Specific Structural Disorder in an Anion Layer and Its Influence on Conducting Properties of New Crystals of the (BEDT-TTF)4A+[M3+(ox)3]G Family, Where G Is 2-Halopyridine; M Is Cr, Ga; A+ Is [K0.8(H3O)0.2]+
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.1.1. β″-(BEDT-TTF)4[K0.8(H3O)0.2][Cr(ox)3]·(2-ClPy) (1)
2.1.2. β″-(BEDT-TTF)4[K0.8(H3O)0.2][Cr(ox)3]·(2-BrPy) (2)
2.1.3. β″-(BEDT-TTF)4[K0.8(H3O)0.2][Ga(ox)3]·(2-ClPy) (3) and α″-(BEDT-TTF)5]·3.4·H2O·0.6 EtOH (5)
- (a)
- 11 mg of BEDT-TTF, 300 mg of K3[Ga(ox)3]·5H2O, 300 mg of 18-crown-6 and the mixture of 2-ClPy (20 mL) with 1,2,4-trichlorobenzene (10 mL) and 96% ethanol (3.5 mL) were used. J = 0.9 μA. Many shining black α″-crystals (5) in the form of thin plates and a few small β″-crystals (3) were collected from the anode after 14 days. After removing these crystals, β″-crystals (3) started to grow and a few of these crystals in the form of elongated plates were collected after 10 days.
- (b)
- 25 mg of BEDT-TTF, 300 mg of K3[Ga(ox)3]·5H2O, 300 mg of 18-krown-6; 20 mL of 2-ClPy, 10 mL of 1,2,4-trichlorobenzene, 3.5 mL of 96% ethanol were used. J = 0.95 μA. A lot of α″-crystals (5) in the form of thick plates were obtained after 15 days.
- (c)
- 15 mg of BEDT-TTF, 350 mg of K3[Ga(ox)3]·5H2O, 700 mg of 18-krown-6; 20 mL of 3-ClPy, 10 mL of 1,2,4-trichlorobenzene, 2 mL of 96% ethanol were used. J = 0.95 μA. A few large thick α″-crystals (5) were obtained after 10 days.
2.1.4. β″-(BEDT-TTF)4[K0.8(H3O)0.2][Ga(ox)3]·(2-BrPy) (4)
2.2. Crystal Structure
2.3. Conducting Properties
3. Results and Discussion
3.1. Synthesis
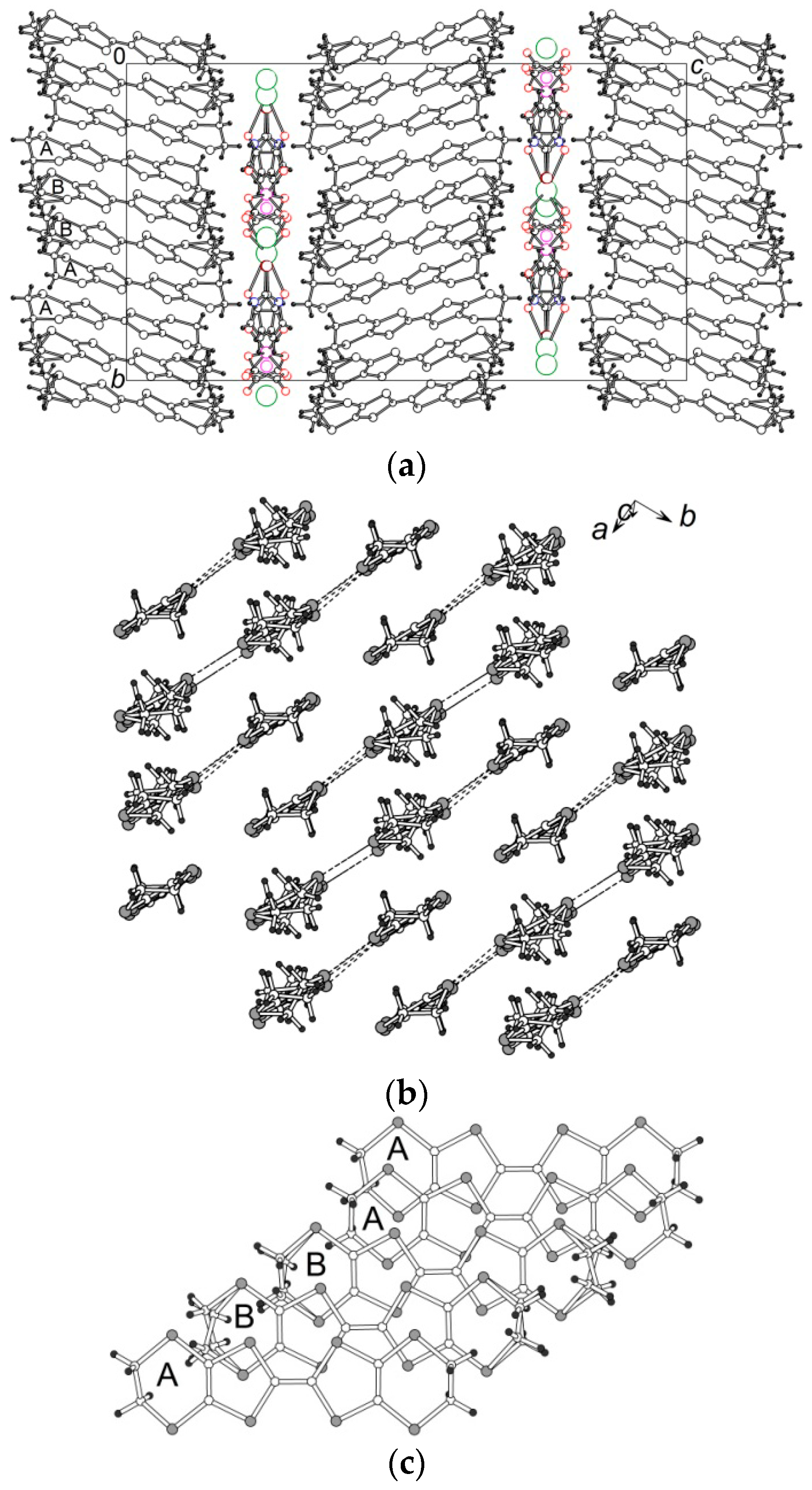
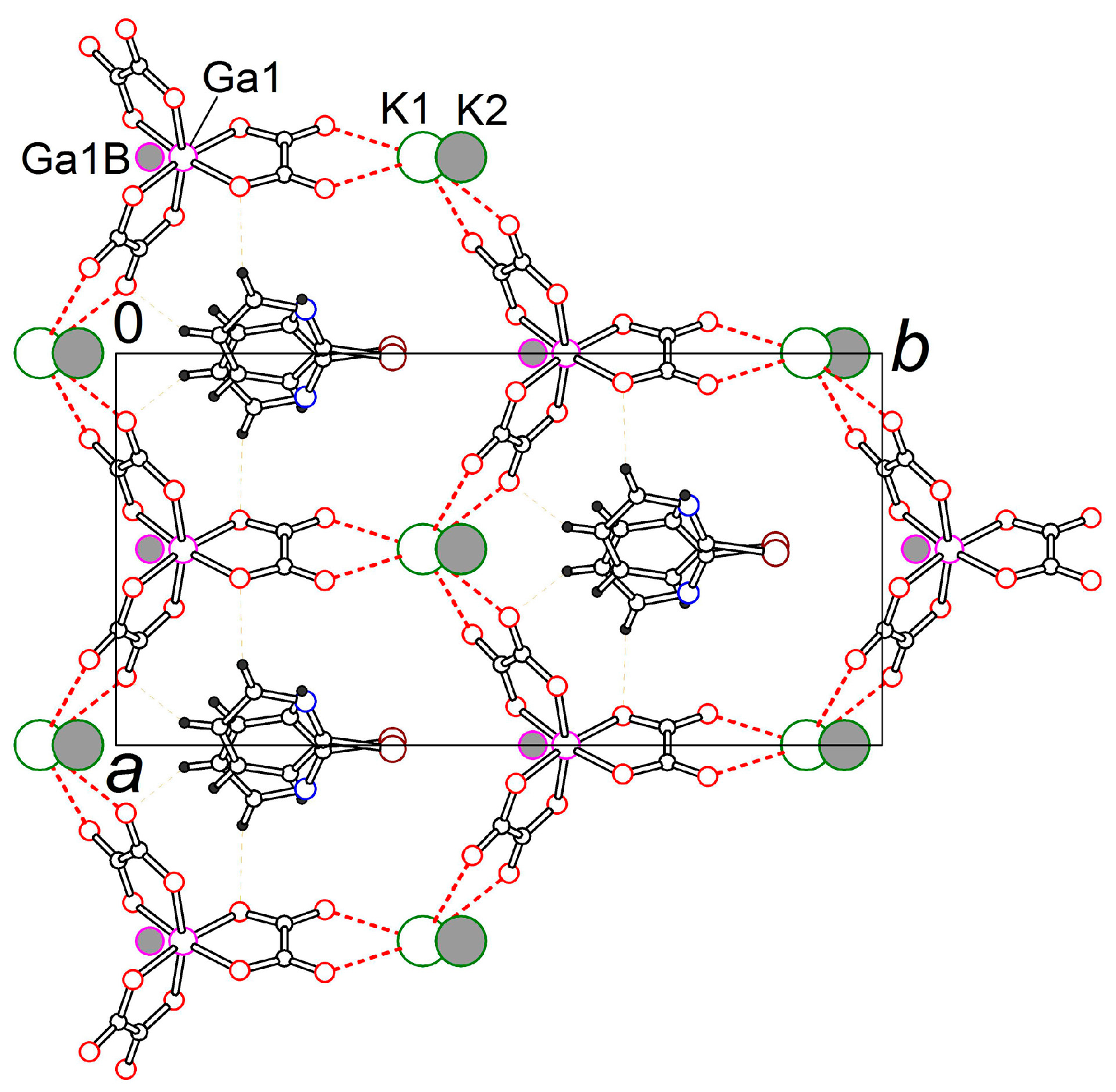
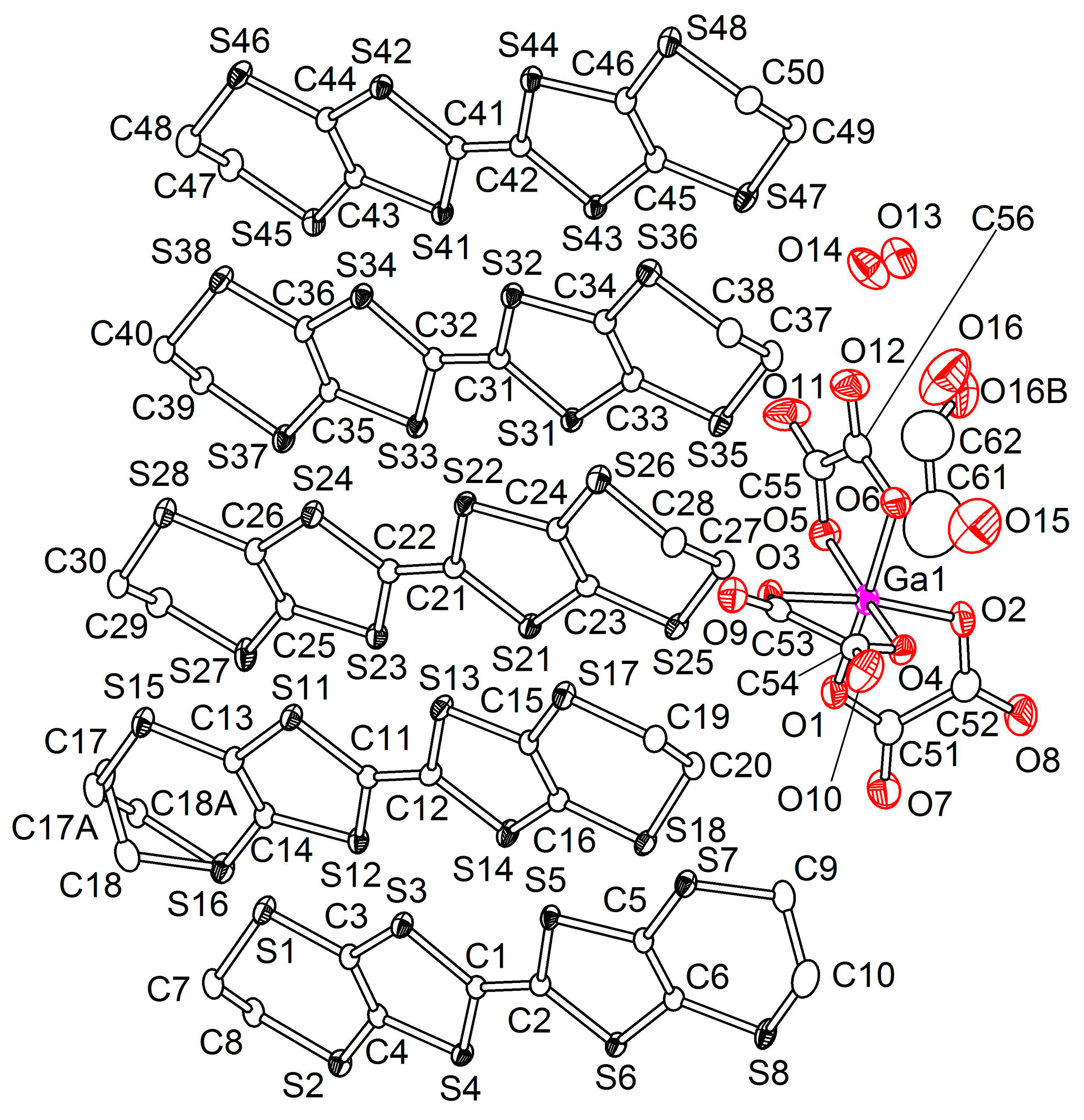
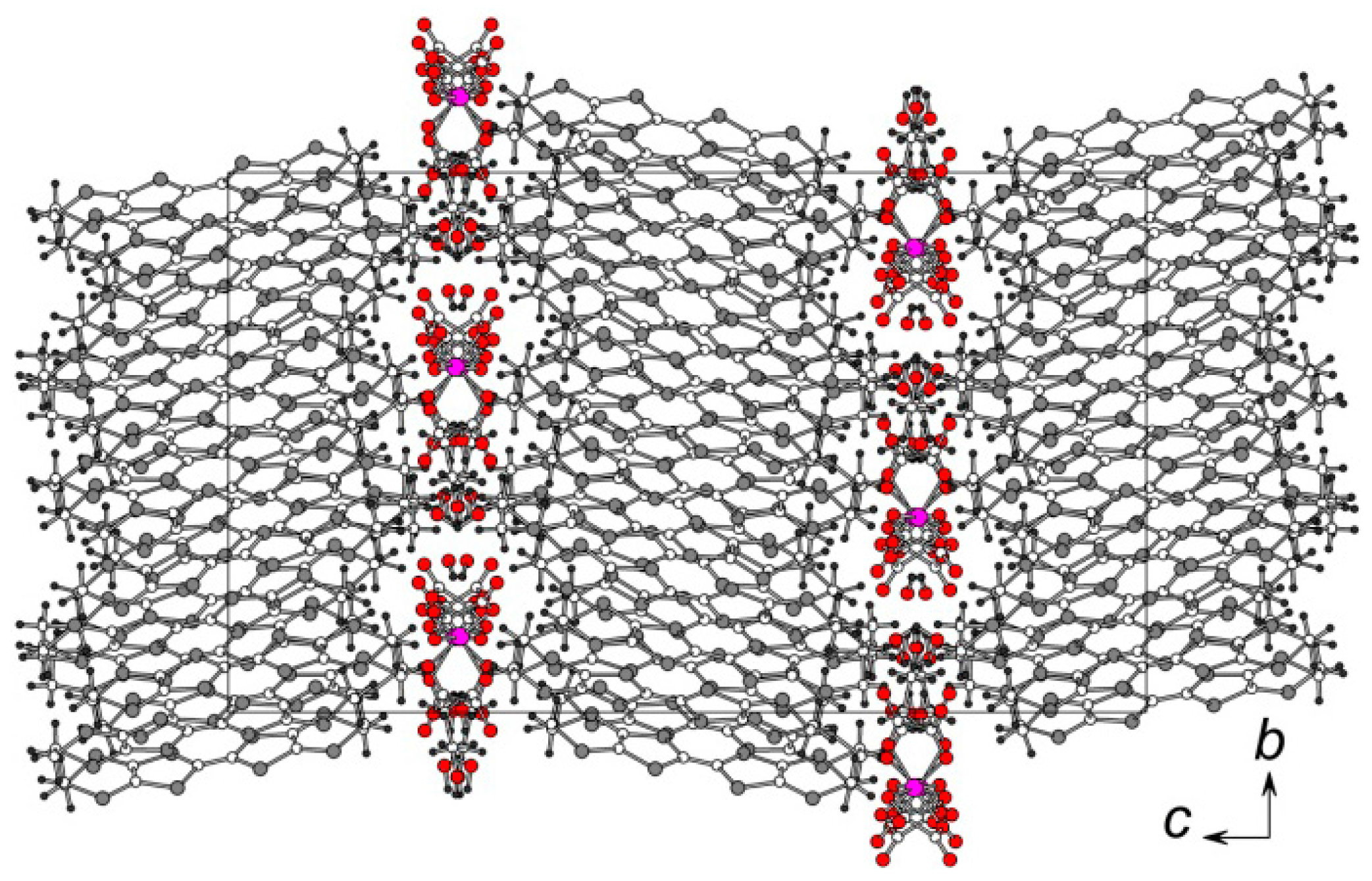
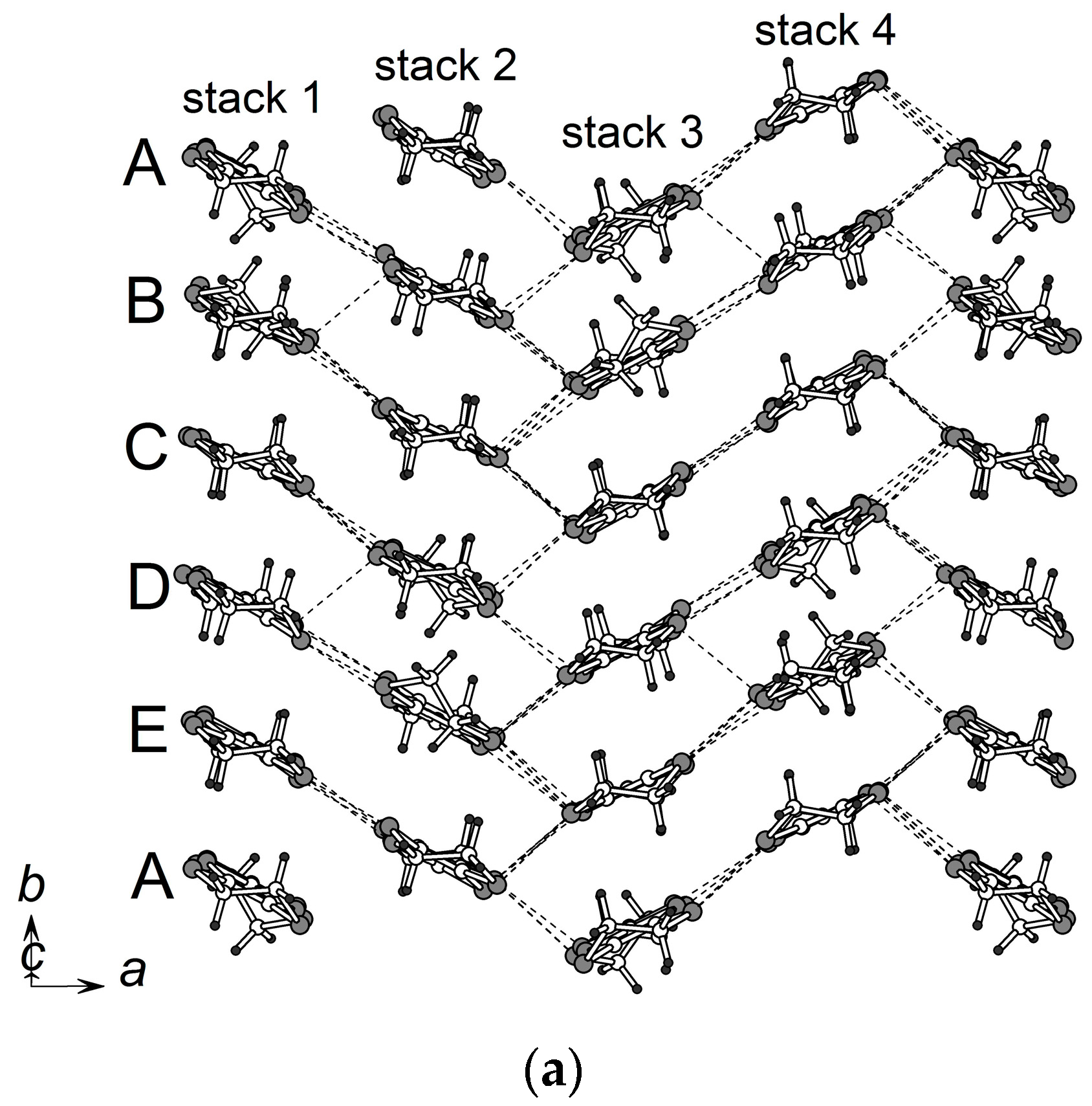
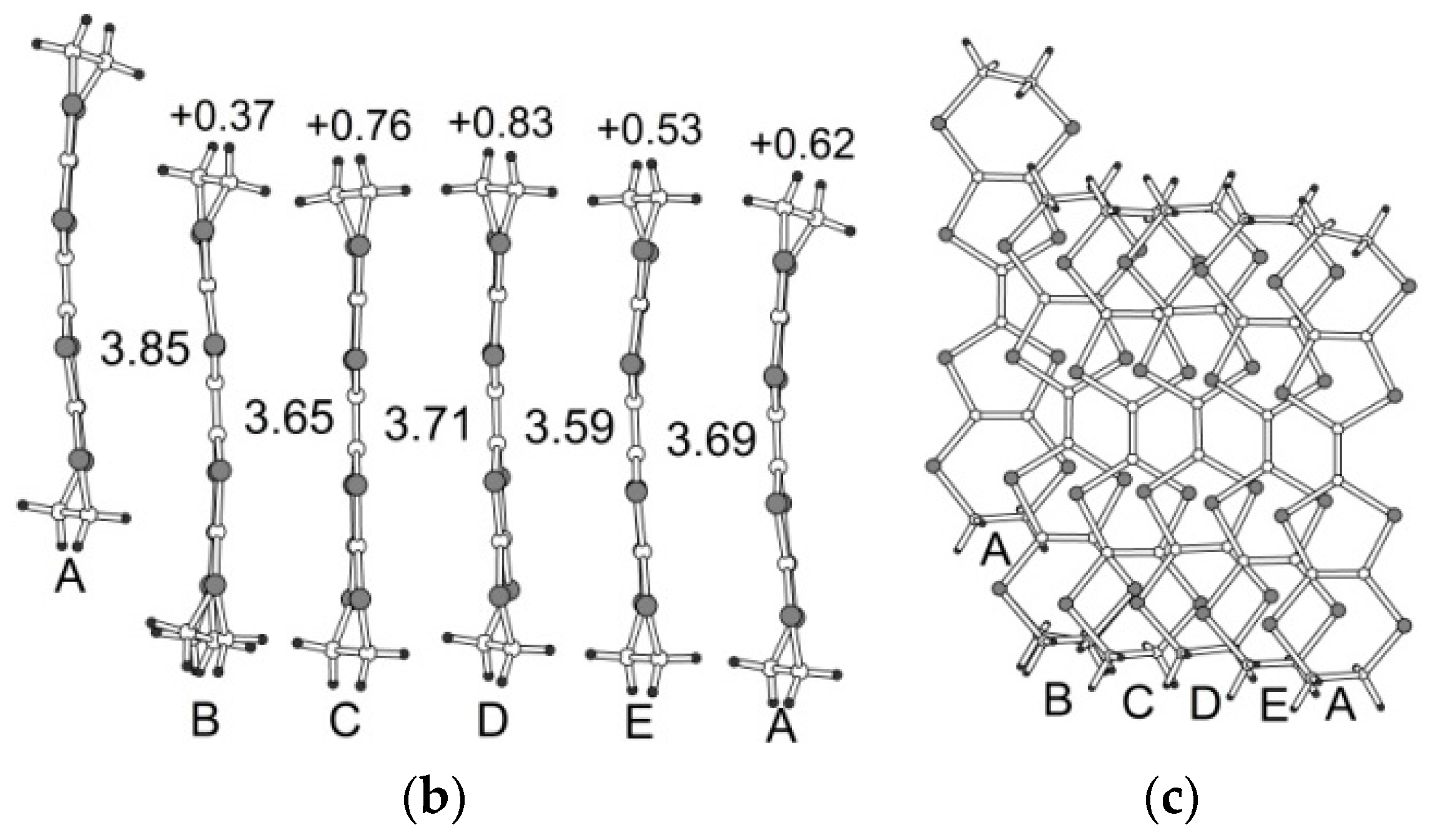
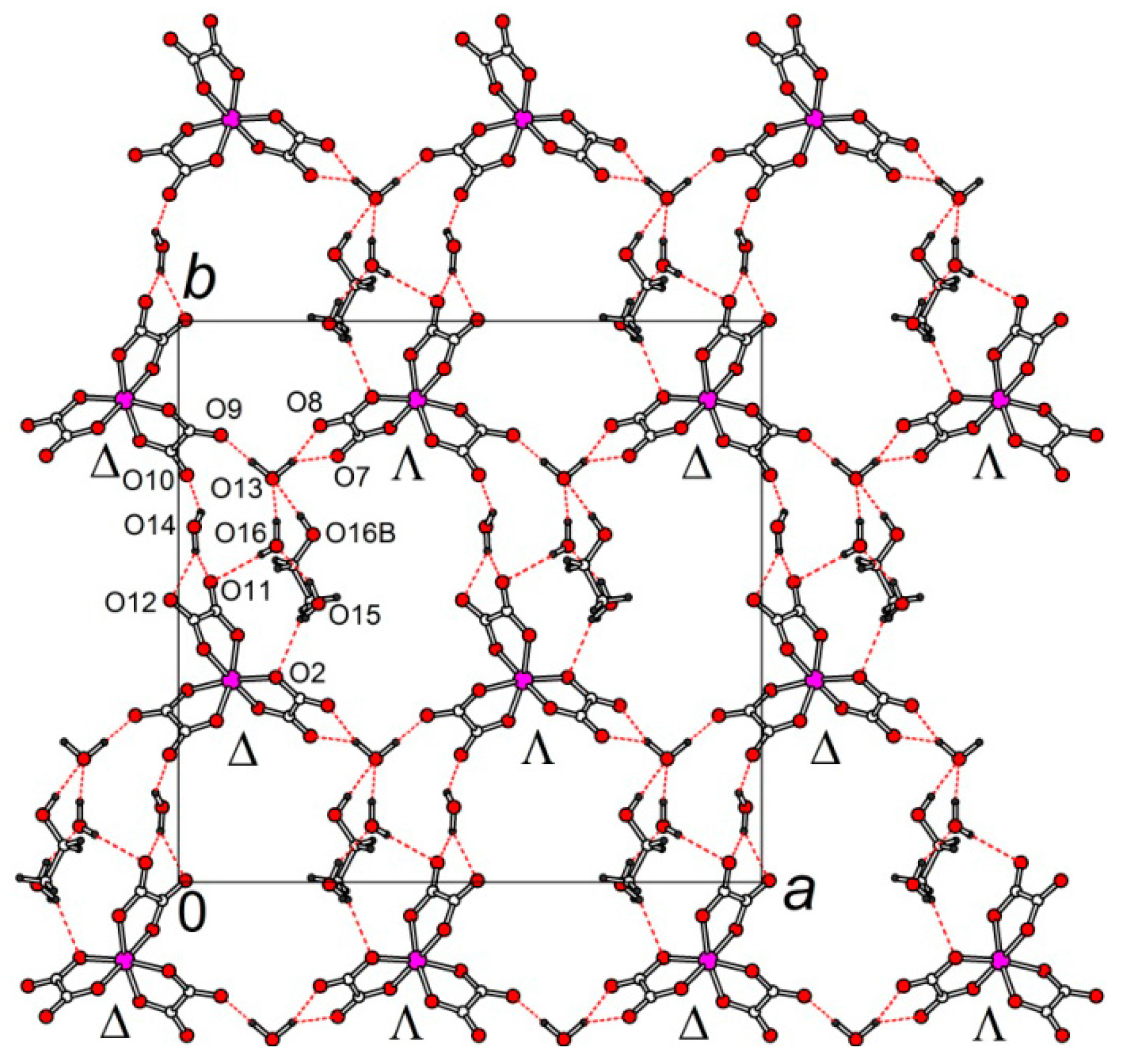
3.2. Crystal Structure
3.2.1. β″-(BEDT-TTF)4[K0.8(H3O)0.2][M(ox)3]·2-HalPy, where M/Hal = Cr/Cl (1), Cr/Br (2), Ga/Cl (3) and Ga/Br (4)
3.2.2. α″-(BEDT-TTF)5[Ga(ox)3]·3.4·H2O·0.6 EtOH (5)
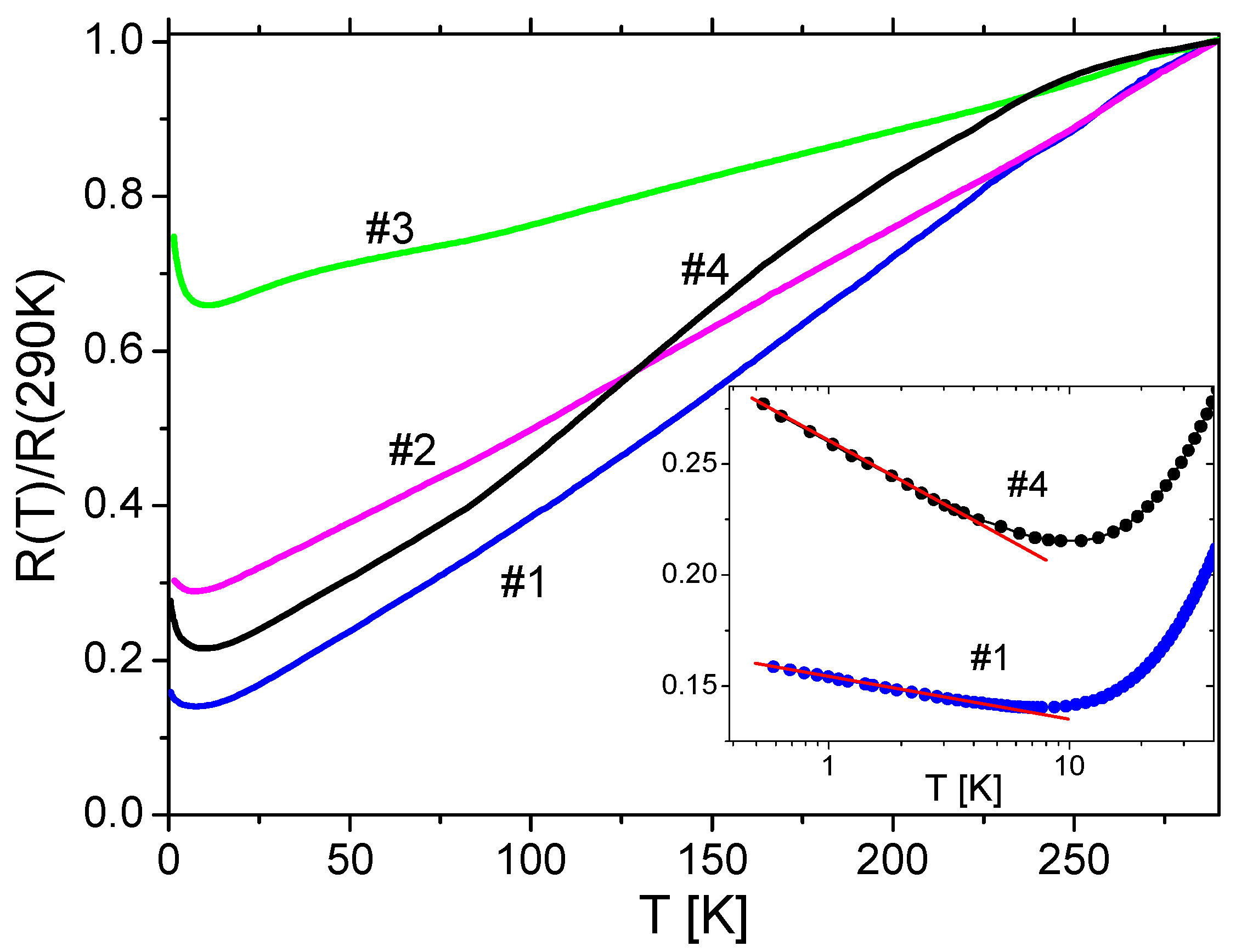
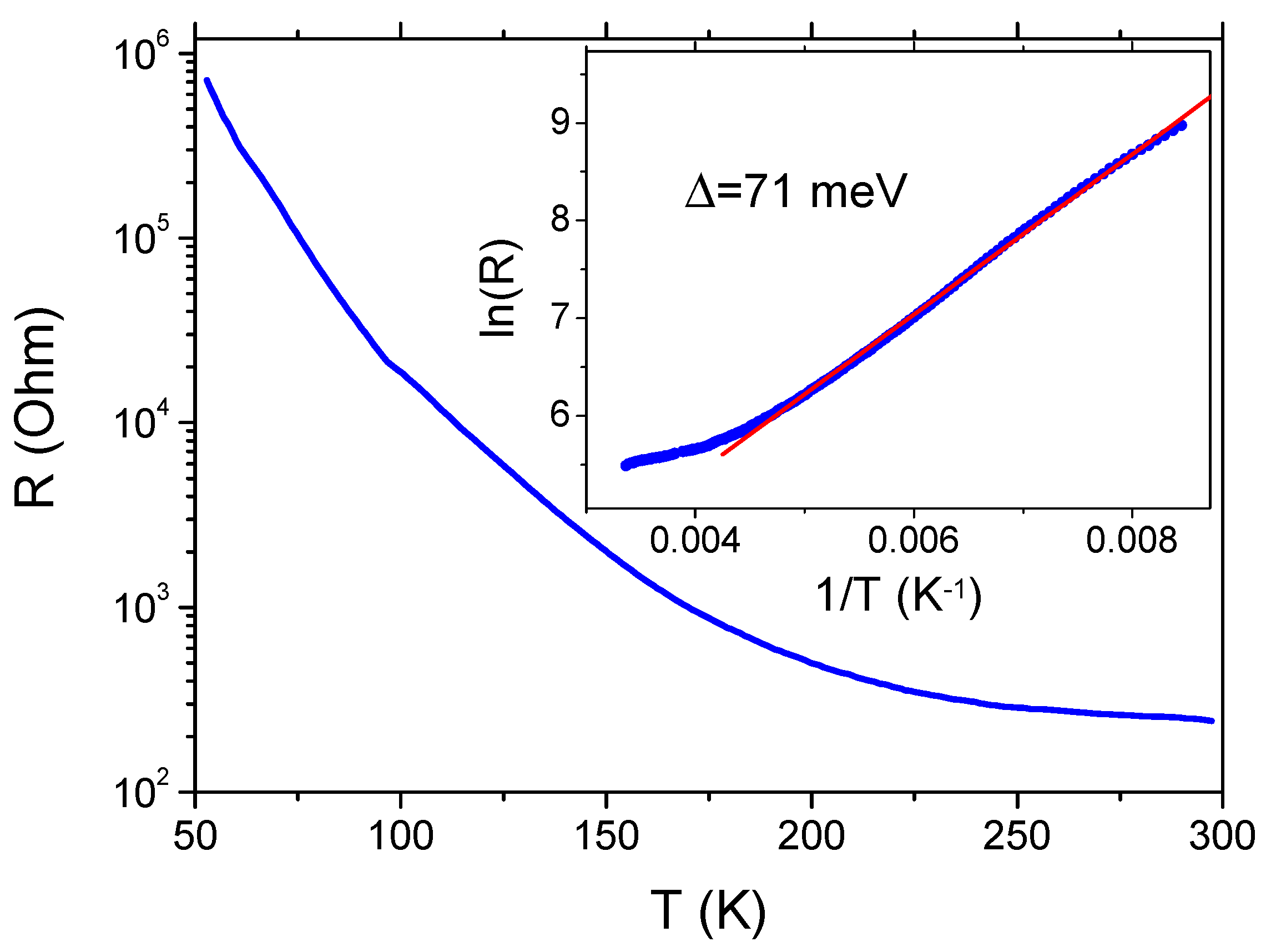
3.3. Conducting Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kurmoo, M.; Graham, A.W.; Day, P.; Coles, S.J.; Hursthouse, M.B.; Caufield, J.L.; Singleton, J.; Pratt, F.L.; Hayes, W.; Ducasse, L.; et al. Superconducting and Semiconducting Magnetic Charge Transfer Salts: (BEDT-TTF)4AFe(C2O4)3 C6H5CN A = H2O, K, NH4. J. Am. Chem. Soc. 1995, 117, 12209–12217. [Google Scholar] [CrossRef]
- Martin, L.; Turner, S.S.; Day, P.; Mabbs, F.E.; McInnes, J.L. New molecular superconductor containing paramagnetic chromium (III) ions. Chem. Commun. 1997, 15, 1367–1368. [Google Scholar] [CrossRef]
- Turner, S.S.; Day, P.; Abdul Malik, K.M.; Hursthouse, M.B.; Teat, S.J.; MacLean, E.J.; Martin, L.; French, S.A. Effect of Included Solvent Molecules on the Physical Properties of the Paramagnetic Charge Transfer Salts β″-(BEDT-TTF)4[(H3O)Fe(C2O4)3]·Solvent (BEDT-TTF = Bis(ethylenedithio)tetrathiafilvalene). Inorg. Chem. 1999, 38, 3543–3549. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Turner, S.S.; Day, P.; Guionneau, P.; Howard, J.A.K.; Hibbs, D.E.; Light, M.E.; Hursthouse, M.B.; Uruichi, M.; Yakushi, K. Crystal Chemistry and Physical Properties of Superconducting and Semiconducting Charge Transfer Salts of the Type (BEDT-TTF)4[AIMIII(C2O4)3]·PhCN (AI = H3O, NH4, K; MIII = Cr, Fe, Co, Al; BEDT-TTF = Bis(ethylenedithio)tetrathiafulvalene). Inorg. Chem. 2001, 40, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Turner, S.S.; Le Pevelen, D.; Day, P.; Light, M.E.; Hursthouse, M.B.; Firth, S.; Clark, R.J.H. β″-BEDT-TTF)4[(H3O)Cr(C2O4)3]CH2Cl2: Effect of Included Solvent on the Structure and Properties of a Conducting Molecular Charge-Transfer Salt. Inorg. Chem. 2001, 40, 5304–5306. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Turner, S.S.; Day, P.; Howard, J.A.K.; Guionneau, P.; McInnes, E.J.L.; Mabbs, F.E.; Clark, R.J.H.; Firth, S.; Biggse, T. New superconducting charge-transfer salts (BEDTTTF)4[AM(C2O4)3]C6H5NO2(A = H3O or NH4, M = Cr or Fe, BEDT-TTF = bis(ethylenedithio)tetrathiafulvalene. Novel charge transfer salts of BEDT-TTF with metal oxalate counterions. J. Mater. Chem. 2001, 11, 2095–2101. [Google Scholar] [CrossRef]
- Rashid, S.S.; Turner, P.; Day, M.E.; Light, M.B.; Hursthouse, P. Guionneau. Synth. Met. 2001, 120, 985–986. [Google Scholar] [CrossRef]
- Akutsu, H.; Akutsu-Sato, A.; Turner, S.S.; Le Pevelen, D.; Day, P.; Laukhin, V.; Klehe, A.-K.; Singleton, J.; Tocher, D.A.; Probert, M.R.; et al. Effect of Included Guest Molecules on the Normal State Conductivity and Superconductivity of β″-(ET)4[(H3O)Ga(C2O4)3]G (G = Pyridine, Nitrobenzene). J. Am. Chem. Soc. 2002, 124, 12430–12431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prokhorova, T.G.; Khasanov, S.S.; Zorina, L.V.; Buravov, L.I.; Tkacheva, V.A.; Baskakov, A.A.; Morgunov, R.B.; Gener, M.; Canadell, E.; Shibaeva, R.P.; et al. Molecular Metals Based on BEDT-TTF Radical Cation Salts with Magnetic Metal Oxalates as Counterions: β"-(BEDT-TTF)4A[M(C2O4)3]DMF (A = K+, NH4+; M = FeIII, CrIII). Adv. Funct. Mater. 2003, 13, 403–411. [Google Scholar] [CrossRef]
- Coldea, A.I.; Bangura, A.F.; Singleton, J.; Ardavan, A.; Akutsu-Sato, A.; Akutsu, H.; Turner, S.S.; Day, P. Fermi-surface topology and the effects of intrinsic disorder in a class of charge-transfer salts containing magnetic ions: β"-(BEDT-TTF)4[(H3O)M(C2O4)3]Y (M = Ga, Cr, Fe; Y = C5H5N. Phys. Rev. B 2004, 69, 085112. [Google Scholar] [CrossRef]
- Akutsu, H.; Akutsu-Sato, A.; Turner, S.S.; Day, P.; Canadell, E.; Firth, S.; Clark, R.J.N.; Yamada, J.; Nakatsuji, S. Superstructures of donor packing arrangements in a series of molecular charge transfer salts. Chem. Commun. 2004, 1, 18–19. [Google Scholar] [CrossRef] [PubMed]
- Audouard, A.; Laukhin, V.N.; Brossard, L.; Prokhorova, T.G.; Yagubskii, E.B.; Canadell, E. Combination frequencies of magnetic oscillations in β"-(BEDT-TTF)4(NH4)[(Fe(C2O4)3]DMF. Phys. Rev. B 2004, 69, 144523. [Google Scholar] [CrossRef]
- Akutsu-Saito, A.; Kobayashi, A.; Mori, T.; Akutsu, H.; Yamada, J.; Nakatsuji, S.; Turner, S.S.; Day, P.; Tocher, D.A.; Light, M.E.; et al. Structures and Physical Properties of New β″-BEDT-TTF Tris-Oxalatometallate (III) Salts Containing Chlorobenzene and Halomethane Guest Molecules. Synth. Met. 2005, 152, 373–376. [Google Scholar] [CrossRef]
- Coronado, E.; Curelli, S.; Giménez-Saiz, C.; Gómez-García, C.J. New magnetic conductors and superconductors based on BEDT-TTF and BEDS-TTF. Synth. Met. 2005, 154, 245–248. [Google Scholar] [CrossRef]
- Coronado, E.; Curelli, S.; Giménez-Saiz, C.; Gómez-García, C.J. A novel paramagnetic molecular superconductor formed by bis(ethylenedithio)tetrathiafulvalene, tris(oxalato)ferrate(III) anions and bromobenzene as guest molecule: (ET)4[(H3O)Fe(C2O4)3]C6H5Br. J. Mater. Chem. 2005, 15, 1429–1436. [Google Scholar] [CrossRef]
- Akutsu-Sato, A.; Akutsu, H.; Yamada, J.; Nakatsuji, S.; Turner, S.S.; Day, P. Suppression of superconductivity in a molecular charge transfer salt by changing quest molecule: β"-(BEDT-TTF)4[(H3O)Fe(C2O4)3](C6H5CN)x(C5H5N)1−x. J. Mater. Chem. 2007, 17, 2497–2499. [Google Scholar] [CrossRef]
- Zorina, L.V.; Prokhorova, T.G.; Simonov, S.V.; Khasanov, S.S.; Shibaeva, R.P.; Manakov, A.I.; Zverev, V.N.; Buravov, L.I.; Yagubskii, E.B. Structure and Magnetotransport Properties of the New Quasi-Two-Dimensional Molecular Metal β″-(BEDT–TFF)4H3O[Fe(C2O4)3]C6H4Cl2. J. Exp. Theor. Phys. 2008, 106, 347–354. [Google Scholar] [CrossRef]
- Martin, L.; Day, P.; Akutsu, H.; Yamada, J.; Nakatsuji, S.; Clegg, W.; Harrington, R.W.; Horton, P.N.; Hursthouse, M.B.; McMillan, P.; et al. Metallic molecular crystals containing chiral or racemic guest molecules. CrystEngComm 2007, 9, 865–867. [Google Scholar] [CrossRef]
- Prokhorova, T.G.; Buravov, L.I.; Yagubskii, E.B.; Zorina, L.V.; Khasanov, S.S.; Simonov, S.V.; Shibaeva, R.P.; Korobenko, A.V.; Zverev, V.N. Effect of electrocrystallization medium on quality, structural features, and conducting properties of single crystals of the (BEDT-TTF)4AI[FeIII(C2O4)3]·G family. CrystEngComm 2011, 13, 537–545. [Google Scholar] [CrossRef]
- Zorina, L.V.; Khasanov, S.S.; Simonov, S.V.; Shibaeva, R.P.; Zverev, V.N.; Canadell, E.; Prokhorova, T.G.; Yagubskii, E.B. Coexistence of two donor packing motifs in the stable molecular metal α-‘pseudo-κ’-(BEDT-TTF)4(H3O)[Fe(C2O4)3]·C6H4Br2. CrystEngComm 2011, 13, 2430–2438. [Google Scholar] [CrossRef]
- Coronado, E.; Curreli, S.; Giménez-Saiz, C.; Gómez-García, C.J. The Series of Molecular Conductors and Superconductors ET4[AFe(C2O4)3]·PhX (ET = bis(ethylenedithio)tetrathiafulvalene; (C2O4)2− = oxalate; A+ = H3O+, K+; X = F, Cl, Br, and I): Influence of the Halobenzene Guest Molecules on the Crystal Structure and Superconducting Properties. Inorg. Chem. 2012, 51, 1111–1126. [Google Scholar] [PubMed]
- Zorina, L.V.; Khasanov, S.S.; Simonov, S.V.; Shibaeva, R.P.; Bulanchuk, P.O.; Zverev, V.N.; Canadell, E.; Prokhorova, T.G.; Yagubskii, E.B. Structural phase transition in the β″-(BEDT-TTF)4H3O[Fe(C2O4)3]·G crystals (where G is a guest solvent molecule). CrystEngComm 2012, 14, 460–465. [Google Scholar] [CrossRef]
- Prokhorova, T.G.; Zorina, L.V.; Simonov, S.V.; Zverev, V.N.; Canadell, E.; Shibaeva, R.P.; Yagubskii, E.B. The first molecular superconductor based on BEDT-TTF radical cation salt with paramagnetic tris(oxalato)ruthenate anion. CrystEngComm 2013, 15, 7048–7055. [Google Scholar] [CrossRef]
- Prokhorova, T.G.; Buravov, L.I.; Yagubskii, E.B.; Zorina, L.V.; Simonov, S.V.; Shibaeva, R.P.; Zverev, V.N. New metallic bi- and monolayered radical cation salts based on BEDT-TTF with tris(oxalato)gallate anion. Eur. J. Inorg. Chem. 2014, 24, 3933–3940. [Google Scholar] [CrossRef]
- Prokhorova, T.G.; Buravov, L.I.; Yagubskii, E.B.; Zorina, L.V.; Simonov, S.V.; Zverev, V.N.; Shibaeva, R.P.; Canadell, E. Effect of Halopyridine Guest Molecules on the Structure and Superconducting Properties of β″-[Bis(ethylenedithio)tetrathiafulvalene]4(H3O)[Fe(C2O4)3]·Guest Crystals. Eur. J. Inorg. Chem. 2015, 34, 5611–5620. [Google Scholar] [CrossRef]
- Martin, L.; Morritt, A.L.; Lopez, J.R.; Nakazawa, Y.; Akutsu, H.; Imajo, S.; Ihara, Y.; Zhang, B.; Zhange, Y.; Guof, Y. Molecular conductors from bis(ethylenedithio)tetrathiafulvalene with tris(oxalato)rhodate. Dalton Trans. 2017, 46, 9542–9548. [Google Scholar] [CrossRef] [PubMed]
- Prokhorova, T.G.; Yagubskii, E.B. Organic conductors and superconductors based on bis(ethylenedithio)tetrathiafulvalene radical cation salts with supramolecular tris(oxalato)metallate anions. Russ. Chem. Rev. 2017, 86, 164–180. [Google Scholar] [CrossRef]
- Coronado, E.; Galan-Mascaros, J.R.; Gomez-Garcia, C.J.; Laukhin, V. Coexistence of ferromagnetism and metallic conductivity in a molecule-based layered compound. Nature 2000, 408, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Day, P.; Horton, P.; Nakatsuji, S.; Yamada, J.; Akutsu, H. Chiral conducting salts of BEDT-TTF containing a single enantiomer of tris(oxalato)chromate(III) crystallized from a chiral solvent. J. Mater. Chem. 2010, 20, 2738–2742. [Google Scholar] [CrossRef]
- Martin, L.; Day, P.; Nakatsuji, S.; Yamada, J.; Akutsu, H.; Horton, P. A molecular charge transfer salt of BEDT-TTF containing a single enantiomer of tris(oxalato)chromate(III) crystallized from a chiral solvent. CrystEngComm 2010, 12, 1369–1372. [Google Scholar] [CrossRef]
- Martin, L.; Akutsu, H.; Hortond, P.N.; Hursthoused, M.B. Chirality in charge-transfer salts of BEDT-TTF of tris(oxalato)chromate (III). CrystEngComm 2015, 17, 2783–2790. [Google Scholar] [CrossRef]
- Martin, L.; Akutsu, H.; Horton, P.N.; Hursthouse, M.B.; Harrington, R.W.; Clegg, W. Chiral Radical-Cation Salts of BEDT-TTF Containing a Single Enantiomer of Tris(oxalato)aluminate(III) and -chromate(III). Eur. J. Inorg. Chem. 2015, 11, 1865–1870. [Google Scholar]
- Martin, L.; Morritt, A.L.; Lopez, J.R.; Akutsu, H.; Nakazawa, Y.; Imajo, S.; Ihara, Y. Ambient-pressure molecular superconductor with a superlattice containing layers of tris(oxalato)rhodate enantiomers and 18-crown-6. Inorg. Chem. 2017, 56, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Mori, T. Structural Genealogy of BEDT-TTF-Based Organic Conductors I. Parallel Molecules: β and β″ Phases. Bull. Chem. Soc. Jpn. 1998, 71, 2509–2526. [Google Scholar] [CrossRef]
- Mori, T.; Mori, H.; Tanaka, S. Structural Genealogy of BEDT-TTF-Based Organic Conductors II. Inclined Molecules: θ, α, and κ Phases. Bull. Chem. Soc. Jpn. 1999, 72, 179–197. [Google Scholar] [CrossRef]
- Olejniczak, I.; Frackowiak, A.; Swietlik, R.; Prokhorova, T.G.; Yagubskii, E.B. Charge Fluctuations and Ethylene-Group-Ordering Transition in β″-(BEDT-TF)4[(H3O)Fe(C2O4)3]·Y Molecular Charge-Transfer Salts. ChemPhysChem 2013, 14, 3925–3935. [Google Scholar] [CrossRef] [PubMed]
- Neogi, P.; Dutt, N.K.J. New compounds of gallium. Part III. Preparation and Resolution of complex oxalate compounds of gallium into optical isomers. Indian Chem. Soc. 1938, 15, 83–86. [Google Scholar]
- CrysAlisPro Software System, Version 1.171.38.41; Rigaku Oxford Diffraction; Rigaku Corporation: Oxford, UK, 2016.
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Guionneau, P.; Kepert, C.J.; Bravic, G.; Chasseau, D.; Truter, M.R.; Kurmoo, M.; Day, P. Determining the charge distribution in BEDT-TTF salts. Synth. Met. 1997, 86, 1973–1974. [Google Scholar] [CrossRef]
- Martin, L.; Engelkamp, H.; Akutsu, H.; Nakatsuji, S.; Yamada, J.; Horton, P.; Hursthouse, M.B. Charge transfer salts of BEDT-TTF with lithium tris(oxalato)metallate(III). Dalton Trans. 2015, 44, 6219–6223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Y.; Liu, F.; Guo, Y. Synthesis, crystal structure, and characterization of charge-transfer salt: (BEDT-TTF)5[Fe(C2O4)3]∙(H2O)2∙CH2Cl2 (BEDT-TTF = bis(ethylenedithio)tetrathiafulvalene). CrystEngComm 2009, 11, 2523–2528. [Google Scholar] [CrossRef]
- Altshuler, B.L.; Aronov, A.G. Electron-Electron Interaction in Disordered Conductors. In Electron-Electron Interactions in Disordered Systems; Efros, A.L., Pollak, M., Eds.; Elsevier: Amsterdam, The Netherlands, 1985; Volume 10, pp. 1–690. ISBN 0444-86916-6. [Google Scholar]
- Gruner, G.; Zawadowski, A. Magnetic impurities in non-magnetic metals. Rep. Prog. Phys. 1974, 37, 1497–1584. [Google Scholar] [CrossRef]

| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| Chemical formula | C51H36.6ClCr K0.8NO12.2S32 | C51H36.6BrCr K0.8NO12.2S32 | C51H36.6ClGa K0.8NO12.2S32 | C51H36.6BrGa K0.8NO12.2S32 | C56.6H48.6Ga O15.7S40 |
| Formula weight | 2003.26 | 2047.72 | 2020.98 | 2065.44 | 919.70 |
| Temperature (K) | 270 | 295 | 295 | 295 | 120 |
| Cell setting | monoclinic | monoclinic | monoclinic | monoclinic | orthorhombic |
| Space group, Z | C2/c, 4 | C2/c, 4 | C2/c, 4 | C2/c, 4 | Pbca, 8 |
| a (Å) | 10.2791(6) | 10.2971(2) | 10.27760(10) | 10.27515(15) | 22.0871(4) |
| b (Å) | 20.0020(9) | 20.0382(5) | 19.9066(3) | 20.0182(4) | 21.0853(4) |
| c (Å) | 35.4485(15) | 35.4621(8) | 35.5504(5) | 35.5972(5) | 35.6286(7) |
| α (°) | 90 | 90 | 90 | 90 | 90 |
| β (°) | 92.948(4) | 93.2943(19) | 92.9970(10) | 93.2026(13) | 90 |
| γ (°) | 90 | 90 | 90 | 90 | 90 |
| Cell volume (Å3) | 7278.7(6) | 7305.0(3) | 7263.4(2) | 7310.5(2) | 16592.7(5) |
| Crystal size (mm) | 0.53 × 0.25 × 0.12 | 0.35 × 0.34 × 0.21 | 0.42 × 0.30 × 0.15 | 0.61 × 0.27 × 0.12 | 0.83 × 0.18 × 0.03 |
| ρ (Mg/m3) | 1.828 | 1.862 | 1.848 | 1.877 | 1.867 |
| μ, cm−1 | 12.17 | 17.23 | 14.41 | 19.42 | 14.02 |
| Refls collected/unique/observed with I > 2σ(I) | 37,634/10,476/8631 | 17,952/9860/8119 | 17,486/9629/8063 | 33,996/10,184/8082 | 64,803/23,232/17,944 |
| Rint | 0.0291 | 0.0154 | 0.0285 | 0.0187 | 0.0263 |
| θmax (°) | 31.12 | 30.36 | 29.93 | 30.60 | 30.72 |
| Parameters refined | 608 | 606 | 612 | 606 | 1076 |
| Final R1(obs), wR2 (all) | 0.0463, 0.1217 | 0.0637, 0.1962 | 0.0445, 0.1203 | 0.0535, 0.1586 | 0.0425, 0.1101 |
| Goodness-of-fit | 1.004 | 1.004 | 1.005 | 1.003 | 1.005 |
| Residual electron density (e Å−3) | 1.188/–0.695 | 1.722/–1.937 | 1.039/–0.728 | 2.068/–1.174 | 2.767/–1.492 |
| CCDC reference | 1818020 | 1818021 | 1818022 | 1818023 | 1818024 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prokhorova, T.G.; Yagubskii, E.B.; Zorina, L.V.; Simonov, S.V.; Zverev, V.N.; Shibaeva, R.P.; Buravov, L.I. Specific Structural Disorder in an Anion Layer and Its Influence on Conducting Properties of New Crystals of the (BEDT-TTF)4A+[M3+(ox)3]G Family, Where G Is 2-Halopyridine; M Is Cr, Ga; A+ Is [K0.8(H3O)0.2]+. Crystals 2018, 8, 92. https://doi.org/10.3390/cryst8020092
Prokhorova TG, Yagubskii EB, Zorina LV, Simonov SV, Zverev VN, Shibaeva RP, Buravov LI. Specific Structural Disorder in an Anion Layer and Its Influence on Conducting Properties of New Crystals of the (BEDT-TTF)4A+[M3+(ox)3]G Family, Where G Is 2-Halopyridine; M Is Cr, Ga; A+ Is [K0.8(H3O)0.2]+. Crystals. 2018; 8(2):92. https://doi.org/10.3390/cryst8020092
Chicago/Turabian StyleProkhorova, Tatiana G., Eduard B. Yagubskii, Leokadiya V. Zorina, Sergey V. Simonov, Vladimir N. Zverev, Rimma P. Shibaeva, and Lev I. Buravov. 2018. "Specific Structural Disorder in an Anion Layer and Its Influence on Conducting Properties of New Crystals of the (BEDT-TTF)4A+[M3+(ox)3]G Family, Where G Is 2-Halopyridine; M Is Cr, Ga; A+ Is [K0.8(H3O)0.2]+" Crystals 8, no. 2: 92. https://doi.org/10.3390/cryst8020092





