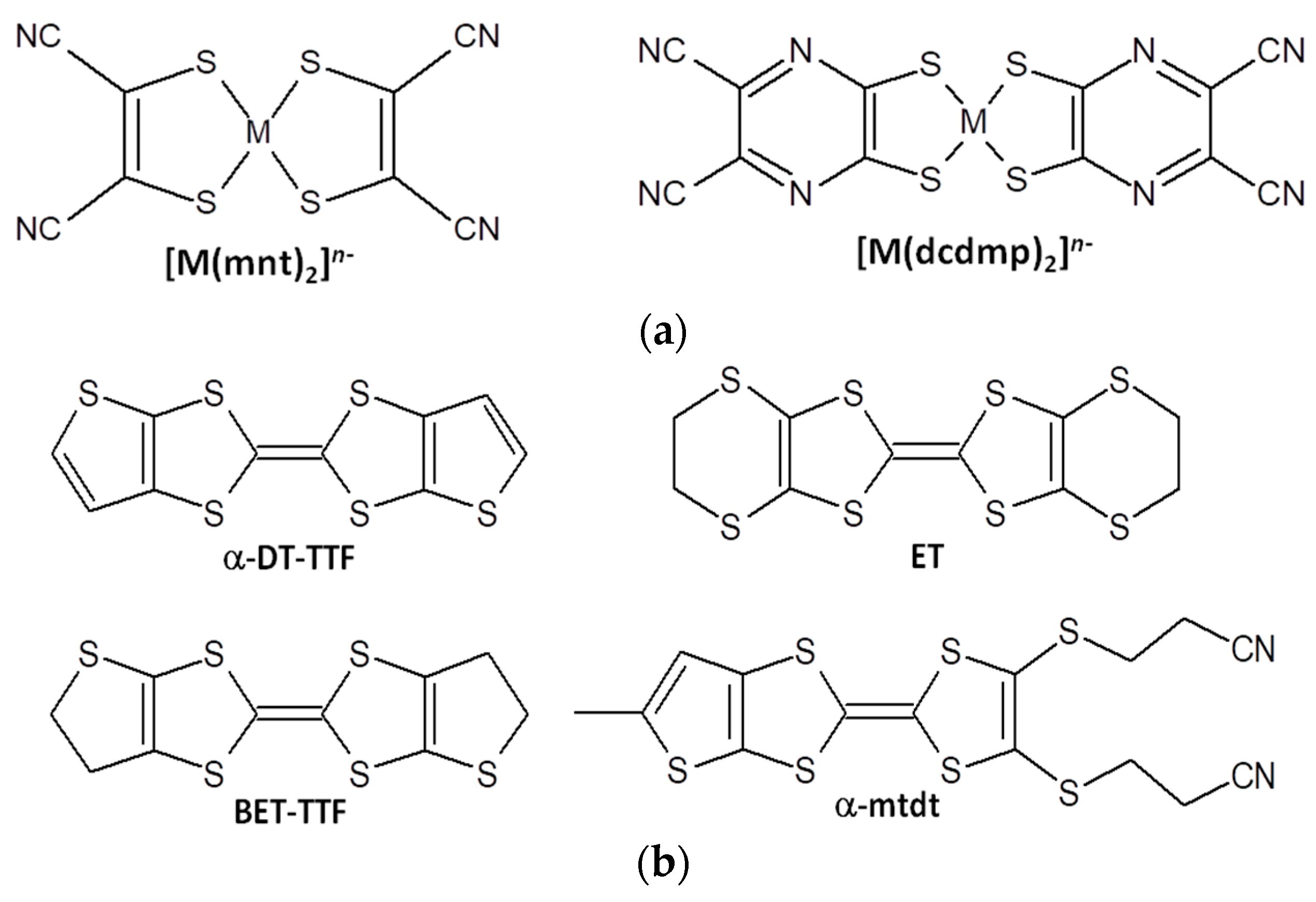
Synthesis and Characterization of Charge Transfer Salts Based on [M(dcdmp)2] (M = Au, Cu and Ni) with TTF Type Donors
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Information
2.2. Synthesis
2.3. X-ray Crystallography
2.4. Electric Transport Properties
3. Results
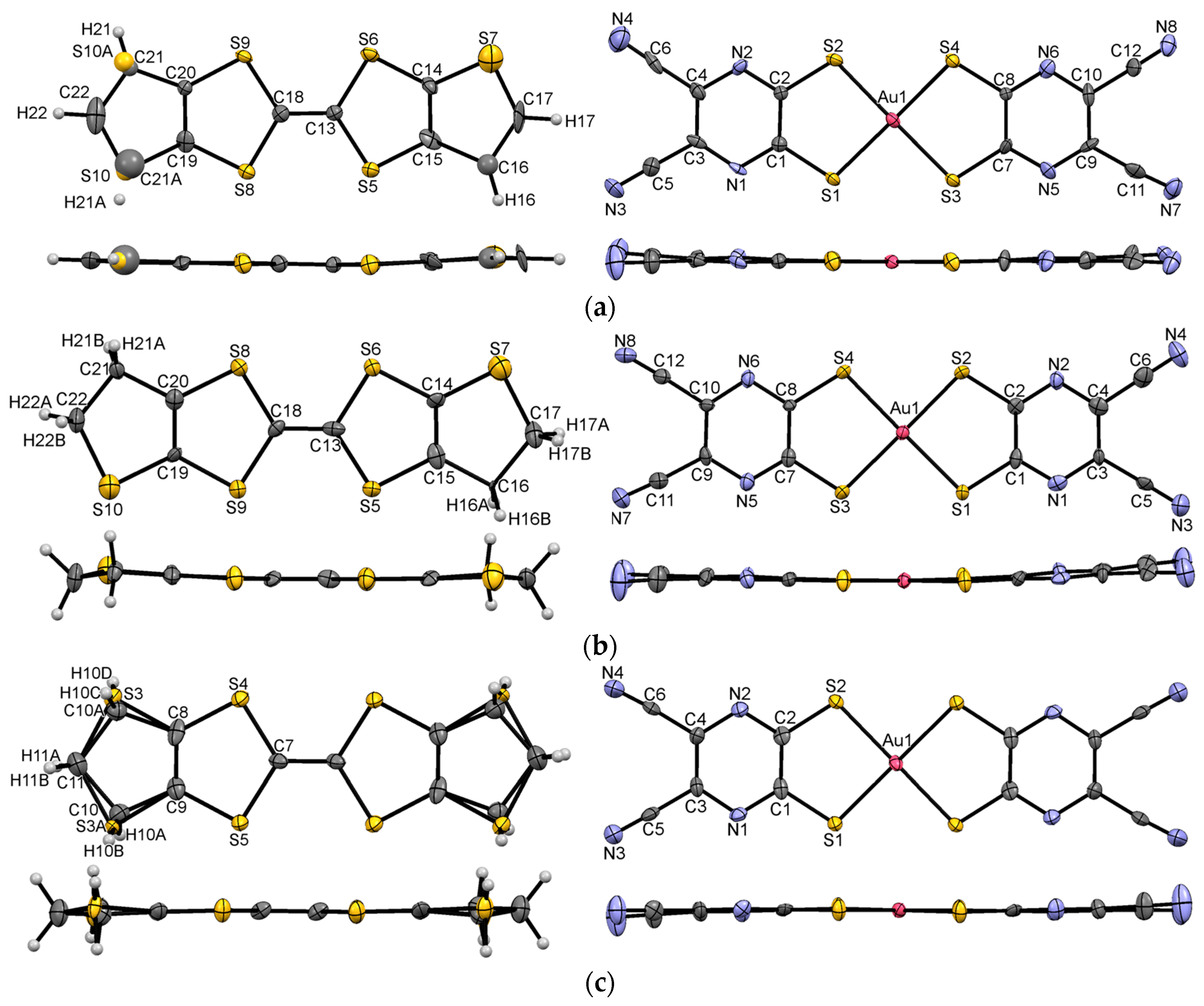
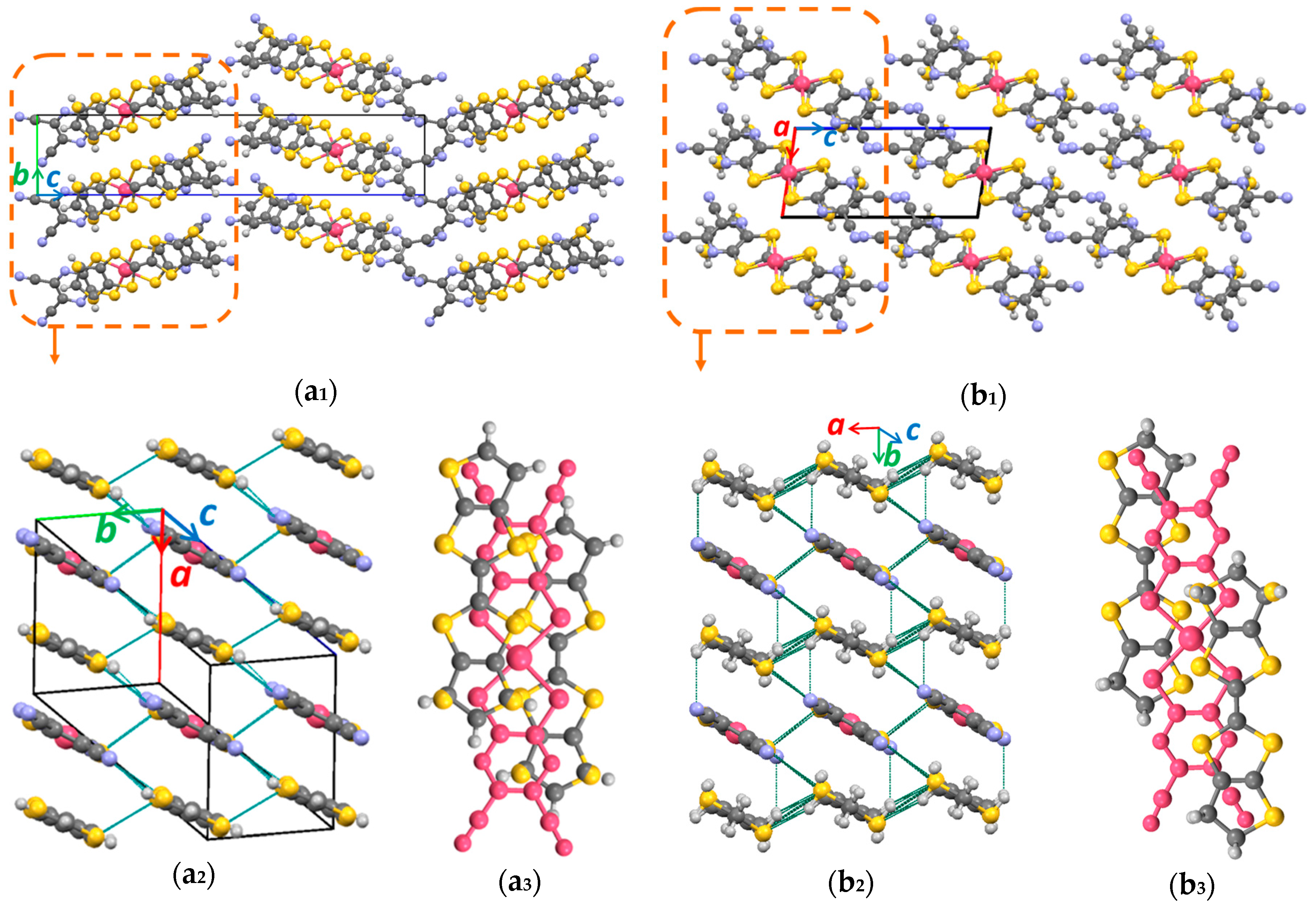
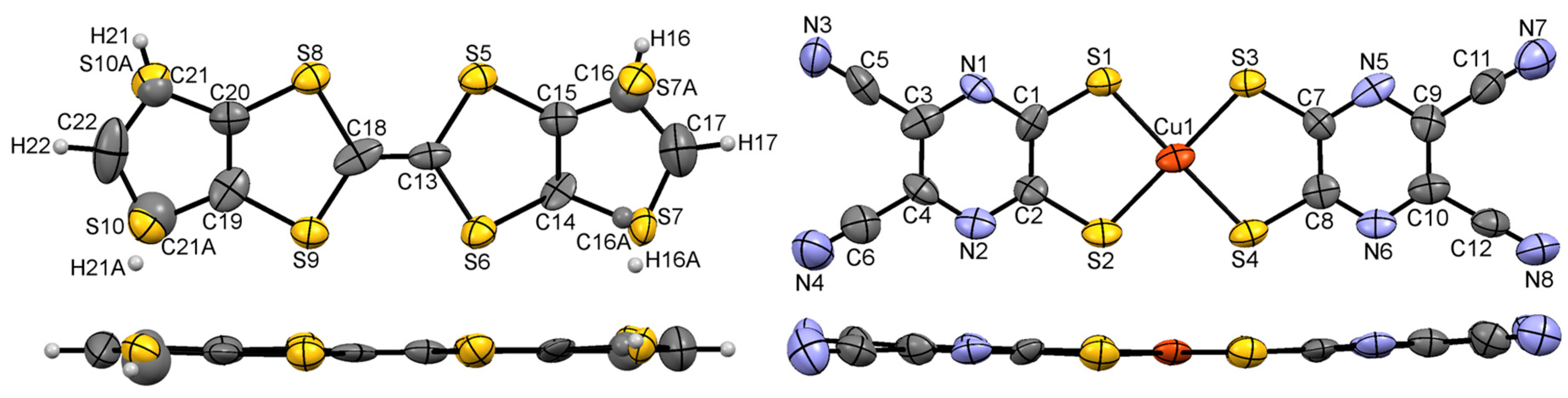
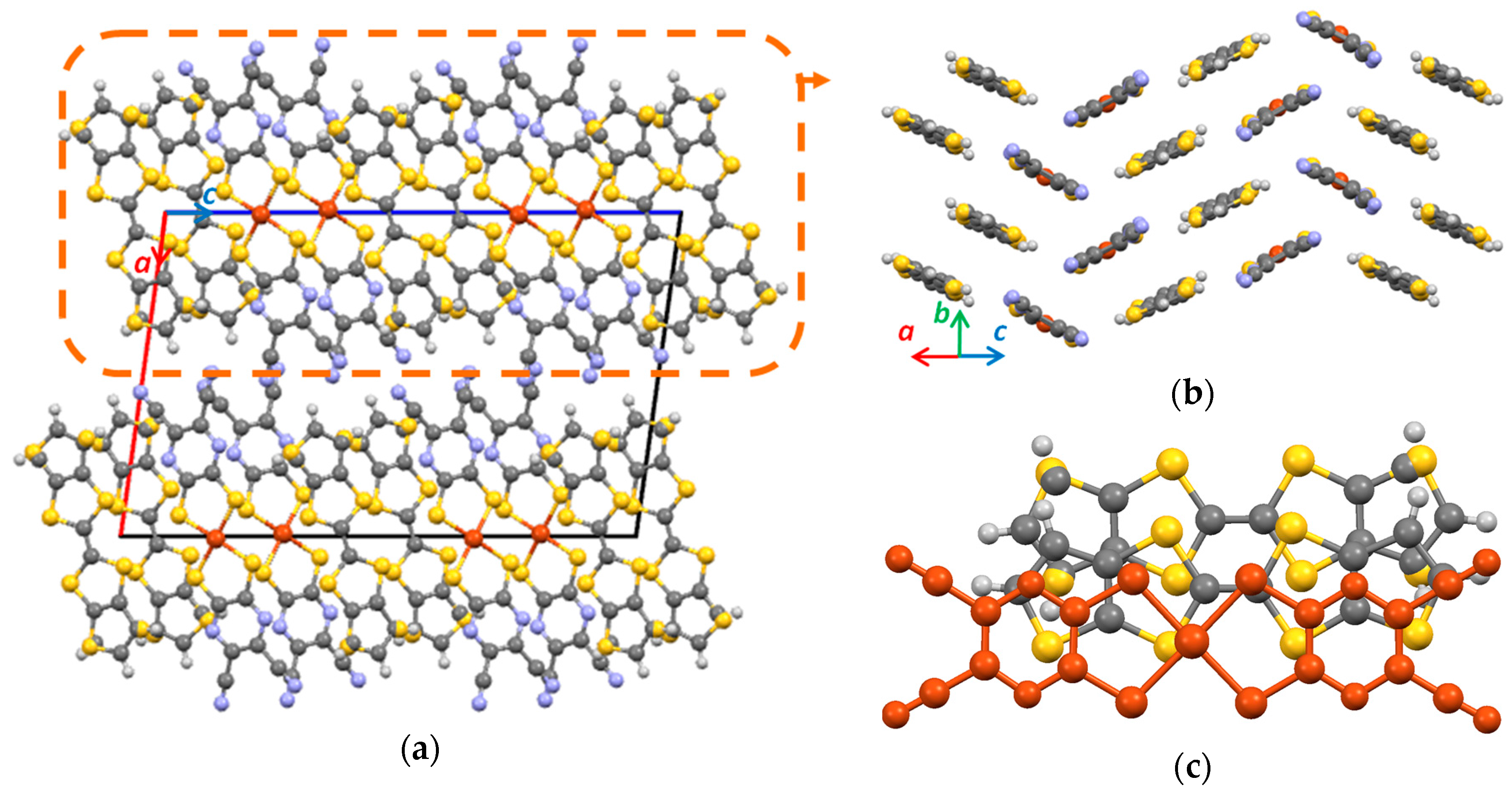
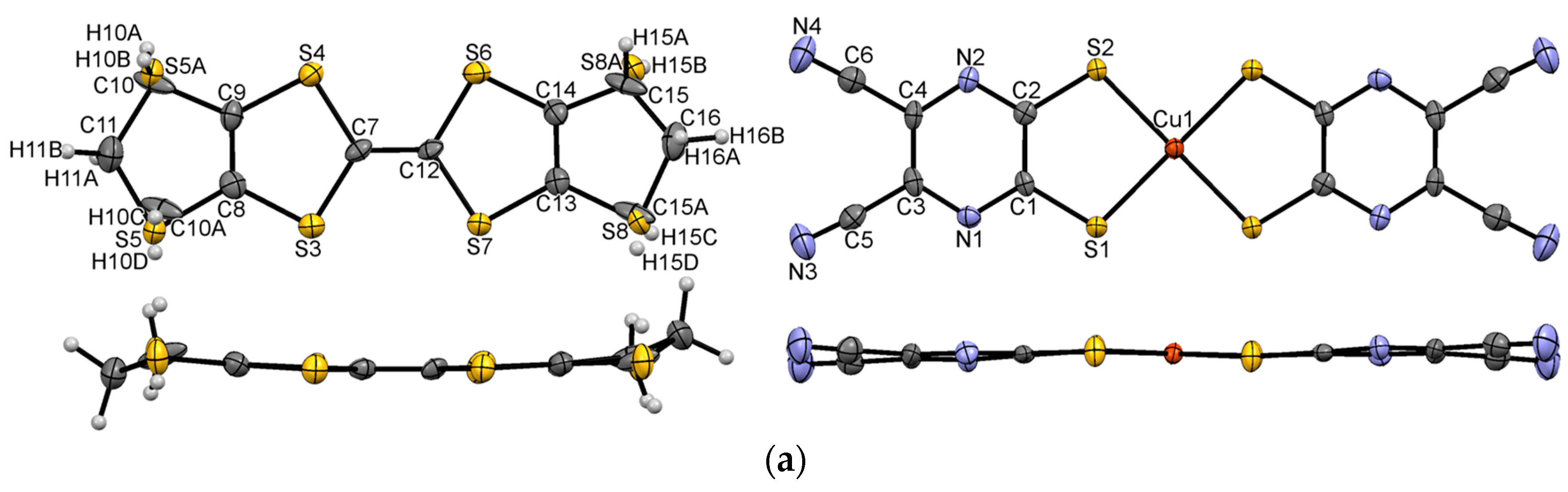
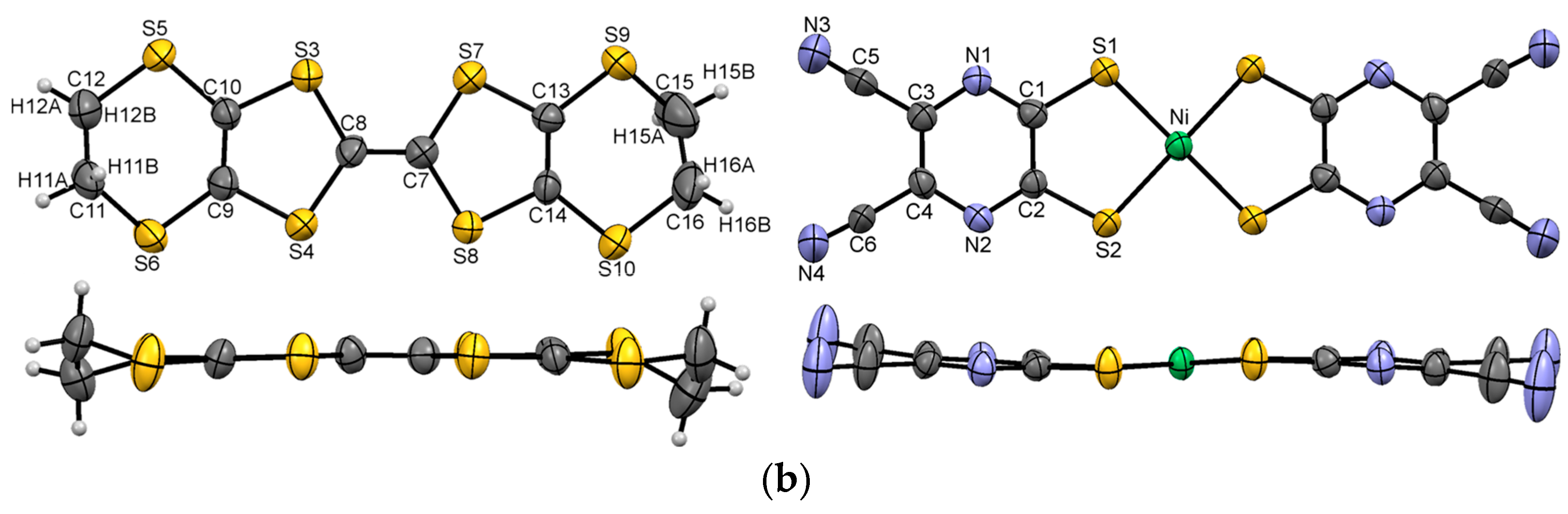
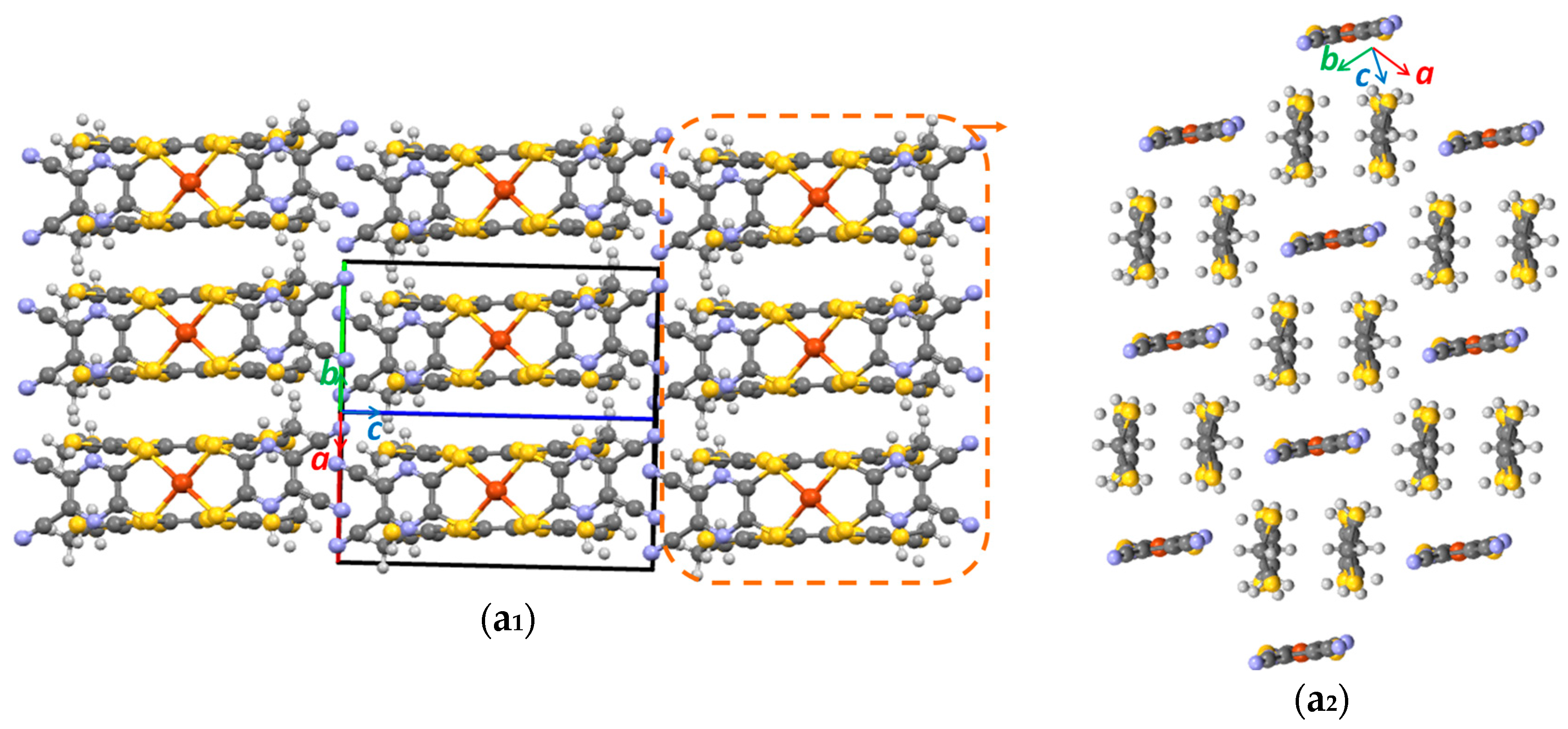
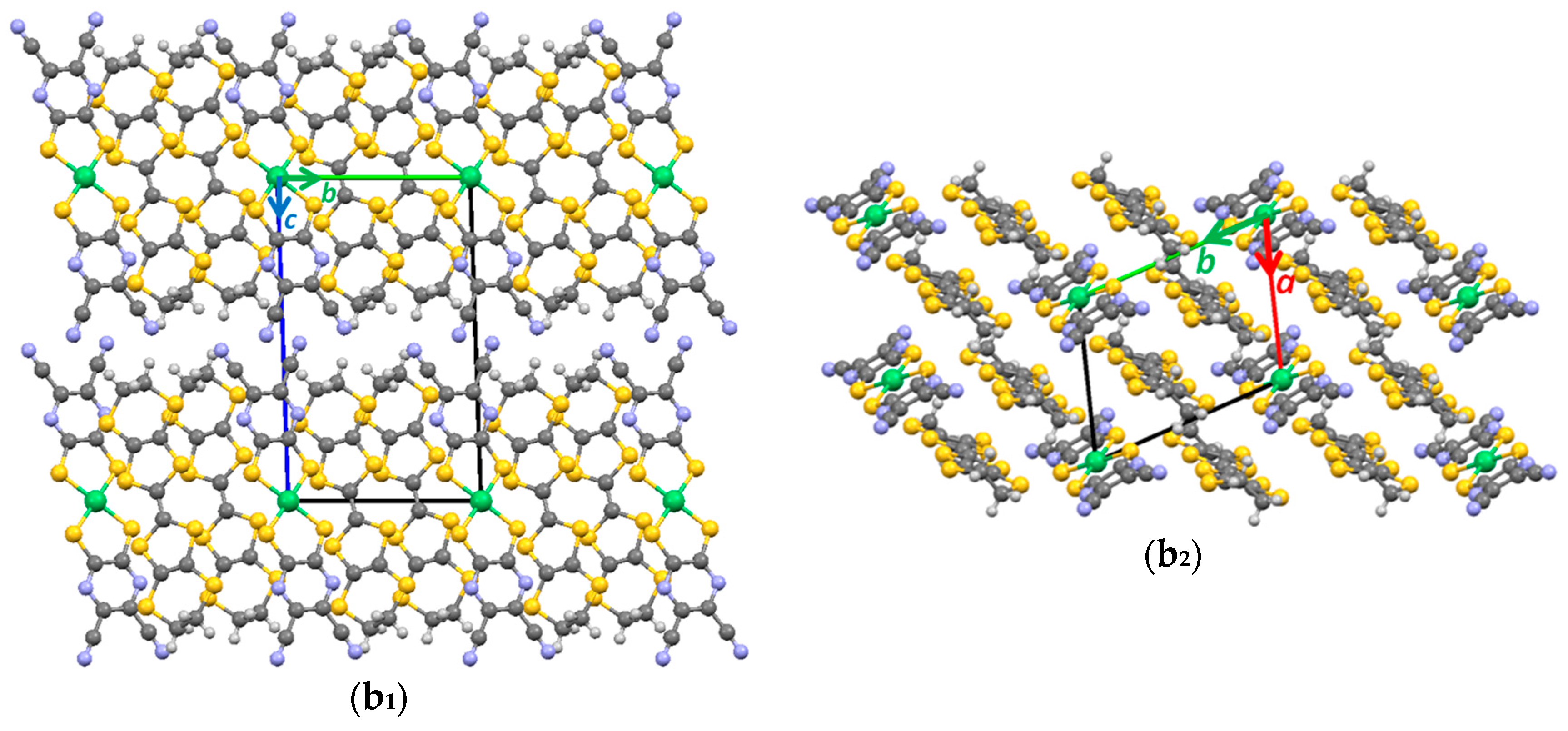
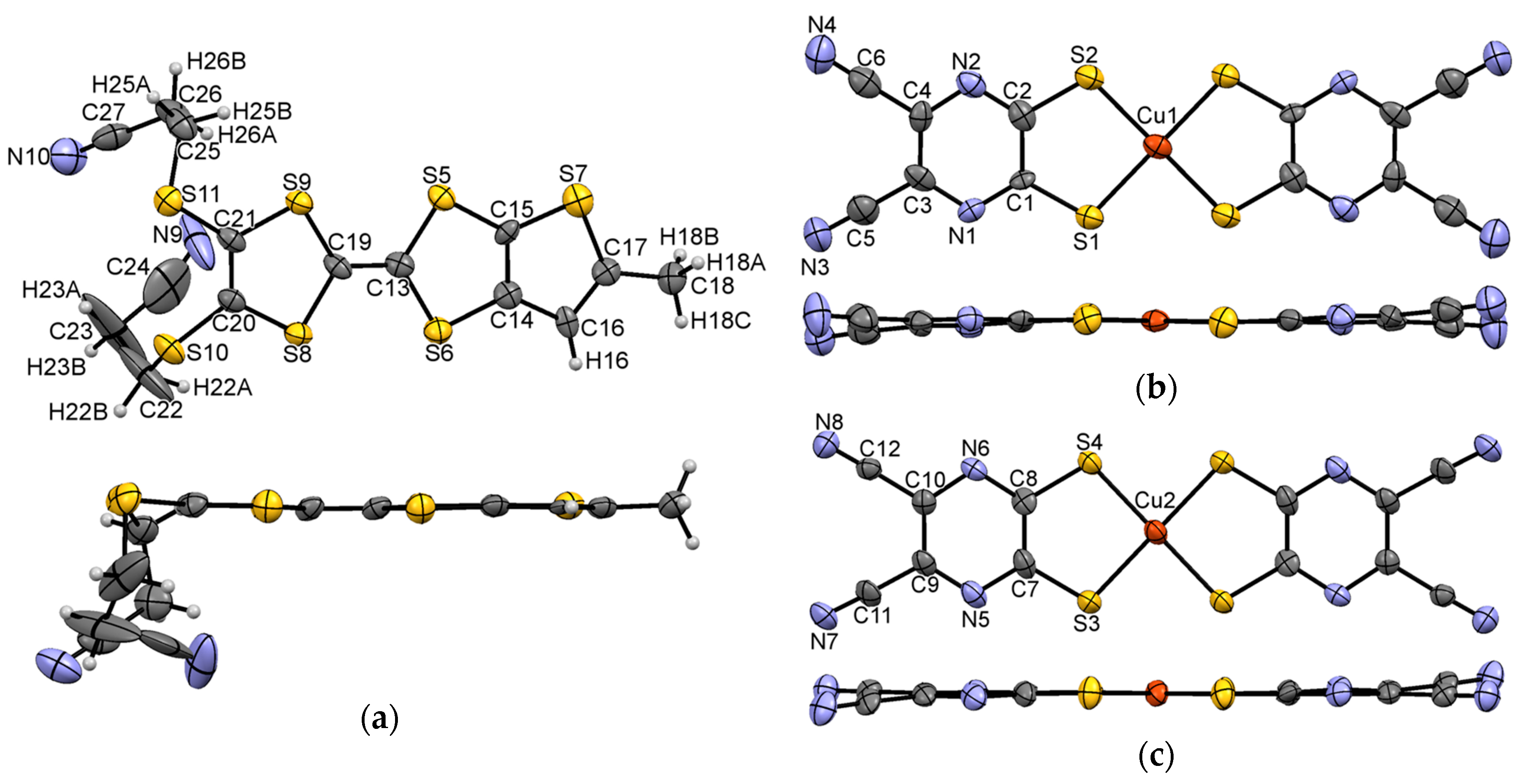
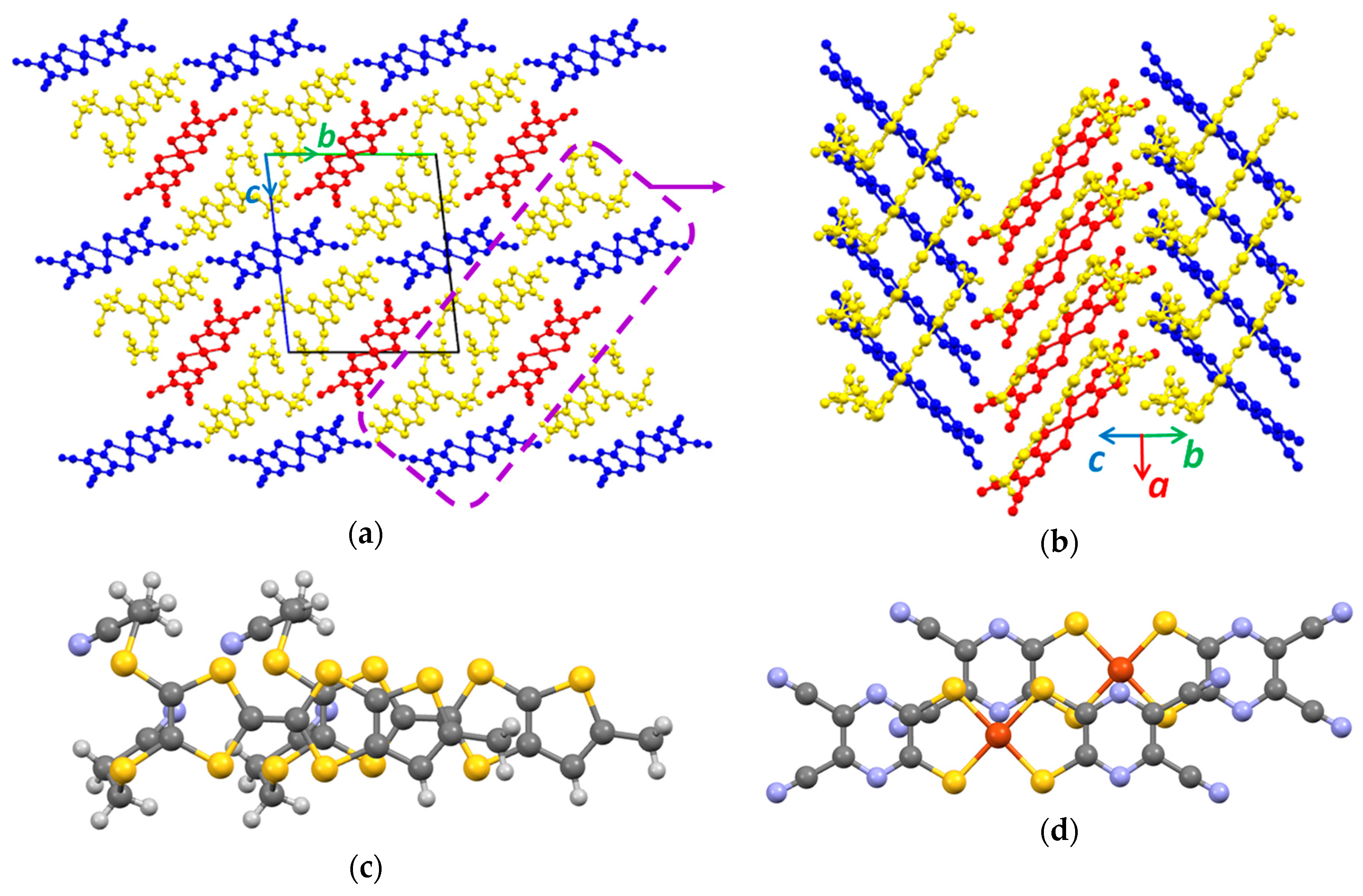
3.1. Single Crystal X-ray Diffraction Analysis
3.2. Electric Transport Properties
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kato, R. Conducting Metal Dithiolene Complexes: Structural and Electronic Properties. Chem. Rev. 2004, 104, 5319–5346. [Google Scholar] [CrossRef] [PubMed]
- Stiefel, E.I. Dithiolene Chemistry. Synthesis, Properties and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Coomber, A.T.; Beljonne, D.; Friend, R.H.; Brédas, J.L.; Charlton, A.; Robertson, N.; Underbill, A.E.; Kurmoo, M.; Day, P. Intermolecular interactions in the molecular ferromagnetic NH4Ni(mnt)2·H2O. Nature 1996, 380, 144–146. [Google Scholar] [CrossRef]
- Fourmigué, M.; Domercq, B.; Jourdain, I.V.; Molinié, P.; Guyon, F. Amaudrut, Interplay of Structural Flexibility and Crystal Packing in a Series of Paramagnetic Cyclopentadienyl/Dithiolene Mo and W Complexes: Evidence for Molecular Spin Ladders. Chem. A Eur. J. 1998, 4, 1714–1723. [Google Scholar] [CrossRef]
- Rovira, C. Molecular Compounds Showing a Spin Ladder Behaviour. In π-Electron Magnetism from Molecules to Magnetic Materials; Veciana, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; Volume 100, pp. 163–188. [Google Scholar]
- Underhill, A.E.; Ahmad, M.M. A new one-dimensional ‘metal’ with conduction through bis (dicyanoethylenedithiolato) platinum anions. J. Chem. Soc. Chem. Commun. 1981, 67–68. [Google Scholar] [CrossRef]
- Ahmad, M.M.; Turner, D.J.; Underhill, A.E.; Jacobsen, C.S.; Mortensen, K.; Carneiro, K. Physical properties and the Peierls instability of Li0.82[Pt(S2C2(CN)2)2]·2H2O. Phys. Rev. B 1984, 29, 4796–4799. [Google Scholar] [CrossRef]
- Brossard, L.; Ribault, M.; Bousseau, M.; Valade, L.; Cassoux, P. Un nouveau type de supraconducteur moléculaire: TTF[Ni(dmit)2]2. Comptes Rendus Acad. Sci. 1986, 302, 205–210. [Google Scholar]
- Cassoux, P. Molecular (super)conductors derived from bis-dithiolate metal complexes. Coord. Chem. Rev. 1999, 185–186, 213–232. [Google Scholar] [CrossRef]
- Robertson, N.; Cronin, L. Metal bis-1,2-dithiolene complexes in conducting or magnetic crystalline assemblies. Coord. Chem. Rev. 2002, 227, 93–127. [Google Scholar] [CrossRef]
- Rabaça, S.; Almeida, M. Dithiolene complexes containing N coordinating groups and corresponding tetrathiafulvalene donors. Coord. Chem. Rev. 2010, 254, 1493–1508. [Google Scholar] [CrossRef]
- Ribas, X.; Dias, J.C.; Morgado, J.; Wurst, K.; Almeida, M.; Veciana, J.; Rovira, C. Novel Cu(III) bis-1,2-diselenolene complex with a highly extended 3D framework through Na+ coordination. CrystEngComm 2002, 4, 564–567. [Google Scholar] [CrossRef]
- Ribas, X.; Dias, J.C.; Morgado, J.; Wurst, K.; Santos, I.C.; Almeida, M.; Vidal-Gancedo, J.; Veciana, J.; Rovira, C. Alkaline Side-Coordination Strategy for the Design of Nickel(II) and Nickel(III) Bis(1,2-diselenolene) Complex Based Materials. Inorg. Chem. 2004, 43, 3631–3641. [Google Scholar] [CrossRef] [PubMed]
- Ribas, X.; Dias, J.C.; Morgado, J.; Wurst, K.; Molins, E.; Ruiz, E.; Almeida, M.; Veciana, J.; Rovira, C. Novel CuIII bis-1,2-dichalcogenene complexes with tunable 3D framework through alkaline cation coordination. A structural and theoretical study. Chem. Eur. J. 2004, 10, 1691–1704. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.A.L.; Santos, I.C.; Lopes, E.B.; Rabaça, S.; Gancedo, V.-J.; Rovira, C.; Almeida, M.; Belo, D. DT–TTF Salts with [Cu(dcdmp)2]−: The Richness of Different Stoichiometries. Cryst. Growth Des. 2016, 16, 3924–3931. [Google Scholar] [CrossRef]
- Belo, D.; Lopes, E.B.; Santos, I.C.; Dias, J.C.; Figueira, M.; Almeida, M.; Fourmigué, M.; Rovira, C. Synthesis and Characterization of Charge Transfer Salts. J. Low Temp. Phys. 2006, 142, 349–354. [Google Scholar] [CrossRef]
- Rovira, C.; Veciana, J.; Santalo, N.; Tarres, J.; Cirujeda, J.; Molins, E.; Llorca, J.; EspinoSa, E. Synthesis of Several Isomeric Tetrathiafulvalene. pi.-Electron Donors with Peripheral Sulfur Atoms. A Study of Their Radical Cations. J. Org. Chem. 1994, 59, 3307–3313. [Google Scholar]
- Silva, R.A.L.; Neves, A.I.; Afonso, M.L.; Santos, I.C.; Lopes, E.B.; Del Pozo, F.; Pfattner, R.; Torrent, M.M.; Rovira, C.; Almeida, M.; et al. α-Dithiophene-tetrathiafulvalene—A Detailed Study of an Electronic Donor and Its Derivatives. Eur. J. Inorg. Chem. 2013, 13, 2440–2446. [Google Scholar] [CrossRef]
- Silva, R.A.L.; Vieira, B.J.C.; Andrade, M.A.; Santos, I.C.; Rabaça, S.; Belo, D.; Almeida, M. TTFs nonsymmetrically fused with alkylthiophenic moieties. Beilstein J. Org. Chem. 2015, 11, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Belo, D.; Santos, I.C.; Almeida, M. 5,6-Dicyano-2,3-dithiopyrazine (dcdmp) chemistry: Synthesis and crystal structure of Au(III)(dcdmp)2 complexes and 2,3,7,8-tetracyano-1,4,6,9-tetraazothianthrene. Polyhedron 2004, 23, 1351–1359. [Google Scholar] [CrossRef]
- Belo, D.; Figueira, M.J.; Santos, I.C.; Gama, V.; Pereira, L.C.; Henriques, R.T.; Almeida, M. Synthesis and characterization of copper complexes with the 2,3-dicyano-5,6-dimercaptopyrazine ligand: Magnetic properties of a ferrocenium salt. Polyhedron 2005, 24, 2035–2042. [Google Scholar] [CrossRef]
- Tomura, M.; Tanaka, S.; Yamashita, Y. Synthesis, structure and properties of the novel conductingdithiolato-metal complexes having dicyanopyrazine moieties. Synth. Met. 1994, 64, 197–202. [Google Scholar] [CrossRef]
- Perrin, D.D.; Armarego, W.L.F. Purification of Laboratory Chemicals, 3rd ed.; Pergamon Press: Oxford, UK, 1988. [Google Scholar]
- Sheldrick, G.M. SADABS; Bruker AXS Inc.: Madison, WI, USA, 2004. [Google Scholar]
- Bruker. SMART and SAINT; Bruker AXS Inc.: Madison, WI, USA, 2008. [Google Scholar]
- Fair, C.K. MoIEN. An Interactive Intelligent System for Crystal Structure Analysis; Enraf-Nonius: Delft, The Netherlands, 1990. [Google Scholar]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.; Giacovazzo, G.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Cryst. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar]
- Almeida, M.; Alcácer, L.; Oostra, S. Anisotropy of thermopower in N-methyl-N-ethylmorpholinium bistetracyanoquinodimethane, MEM(TCNQ)2, in the region of the high-temperature phase transitions. Phys. Rev. B 1984, 30, 2839–2844. [Google Scholar] [CrossRef]
- Lopes, E.B. Internal Report; INETI-Sacavém: Lisbon, Portugal, 1991. [Google Scholar]
- Chaikin, P.M.; Kwak, J.F. Apparatus for Thermopower Measurements on Organic Conductors. Rev. Sci. Instrum. 1975, 46, 218–220. [Google Scholar] [CrossRef]
- Schaffer, P.E.; Wudl, F.; Thomas, G.A.; Ferraris, J.P.; Cowan, D.O. Apparent giant conductivity peaks in an anisotropic medium: TTF-TCNQ. Solid State Commun. 1974, 14, 347–351. [Google Scholar] [CrossRef]
- Belo, D.; Morgado, J.; Lopes, E.B.; Santos, I.C.; Rabaça, S.; Duarte, M.T.; Gama, V.; Henriques, R.T.; Almeida, M. Synthesis and Characterisation of Charge Transfer Salts Based on Au(dcdmp), and TTF Type Donors. Synth. Met. 1999, 102, 1751–1752. [Google Scholar] [CrossRef]
- Tomura, M. Bis(tetra-n-butylammonium) bis(5,6-dicyanopyrazine-2,3-dithiolato-κ2S,S’)nickelate(II). IUCrData 2017, 2, x171059. [Google Scholar] [CrossRef]
- Kobayashi, H.; Kobayashi, A.; Sasaki, Y.; Saito, G.; Inokuchi, H. The Crystal and Molecular Structures of Bis(ethylenedithio)tetrathiafulvalene. Bull. Chem. Soc. Jpn. 1986, 59, 301–302. [Google Scholar] [CrossRef]

| CT Salts | σRT (Scm) | Ea (meV) |
|---|---|---|
| (ET)2[Ni(dcdmp)2] (6) | 9.4 × 10−5 | 128 |
| (ET)[Cu(dcdmp)2] (4) | 4.2 × 10−4 | 190 |
| (BET-TTF)2[Cu(dcdmp)2] (5) | 7.4 × 10−4 | 134 |
| (α-mtdt)[Cu(dcdmp)2] (7) | 1.1 × 10−3 | 207 |
| (BET-TTF)[Au(dcdmp)2] (2) | 5.4 × 10−3 | 95 |
| (α-DT-TTF)[Au(dcdmp)2] (1) | 5.2 × 10−2 a; 1.6 × 10−1 b | 105 a; 59 b |
| (α-DT-TTF)[Cu(dcdmp)2] (3) | 1.1 × 10−2 | 107 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, R.A.L.; Santos, I.C.; Rabaça, S.; Lopes, E.B.; Gama, V.; Almeida, M.; Belo, D. Synthesis and Characterization of Charge Transfer Salts Based on [M(dcdmp)2] (M = Au, Cu and Ni) with TTF Type Donors. Crystals 2018, 8, 141. https://doi.org/10.3390/cryst8030141
Silva RAL, Santos IC, Rabaça S, Lopes EB, Gama V, Almeida M, Belo D. Synthesis and Characterization of Charge Transfer Salts Based on [M(dcdmp)2] (M = Au, Cu and Ni) with TTF Type Donors. Crystals. 2018; 8(3):141. https://doi.org/10.3390/cryst8030141
Chicago/Turabian StyleSilva, Rafaela A. L., Isabel C. Santos, Sandra Rabaça, Elsa B. Lopes, Vasco Gama, Manuel Almeida, and Dulce Belo. 2018. "Synthesis and Characterization of Charge Transfer Salts Based on [M(dcdmp)2] (M = Au, Cu and Ni) with TTF Type Donors" Crystals 8, no. 3: 141. https://doi.org/10.3390/cryst8030141