Structural and Magnetic Behavior of Oxidized and Reduced Fe Doped LiNbO3 Powders
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis
2.2. Characterization and Magnetic Measurements
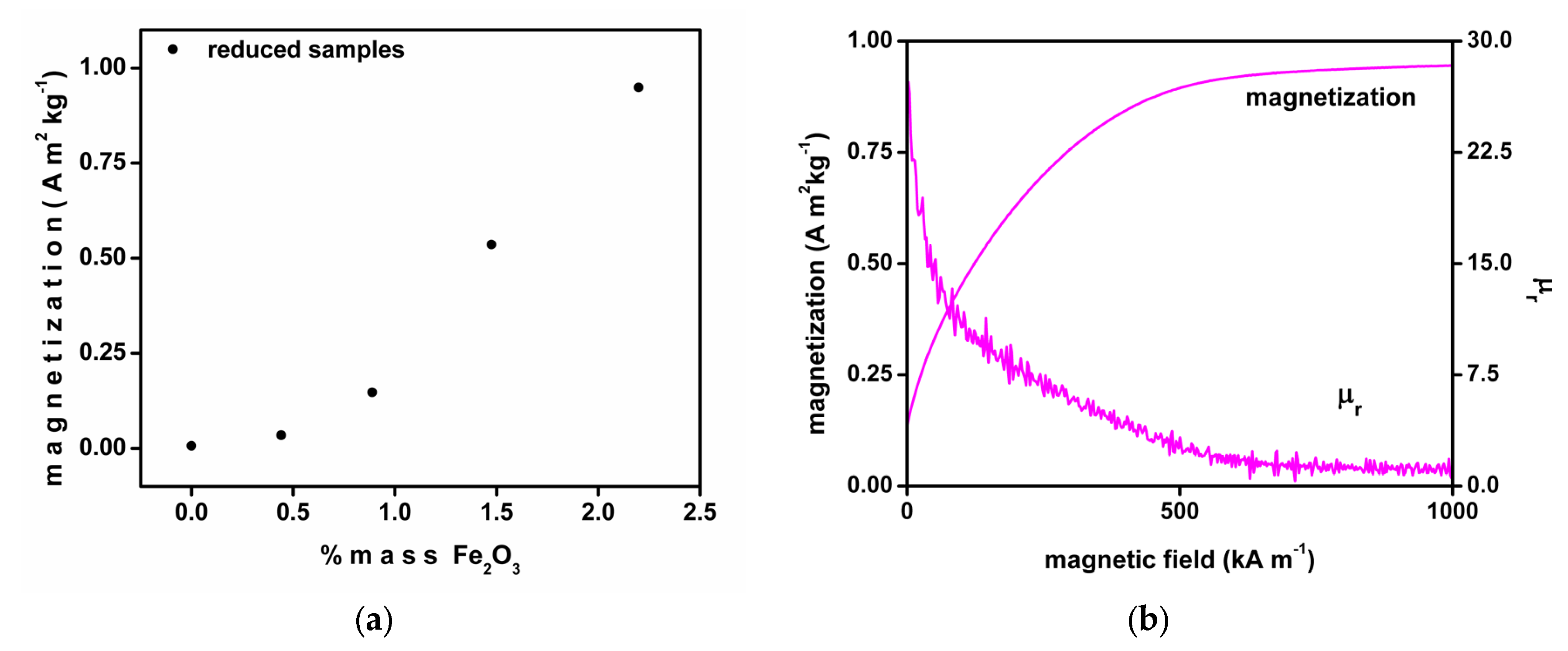
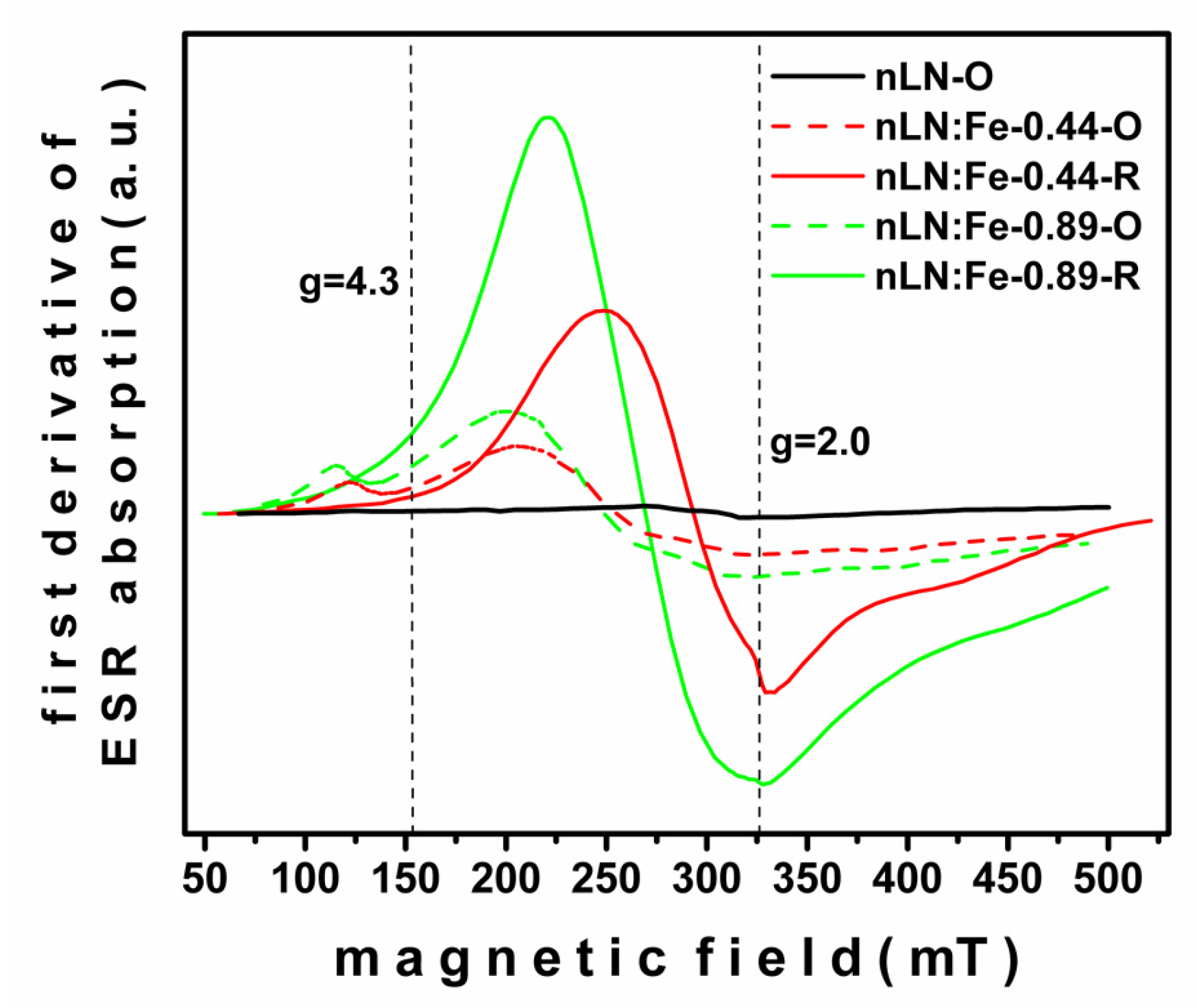
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Nassau, K.; Levinstein, H.J.; Loiacono, G.M. Ferroelectric Lithium Niobate. 1. Growth, domain structure, dislocations and etching. J. Chem. Phys. Solids 1966, 27, 839–888. [Google Scholar] [CrossRef]
- Nassau, K.; Levinstein, H.J.; Loiacono, G.M. Ferroelectric Lithium Niobate. 2. Preparation of single domain crystals. J. Chem. Phys. Solids 1966, 27, 989–996. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Reddy, J.M.; Bernstein, J.L. Ferroelectric Lithium Niobate. 3. Single crystal X-ray diffraction study at 24 °C. J. Chem. Phys. Solids 1966, 27, 971–1012. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Hamilton, W.C.; Reddy, J.M. Ferroelectric Lithium Niobate. 4. Single crystal neutron diffraction study at 24 °C. J. Chem. Phys. Solids 1966, 27, 1013–1018. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Levinstein, H.J.; Reddy, J.M. Ferroelectric Lithium Niobate. 5. Polycrystal X-ray diffraction study between 24 °C and 1200 °C. J. Chem. Phys. Solids 1966, 27, 1019–1026. [Google Scholar] [CrossRef]
- Weis, R.S.; Gayklord, T.K. Lithium Niobate: Summary of physical properties and crystal structure. Appl. Phys. A 1985, 37, 191–203. [Google Scholar] [CrossRef]
- Arizmendi, L. Photonic applications of lithium niobate crystals. Phys. Status Solidi (a) 2004, 201, 253–283. [Google Scholar] [CrossRef]
- Peach, R.C.; Craig, D.; Lewis, M.F.; West, C.L.; Morgan, D.F.; Fedorov, V.A.; Korkishko, Y.N.; Ciplys, D.; Rimeika, R.; Hinkov, V. Acoustic wave propagation and properties. In EMIS Datareviews Series. Properties of Lithium Niobate, 1st ed.; Wong, K.K., Northstar Photonics Inc. USA, Eds.; The Institution of Electrical Engineers (INSPEC): London, UK, 2002; Volume 28, pp. 213–270. ISBN 0-85296-799-3. [Google Scholar]
- Sumets, M.P.; Dybov, V.A.; Ievlev, V.M. LiNbO3 Films: Potential application, synthesis techniques, structure, properties. Inorg. Mater. 2017, 53, 1361–1377. [Google Scholar] [CrossRef]
- Luo, R.; Jiang, H.; Rogers, S.; Liang, H.; He, Y.; Lin, Q. On-chip second-harmonic generation and broadband parametric down-conversion in a lithium niobate microresonator. Opt. Exp. 2017, 25, 24531–24539. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Dena, O.; García-Ramírez, E.V.; Fierro-Ruiz, C.D.; Vigueras-Santiago, E.; Farías, R.; Reyes-Esqueda, J.A. Effect of size and composition on the second harmonic generation from lithium niobate powders at different excitation wavelengths. Mater. Res. Express 2017, 4. [Google Scholar] [CrossRef]
- Volk, T.; Wöhlecke, M. Point defects in LiNbO3. In Springer Series in Materials Science. Lithium Niobate. Defects, Photorefraction and Ferroelectric Switching, 1st ed.; Hull, R., Osgood, R.M., Jr., Parisi, J., Warlimont, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 115, pp. 9–50. ISBN 978-3-540-70765-3. [Google Scholar]
- Schirmer, O.F.; Thiemann, O.; Wöhlecke, M. Defects in LiNbO3—I. Experimental aspects. J. Phys. Chem. Solids 1991, 52, 185–200. [Google Scholar] [CrossRef]
- Malovichko, G.; Grachev, V.; Schirmer, O. Interrelation of intrinsic and extrinsic defects—Congruent, stoichiometric and regularly ordered lithium niobate. Appl. Phys. B 1999, 68, 785–793. [Google Scholar] [CrossRef]
- Kovács, L.; Kocksor, L.; Szaller, Z.; Hajdara, I.; Dravecz, G.; Lengyel, K.; Corradi, G. Lattice site of rare-earth ions in stoichiometric lithium niobate probed by OH− vibrational spectroscopy. Crystals 2017, 7, 230. [Google Scholar] [CrossRef]
- Song, C.; Zeng, F.; Shen, Y.X.; Geng, K.W.; Xie, Y.N.; Wu, Z.Y.; Pan, F. Local Co structure and ferromagnetism in ion-implanted Co-doped LiNbO3. Phys. Rev. B 2006, 73. [Google Scholar] [CrossRef]
- Song, C.; Wang, C.Z.; Yang, Y.C.; Liu, X.J.; Zeng, F.; Pan, F. Room temperature ferromagnetism and ferroelectricity in cobalt-doped LiNbO3 film. Appl. Phys. Lett. 2008, 92. [Google Scholar] [CrossRef]
- Zeng, F.; Sheng, P.; Tang, G.S.; Pan, F.; Yan, W.S.; Hu, F.C.; Zou, Y.; Huang, Y.Y.; Jiang, Z.; Guo, D. Electronic structure and magnetism of Fe-doped LiNbO3. Mater. Chem. Phys. 2012, 136, 783–788. [Google Scholar] [CrossRef]
- Yan, T.; Ye, N.; Xu, L.; Sang, Y.; Chen, Y.; Song, W.; Long, X.; Wang, J.; Liu, H. Ferromagnetism in chemically reduced LiNbO3 and LaTiO3 crystals. J. Phys. D Appl. Phys. 2016, 49. [Google Scholar] [CrossRef]
- Díaz-Moreno, C.; Farias, R.; Hurtado-Macias, A.; Elizalde-Galindo, J.; Hernandez-Paz, J. Multiferroic response of nanocrystalline lithium niobate. J. Appl. Phys. 2012, 111. [Google Scholar] [CrossRef]
- Li, Y.Y.; Chen, H.L.; Kuo, C.L.; Hsieh, P.H.; Hwang, W.S. Raman spectra and ferromagnetism of nanocrystalline Fe-doped Li0.43Nb0.57O3+δ. Ceram. Int. 2016, 42, 10764–10769. [Google Scholar] [CrossRef]
- Kong, L.B.; Chang, T.S.; Ma, J.; Boey, F. Progress in synthesis of ferroelectric ceramic materials via high-energy mechanochemical technique. Prog. Mater. Sci. 2008, 53, 207–322. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 11–84. [Google Scholar] [CrossRef]
- Crystallographic Open Database. Information Card for Entry 2101175. Available online: http://www.crystallography.net/cod/2101175.html (accessed on 15 December 2017).
- Maeda, K.; Mizubayashi, H.B. Nanoscopic Architecture and Microstructure. In Springer Handbook of Materials Measurement Methods, 1st ed.; Czichos, H., Saito, T., Smith, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; p. 221. ISBN 978-3-540-20785-6. [Google Scholar]
- Repelin, Y.; Husson, E.; Bennani, F.; Proust, C. Raman spectroscopy of lithium niobate and lithium tantalite. Force field calculation. J. Phys. Chem. Solids 1999, 60, 819–825. [Google Scholar] [CrossRef]
- Pezzotti, G. Raman spectroscopy of piezoelectrics. J. Appl. Phys. 2013, 113. [Google Scholar] [CrossRef]
- Schlarb, U.; Klauer, S.; Wesselmann, M.; Betzler, K.; Wöhlecke, M. Determination of the Li/Nb ratio in Lithium Niobate by Means of Birefringence and Raman Measurements. Appl. Phys. A 1993, 56, 311–315. [Google Scholar] [CrossRef]
- fytyk. Curve Fitting and Data Analysis. Available online: Fytyk.nieto.pl (accessed on 13 December 2017).
- Knabe, B.; Schütze, D.; Jungk, T.; Svete, M.; Assenmacher, W.; Mader, W.; Buse, K. Synthesis and characterization of Fe-doped LiNbO3 nanocrystals from a triple-alkoxide method. Phys. Status Solidi (a) 2011, 208, 857–862. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Ciampolillo, M.V.; Zaltron, A.; Bazzan, M.; Argiolas, N.; Sada, C.; Bianconi, M. Lithium niobate crystals doped with iron by thermal diffusion: Relation between lattice deformation and reduction degree. J. Appl. Phys. 2010, 107. [Google Scholar] [CrossRef]
- Rebouta, L.; da Silva, M.F.; Soares, J.C.; Hage-Ali, M.; Stoquert, J.P.; Siffert, P.; Sanz-García, J.A.; Diéguez, E.; Agulló-López, F. Lattice site of Iron in LiNbO3(Fe3+) by the PIXE/Chanelling Technique. EPL 1991, 14, 557–561. [Google Scholar] [CrossRef]
- Shannon, R.D.; Prewitt, C.T. Effective Ionic Radii in Oxides and Fluorides. Acta Cryst. 1969, B25, 925–946. [Google Scholar] [CrossRef]
- Towner, H.H.; Kim, Y.M.; Story, H.S. EPR studies of crystal field parameters in Fe3+:LiNbO3. J. Chem. Phys. 1971, 56, 3676–3679. [Google Scholar] [CrossRef]
- Griscom, D.L.; Beltrán-López, V.; Merzbacher, C.I.; Bolden, E. Electron spin resonance of 65-million-year-old glasses and rocks from the Cretaceous-Tertiary boundary. JNCS 1999, 253, 1–22. [Google Scholar] [CrossRef]
- Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, W. The high score suite. Powder Diffr. 2014, 29, S13–S18. [Google Scholar] [CrossRef]
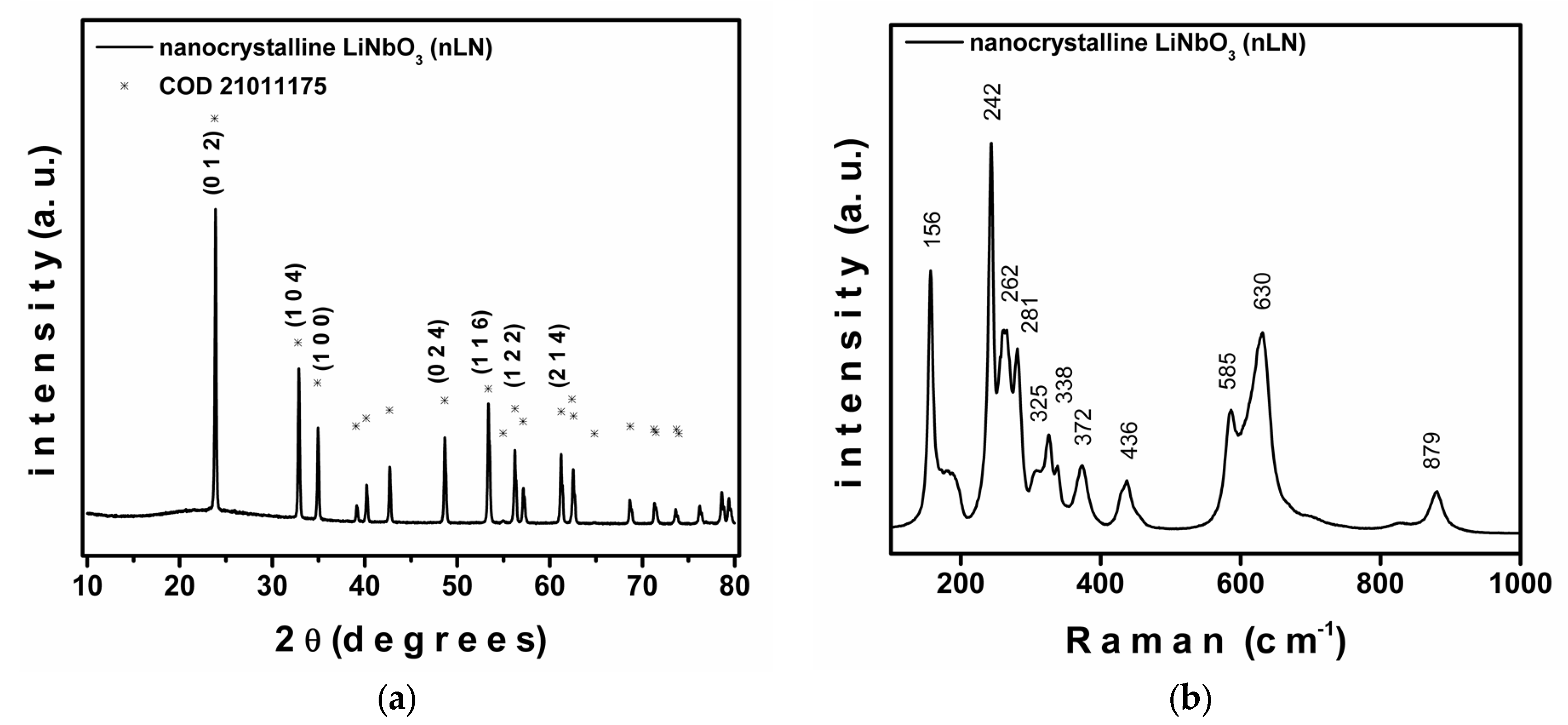
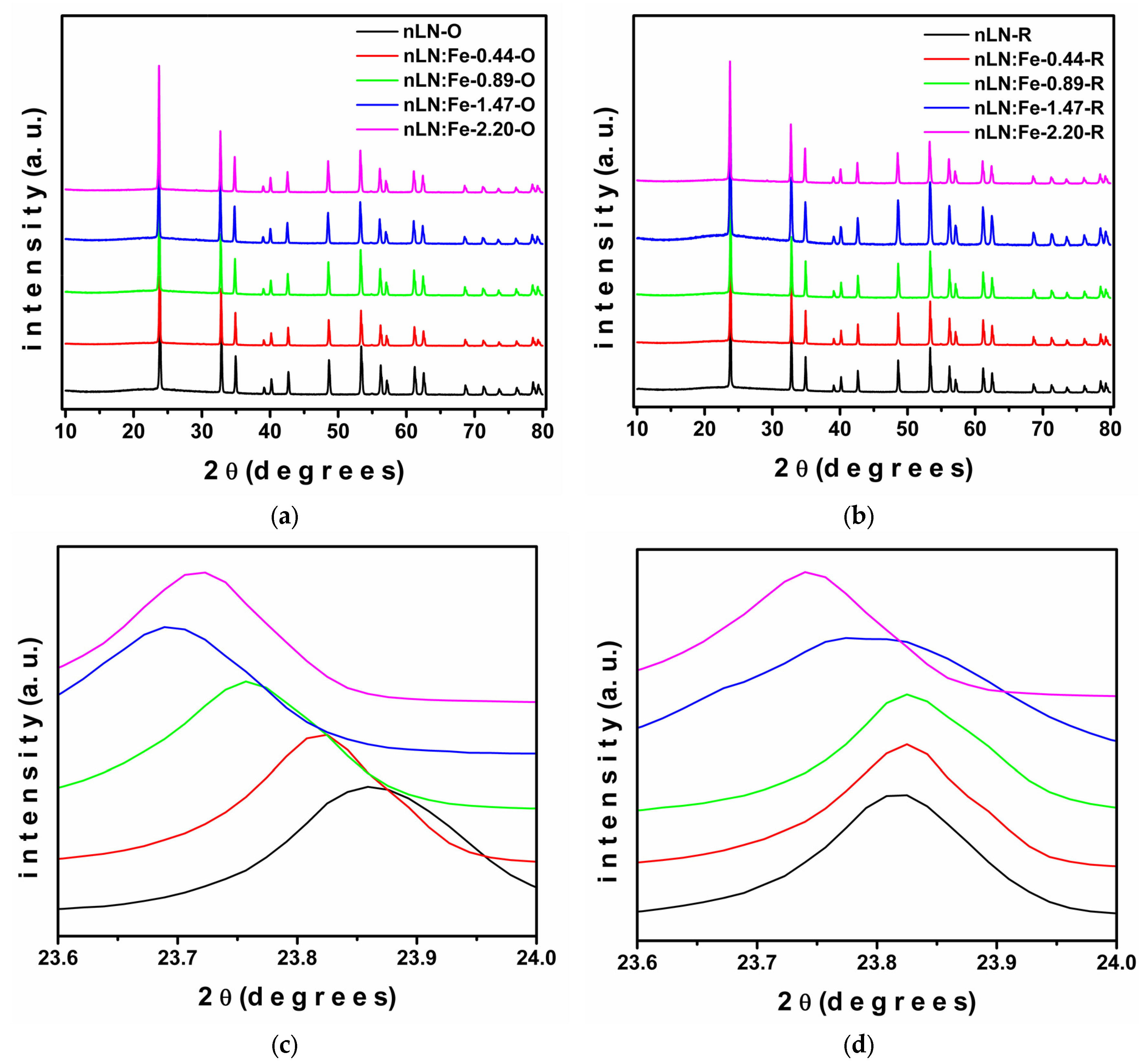
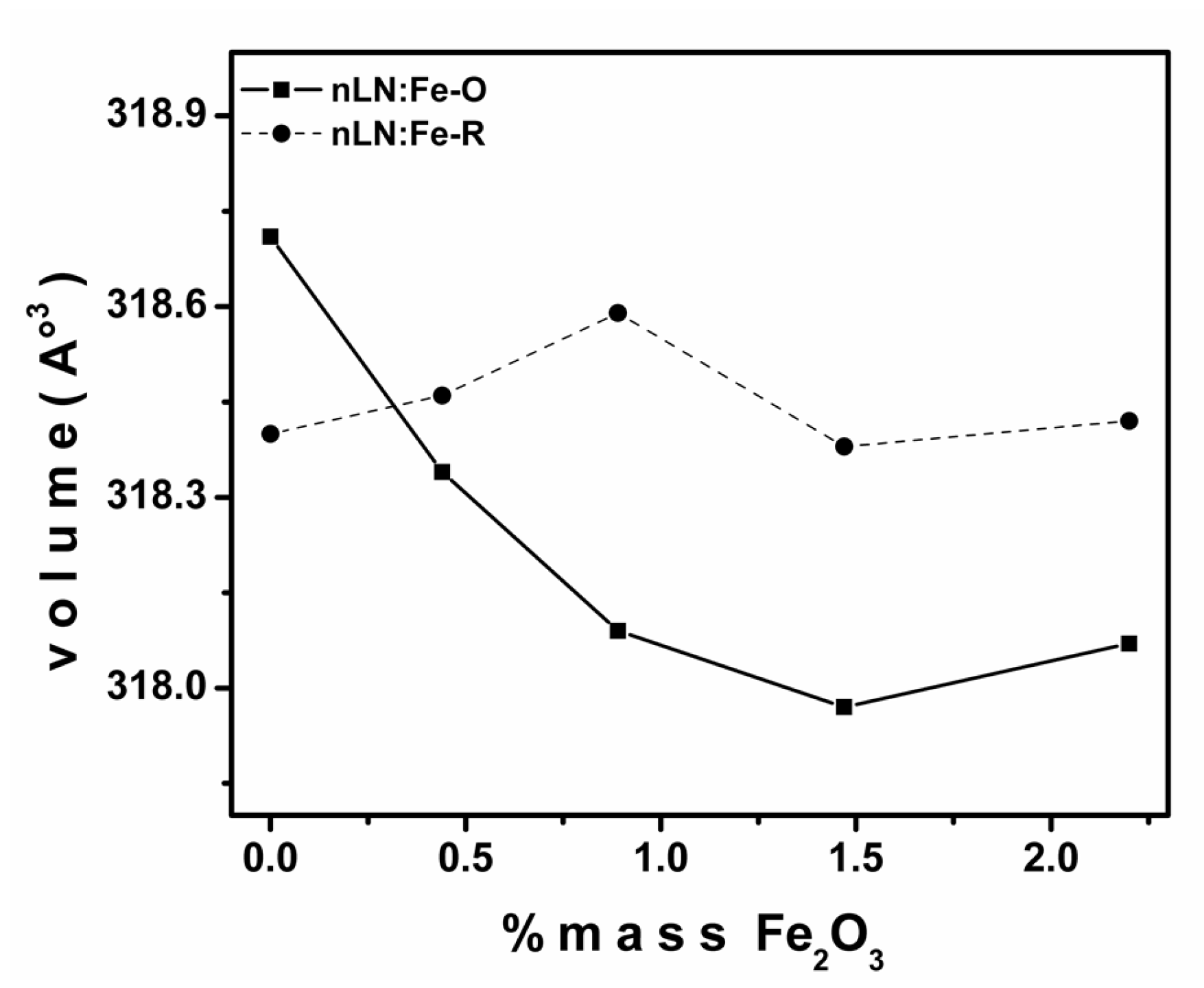
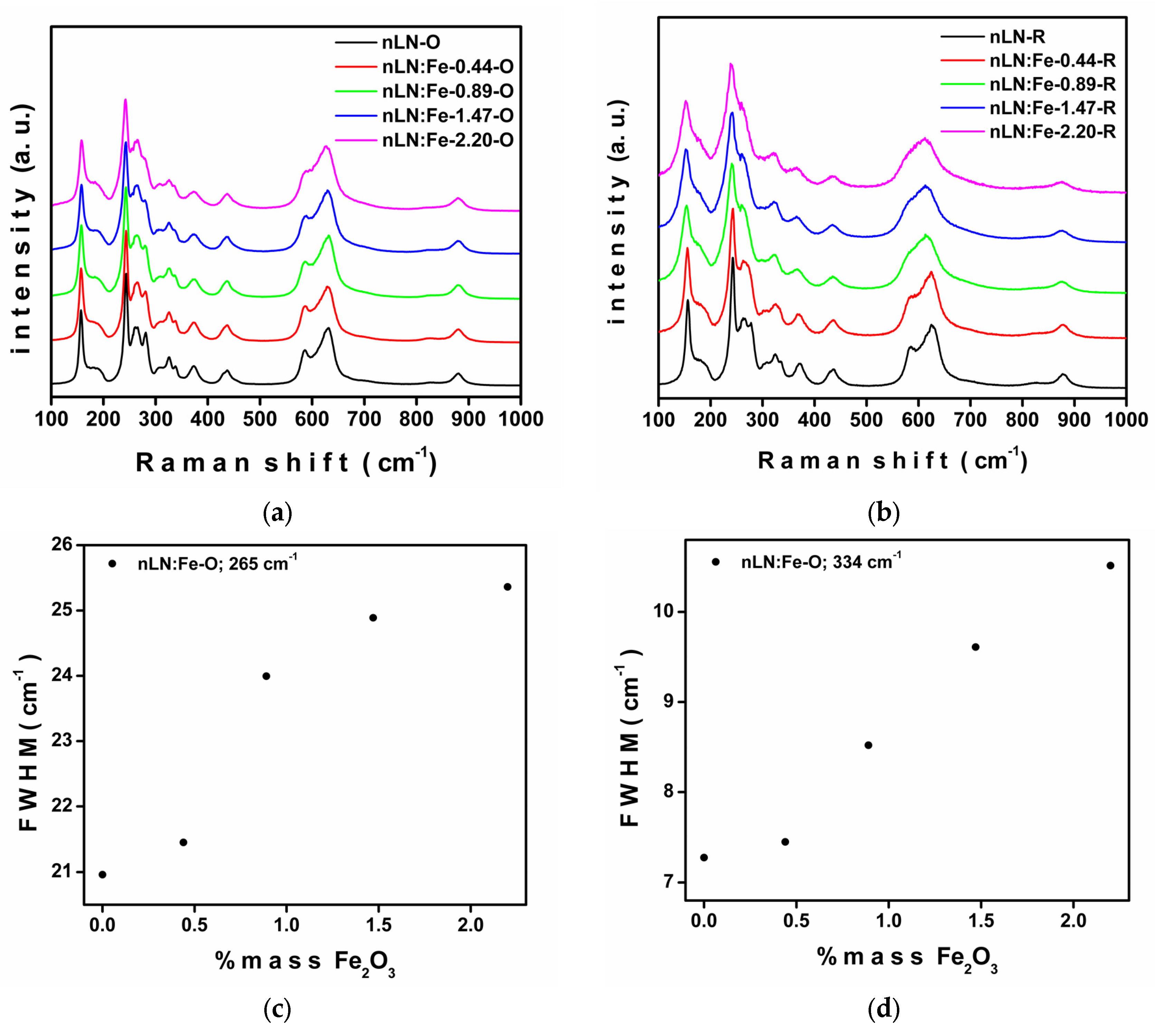

| Sample | a () = b () | c () | Cell Volume (3) | Weighted R Profile | Goodness of Fit |
|---|---|---|---|---|---|
| nLN-O | 5.15351 | 13.85655 | 318.70730 | 12.97 | 21.07 |
| nLN:Fe-0.44-O | 5.15117 | 13.85305 | 318.33790 | 12.89 | 24.94 |
| nLN:Fe-0.89-O | 5.15023 | 13.84717 | 318.08590 | 13.53 | 26.14 |
| nLN:Fe-1.47-O | 5.14999 | 13.84339 | 317.96950 | 13.03 | 22.69 |
| nLN:Fe-2.20-O | 5.15042 | 13.84527 | 318.06570 | 12.52 | 23.37 |
| nLN-R | 5.15154 | 13.85371 | 318.39860 | 14.25 | 27.32 |
| nLN:Fe-0.44-R | 5.15196 | 13.85424 | 318.46240 | 14.87 | 32.88 |
| nLN:Fe-0.89-R | 5.15349 | 13.84612 | 318.59460 | 13.45 | 24.15 |
| nLN:Fe-1.47-R | 5.15245 | 13.84793 | 318.37750 | 13.42 | 23.53 |
| nLN:Fe-2.20-R | 5.15265 | 13.84850 | 318.41600 | 12.48 | 23.14 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fierro-Ruiz, C.D.; Sánchez-Dena, O.; Cabral-Larquier, E.M.; Elizalde-Galindo, J.T.; Farías, R. Structural and Magnetic Behavior of Oxidized and Reduced Fe Doped LiNbO3 Powders. Crystals 2018, 8, 108. https://doi.org/10.3390/cryst8030108
Fierro-Ruiz CD, Sánchez-Dena O, Cabral-Larquier EM, Elizalde-Galindo JT, Farías R. Structural and Magnetic Behavior of Oxidized and Reduced Fe Doped LiNbO3 Powders. Crystals. 2018; 8(3):108. https://doi.org/10.3390/cryst8030108
Chicago/Turabian StyleFierro-Ruiz, Cesar D., Oswaldo Sánchez-Dena, Eva M. Cabral-Larquier, José T. Elizalde-Galindo, and Rurik Farías. 2018. "Structural and Magnetic Behavior of Oxidized and Reduced Fe Doped LiNbO3 Powders" Crystals 8, no. 3: 108. https://doi.org/10.3390/cryst8030108






