Role of Bis(triphenylphosphine)iminium Cation [PNP]+ on the Crystal Packing of [PNP]+[HSeO3]− Solvate Salt
Abstract
:1. Introduction
2. Results and Discussion
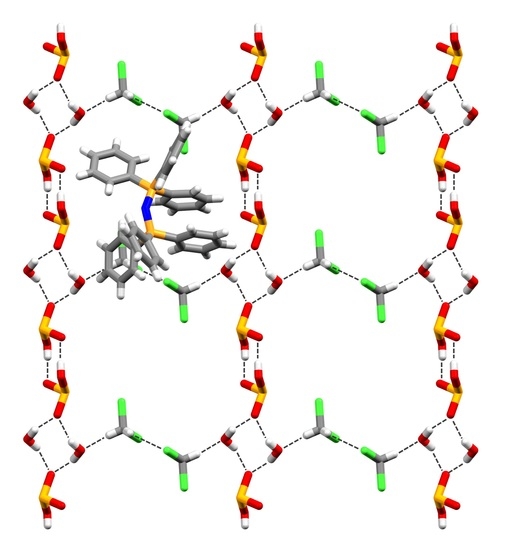
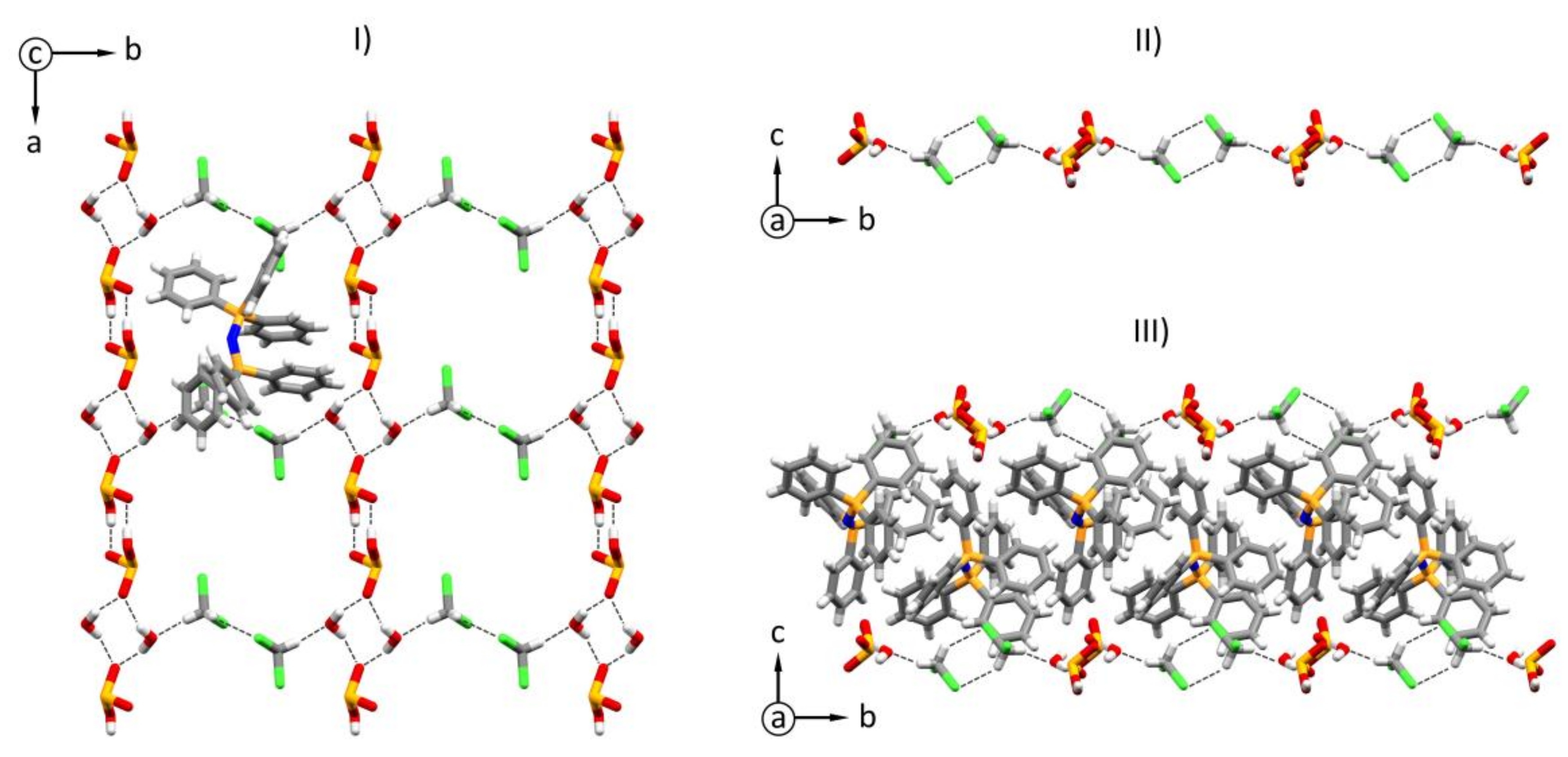
2.1. Crystal and Molecular Structure of Bis(triphenylphosphine)iminium Hydrogen Selenate(IV) Solvate (1)
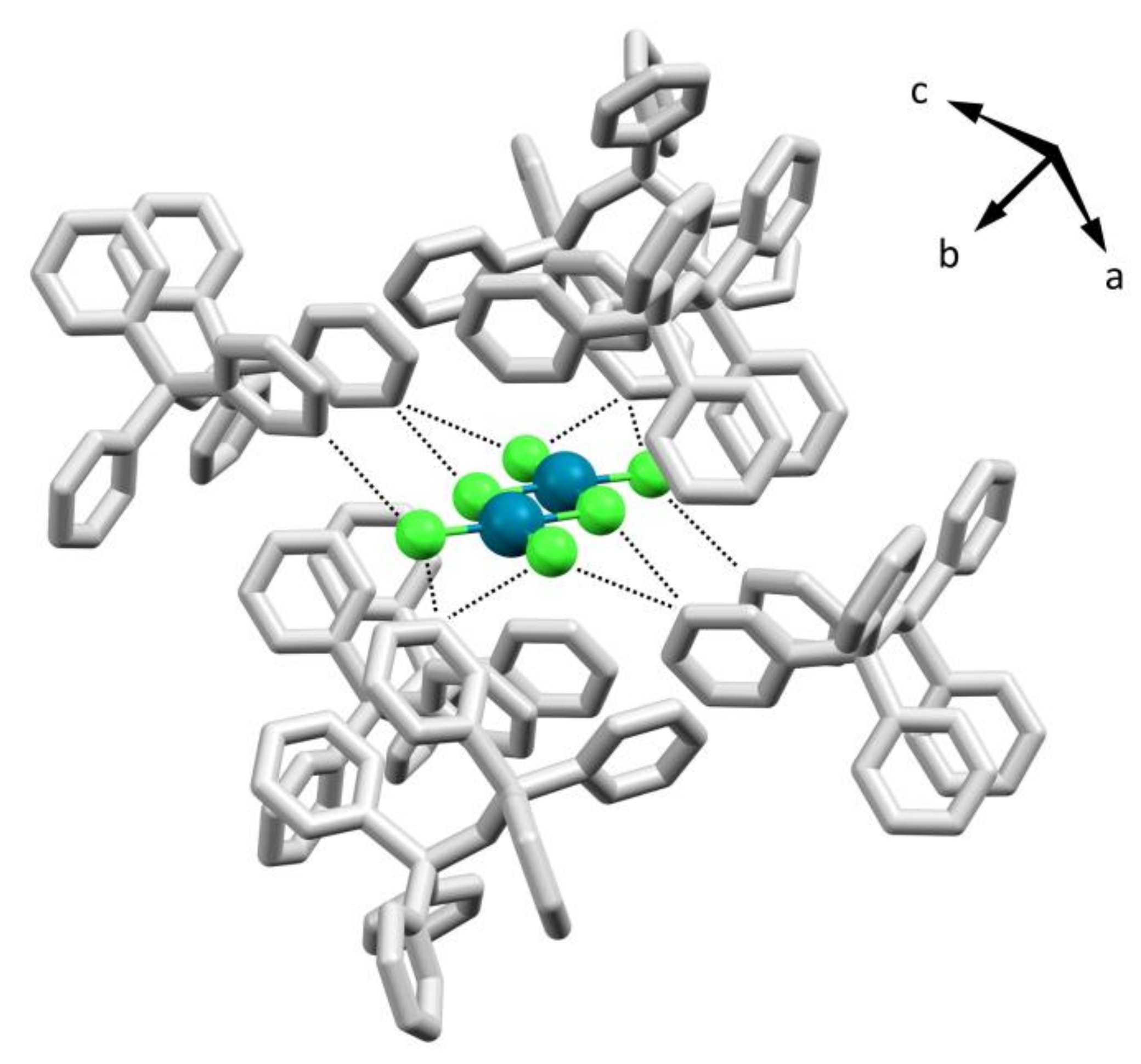
2.2. Reaction of the Crude Product of Bis(triphenylphosphine)iminium Chloride and Sodium Selenate(IV) with Bis(acetonitrile)dichloropalladium(II). Identification of Bis(triphenylphosphine)iminium Hexachloropalladate (2)
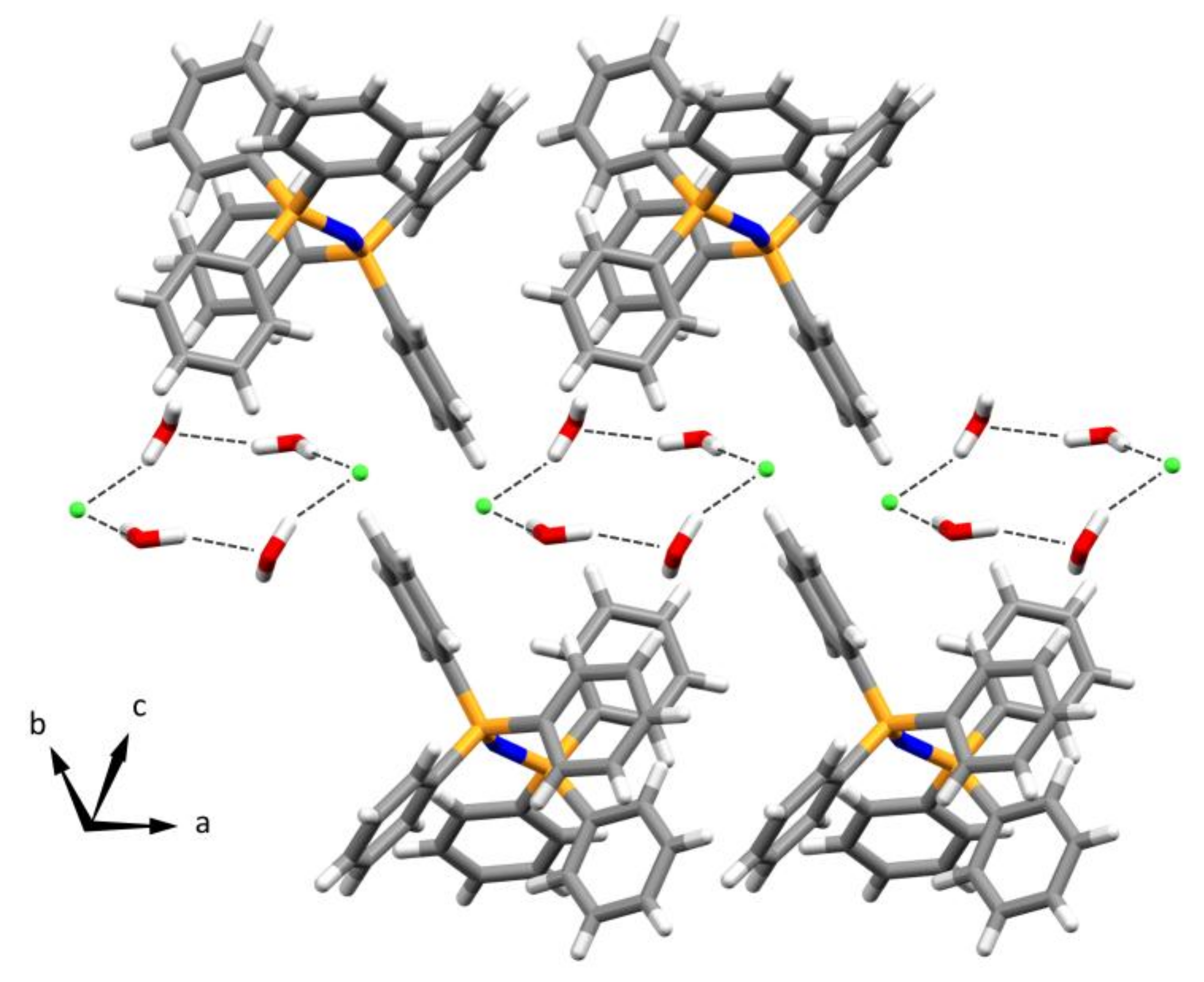
2.3. Crystal and Molecular Structure of a Novel Phase of Bis(triphenylphosphine)iminium Chloride Bihydrate (3)
3. Conclusions
4. Materials and Methods
4.1. General Remarks
4.1.1. Preparation of 1
4.1.2. Preparation of 2
4.2. X-ray Data Collection, Structure Solution, and Refinement
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Paterson, B.; Harrison, W.T.A. Synthesis and Crystal Structure of In(OH)(SeO3). Zeitschrift fur Anorganische und Allgemeine Chemie 2007, 633, 158–161. [Google Scholar] [CrossRef]
- Wontcheu, J.; Schleid, T. Tb3O2Cl[SeO3]2 and Tb5O4Cl3[SeO3]2: Oxide chloride oxoselenates(IV) of trivalent terbium with “lone-pair” channel or layer structures. Zeitschrift fur Anorganische und Allgemeine Chemie 2005, 631, 309–315. [Google Scholar] [CrossRef]
- Albrecht-Schmitt, T.E.; Almond, P.M.; Sykora, R.E. Cation-cation interactions in neptunyl(V) compounds: Hydrothermal preparation and structural characterization of NpO2(IO3) and α- and β-AgNpO2(SeO3). Inorg. Chem. 2003, 42, 3788–3795. [Google Scholar] [CrossRef] [PubMed]
- Porter, Y.; Halasyamani, P.S. New alkali-metal-molybdenum(VI)-selenium(IV) oxides: Syntheses, structures, and characterization of A2SeMoO6(A=Na+, K+, or Rb+). J. Solid State Chem. 2003, 174, 441–449. [Google Scholar] [CrossRef]
- Almond, P.M.; Peper, S.M.; Bakker, E.; Albrecht-Schmitt, T.E. Variable dimensionality and new uranium oxide topologies in the alkaline-earth metal uranyl selenites AE[(UO2)(SeO3)2] (AE=Ca, Ba) and Sr[(UO2)(SeO3)2] 2H2O. J. Solid State Chem. 2002, 168, 358–366. [Google Scholar] [CrossRef]
- Harrison, W.T.A. Caesium vanadium selenite, Cs(VO2)3(SeO3)2. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2000, C56, e422. [Google Scholar] [CrossRef]
- Halasyamani, P.S.; Poeppelmeier, K.R. Noncentrosymmetric Oxides. Chem. Mater. 1998, 10, 2753–2769. [Google Scholar] [CrossRef]
- Ra, H.-S.; Ok, K.M.; Halasyamani, P.S. Combining second-order Jahn-Teller distorted cations to create highly efficient SHG materials: Synthesis, characterization, and NLO properties of BaTeM2O9 (M = Mo6+ or W6+). J. Am. Chem. Soc. 2003, 125, 7764–7765. [Google Scholar] [CrossRef] [PubMed]
- Ok, K.M.; Halasyamani, P.S. Asymmetric Cationic Coordination Environments in New Oxide Materials: Synthesis and Characterization of Pb4Te6M10O41(M = Nb5+ or Ta5+). Inorg. Chem. 2004, 43, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Ok, K.M.; Orzechowski, J.; Halasyamani, P.S. Synthesis, Structure, and Characterization of Two New Layered Mixed-Metal Phosphates, BaTeMO4(PO4) (M = Nb5+ or Ta 5+). Inorg. Chem. 2004, 43, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Goodey, J.; Ok, K.M.; Broussard, J.; Hofmann, C.; Escobedo, F.V.; Halasyamani, P.S. Syntheses, structures, and second-harmonic generating properties in new quaternary tellurites: A2TeW3O12(A=K, Rb, or Cs). J. Solid State Chem. 2003, 175, 3–12. [Google Scholar] [CrossRef]
- Mao, J.-G.; Jiang, H.-L.; Kong, F. Structures and Properties of Functional Metal Selenites and Tellurites. Inorg. Chem. 2008, 47, 8498–8510. [Google Scholar] [CrossRef] [PubMed]
- Delferro, M.; Graiff, C.; Elviri, L.; Predieri, G. Self-assembly of polyoxoselenitopalladate nanostars [Pd₁₅(μ₃-SeO₃)₁₀(μ₃-O)₁₀Na]9− and their supramolecular pairing in the solid state. Dalton Trans. 2010, 39, 4479–4481. [Google Scholar] [CrossRef] [PubMed]
- Savedoff, L.G. Conductance of Electrolytes in Anhydrous Acetone. J. Am. Chem. Soc. 1966, 88, 664–667. [Google Scholar] [CrossRef]
- Springer, C.H.; Coetzee, J.F.; Kay, R.L. Transference number measurements in acetonitrile as solvent. J. Phys. Chem. 1969, 73, 471–476. [Google Scholar] [CrossRef]
- Austad, T.; Engemyr, L.B.; Songstad, J. The Nucleophilicity of the Cyanate Ion. Acta Chem. Scand. 1971, 25, 3535–3536. [Google Scholar] [CrossRef]
- De Lorentiis, L.; Graiff, C.; Predieri, G. Bis(triphenylphosphanylidene)iminium dichloridotriphenylstannate(IV). Acta Crystallogr. Sect. E Struct. Rep. Online 2011, E67, m1356. [Google Scholar] [CrossRef] [PubMed]
- Martinsen, A.; Songstad, J.; Larsson, R.; Pouchard, M.; Hagenmuller, P.; Andresen, A.F. Preparation and Properties of Some Bis(triphenylphosphine)iminium Salts, [(Ph3P)2N]X. Acta Chem. Scand. 1977, A31, 645–650. [Google Scholar] [CrossRef]
- Weil, M. Ammonium hydrogenselenate(IV). Acta Crystallogr. Sect. E Struct. Rep. Online 2006, E62, i38–i40. [Google Scholar] [CrossRef]
- Chomnilpan, B.Y.S.; Liminga, R. Lithium Hydrogenselenite. Acta Crystallogr. 1979, B35, 3011–3013. [Google Scholar] [CrossRef]
- Chomnilpan, S.; Liminga, R.; Sonnenveld, E.J.; Visser, J.W. A Reinvestigation of the Structure of Sodium Hydrogenselenite. Acta. Cryst. 1981, B37, 2220–2223. [Google Scholar] [CrossRef]
- Němec, I.; Chudoba, V.; Havlíček, D.; Císařová, I.; Mička, Z. Preparation, crystal structure, vibrational spectra, and thermal behavior of N, N′-dimethylpiperazinium(2+) hydrogen selenite. J. Solid State Chem. 2001, 161, 312–318. [Google Scholar] [CrossRef]
- Takouachet, R.; Benali-Cherif, R.; Bendeif, E.E.; Benali-Cherif, N.; Pillet, S.; Schaniel, D. Structural analysis and IR-spectroscopy of a new anilinium hydrogenselenite hybrid compound: A subtle structural phase transition. Inorg. Chim. Acta 2016, 446, 6–12. [Google Scholar] [CrossRef]
- Ritchie, L.K.; Harrison, W.T.A. 1-Carbamoylguanidinium hydrogenselenite. Acta Crystallogr. Sect. E Struct. Rep. Online 2003, 59, o1296–o1298. [Google Scholar] [CrossRef]
- De Matos Gomes, E.; Nogueira, E.; Fernandes, I.; Belsley, M.; Paixao, J.A.; Matos Beja, A.; Ramos Silva, M.; Martín-Gil, J.; Mano, J.F. Synthesis, structure, thermal and non-linear optical properties of L-argininium hydrogen selenite. Acta Crystallogr. Sect. B Struct. Sci. 2001, B57, 828–832. [Google Scholar] [CrossRef] [PubMed]
- Paixão, J.A.; Matos Beja, A.; Ramos Silva, M.; de Matos Gomes, E.; Martín-Gil, J.; Martín-Gil, F.J. N,N′-Diphenylguanidinium Hydrogenselenite Monohydrate. Acta Crystallogr. Sect. C Crystal Struct. Commun. 1997, 53, 1113–1115. [Google Scholar] [CrossRef]
- Takouachet, R.; Benali-Cherif, R.; Benali-Cherif, N. Cytosinium hydrogen selenite. Acta Crystallogr. Sect. E Struct. Rep. Online 2014, 70, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Lukevics, E.; Arsenyan, P.; Shestakova, I.; Domracheva, I.; Kanepe, I.; Belyakov, S.; Popelis, J.; Pudova, O. Synthesis, structure and cytotoxicity of organoammonium selenites. Appl. Organomet. Chem. 2002, 16, 228–234. [Google Scholar] [CrossRef]
- Paixão, J.A.; Silva, M.R.; Beja, A.M.; Eusébio, E. Crystal structure and properties of l-tryptophanium hydrogen selenite. Polyhedron 2006, 25, 2021–2025. [Google Scholar] [CrossRef]
- Hasselgren, C.; Jagner, S.; Dance, I. Three-Coordinate [CuIIX3]− (X=Cl, Br), Trapped in a Molecular Crystal. Chem. A Eur. J. 2002, 8, 1270–1278. [Google Scholar] [CrossRef]
- Andrews, S.J.; Robbt, D.A.; Welch, A.J. Structure of Bis(triphenylphosphoranediyl)ammonium Chloride-Boric Acid Adduct (1:1), C36H30NP2+.CI-.BH303. Acta Crystallogr. 1983, C39, 880–882. [Google Scholar]
- Bruker. SAINT; Version 7.06a; Bruker AXS Inc.: Madison, WI, USA, 2003. [Google Scholar]
- Sheldrick, G.M. SADABS—Bruker Nonius Area Detector Scaling and Absorption Correction, V2016/2; Bruker AXS Inc.: Madison, WI, USA, 2016. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]


| [PNP][HSeO3]∙H2O∙CH2Cl2 1 | [PNP]2[Pd2Cl6] 2 | [PNP]Cl∙2H2O 3 | |
|---|---|---|---|
| Formula | C37 H35 Cl2 N O4 P2 Se | C72H60N2P4Pd2Cl6 | C36H34ClNO2P2 |
| FW | 769.46 | 1502.6 | 610.03 |
| crystal system | monoclinic | monoclinic | monoclinic |
| space group | P21/c | P21/c | P21/c |
| a, Å | 10.8664(11) | 9.1894(4) | 10.6243(9) |
| b, Å | 12.6743(13) | 22.3661(10) | 12.6404(11) |
| c, Å | 26.029(3) | 16.2595(7) | 23.595(2) |
| β, deg | 95.727(2) | 103.657(1) | 101.558(2) |
| V, Å3 | 3567.0(6) | 3247.4(2) | 3104.4(5) |
| Z | 4 | 2 | 4 |
| Dcalcd, g cm−3 | 1.433 | 1.537 | 1.305 |
| F(000) | 1576.0 | 1520.0 | 1280.0 |
| µ, cm−1 | 1.333 | 0.944 | 0.260 |
| rflns collected | 34915 | 50951 | 49398 |
| rflns unique | 5605 | 9552 | 9461 |
| rflns observed [I>2σ(I)] | 4326 [Rint = 0.0335] | 7832 [Rint = 0.0334] | 6719 [Rint = 0.0435] |
| Parameters | 428 | 388 | 385 |
| R indices [I>2σ(I)] | R1 = 0.0963; wR2 = 0.3612 | R1 = 0.0319; wR2 = 0.0817 | R1 = 0.0652; wR2 = 0.1825 |
| R indices (all data) | R1 = 0.1141; wR2 = 0.3953 | R1 = 0.0425; wR2 = 0.0867 | R1 = 0.0918; wR2 = 0.2039 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canossa, S.; Graiff, C. Role of Bis(triphenylphosphine)iminium Cation [PNP]+ on the Crystal Packing of [PNP]+[HSeO3]− Solvate Salt. Crystals 2018, 8, 151. https://doi.org/10.3390/cryst8040151
Canossa S, Graiff C. Role of Bis(triphenylphosphine)iminium Cation [PNP]+ on the Crystal Packing of [PNP]+[HSeO3]− Solvate Salt. Crystals. 2018; 8(4):151. https://doi.org/10.3390/cryst8040151
Chicago/Turabian StyleCanossa, Stefano, and Claudia Graiff. 2018. "Role of Bis(triphenylphosphine)iminium Cation [PNP]+ on the Crystal Packing of [PNP]+[HSeO3]− Solvate Salt" Crystals 8, no. 4: 151. https://doi.org/10.3390/cryst8040151