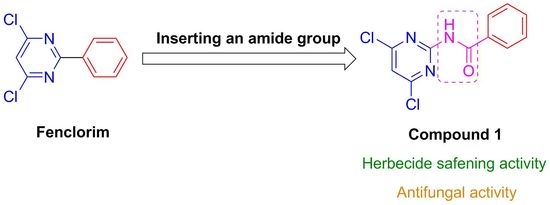
Synthesis, Crystal Structure, Herbicide Safening, and Antifungal Activity of N-(4,6-Dichloropyrimidine-2-Yl)Benzamide
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Techniques
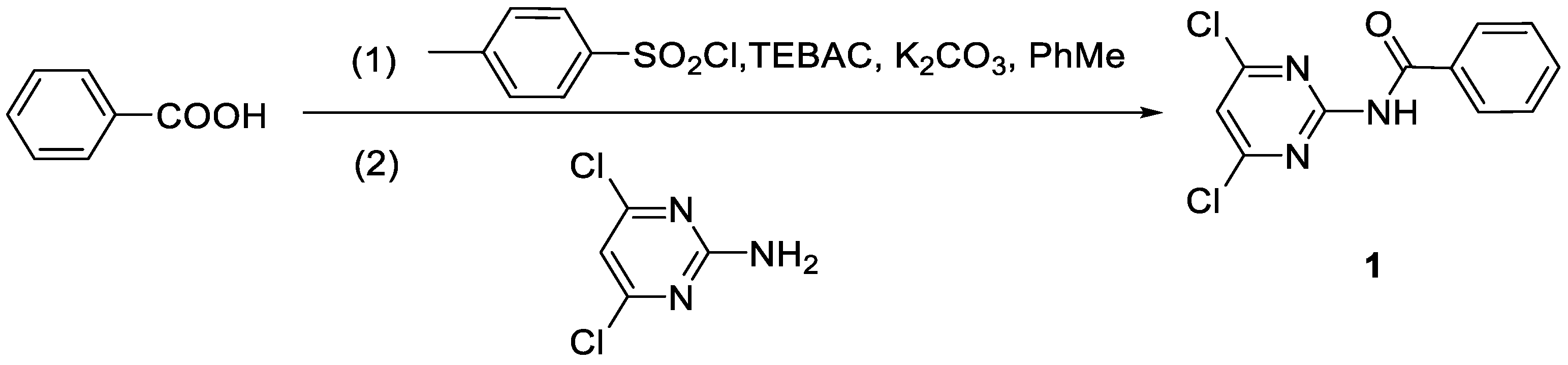
2.2. Synthetic Precedure
2.3. Structure Determination
2.4. Herbicide Safener Activity
2.5. Antifungal Activity
3. Results and Discussion
3.1. Crystal Structure
3.2. Spectroscopic Properties
3.3. Biological Activity
3.3.1. Evaluation of Herbicide Safener Activity
3.3.2. Evalution of Antifungal Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, H.J.; Xiong, M.Y.; Tian, B.L. Comparative phytotoxicity of Rac-metolachlor and S-metolachlor on rice seedlings. J. Environ. Sci. Health B 2012, 47, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.O.D.; Frova, C.; Schröder, P.; Tissut, M. Exploiting plant metabolism for the phytoremediation of persistent herbicides. Environ. Sci. Pollut. Res. 2002, 9, 18–28. [Google Scholar] [CrossRef]
- Rosinger, C.; Evans, P.; Hacker, E. Crop Plant-Compatible Herbicidal Compositions Comprising Herbicides and Safeners. U.S. Patent 20070010399A1, 11 January 2007. [Google Scholar]
- Zheng, Y.; Liu, B.; Gou, Z.; Li, Y.; Zhang, X.; Wang, Y.; Yu, S.; Li, Y.; Sun, D. Design of novel CSA analogues as potential safeners and fungicides. Bioorg. Med. Chem. Lett. 2015, 25, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.X.; Qu, H.T.; Fu, Y.; Gao, S.; Ye, F. Alleviation of injury from chlorimuron-ethyl in maize treated with safener 3-dichloroacetyl oxazolidine. Can. J. Plant Sci. 2015, 95, 897–903. [Google Scholar] [CrossRef]
- Mahoney, K.J.; Nurse, R.E.; Everman, W.J.; Sprague, C.L.; Sikkema, P.H. Tolerance of corn (L.) to early and late glyphosate applications. Am. J. Plant Sci. 2014, 5, 2748–2754. [Google Scholar] [CrossRef]
- Satchivi, N.; Schmitzer, P. Safening 6-(Trisubstituted Phenyl)-4-Amino-2-Pyridinecarboxylate Herbicide Injury on Cereal Crops (Wheat and Barley). Patent WO 2010059676A2, 27 May 2010. [Google Scholar]
- Rosinger, C. Herbicide safeners: An overview. Julius Kühn Archiv 2014, 443, 516–525. [Google Scholar]
- Jablonkai, I. Herbicide safeners: Effective tools to improve herbicide selectivity. In Herbicides-Current Research and Case Studies in Use; Andrew, J.P., Jessica, A.K., Eds.; Intech: Rijeka, Coratia, 2013; pp. 589–620. [Google Scholar]
- Parker, C. Herbicide protectants and antidotes—A review. Pans 1976, 22, 65–74. [Google Scholar]
- Hatzios, K.K. Herbicide antidotes: Development, chemistry, and mode of action. Adv. Agron. 1983, 36, 265–316. [Google Scholar]
- Hoffmann, O.L. Herbicide antidotes: From concept to practice. In The Chemistry and Action of Herbicide Antidotes; Pallos, F.M., Casida, J.E., Eds.; Academic Press: London, UK, 1978; pp. 1–13. [Google Scholar]
- Hoffmann, O.L. 1,8-Naphthalic Anhydride Protective Coatings of Cereals against Herbicidal S-ethyl N,N-Dipropylthiocarbamate. Patent DE 1952910A, 25 June 1970. [Google Scholar]
- Fedtke, C.; Strang, R.H. Synergistic activity of the herbicide safener dichlormid with herbicides affecting photosynthesis. Z. Naturforsch. C 1990, 45, 565–568. [Google Scholar]
- Chang, T.S.; Merkle, M.G. Oximes as seed safeners for grain sorghum (Sorghum bicolor) to herbicides. Weed Sci. 1982, 30, 70–73. [Google Scholar]
- Hall, J.C.; Stephenson, G.R. The basis for the synergizing and safening action of fenchlorazole-ethyl on the herbicidal activity of fenoxaprop-ethyl: A review. In Proceedings of the Crop Protection Conference-Weeds, Brington, UK, 20–23 November 1995; British Crop Protection Council: Surrey, UK, 1995; pp. 261–268. [Google Scholar]
- Hatzios, K.K. Interactions of the safener flurazole with chloroacetanilide and thiocarbamate herbicides on maize (Zea mays L.). Can. J. Plant Sci. 1986, 66, 353–359. [Google Scholar] [CrossRef]
- Tsukuda, K.; Ichizen, N.; Konnai, M.; Takematsu, T. Effect of seeding depth on crop injury by pyributicarb in direct-seeded rice plants and evaluation of dymron as a safener. J. Weed Sci. Technol. 2000, 45, 1–6. [Google Scholar] [CrossRef]
- Brazierhicks, M.; Evans, K.M.; Cunningham, O.D.; Hodgson, D.R.; Steel, P.G.; Edwards, R. Catabolism of glutathione conjugates in Arabidopsis thaliana. Role in metabolic reactivation of the herbicide safener fenclorim. J. Biochem. Chem. 2008, 283, 21102–21112. [Google Scholar]
- Deng, F.; Hatzios, K.K. Purification and characterization of two glutathione S-transferase isozymes from indica-type rice involved in herbicide detoxification. Pestic. Biochem. Physiol. 2002, 72, 10–23. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, X.; Fang, Y. Fenclorim effects on rice germination and yield. Can. J. Plant Sci. 2013, 93, 237–241. [Google Scholar] [CrossRef]
- Miyauchi, N.; Kobayashi, K.; Usui, K. Differential safening activity of dymron and fenclorim on pretilachlor injury in rice seedlings in soil. Weed Biol. Manag. 2002, 2, 46–51. [Google Scholar] [CrossRef]
- Montes, R.C.; Freitas, T.S.D.; Costa, M.D.S.; Oliveira, F.D.S.; Campina, F.F.; Ferreira, A.R.; Ferreira, S.D.O.; Melo, H.D.D.; Damião, P.D. Antimicrobial evaluation of cinnamic and benzoic haloamides. J. Chem. Pharm. Res. 2016, 8, 311–320. [Google Scholar]
- Lan, X.; Xie, D.; Yin, L.; Wang, Z.; Chen, J.; Zhang, A.; Song, B.; Hu, D. Novel α,β-unsaturated amide derivatives bearing α-amino phosphonate moiety as potential antiviral agents. Bioorg. Med. Chem. Lett. 2017, 27, 4270–4273. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.M.; Lu, S.; Chen, Y.D.; Lu, T.; Liu, H.C. Molecular docking and 3D-QSAR studies on a series of fused heterocyclic amides as B-Raf inhibitors. Chin. J. Struct. Chem. 2017, 36, 1568–1585. [Google Scholar]
- Deng, X.L.; Xie, J.; Li, Y.Q.; Yuan, D.K.; Zhang, X.P.; Wang, Q.M.; Chi, M.; Yang, X.L. Design, synthesis and biological activity of novel substituted pyrazole amide derivatives targeting EcR/USP receptor. Chin. Chem. Lett. 2016, 27, 566–570. [Google Scholar] [CrossRef]
- Deng, X.L.; Zhang, L.; Hu, X.P.; Yin, B.; Liang, P.; Yang, X.L. Target-based design, synthesis and biological activity of new pyrazole amide derivatives. Chin. Chem. Lett. 2016, 27, 251–255. [Google Scholar] [CrossRef]
- Sun, M.; Yang, H.H.; Tian, L.; Li, J.Q.; Zhao, W.G. Design, synthesis, and fungicidal activities of imino diacid analogs of valine amide fungicides. Bioorg. Med. Chem. Lett. 2015, 25, 5729–5731. [Google Scholar] [CrossRef] [PubMed]
- Gonec, T.; Kos, J.; Zadrazilova, I.; Pesko, M.; Keltosova, S.; Tengler, J. Antibacterial and herbicidal activity of ring-substituted 1-hydroxynaphthalene-2-carboxanilides. Bioorg. Med. Chem. Lett. 2013, 21, 6531–6541. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Hatzios, K.K. Effects of the herbicide pretilachlor and the safener fenclorim on glutathione content and glutathione-dependent enzyme activity of rice. Z. Naturforsch. C 1991, 46, 861–865. [Google Scholar]
- Hassan, H.M.; Farrag, A.A. Synthesis, and phytopathological application of some novel amino acid, dipeptide and diphenylphosphonic acid derivatives of 2-aminopyrimidine. J. Chem. Pharm. Res. 2011, 3, 776–785. [Google Scholar]
- Agha, K.A.; Abo-Dya, N.E.; Ibrahim, T.S.; Abdel-Aal, E.H. Efficient synthesis of N-acylbenzotriazoles using tosyl chloride: En route to suberoylanilide hydroxamic acid. ARKIVOC 2016, 3, 161–170. [Google Scholar]
- Ananthalakshmi, S.; Nallu, M. Kinetic investigation on the reactions of p-toluenesulfonyl chloride with p-substituted benzoic acid(s) in the presence of triethylamine in aprotic solvents. Int. J. Chem. Kinet. 2010, 2, 303–308. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Hatzios, K.K. Characterization and safener induction of multiple glutathione S-transferases in three genetic lines of rice. Pestic. Biochem. Physiol. 2002, 72, 24–39. [Google Scholar]
- Ma, M.; Feng, J.; Li, R.; Chen, S.W.; Xu, H. Synthesis and antifungal activity of ethers, alcohols, and iodohydrin derivatives of sclareol against phytopathogenic fungi in vitro. Bioorg. Med. Chem. Lett. 2015, 25, 2773–2777. [Google Scholar] [CrossRef] [PubMed]
- Thanusu, J.; Kanagarajan, V.; Gopalakrishnan, M. Synthesis, spectral analysis and in vitro microbiological evaluation of 3-(3-Alkyl-2,6-diarylpiperidin-4-ylidene)-2-thioxoimidazolidin-4-ones as a new class of antibacterial and antifungal agents. Bioorg. Med. Chem. Lett. 2010, 20, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.X.; Zhai, Z.W.; Yang, M.Y.; Sun, Z.H.; Wu, H.K.; Liu, X.H. Synthesis, crystal structure, DFT study and antifungal activity of 4-(5-((4-bromobenzyl)thio)-4-phenyl-4H-1,2,4-triazol-3-yl)pyridine. Crystals 2016, 6, 4. [Google Scholar] [CrossRef]
- Kwon, E.; Kim, J.; Kang, G.; Kim, T.H. Crystal structure of fenclorim. Acta Cryst. 2015, E71, o714. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, M.; Viswanathan, V.; Timiri, A.K.; Sinha, B.N.; Jayaprakash, V.; Velmurugan, D. Crystal structures of 2-[(4,6-di-amino-pyrimidin-2-yl)sulfan-yl]-N-(2,4-di-methyl-phen-yl)acetamide and 2-[(4,6-di-amino-pyrimidin-2-yl)sulfan-yl]-N-(3-meth-oxy-phen-yl)acetamide. Acta Cryst. 2017, 73, 996–1000. [Google Scholar]
- Weng, J.Q.; Wang, L.; Liu, X.H. Synthesis, crystal structure and herbicidal activity of a 1,2,4-triazol-5-(4H)-one derivative. J. Chem. Soc. Pak. 2012, 34, 1248–1252. [Google Scholar]
- Shen, Z.H.; Shi, Y.X.; Yang, M.Y.; Sun, Z.H.; Weng, J.Q.; Tan, C.X.; Liu, X.H.; Li, B.J.; Zhao, W.G. Synthesis, crystal structure, DFT studies and biological activity of a novel schiff base containing triazolo [4,3-a]pyridine moiety. Chin. J. Struct. Chem. 2016, 35, 457–464. [Google Scholar]
- Löser, R.; Pitzschler, R.; Köckerling, M.; Löser, R.; Pitzschler, R.; Köckerling, M. Synthesis and X-ray crystal structure of N′-cyano-N,N′-dimethyl-4-nitrobenzohydrazide. Crystals 2017, 7, 290. [Google Scholar] [CrossRef]
- Manchado, A.; Salgado, M.M.; Vicente, A.; Díez, D.; Sanz, F.; Garrido, N.M. Crystal structure of methyl (4R)-4-(4-meth-oxy-benzo-yl)-4-{[(1R)-1-phenyl-eth-yl]carbamo-yl}butano-ate. Acta Cryst. 2017, 73, 503–506. [Google Scholar]
- Tang, X.K.; Zhou, X.M.; Wu, J.; Li, J.B.; Bai, L.Y. A novel function of sanshools: The alleviation of injury from metolachlor in rice seedlings. Pestic. Biochem. Physiol. 2014, 110, 44–49. [Google Scholar] [CrossRef] [PubMed]
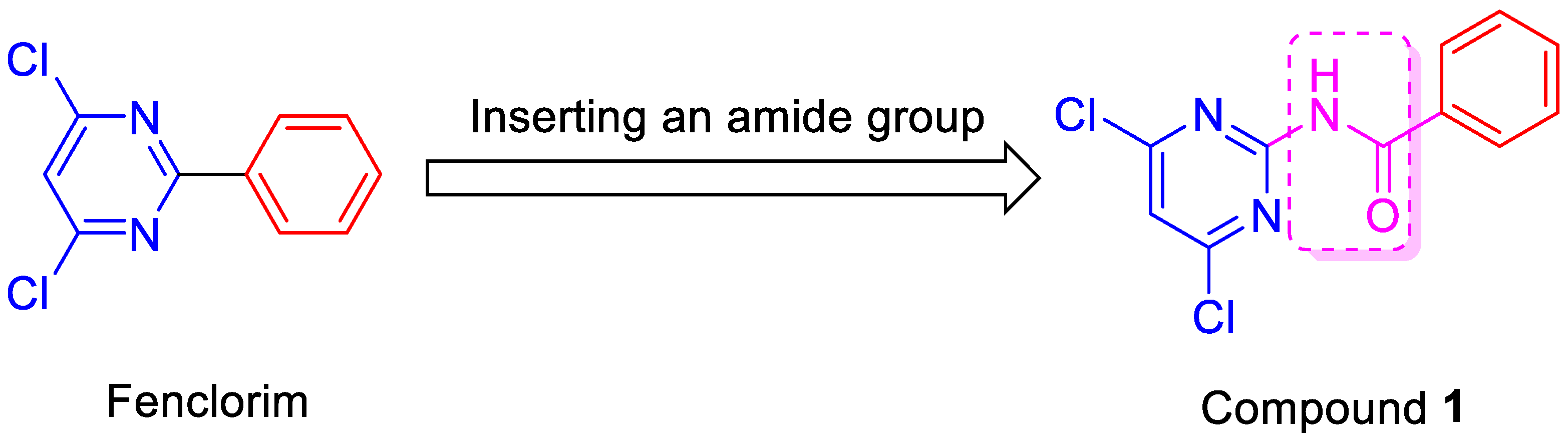


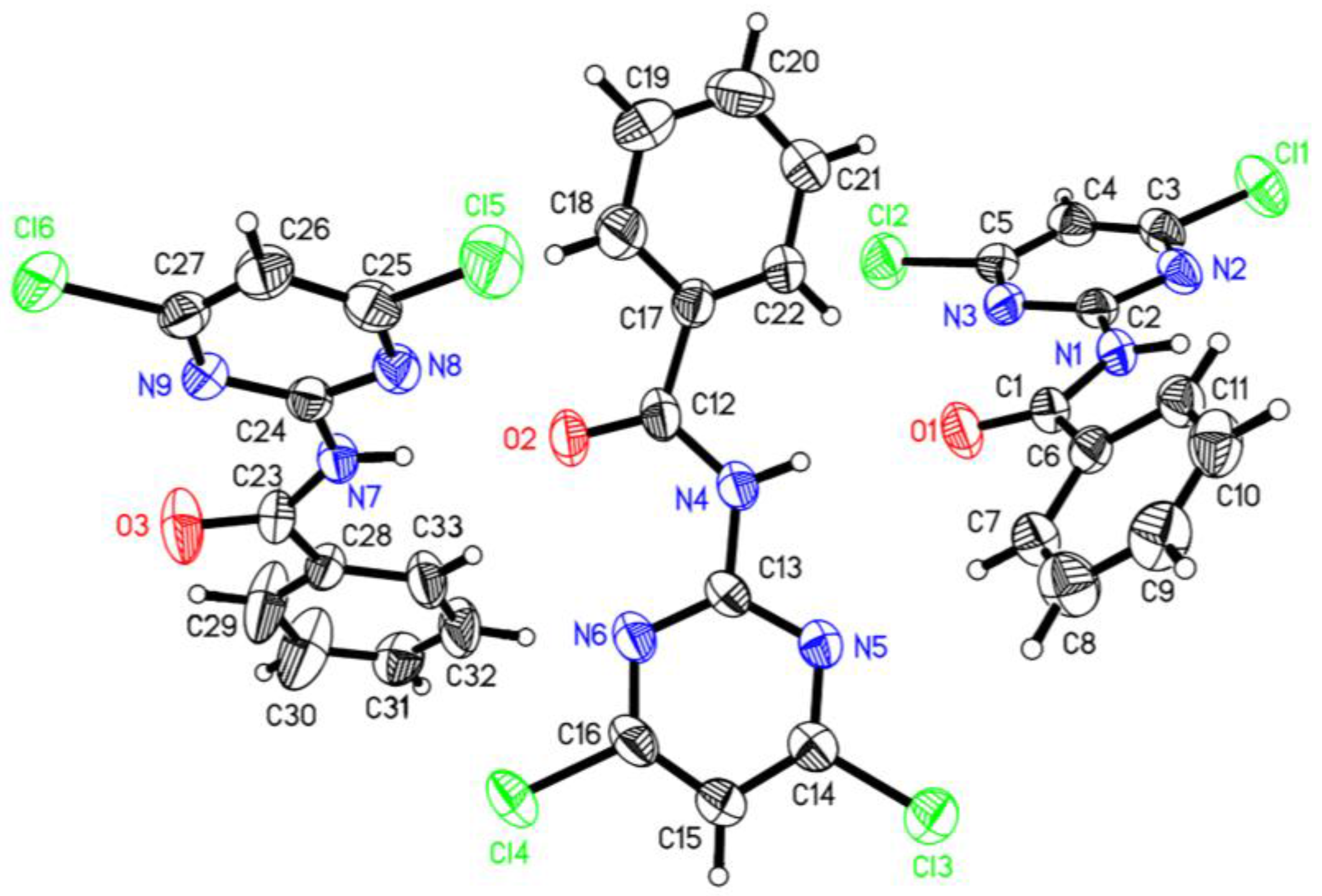
| Compound | 1 |
|---|---|
| CCDC No. | 1810908 |
| Empirical formula | C11H7Cl2N3O |
| Formula weight | 268.10 |
| Crystal system | Monoclinic |
| Space group | P 21/c |
| Unit cell dimensions | a = 14.9156(6) Å, α = 90° b = 16.6291(8) Å, β = 95.160(2)° c = 14.4740(6) Å, γ = 90° |
| Volume/Å3 | 3575.5(3) |
| Z | 12 |
| Dc/g∙cm−3 | 1.494 |
| µ/mm−1 | 3.182 |
| F(000) | 1632 |
| Crystal size/mm3 | 0.170 × 0.100 × 0.040 |
| θmin/θmax/◦ | 3.471/54.979 |
| Limiting indices | −18 ≤ h ≤ 15, −20 ≤ k ≤ 20, −17 ≤ l ≤ 17 |
| Reflections collected | 37,976 |
| Independent reflections | 6797 [R(int) = 0.0675] |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 6797/0/449 |
| Goodness-of-fit on F2 | 1.049 |
| R1/wR2[I > 2σ(I)] | 0.0870/0.2337 |
| R1/wR2(all data) | 0.1110/0.2540 |
| Largest diff. peak and hole/e.Å−1 | 0566/−0.346 |
| Bond | Distance (Å) | Bond | Distance (Å) |
| Cl(1)–C(3) | 1.735(5) | Cl(2)–C(5) | 1.732(6) |
| N(1)–C(1) | 1.370(6) | N(1)–C(2) | 1.376(7) |
| N(1)–H(1) | 0.8600 | N(2)–C(3) | 1.307(7) |
| O(1)–C(1) | 1.223(6) | C(3)–C(4) | 1.387(8) |
| C(4)–C(5) | 1.373(7) | C(4)–H(4) | 0.9300 |
| C(7)–C(8) | 1.359(8) | N(2)–C(2) | 1.350(6) |
| C(9)–C(10) | 1.360(9) | C(9)–H(9) | 0.9300 |
| C(10)–C(11) | 1.383(8) | N(3)–C(5) | 1.316(7) |
| Angle | (°) | Angle | (°) |
| C(1)–N(1)–C(2) | 128.7(4) | O(1)–C(1)–N(1) | 120.6(5) |
| C(8)–C(7)–C(6) | 120.2(5) | C(3)–N(2)–C(2) | 115.0(4) |
| O(1)–C(1)–C(6) | 122.0(4) | N(1)–C(1)–C(6) | 117.4(4) |
| C(7)–C(8)–C(9) | 119.8(6) | C(11)–C(6)–C(1) | 123.5(5) |
| C(10)–C(9)–C(8) | 121.7(6) | C(8)–C(7)–C(6) | 120.2(5) |
| Torsion | (°) | Torsion | (°) |
| C(2)–N(1)–C(1)-O(1) | 3.6(8) | C(2)–N(1)–C(1)–C(6) | −177.2(4) |
| C(3)–N(2)–C(2)–N(1) | −178.9(4) | C(11)–C(6)–C(7)–C(8) | −0.7(8) |
| C(2)–N(2)–C(3)–C(4) | −0.4(7) | C(3)–C(4)–C(5)–Cl(2) | −179.4(4) |
| O(1)–C(1)–C(6)–C(7) | 19.3(7) | C(7)–C(6)–C(11)–C(10) | −0.7(7) |
| D–H···A | d(D–H)/(Å) | d(H···A)/(Å) | d(D···A)/(Å) | <(DHA)/(°) |
|---|---|---|---|---|
| N(7)–H(7A)···O(2) | 0.86 | 2.15 | 2.981(5) | 163.4 |
| N(4)–H(4A)···O(1) | 0.86 | 2.12 | 2.968(5) | 170.0 |
| C(26)–H(26)···Cl(1) #1 | 0.93 | 2.96 | 3.874(6) | 166.8 |
| C(15)–H(15)···Cl(2) #2 | 0.93 | 2.98 | 3.893(6) | 167.4 |
| N(1)–H(1)···O(3) #3 | 0.86 | 2.05 | 2.851(5) | 155.3 |
| Compd. | Safening Effect (% of Non-Treated Control) | |||
|---|---|---|---|---|
| Plant Height | Root Length | Fresh Weight | Emergence Rate | |
| M | 51.17 ± 0.75 | 48.46 ± 0.38 | 65.42 ± 0.86 | 57.67 ± 1.15 |
| C + M | 82.26 ± 0.21 | 91.03 ± 0.72 | 78.52 ± 0.68 | 87.47 ± 0.92 |
| F + M | 86.18 ± 0.23 | 95.10 ± 0.70 | 82.22 ± 0.74 | 94.00 ± 1.00 |
| Compound | IC50 (±SD) mg∙L−1 | |||
|---|---|---|---|---|
| S. sclerotiorum | F. oxysporum | F. graminearu | T. cucumeris | |
| 1 | 1.23 ± 1.24 | 9.97 ± 0.15 | 33.50 ± 0.43 | 21.72 ± 0.25 |
| fenclorim | 18.11 ± 1.08 | 27.33 ± 0.03 | 39.53 ± 0.31 | 28.46 ± 0.30 |
| pyrimethanil | 8.39 ± 0.45 | 23.44 ± 0.57 | 30.68 ± 0.04 | 7.59 ± 0.15 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, W.-N.; Zhu, Z.-Y.; Deng, Y.-N.; Wu, Z.-C.; Zhou, Y.; Zhou, X.-M.; Bai, L.-Y.; Deng, X.-L. Synthesis, Crystal Structure, Herbicide Safening, and Antifungal Activity of N-(4,6-Dichloropyrimidine-2-Yl)Benzamide. Crystals 2018, 8, 75. https://doi.org/10.3390/cryst8020075
Zheng W-N, Zhu Z-Y, Deng Y-N, Wu Z-C, Zhou Y, Zhou X-M, Bai L-Y, Deng X-L. Synthesis, Crystal Structure, Herbicide Safening, and Antifungal Activity of N-(4,6-Dichloropyrimidine-2-Yl)Benzamide. Crystals. 2018; 8(2):75. https://doi.org/10.3390/cryst8020075
Chicago/Turabian StyleZheng, Wen-Na, Zhe-Yuan Zhu, Ya-Nan Deng, Zhong-Chi Wu, Yong Zhou, Xiao-Mao Zhou, Lian-Yang Bai, and Xi-Le Deng. 2018. "Synthesis, Crystal Structure, Herbicide Safening, and Antifungal Activity of N-(4,6-Dichloropyrimidine-2-Yl)Benzamide" Crystals 8, no. 2: 75. https://doi.org/10.3390/cryst8020075