1. Introduction
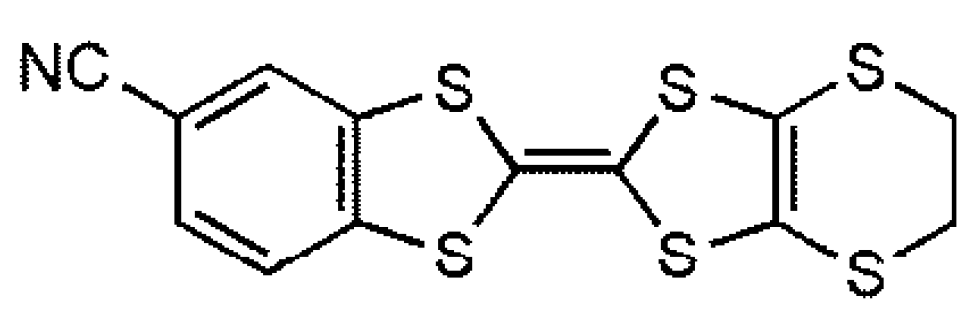
It has been recently shown that the dissymmetrical TTF derivative cyanobenzene-ethylenedithio-tetrathiafulvalene (CNB-EDT-TTF) (
Scheme 1) is an electron donating molecule that when combined with small anions X such as ClO
4−, PF
6−, and I
3−, leads to a family of radical cation salts of composition (CNB-EDT-TTF)
4X and characterized by a unique donor bilayer structure [
1]. This bilayer arrangement is promoted by an effective arrangement of hydrogen bond dimeric C–N···H–C interactions involving the cyano groups, as a combination of R
22(10) and R
22(8) synthons in a two-dimensional network. These (CNB-EDT-TTF)
4X compounds have been obtained in several polymorphic phases associated with different donor packing patterns or anion ordering schemes [
2,
3] and for
κ-type packing pattern some of these 2D metals have shown superconductivity at low temperatures [
2].
The donor bilayer structure confers to these salts unusual metallic properties associated to high band filling, large electronic effective masses and quasi degenerated 2D Fermi surfaces, making the study of this type of bilayer metallic systems quite attractive. However the range of stability of the (CNB-EDT-TTF)
4X salts was found so far restricted to linear, tetrahedral or octahedral anions of size comparable to ClO
4−, PF
6−, and I
3−, and for instance with a larger anion such as FeBr
4−, a salt of different stoichiometry (CNB-EDT-TTF)
2FeBr
4 and distinct structure is obtained instead [
4]. These findings directed our attention towards exploring new CNB-EDT-TTF salts with comparable small anions and in this contribution we report the salts obtained with the smaller tetrahedral anion BF
4−. With this small tetrahedral anion salts with both 4:1 and 1:1 stoichiometries were obtained.
2. Materials and Methods
2.1. General Information
CNB-EDT-TTF was prepared following a previous described procedure [
5] which was more recently improved [
3].
n-Bu
4N BF
4 (Sigma Aldrich, Darmstadt, Germany) was purified by recrystallization from ethyl acetate. Dichloromethane and tetrahydrofuran (THF) were freshly distilled immediately before use.
2.2. Synthesis
[CNB-EDT-TTF] BF4 (1). A THF solution of the donor CNB-EDT-TTF (2.2 × 10−3 M) and n-Bu4N BF4 (1.1 × 10−2 M) was added to an H-shaped two-compartment cells separated by frit glass with Pt electrodes and sealed under nitrogen. After applying initially a current density of 0.5 μA/cm2, during the first 3 days, then increased to 0.7 μA/cm2 during more 20 days, black bock shaped crystals grown on the anode were collected and washed with THF and dichloromethane.
β”-[CNB-EDT-TTF]4 BF4 (2). A dichloromethane solution of the donor CNB-EDT-TTF and n-Bu4N BF4 (both 2 × 10−3 M) was added to an H-shaped two-compartment cells separated by frit glass with Pt electrodes and sealed under nitrogen. After approximately 15 days of applying a constant current density of 1 μA/cm2, dark brown elongated platelet shaped crystals grown on the anode were collected and washed with dichloromethane/ether.
2.3. X-ray Crystallography
Selected single crystals were mounted on a loop with protective oil and X-ray data was collected on a APEX II CCD detector diffractometer (Bruker, Billerica, Massachusetts, MA, USA) using graphite monochromated MoK
α radiation (
λ = 0.71073 Å) and operating in a
φ and
ω scans mode. A semi empirical absorption correction was carried out using SADABS [
6]. Data collection, cell refinement and data reduction were done with the SMART and SAINT programs [
7]. The structures were solved by direct methods using SIR97 [
8] and refined by full matrix least-squares methods using the program SHELXL97 [
9] using the winGX software package [
10]. Non-hydrogen atoms were refined with anisotropic thermal parameters whereas H-atoms were placed in idealized positions and allowed to refine riding on the parent C atom. Molecular graphics were prepared using Mercury [
11]. CCDC 1825988-1825989 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via
www.ccdc.cam.ac.uk/data_request/cif.
Crystallographic data for compound 1, (CNB-EDT-TTF)BF4: C13H7BF4NS6, M = 456.37 g·mol−1, monoclinic, space group P21/c, a = 9.5066(9)) Å, b = 8.3629(9) Å, c = 21.1593(13)Å, β = 95.984(6)°, V = 1673.1(3) Å3, Z = 4, ρcalc = 1.812 g·cm−3, μ(Mo Kα) = 0.855 mm−1, 5429 reflections measured, 2887 unique Rint = 0.0734, θmax = 25.02°, R1 = 0.0618 using 2887 Refl. > 2σ(I), ωR2 = 0.1189, T = 150(2) K. CCDC 1825989.
Crystallographic data for compound 2, ”-(CNB-EDT-TTF)4BF4: C13H7NS6, M = 832.62 g·mol−1, monoclinic, space group P21/m, a = 4.8418(13) Å, b = 55.724(15)Å, c = 5.9081(16)Å, β = 94.208(18)°, V = 1589.7(7)Å3, Z = 4, ρcalc = 1.544 g·cm−3, μ(Mo Kα) = 0.847 mm−1, 8106 reflections measured, 1584 unique Rint = 0.0747, θmax = 20.64°, R1 = 0.0764 using 1584 Refl. > 2σ(I), ωR2 = 0.1727, T = 150(2) K. CCDC 1825988.
2.4. Electronic Band Structure Calculations
The tight-binding band structure calculations [
12] were of the extended Hückel type and a modified Wolfsberg–Helmholtz formula was used to calculate the non-diagonal H
μν values [
13]. All valence electrons were taken into account in the calculations and the basis set consisted of Slater-type orbitals of double-
ζ quality for C and N 2s and 2p, S 3s and 3p and of single-
ζ quality for H1s. The ionization potentials, contraction coefficients and exponents were taken exactly as in our previous work for (CN-BEDT-TTF)
4I
3 [
2]. Because of the different crystallographic cells the four different HOMO···HOMO intralayer interactions were defined as in the previous work on disordered (CN-BEDT-TTF)
4I
3 [
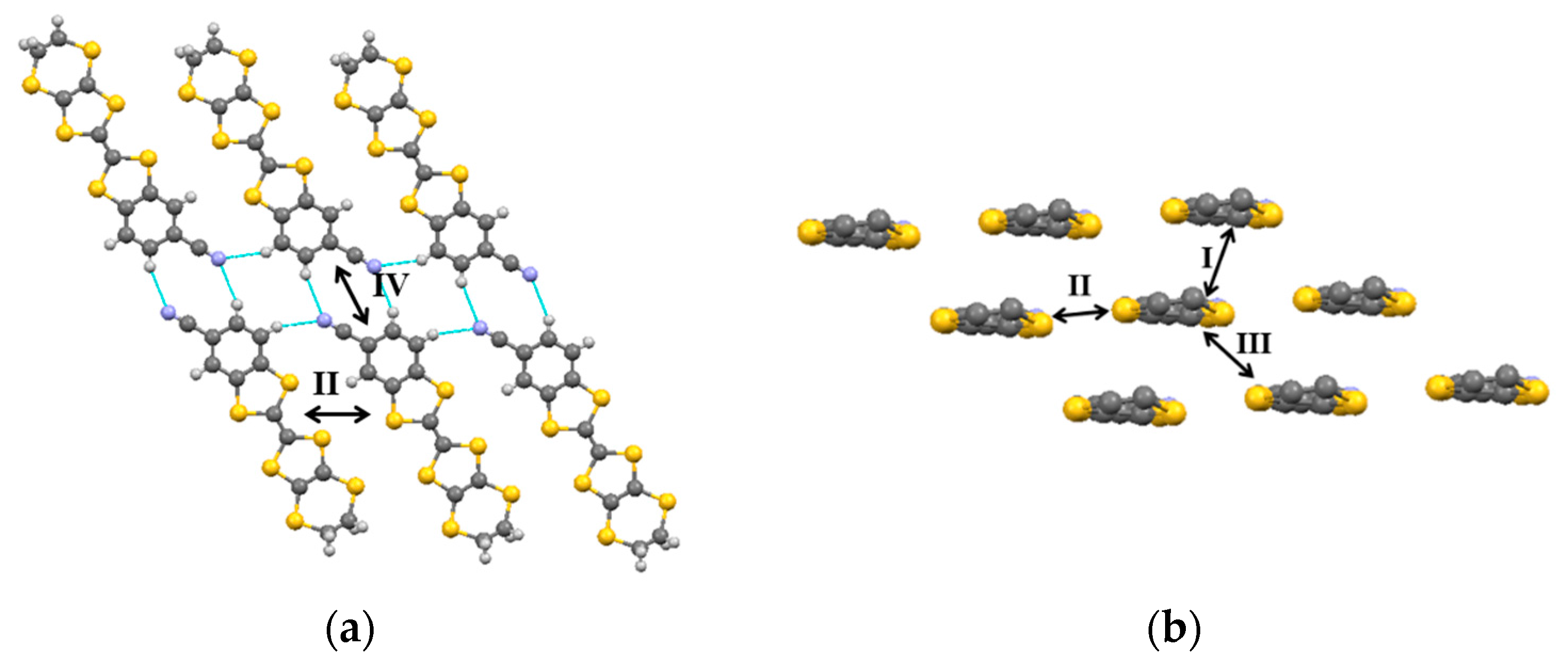
2]: I, interaction along the stacks; II, lateral
π-type interaction; III, interaction along the step-chains and IV, head to head interlayer interaction .
2.5. Electric Transport Properties
Electrical conductivity and thermoelectric power were measured in single crystals in the temperature range of 20–320 K, in a cell attached to the cold stage of a closed cycle helium refrigerator. In the first step, the thermoelectric power was measured by using a slow AC (ca. 10
−2 Hz) technique [
14], by attaching two
φ = 25 μm diameter 99.999% pure Au wires (Goodfellow), thermally anchored to two quartz blocks, with Pt paint (Demetron 308A), to the extremities of an elongated sample as in a previously described apparatus [
15], under computer control [
16]. The oscillating thermal gradient was kept below 1 K and it was measured with a differential Au-0.05 at. % Fe vs. chromel thermocouple, and the sample temperature measured with a calibrated thermocouple of the same type. The absolute thermoelectric power of the samples was obtained after correction for the absolute thermopower of the Au leads, by using the data of Huebner [
17]. In a second step two additional contacts between the previous ones, were similarly made in order to achieve a four-in-line contact configuration and electrical resistivity was measured imposing an AC current of 1 μA at 70 HZ and the voltage drop measured using a lock-in technique. Sample electrodes configuration was checked for unnested to nested voltage ratio, as defined by Schaffer et al. [
18], which was kept below 5%.
3. Results
As previously observed with the anion ClO
4− depending on the solvent and electrocrystallization conditions salts with 1:1 and 4:1 donor:acceptor stoichiometries; (CNB-EDT-TTF)BF
4 (
1) and (CNB-EDT-TTF)
4BF
4 (
2) can be obtained, the first one as black block shaped crystals from tetrahydrofuran (THF) and the last one as thin dark brown platelet shaped crystals from dichloromethane solutions. For the 4:1 salt, among the several crystals tested only the monoclinic phase
β”M-(CNB-EDT-TTF)
4BF
4 could be observed with so far no evidence for the triclinic one described for ClO
4 [
3]. These two BF
4 salts are isostructural with the analogues with ClO
4− anions [
3] and therefore the description of their crystal structures will be only briefly summarized.
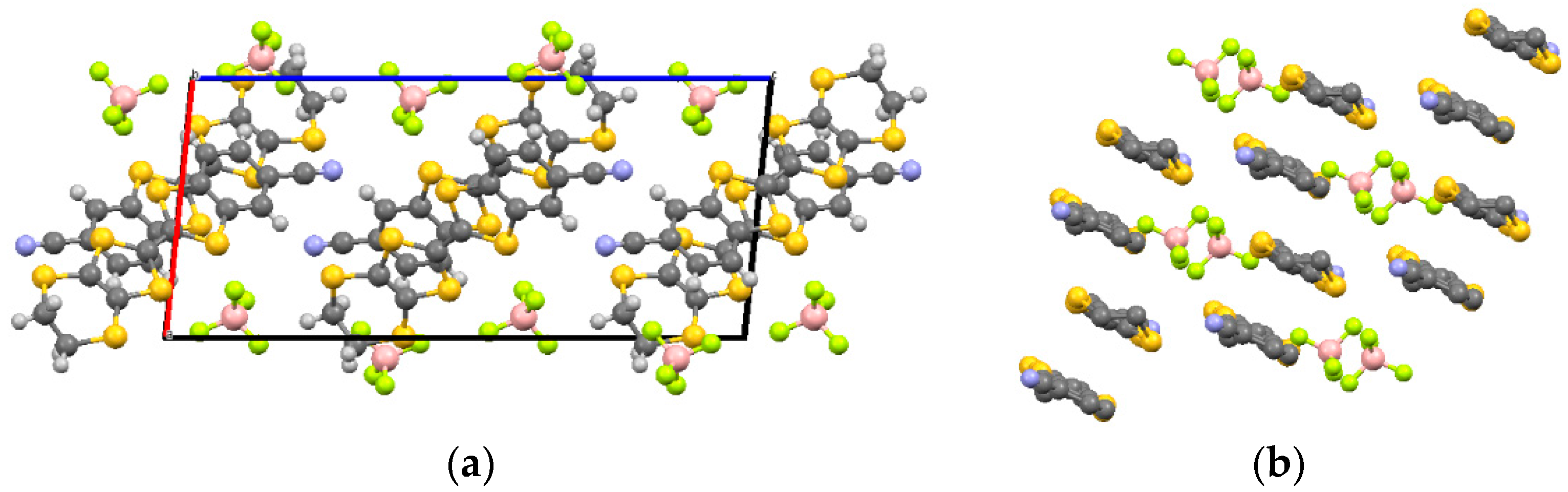
(CNB-EDT-TTF)BF
4 (
1) crystallizes in the monoclinic system, space group P2
1/c. The asymmetric unit contains one independent donor molecule and one tetrahedral BF
4− anion located in general positions (
Figure S1). The donor geometry is almost planar with exception of the dihydrodithiin ring which presents the usual half-chair conformation with bond lengths (central C = C:C5−C6 = 1.374(9) Å) identical within experimental uncertainty to those observed in other 1:1 salts with this donor in monocationic state [
3,
4] (
Table S1). The crystal structure of
1 consists in head-to-tail chains of CNB-EDT-TTF donors dimers stacked along
b, as shown in
Figure 1. Donors in nearby stacks with reversed tilting (related by a screw axis) are connected by a helical network of short C−N···H−C contacts at 2.546 Å. In addition there are several short S···S, S···C and S···F intermolecular contacts and hydrogen bonds listed in
Table S2.
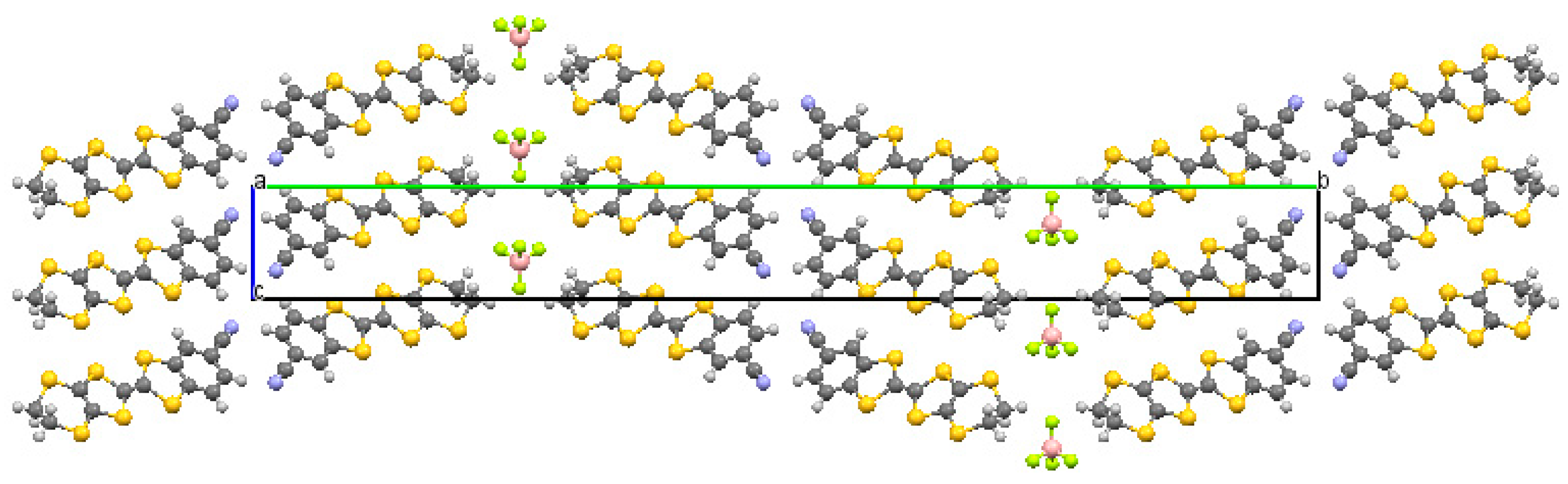
(CNB-EDT-TTF)
4BF
4 (
2) crystallizes in the monoclinic system, space group P2
1/m, it is isostructural with the previously described monoclinic phase of the analogue with ClO
4 [
3] but with cell parameters slightly larger.
Figure 2 shows a projection of the crystal structure of
2 along the stacking axis (corresponding to
a). The refinement of the crystal structure from different crystals measured was always severely limited by a large disorder associated with the anions which could not be clearly identified in several possible positions placed in a channel along
c between the donor bilayers. However when a squeeze was applied leaving only the donors to be considered in the structural refinement, a fair agreement could be achieved. The asymmetric unit of
2 (
Figure S2) contains one independent CNB-EDT-TTF donor molecule with all atoms located at general positions. The donor geometry and bond lengths (
Table S3) are within experimental uncertainty identical to that previously observed in
β”-(CNB-EDT-TTF)
4X salts. The donor crystal packing consist in the previously described
β”-type bilayer structure of donors, with similar short intermolecular contacts listed in
Table S4, and alternated tilting orientations of bilayers along
b, the large cell parameter. A relevant difference is on the cell parameters
a and
c, slightly larger than those observed for ClO
4 in spite of the smaller anion size. This difference is certainly due to the severe anion disorder and it corresponds to a not so tight packing of the molecules in the layers.
The donor bilayer structure is due to a network of bifurcated C-N
…H interactions in an efficient combination of R
22(10) and R
22(8) synthons between coplanar molecules (
Figure 3a) with contact distances (N1···H3 at 2.624 Å, N1···H5 at 2.505 Å) comparable to those previously found in
β”-(CNB-EDT-TTF)
4X salts [
1,
2,
3]. However the stacking distance between successive planes of molecules interconnected by R
22(10) and R
22(8) synthons is slightly larger in this BF
4 salt. The three different intermolecular intralayer interactions I, II and III (I, along the stacks; II, lateral
π-type and III, along the step-chains) are shown in
Figure 3b.
Figure 3a also shows the interaction IV between a pair of head to head molecules in adjacent layers. The magnitude of these interactions was evaluated under the extended Hückel approach and values obtained are listed in
Table 1 and compared with those in the ClO
4, PF
6 and I
3 analogues. Interactions II and IV are practically identical, as a consequence of a quite similar network of C-N···H interactions, but interactions I and III are reduced as a consequence of a larger intermolecular spacing.
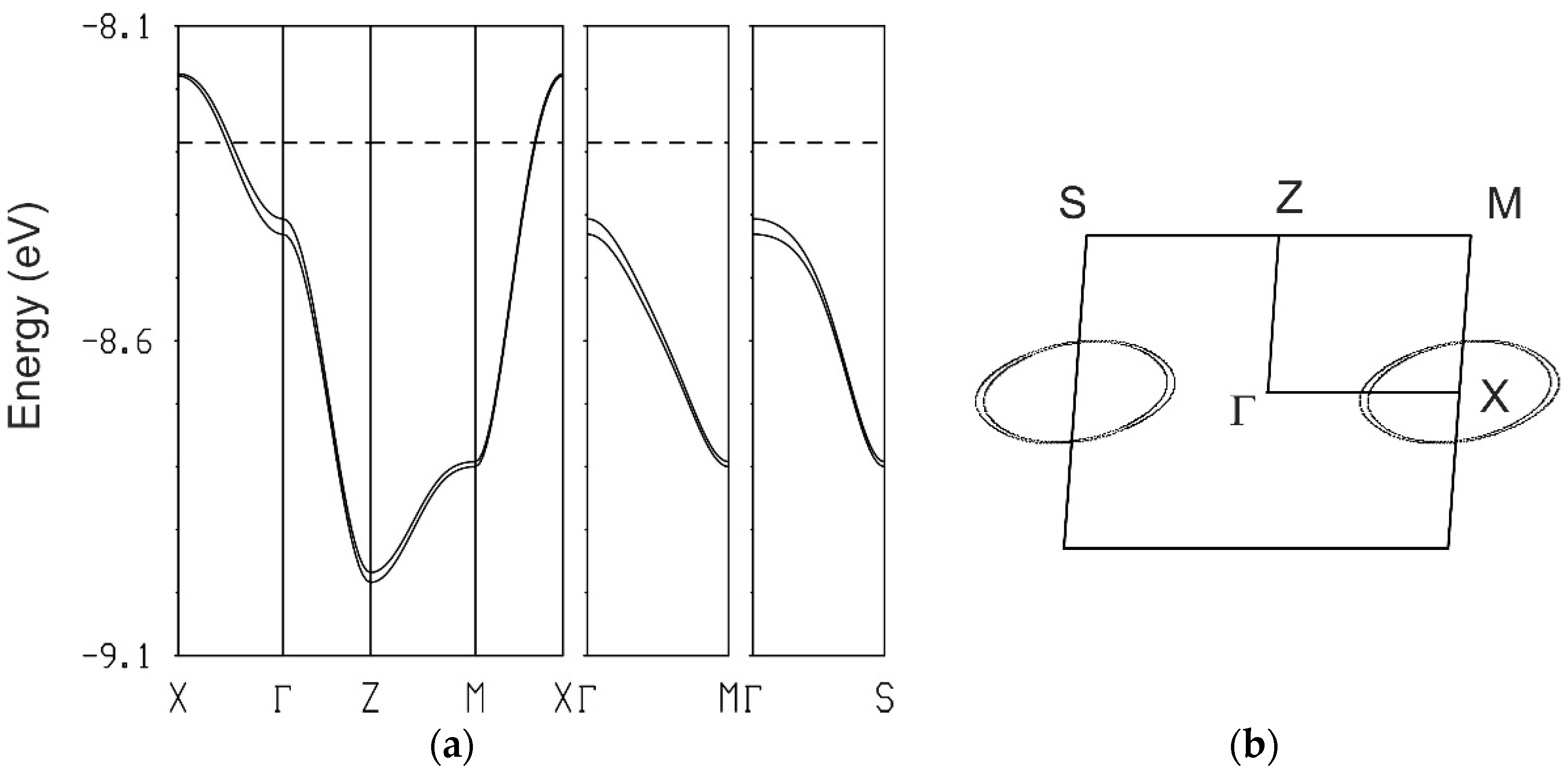
The electronic band structure predicted on the basis of these calculated intermolecular interactions and the corresponding Fermi surface assuming an average charge ¼+ for each donor molecule are depicted in
Figure 4. Due to the small interlayer interaction IV, associated with head to head intermolecular contacts, the bands are split in two almost degenerated ones. The Fermi surface corresponds to two elliptical cylinders, as typical of
β”-salts [
19], and their cross section area corresponds to about 1/8 of the first Brillouin Zone as previously calculated for identical
β”-(CNB-EDT-TTF)
4X salts [
1,
2].
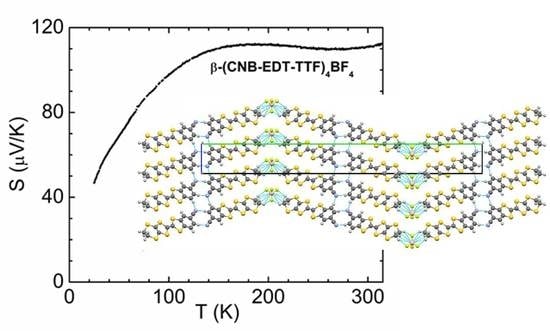
The metallic properties predicted by these electronic band structure calculations are confirmed by the measurements of electrical transport properties in single crystals, shown in
Figure 5 where they are compared with those previously reported for the ClO
4 and PF
6 analogues. The electrical resistivity,
ρ (~0.05–0.06 Ω cm at room temperatures) upon cooling shows an almost linear decrease until about 50 K, reaches a broad and sample dependent minimum at ~25–30 K, followed by a gradual increase upon further cooling, reaching at 1.7 K values comparable to those at room temperature. This increase of resistivity at low temperatures, which was found slightly sample dependent, can be due electron repulsion or most likely to localization effects induced by anion disorder [
20]. Indeed no such minima of electrical resistivity was observed at this temperature range in the ClO
4 and PF
6 analogues where the anions were found not so severely disordered, and where the electrical resistivity presents a stronger temperature dependence in a more extended linear regime.
The thermopower S presents rather large positive values of ~110 μV/K at room temperature, and it remains almost temperature independent down to 150 K. Upon cooling below this temperature the thermopower values start decreasing towards zero in a fashion approximately proportional to the temperature T, confirming the metallic nature of the compound and indicating that the conduction takes place in a continuum of states around the Fermi-level. The large positive values are consistent with the large band filling (7/8) predicted by the band structure calculations described above assuming an average ¼+ charge of each donor molecule. The thermopower values significantly larger than in the ClO4 and PF6 analogues are also consistent with the predicted smaller bandwidth of the BF4 salt as a consequence of larger intermolecular spacing, which results in a larger density of states at the Fermi-level.
The high temperature regime of large and almost temperature independent thermopower appears as a common feature of the
β”-salts of this donor. In this respect it is worth consider that the thermopower, which is the entropy per charge carrier weighted by their contribution to the total electrical conductivity, is directly related to the density of states at the Fermi level and the contribution
σ(
E) of states with energy
E close to the Fermi level, E
F, to the total conductivity
σ [
21]:
Therefore the large thermopower of these 4:1
β”-salts is a consequence of the large band filling placing the Fermi level in region of large effective mass. In contrast to the low temperature regime, with a
SαT behavior typical of metals, the high temperature regime with almost temperature independent thermopower can result from correlation effects in a narrow band, when the carrier mean free path becomes comparable to the lattice parameters and the transport mechanism becomes diffusive rather than coherent. Finally it should be mentioned that the measured thermopower value of ~110 μV/K in
β”-(CNB-EDT-TTF)
4BF
4 is to the best of our knowledge the largest one observed in a molecular metallic system. Although the thermal conductivity of these molecular compounds was not yet measured, it is expected to be modest in view of the complex molecular structure (~1 W/Km) [
22,
23], the large thermopower values of these bilayer systems place them as good candidates for efficient thermoelectric materials. In fact from an electrical conductivity
σ = 20 S/cm and thermoelectric power
S = 110 μV/K, a power factor
PF =
S2σ = 24 μW/K
2m can be deduced, which is comparable to the best values reported so far for molecular materials such as 387 μW/K
2m in (TTT)I
3 or 110 μW/K
2m in Cu(DMDCNQI)
2 [
1].