/cryst8020059
Quiñones-Jurado ZV, Ávila-Orta CA, Castillo-Reyes BE, Mata-Padilla JM, Hsiao BS, Medellín-Rodríguez FJ, Waldo-Mendoza MA. Effect of Sorbitol Templates on the Preferential Crystallographic Growth of Isotactic Polypropylene Wax. Crystals. 2018; 8(2):59. https://doi.org/10.3390/cryst8020059
Chicago/Turabian StyleQuiñones-Jurado, Zoe V., Carlos A. Ávila-Orta, Blanca E. Castillo-Reyes, José M. Mata-Padilla, Benjamin S. Hsiao, Francisco J. Medellín-Rodríguez, and Miguel A. Waldo-Mendoza. 2018. "Effect of Sorbitol Templates on the Preferential Crystallographic Growth of Isotactic Polypropylene Wax" Crystals 8, no. 2: 59. https://doi.org/10.3390/cryst8020059





