The Jahn-Teller Distortion at High Pressure: The Case of Copper Difluoride
Abstract
:1. Introduction
2. Materials and Methods
3. Results
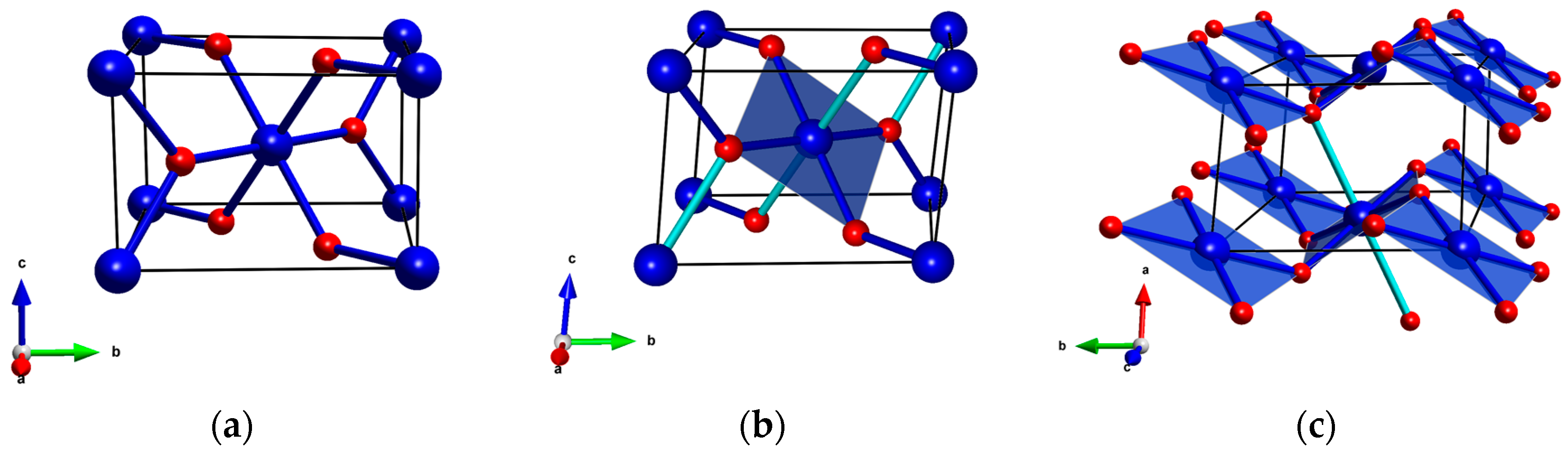
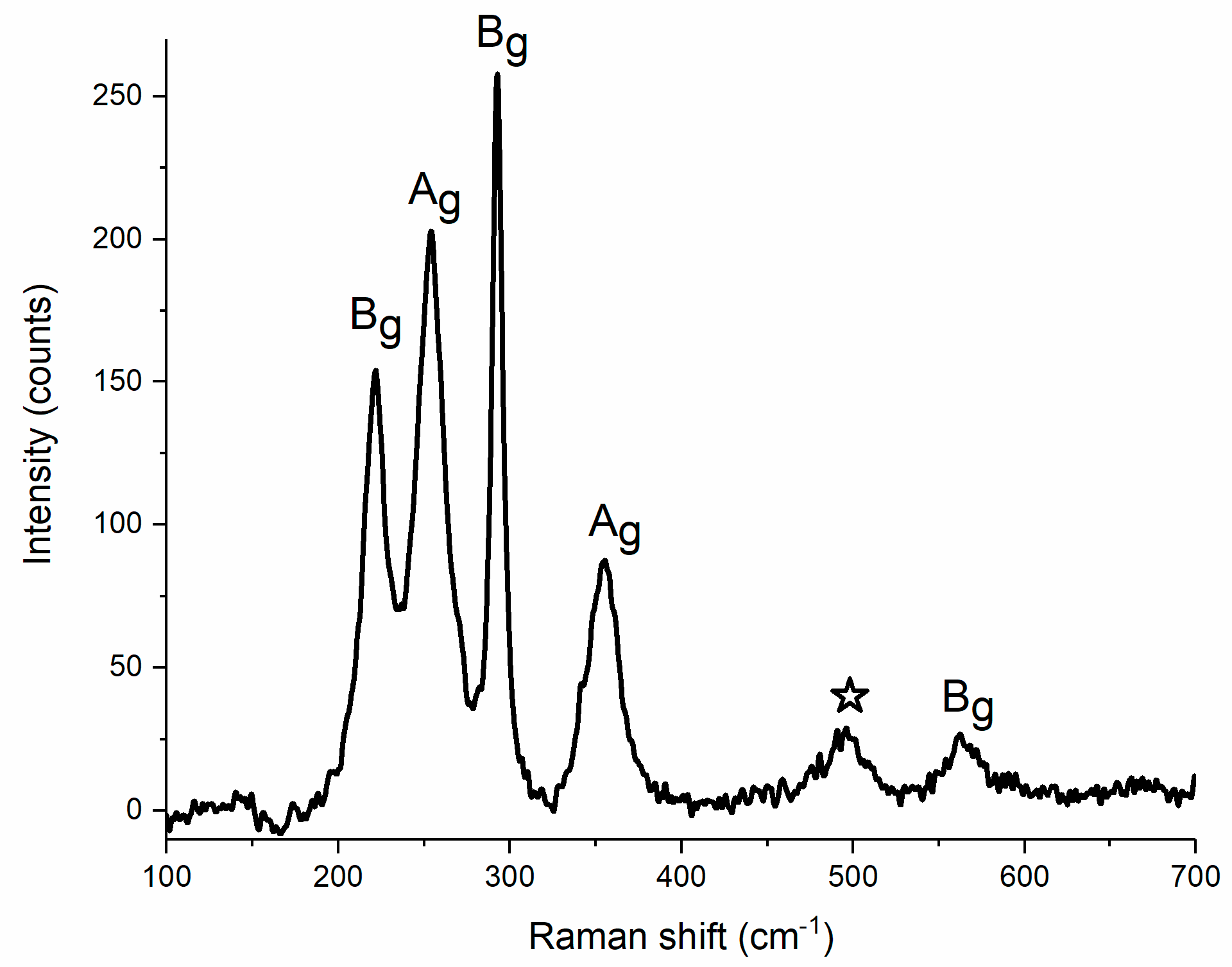
3.1. Ambient Pressure
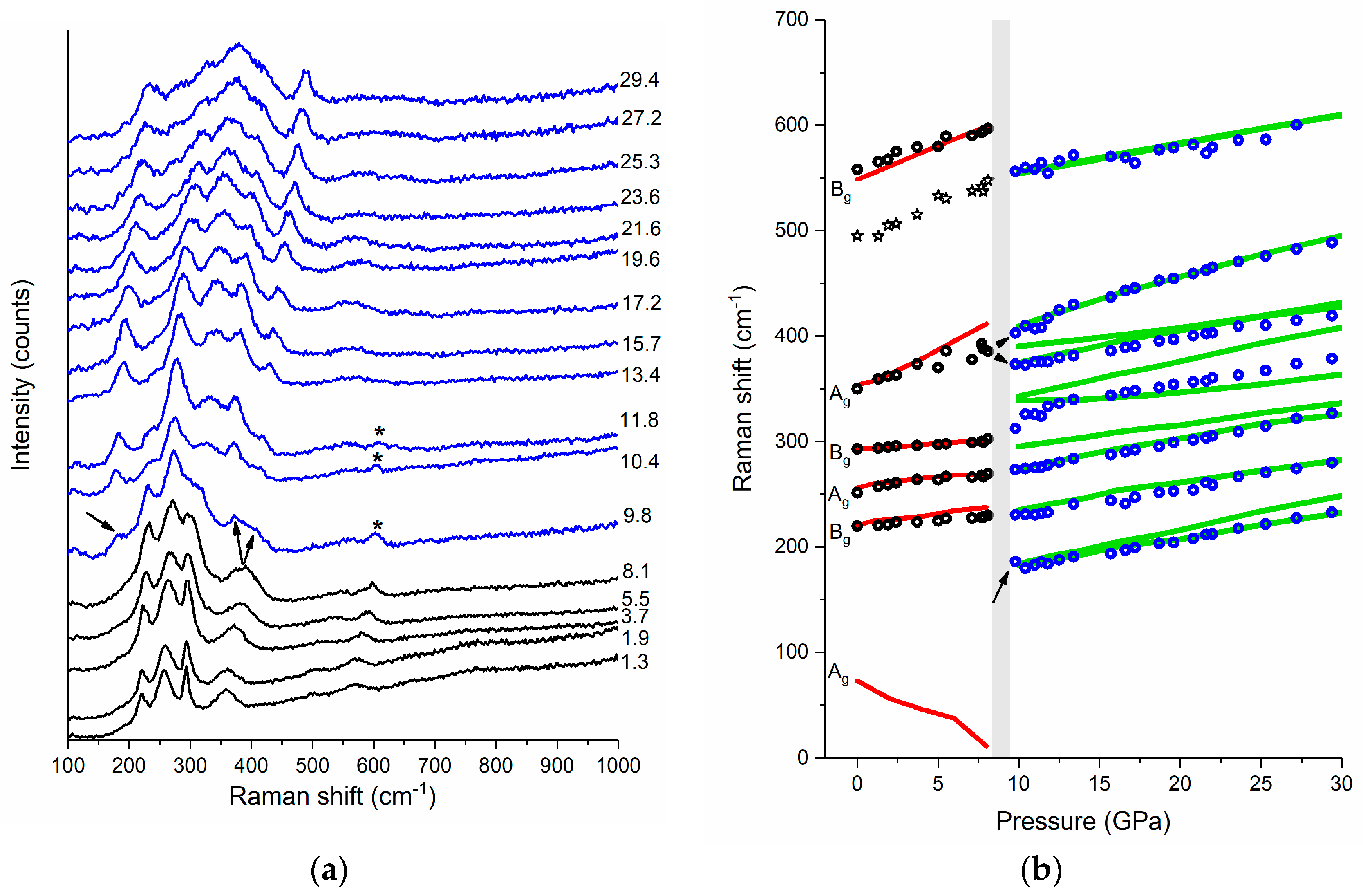
3.2. Raman Scattering up to 29 GPa
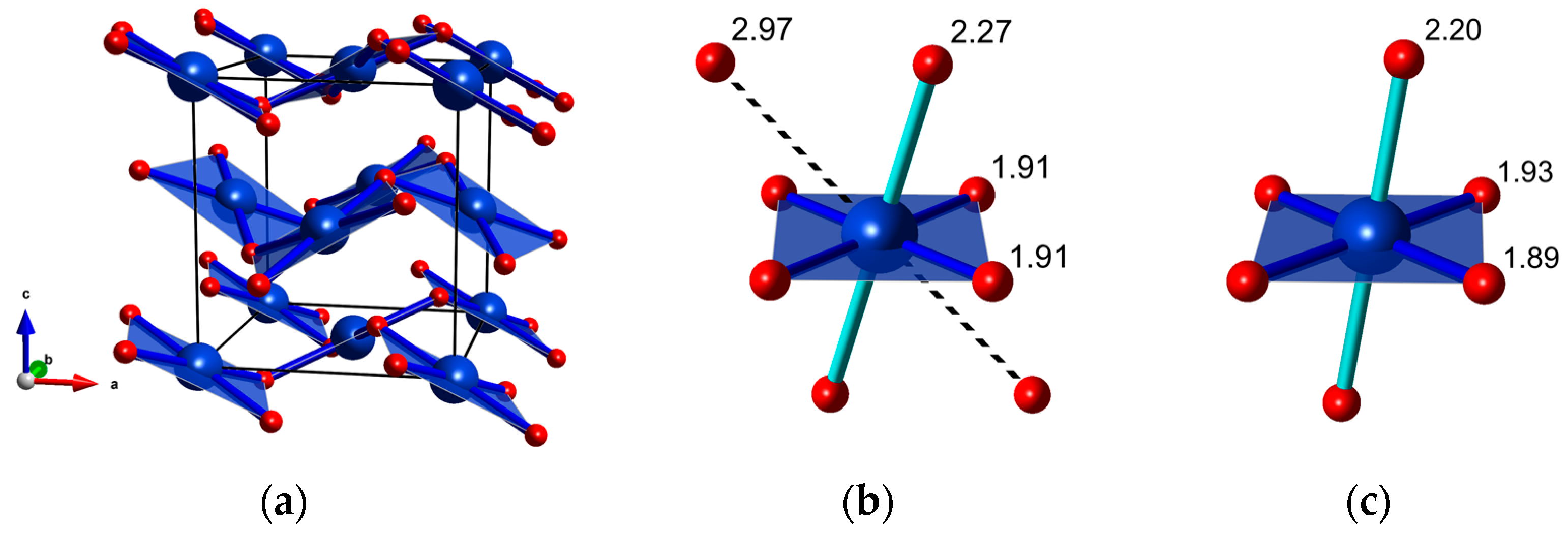
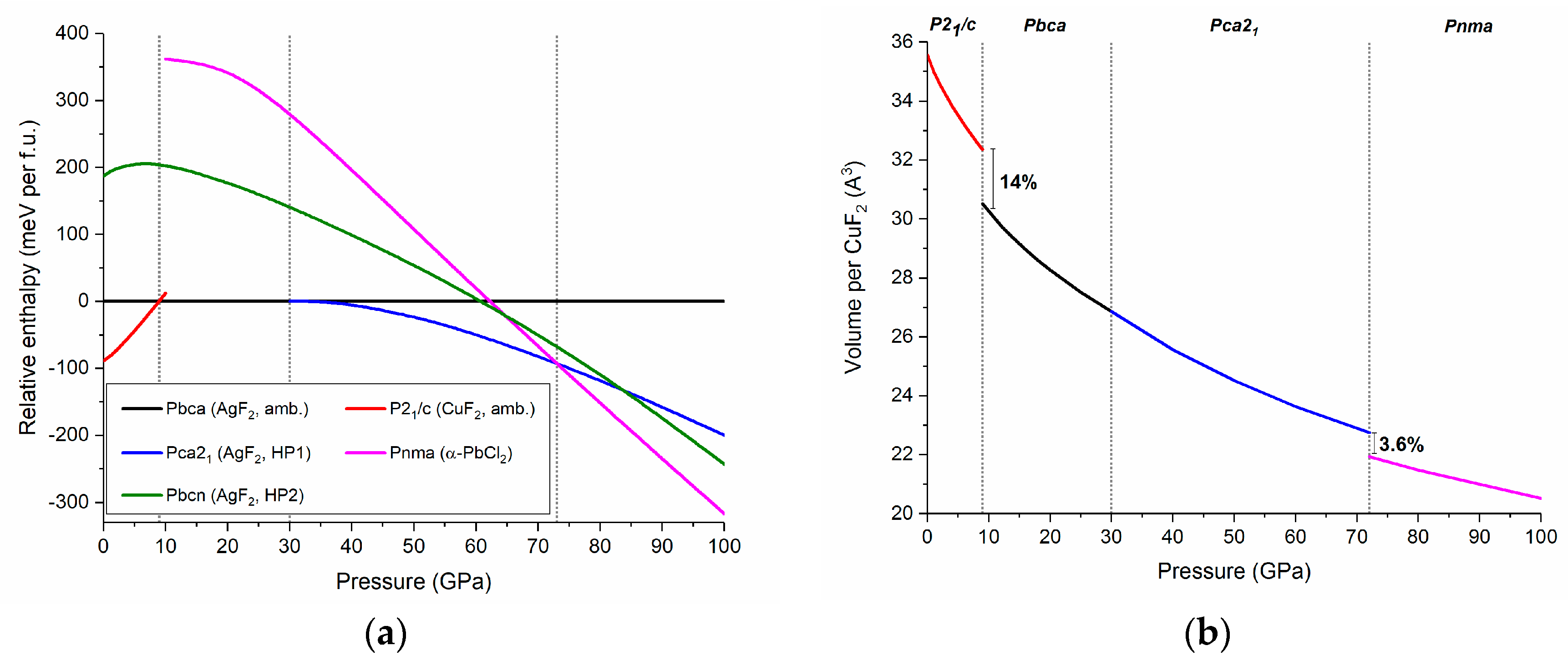
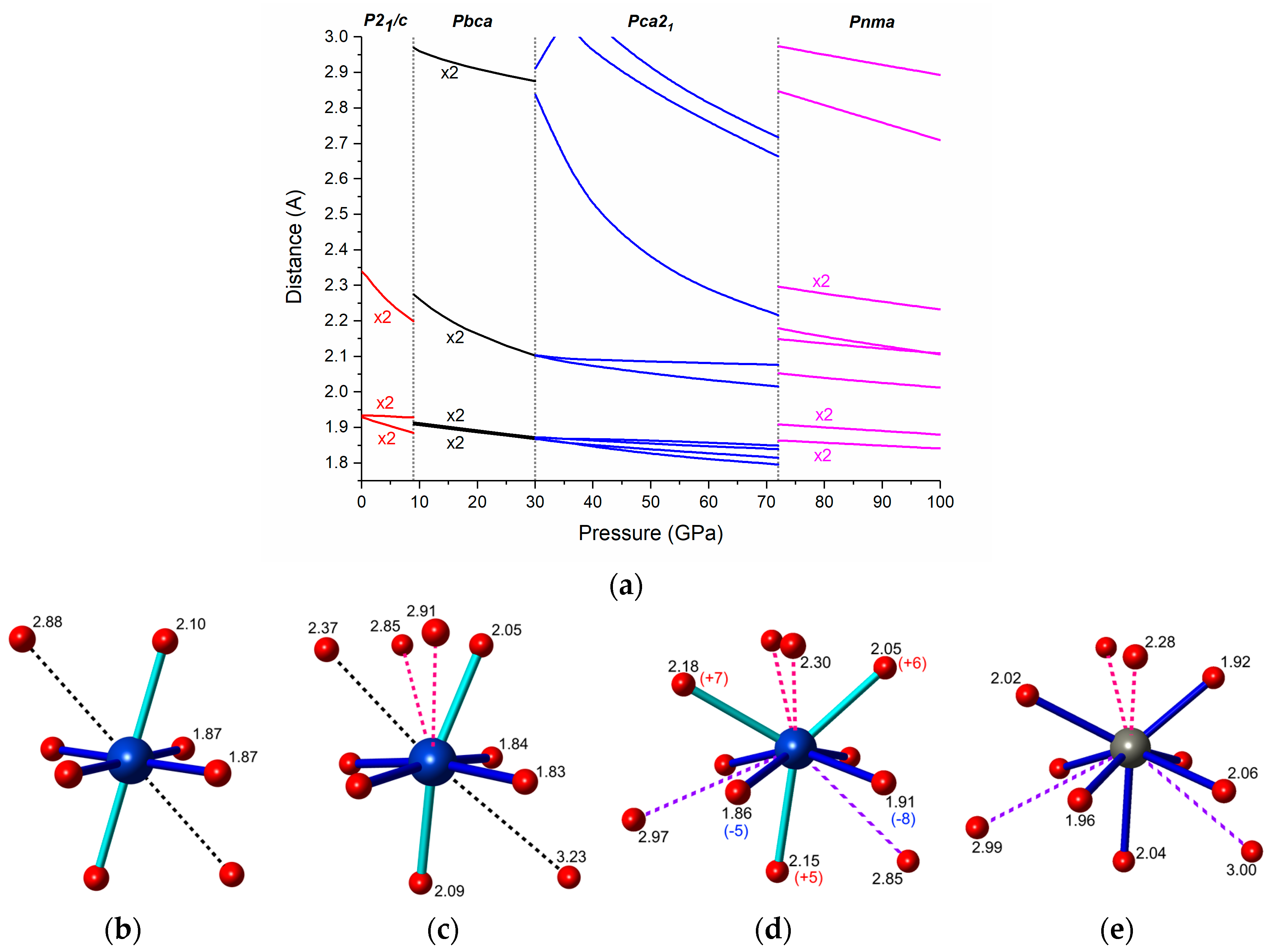
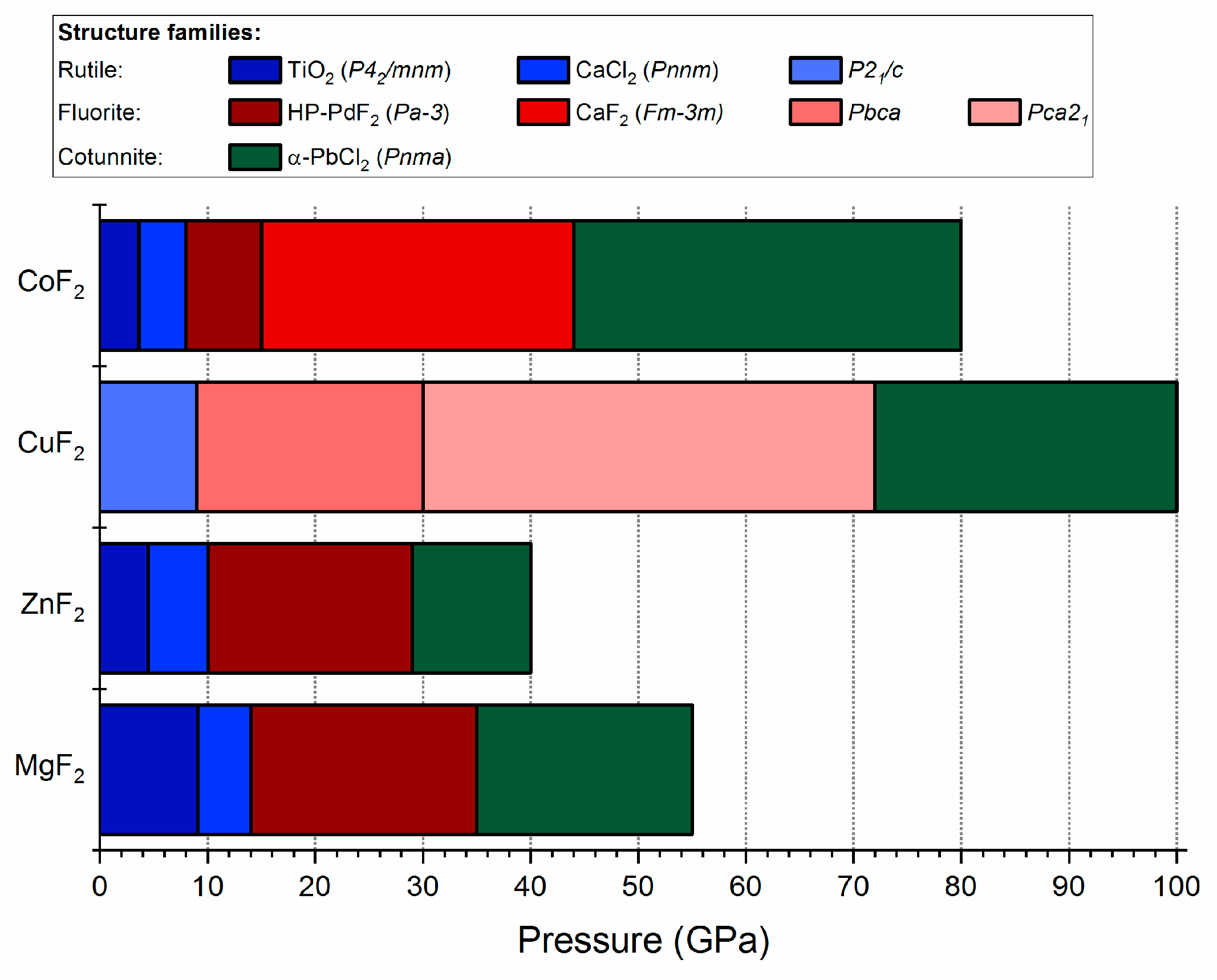
3.3. Calculations up to 100 GPa
4. Discussion
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Jahn, H.A.; Teller, E. Stability of Polyatomic Molecules in Degenerate Electronic States. I. Orbital Degeneracy. Proc. R. Soc. A Math. Phys. Eng. Sci. 1937, 161, 220–235. [Google Scholar] [CrossRef]
- Reinen, D.; Friebel, C. Local and Cooperative Jahn-Teller Interactions in Model Structures Spectroscopic and Structural Evidence. In Structural Problems; Springer: Berlin/Heidelberg, Germany, 1979; Volume 37, pp. 1–60. [Google Scholar]
- Falvello, L.R. Jahn–Teller effects in solid-state co-ordination chemistry. Dalton Trans. 1997, 4463–4476. [Google Scholar] [CrossRef]
- Halcrow, M.A. Jahn-Teller distortions in transition metal compounds, and their importance in functional molecular and inorganic materials. Chem. Soc. Rev. 2013, 42, 1784–1795. [Google Scholar] [CrossRef] [PubMed]
- Mazej, Z.; Kurzydłowski, D.; Grochala, W. Unique Silver(II) Fluorides: The Emerging Electronic and Magnetic Materials. In Photonic and Electronic Properties of Fluoride Materials; Tressaud, A., Poeppelmeier, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 231–260. ISBN 9780128016398. [Google Scholar]
- Kugel’, K.I.; Khomskii, D.I. The Jahn-Teller effect and magnetism: Transition metal compounds. Sov. Phys. Uspekhi 1982, 25, 231–256. [Google Scholar] [CrossRef]
- Guennou, M.; Bouvier, P.; Toulemonde, P.; Darie, C.; Goujon, C.; Bordet, P.; Hanfland, M.; Kreisel, J. Jahn-Teller, Polarity, and Insulator-to-Metal Transition in BiMnO3 at High Pressure. Phys. Rev. Lett. 2014, 112, 75501. [Google Scholar] [CrossRef] [PubMed]
- Reinen, D. The Modulation of Jahn–Teller Coupling by Elastic and Binding Strain Perturbations—A Novel View on an Old Phenomenon and Examples from Solid-State Chemistry†. Inorg. Chem. 2012, 51, 4458–4472. [Google Scholar] [CrossRef] [PubMed]
- Bersuker, I.B. The Jahn-Teller and pseudo Jahn-Teller effect in materials science. J. Phys. Conf. Ser. 2017, 833, 12001. [Google Scholar] [CrossRef]
- McLain, S.E.; Dolgos, M.R.; Tennant, D.A.; Turner, J.F.C.; Barnes, T.; Proffen, T.; Sales, B.C.; Bewley, R.I. Magnetic behaviour of layered Ag(II) fluorides. Nat. Mater. 2006, 5, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Kurzydłowski, D.; Derzsi, M.; Mazej, Z.; Grochala, W. Crystal, electronic, and magnetic structures of M2AgF4 (M = Na–Cs) phases as viewed from the DFT+U method. Dalton Trans. 2016, 45, 16255–16261. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.A.; Martínez-Lope, M.J.; Casais, M.T.; Fernández-Dáz, M.T. Evolution of the Jahn-Teller distortion of MnO6 octahedra in RMnO3 perovskites (R = Pr, Nd, Dy, Tb, Ho, Er, Y): A neutron diffraction study. Inorg. Chem. 2000, 39, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Lufaso, M.W.; Woodward, P.M. Jahn-Teller distortions, cation ordering and octahedral tilting in perovskites. Acta Crystallogr. B. 2004, 60, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B. Electronic and ionic transport properties and other physical aspects of perovskites. Rep. Prog. Phys. 2004, 67, 1915–1993. [Google Scholar] [CrossRef]
- Kurzydłowski, D.; Mazej, Z.; Grochala, W. Na2AgF4: 1D antiferromagnet with unusually short Ag2+···Ag2+ separation. Dalton Trans. 2013, 42, 2167–2173. [Google Scholar] [CrossRef] [PubMed]
- Kurzydłowski, D.; Jaroń, T.; Ozarowski, A.; Hill, S.; Jagličić, Z.; Filinchuk, Y.; Mazej, Z.; Grochala, W. Local and Cooperative Jahn–Teller Effect and Resultant Magnetic Properties of M2AgF4 (M = Na–Cs) Phases. Inorg. Chem. 2016, 55, 11479–11489. [Google Scholar] [CrossRef] [PubMed]
- Aguado, F.; Rodríguez, F.; Núñez, P. Pressure-induced Jahn-Teller suppression and simultaneous high-spin to low-spin transition in the layered perovskite CsMnF4. Phys. Rev. B 2007, 76, 94417. [Google Scholar] [CrossRef]
- Baldini, M.; Struzhkin, V.V.; Goncharov, A.F.; Postorino, P.; Mao, W.L. Persistence of Jahn-Teller Distortion up to the Insulator to Metal Transition in LaMnO3. Phys. Rev. Lett. 2011, 106, 66402. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Fuertes, J.; Segura, A.; Rodríguez, F.; Errandonea, D.; Sanz-Ortiz, M.N. Anomalous High-Pressure Jahn-Teller Behavior in CuWO4. Phys. Rev. Lett. 2012, 108, 166402. [Google Scholar] [CrossRef] [PubMed]
- Aguado, F.; Rodríguez, F.; Valiente, R.; Itiè, J.-P.; Hanfland, M. Pressure effects on Jahn-Teller distortion in perovskites: The roles of local and bulk compressibilities. Phys. Rev. B 2012, 85, 100101. [Google Scholar] [CrossRef]
- Calestani, G.; Orlandi, F.; Mezzadri, F.; Righi, L.; Merlini, M.; Gilioli, E. Structural evolution under pressure of BiMnO3. Inorg. Chem. 2014, 53, 8749–8754. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, A.; Winkler, B.; Morgenroth, W.; Perlov, A.; Milman, V. Pressure-induced spin collapse of octahedrally coordinated Mn3+ in the tetragonal hydrogarnet henritermierite Ca3Mn2[SiO4]2 [O4 H4]. Phys. Rev. B 2015, 92, 014117. [Google Scholar] [CrossRef]
- Haines, J.; Léger, J.M.; Gorelli, F.; Klug, D.D.; Tse, J.S.; Li, Z.Q. X-ray diffraction and theoretical studies of the high-pressure structures and phase transitions in magnesium fluoride. Phys. Rev. B 2001, 64, 134110. [Google Scholar] [CrossRef]
- Perakis, A.; Lampakis, D.; Boulmetis, Y.C.; Raptis, C. High-pressure Raman study of the ferroelastic rutile-to-CaCl2 phase transition in ZnF2. Phys. Rev. B 2005, 72, 144108. [Google Scholar] [CrossRef]
- Wu, X.; Wu, Z. Theoretical calculations of the high-pressure phases of ZnF2 and CdF2. Eur. Phys. J. B 2006, 50, 521–526. [Google Scholar] [CrossRef]
- Kusaba, K.; Kikegawa, T. In situ X-ray observation of phase transitions in under high pressure and high temperature. Solid State Commun. 2008, 145, 279–282. [Google Scholar] [CrossRef]
- Dorfman, S.M.; Jiang, F.; Mao, Z.; Kubo, A.; Meng, Y.; Prakapenka, V.B.; Duffy, T.S. Phase transitions and equations of state of alkaline earth fluorides CaF2, SrF2, and BaF2 to Mbar pressures. Phys. Rev. B 2010, 81, 174121. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Li, Y.; Liu, Y.; Ma, Y. First-principles study of phase transitions in antiferromagnetic XF2 (X = Fe, Co and Ni). Solid State Commun. 2011, 151, 1475–1478. [Google Scholar] [CrossRef]
- Liu, G.; Wang, H.; Ma, Y.; Ma, Y. Phase transition of cadmium fluoride under high pressure. Solid State Commun. 2011, 151, 1899–1902. [Google Scholar] [CrossRef]
- López-Moreno, S.; Romero, A.H.; Mejía-López, J.; Muñoz, A.; Roshchin, I.V. First-principles study of electronic, vibrational, elastic, and magnetic properties of FeF2 as a function of pressure. Phys. Rev. B 2012, 85, 134110. [Google Scholar] [CrossRef]
- Barreda-Argüeso, J.A.; López-Moreno, S.; Sanz-Ortiz, M.N.; Aguado, F.; Valiente, R.; González, J.; Rodríguez, F.; Romero, A.H.; Muñoz, A.; Nataf, L.; et al. Pressure-induced phase-transition sequence in CoF2: An experimental and first-principles study on the crystal, vibrational, and electronic properties. Phys. Rev. B 2013, 88, 214108. [Google Scholar] [CrossRef]
- Torabi, S.; Hammerschmidt, L.; Voloshina, E.; Paulus, B. Ab initio investigation of ground-state properties of group-12 fluorides. Int. J. Quantum Chem. 2014, 114, 943–951. [Google Scholar] [CrossRef]
- López-Moreno, S.; Romero, A.H.; Mejía-López, J.; Muñoz, A. First-principles study of pressure-induced structural phase transitions in MnF2. Phys. Chem. Chem. Phys. 2016, 18, 33250–33263. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, E.; Yao, Y.; Goncharov, A.F.; Konôpková, Z.; Raptis, C. High-pressure structural study of MnF2. Phys. Rev. B 2016, 93, 54101. [Google Scholar] [CrossRef]
- Barreda-Argüeso, J.A.; Aguado, F.; González, J.; Valiente, R.; Nataf, L.; Sanz-Ortiz, M.N.; Rodríguez, F. Crystal-Field Theory Validity Through Local (and Bulk) Compressibilities in CoF2 and KCoF3. J. Phys. Chem. C 2016, 120, 18788–18793. [Google Scholar] [CrossRef]
- Grzelak, A.; Gawraczyński, J.; Jaroń, T.; Kurzydłowski, D.; Mazej, Z.; Leszczyński, P.J.; Prakapenka, V.B.; Derzsi, M.; Struzhkin, V.V.; Grochala, W. Metal fluoride nanotubes featuring square-planar building blocks in a high-pressure polymorph of AgF2. Dalton Trans. 2017, 46, 14742–14745. [Google Scholar] [CrossRef] [PubMed]
- Grzelak, A.; Gawraczyński, J.; Jaroń, T.; Kurzydłowski, D.; Budzianowski, A.; Mazej, Z.; Leszczyński, P.J.; Prakapenka, V.B.; Derzsi, M.; Struzhkin, V.V.; et al. High-Pressure Behavior of Silver Fluorides up to 40 GPa. Inorg. Chem. 2017, 56, 14651–14661. [Google Scholar] [CrossRef] [PubMed]
- Maitland, R.; Jack, K.H. The Crystal Structure and Interatomic Bonding of Chromous and Chromic Fluorides. Proc. Chem. Soc. 1957, 1957, 232–233. [Google Scholar]
- Dewaele, A.; Torrent, M.; Loubeyre, P.; Mezouar, M. Compression curves of transition metals in the Mbar range: Experiments and projector augmented-wave calculations. Phys. Rev. B 2008, 78, 104102. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk : A general-purpose peak fitting program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Liechtenstein, A.I.; Zaanen, J. Density-functional theory and strong interactions: Orbital ordering in Mott-Hubbard insulators. Phys. Rev. B 1995, 52, R5467. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Legut, D.; Wdowik, U.D. Vibrational properties and the stability of the KCuF3 phases. J. Phys. Condens. Matter 2013, 25, 115404. [Google Scholar] [CrossRef] [PubMed]
- Pavarini, E.; Koch, E.; Lichtenstein, A.I. Mechanism for Orbital Ordering in KCuF3. Phys. Rev. Lett. 2008, 101, 266405. [Google Scholar] [CrossRef] [PubMed]
- Binggeli, N.; Altarelli, M. Orbital ordering, Jahn-Teller distortion, and resonant x-ray scattering in KCuF3. Phys. Rev. B 2004, 70, 85117. [Google Scholar] [CrossRef]
- Fischer, P.; Hälg, W.; Schwarzenbach, D.; Gamsjäger, H. Magnetic and crystal structure of copper(II) fluoride. J. Phys. Chem. Solids 1974, 35, 1683–1689. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Falls, Z.; Lonie, D.C.; Avery, P.; Shamp, A.; Zurek, E. XtalOpt version r9: An open-source evolutionary algorithm for crystal structure prediction. Comput. Phys. Commun. 2016, 199, 178–179. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA: A three-dimensional visualization system for electronic and structural analysis. J. Appl. Crystallogr. 2008, 41, 653–658. [Google Scholar] [CrossRef]
- Stokes, H.T.; Hatch, D.M. FINDSYM : Program for identifying the space-group symmetry of a crystal. J. Appl. Crystallogr. 2005, 38, 237–238. [Google Scholar] [CrossRef]
- Kroumova, E.; Aroyo, M.I.; Perez-Mato, J.M.; Kirov, A.; Capillas, C.; Ivantchev, S.; Wondratschek, H. Bilbao Crystallographic Server: Useful Databases and Tools for Phase-Transition Studies. Phase Transit. 2003, 76, 155–170. [Google Scholar] [CrossRef]
- Billy, C.; Haendler, H.M. The Crystal Structure of Copper(II) Fluoride. J. Am. Chem. Soc. 1957, 79, 1049–1051. [Google Scholar] [CrossRef]
- Taylor, J.C.; Wilson, P.W. The structures of fluorides VI. Precise structural parameters in copper difluoride by neutron diffraction. J. Less Common Met. 1974, 34, 257–259. [Google Scholar] [CrossRef]
- Chatterji, T.; Hansen, T.C. Magnetoelastic effects in Jahn–Teller distorted CrF2 and CuF2 studied by neutron powder diffraction. J. Phys. Condens. Matter 2011, 23, 276007. [Google Scholar] [CrossRef] [PubMed]
- Burns, P.C.; Hawthorne, F.C. Rietveld Refinement of the Crystal Structure of CuF2. Powder Diffr. 2013, 6, 156–158. [Google Scholar] [CrossRef]
- Reinhardt, P.; Moreira, I.D.P.R.; de Graaf, C.; Dovesi, R.; Illas, F. Detailed ab-initio analysis of the magnetic coupling in CuF2. Chem. Phys. Lett. 2000, 319, 625–630. [Google Scholar] [CrossRef]
- Joenk, R.J.; Bozorth, R.M. Magnetic Properties of CuF2. J. Appl. Phys. 1965, 36, 1167–1168. [Google Scholar] [CrossRef]
- Boo, W.O.J.; Stout, J.W. Heat capacity and entropy of CuF2 and CrF2 from 10 to 300 °K. Anomalies associated with magnetic ordering and evaluation of magnetic contributions to the heat capacity. J. Chem. Phys. 1979, 71, 9–16. [Google Scholar] [CrossRef]
- Fischer, P.; Roult, G.; Schwarzenbach, D. Crystal and magnetic structure of silver difluoride-II. Weak 4d-ferromagnetism of AgF2. J. Phys. Chem. Solids 1971, 32, 1641–1647. [Google Scholar] [CrossRef]
- Jesih, A.; Lutar, K.; Žemva, B.; Bachmann, B.; Becker, S.; Müller, B.G.; Hoppe, R. Einkristalluntersuchungen an AgF2. Z. Anorg. Allg. Chem. 1990, 588, 77–83. [Google Scholar] [CrossRef]
- Birch, F. Finite Elastic Strain of Cubic Crystals. Phys. Rev. 1947, 71, 809–824. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]

| Symmetry | ωth | ωexp |
|---|---|---|
| Bg | 524 | 566 |
| Ag | 338 | 355 |
| Bg | 280 | 293 |
| Ag | 245 | 254 |
| Bg | 210 | 221 |
| Ag | 70 | n.d. |
| Phase | B0 | B0’ |
|---|---|---|
| P21/c | 75 | 6.1 |
| Pbca | 71 | 5.4 |
| ZnF2 (P42/mnm) | 101 (105) 1 | 4.3 |
| ZnF2 (Fm-3m) | 116 (120) 1 | 4.7 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurzydłowski, D. The Jahn-Teller Distortion at High Pressure: The Case of Copper Difluoride. Crystals 2018, 8, 140. https://doi.org/10.3390/cryst8030140
Kurzydłowski D. The Jahn-Teller Distortion at High Pressure: The Case of Copper Difluoride. Crystals. 2018; 8(3):140. https://doi.org/10.3390/cryst8030140
Chicago/Turabian StyleKurzydłowski, Dominik. 2018. "The Jahn-Teller Distortion at High Pressure: The Case of Copper Difluoride" Crystals 8, no. 3: 140. https://doi.org/10.3390/cryst8030140





