Tuning of Luminescent and Magnetic Properties via Metal Doping of Zn-BTC Systems
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Characterization
2.3. Magnetic Experiments
2.4. Synthesis
3. Results and Discussion
3.1. Metal Ion Doping
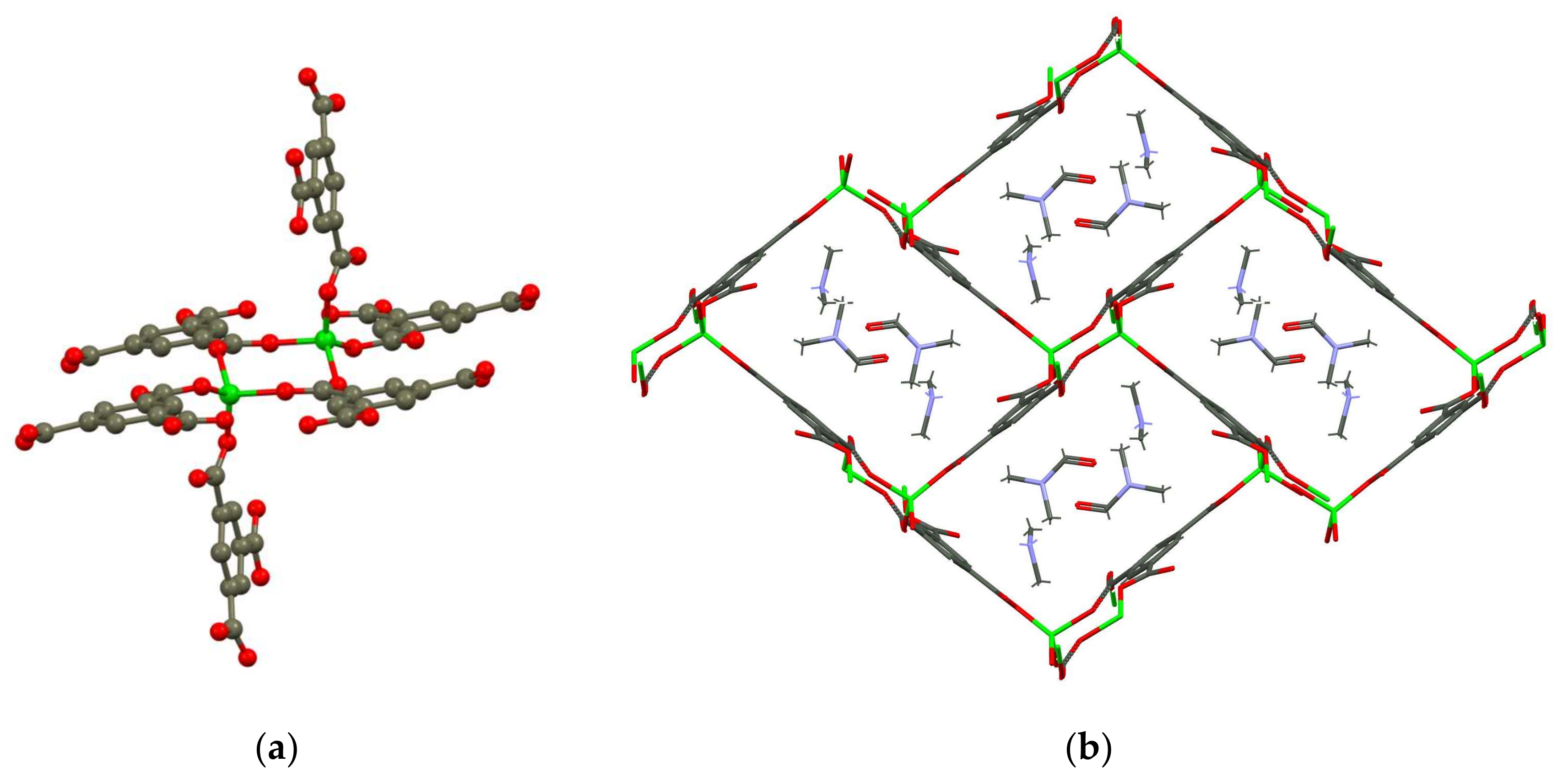
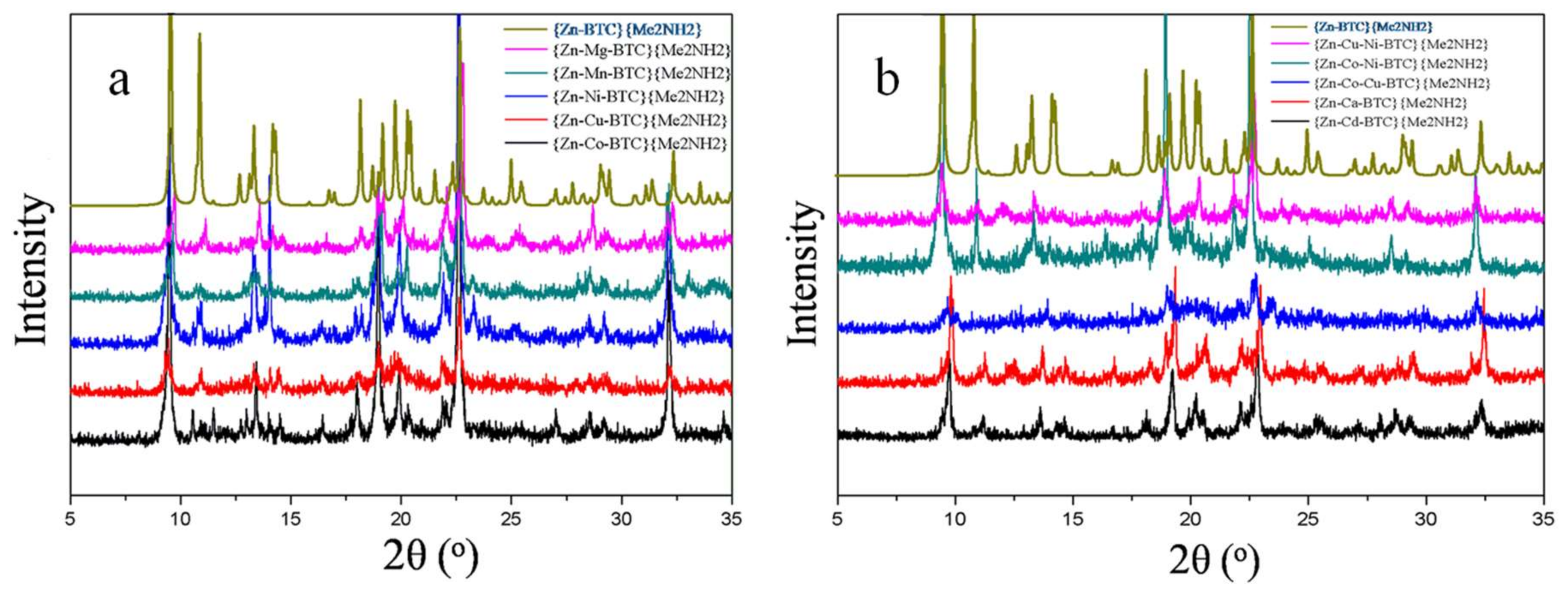
3.2. Single Crystal and Powder X-ray Diffraction Analysis
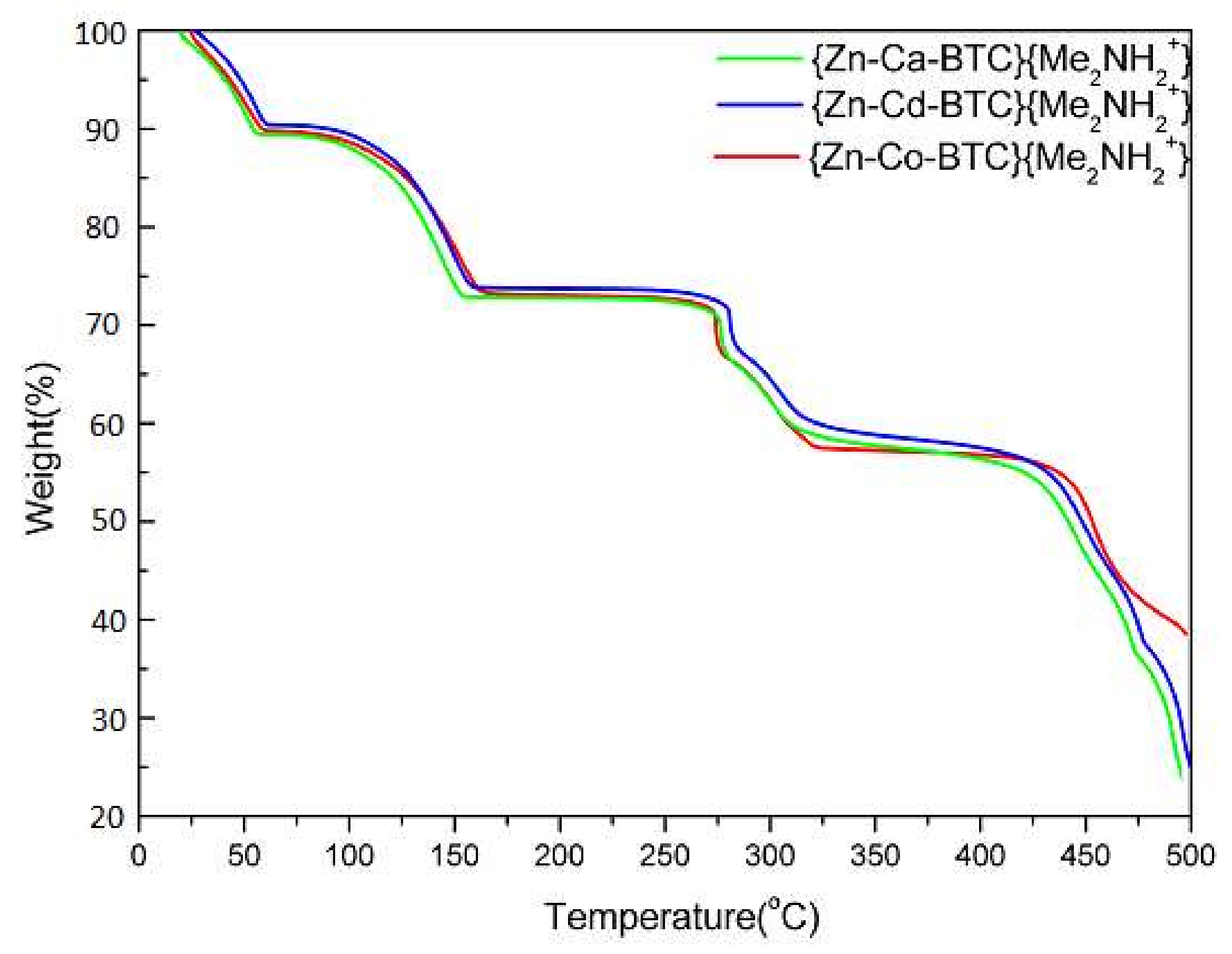
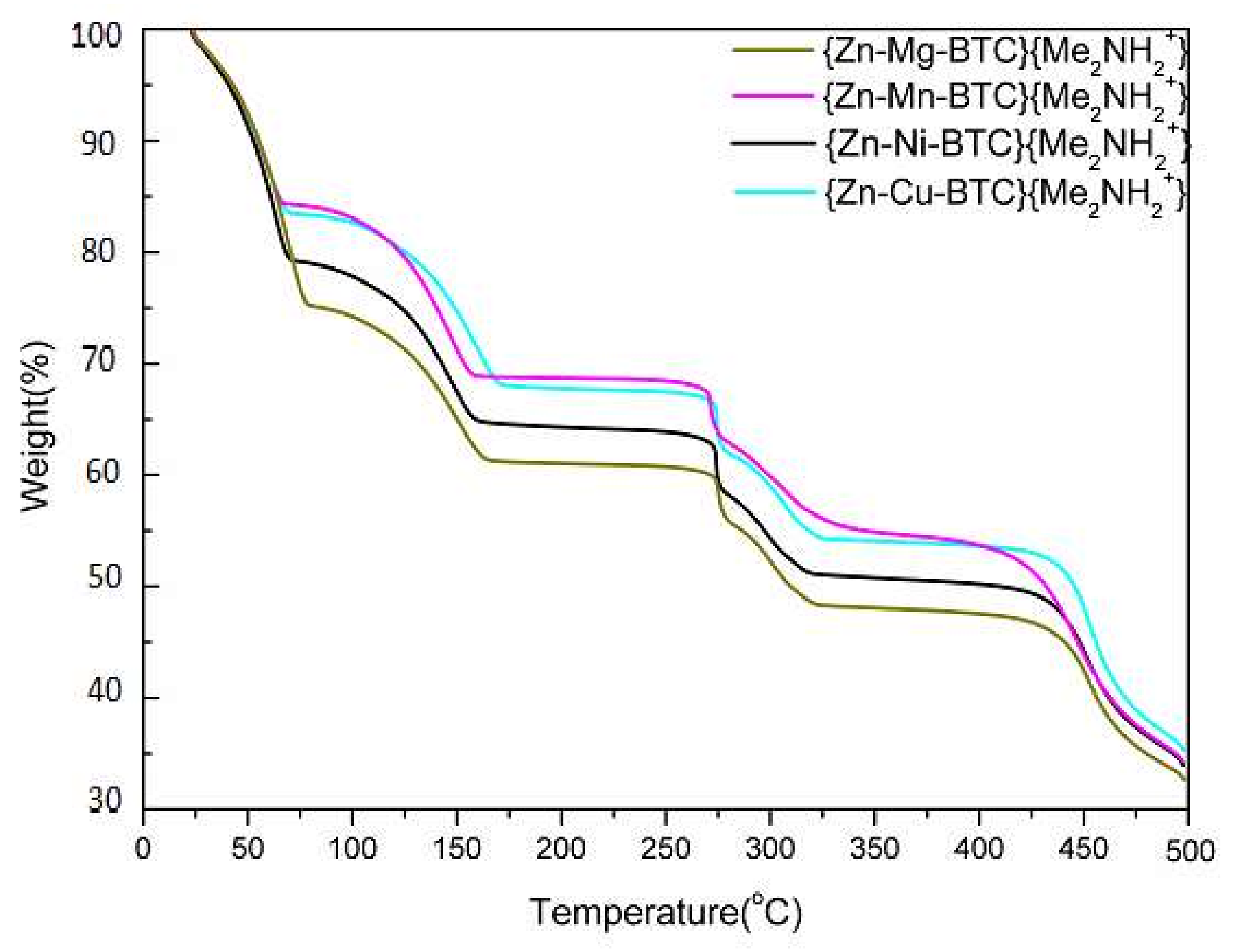
3.3. Thermal Gravimetric Analysis
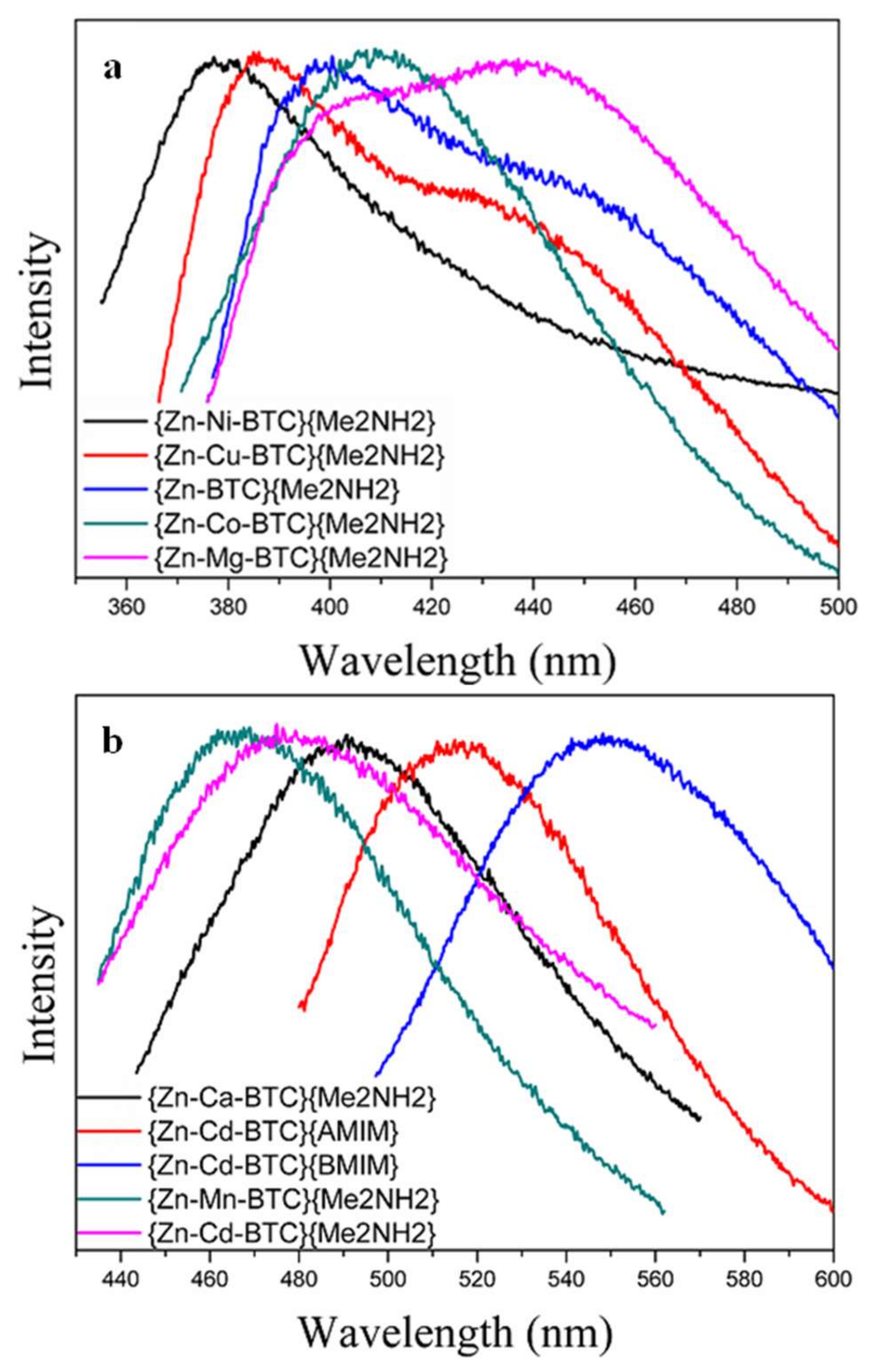
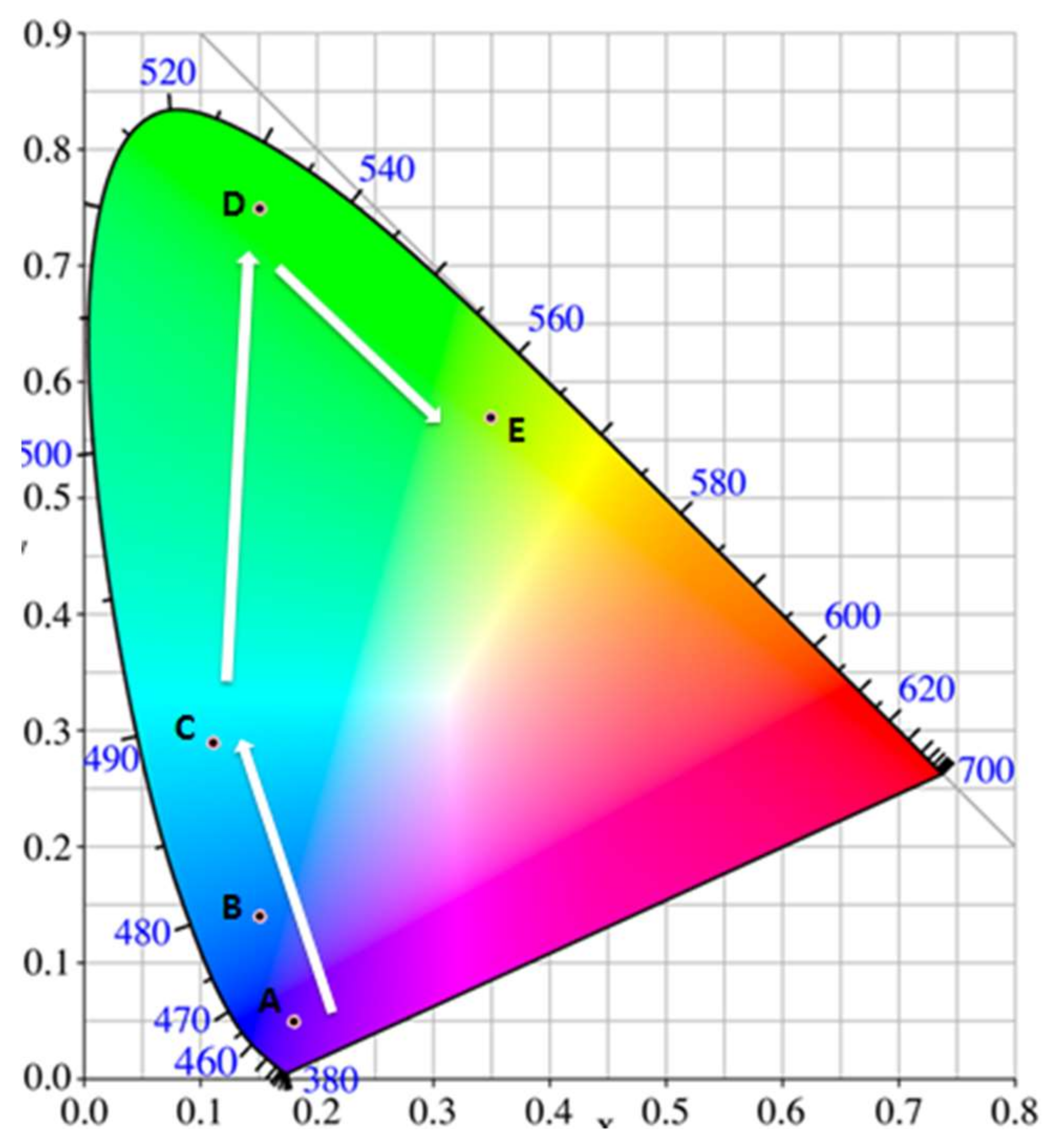
3.4. Effects of Metal Doping on Photoluminescence
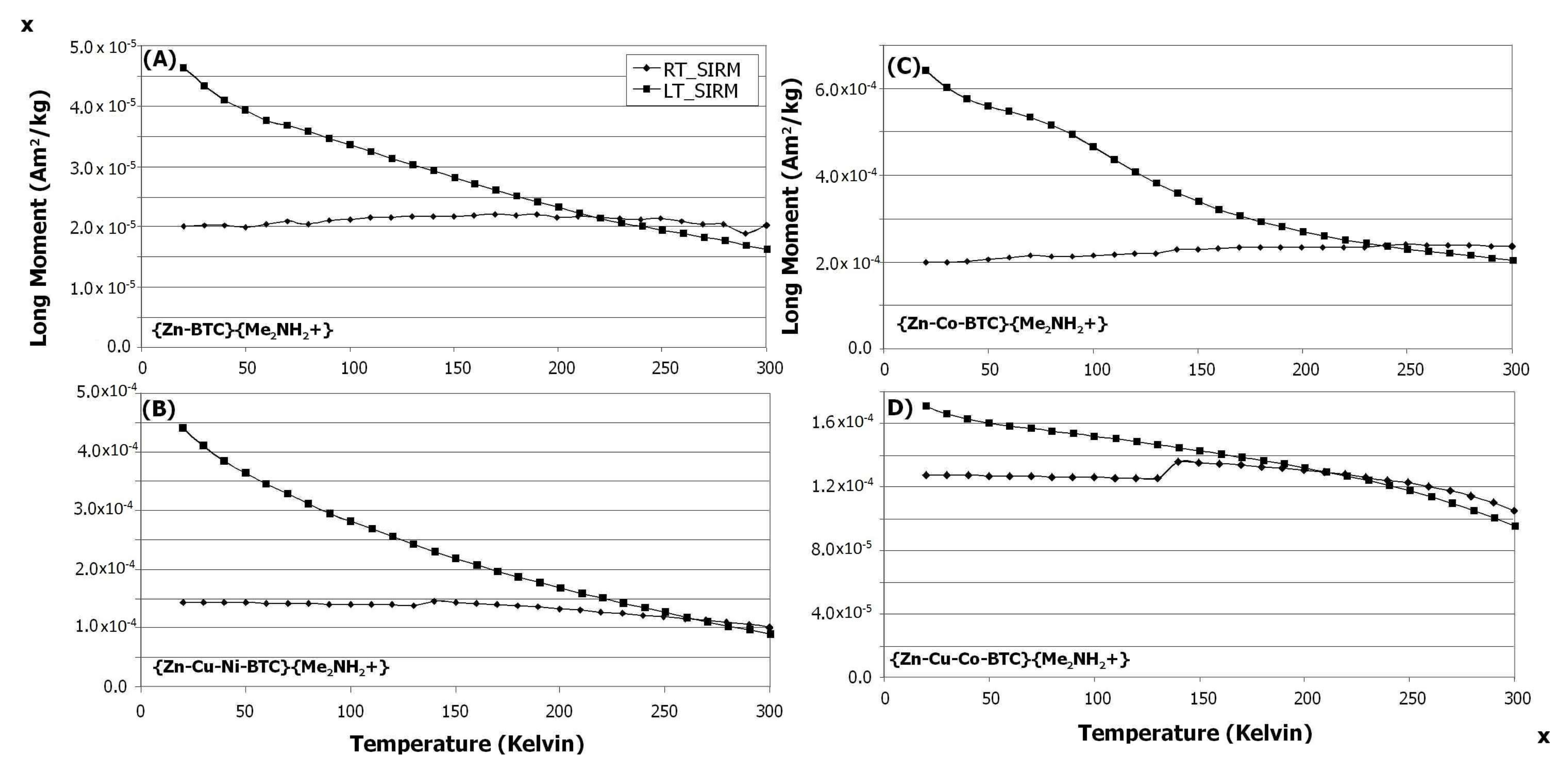
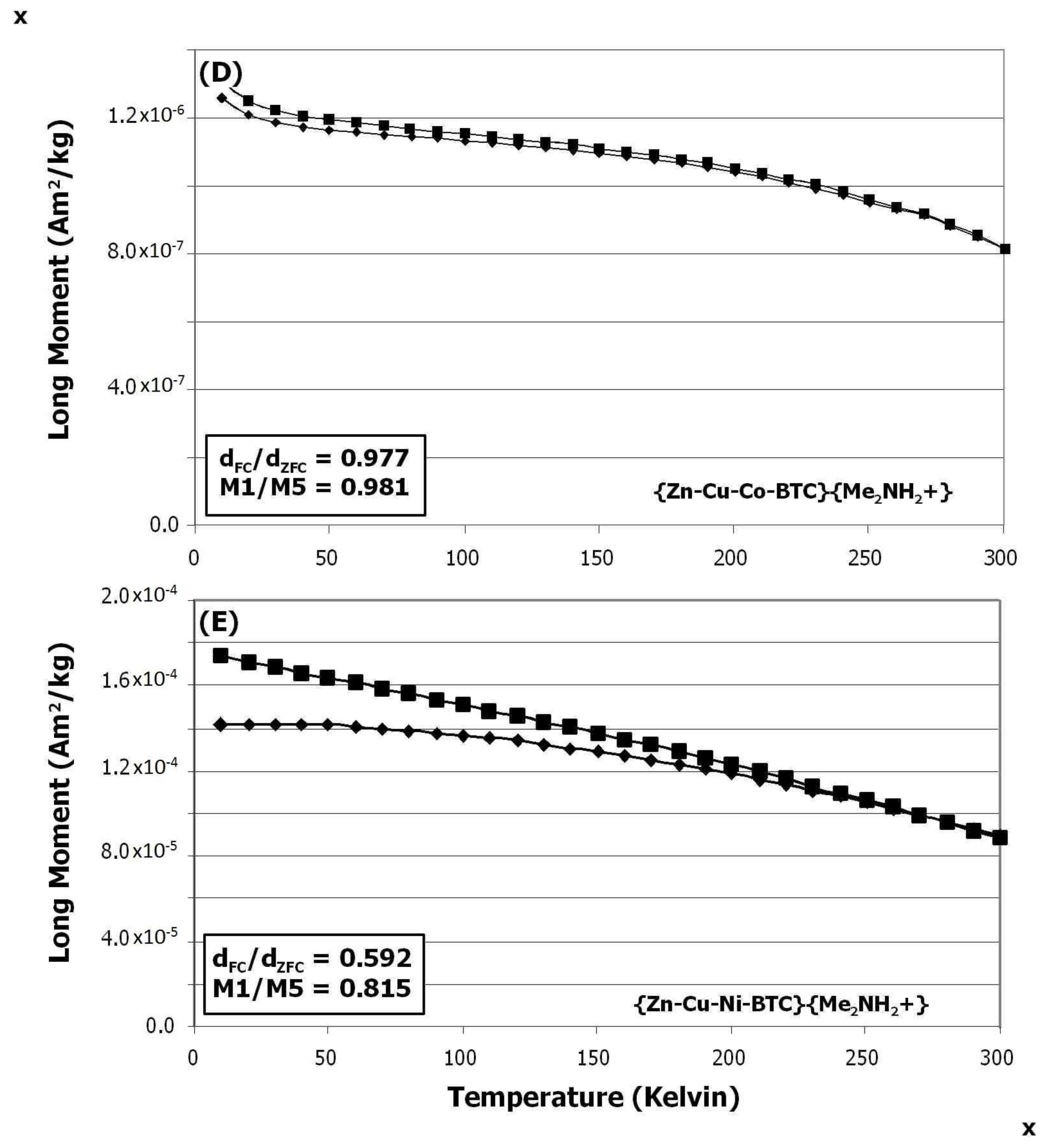
3.5. Effects of Metal Doping on Magnetism
3.6. Remanence Results
3.6.1. Superparamagnetic Ordering Behavior
3.6.2. Induced Magnetization Data
3.6.3. Ferromagnetic Ordering Behavior: Little Field Dependence on Warming
3.6.4. Ferromagnetic Ordering Behavior: Slight Field Dependence on Warming
3.6.5. Paramagnetic Behavior—Lacks Magnetic Ordering
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kaye, S.S.; Dailly, A.; Yaghi, O.M.; Long, J.R. Impact of preparation and handling on the hydrogen storage properties of Zn4O (1,4-benzenedicarboxylate) 3 (MOF-5). J. Am. Chem. Soc. 2007, 129, 14176–14177. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.K.; Siberio-Pérez, D.Y.; Kim, J.; Go, Y.; Eddaoudi, M.; Matzger, A.J.; O’Keeffe, M.; Yaghi, O.M. A route to high surface area, porosity and inclusion of large molecules in crystals. Nature 2004, 427, 523–527. [Google Scholar] [CrossRef] [PubMed]
- O’Keeffe, M.; Yaghi, O.M. Deconstructing the crystal structures of metal-organic frameworks and related materials into their underlying nets. Chem. Rev. 2012, 112, 675–702. [Google Scholar] [CrossRef] [PubMed]
- Getman, R.B.; Bae, Y.S.; Wilmer, C.E.; Snurr, R.Q. Review and Analysis of molecular simulations of methane, hydrogen, and acetylene storage in metal–organic frameworks. Chem. Rev. 2012, 112, 703–723. [Google Scholar] [CrossRef] [PubMed]
- Kurmoo, M. Magnetic metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1353–1379. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhao, Y.; Xu, H.; Kinnibrugh, T.L.; Yang, D.; Timofeeva, T.V.; Daemen, L.L.; Zhang, J.; Bao, W.; Thompson, J.D.; et al. A Novel Helical Double-Layered Cobalt(II)–Organic Framework with Tetranuclear [Co4(µ3-OH)2] Clusters Linked by an Unsymmetrical Pyridylbenzoate Ligand. Inorg. Chem. 2007, 46, 9021–9023. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, T.; Lin, W. Rational synthesis of noncentrosymmetric metal-organic frameworks for second-order nonlinear optics. Chem. Rev. 2012, 112, 1084–1104. [Google Scholar] [CrossRef] [PubMed]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal-organic framework materials as chemical sensors. Chem. Rev. 2011, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Xu, H.; Liu, Y.; Zhao, Y.; Daemen, L.L.; Brown, C.; Timofeeva, T.V.; Ma, S.; Zhou, H.-C. Hydrogen Adsorption in a Highly Stable Porous Rare-Earth Metal-Organic Framework: Sorption Properties and Neutron Diffraction Studies. J. Am. Chem. Soc. 2008, 130, 9626–9627. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal-organic frameworks in biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent Functional Metal–Organic Frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-D.; Zhang, K.; Wang, Y.; Long, W.-W.; Sa, R.-J.; Liu, T.-F.; Lu, J. Fluorescent Metal–Organic Framework (MOF) as a Highly Sensitive and Quickly Responsive Chemical Sensor for the Detection of Antibiotics in Simulated Wastewater. Inorg. Chem. 2018, 57, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Chakraborty, A.; Jayaramulu, K.; Hazra, A.; Maji, T.K. A bimodal anionic MOF: Turn-off sensing of Cu-II and specific sensitization of Eu-III. Chem. Commun. 2014, 50, 13567–13570. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Feng, X.; Han, T.; Wang, S.; Lin, Z.; Dong, Y.; Wang, B. Tuning the luminescence of metal-organic frameworks for detection of energetic heterocyclic compounds. J. Am. Chem. Soc. 2014, 136, 15485–15488. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Pei, X.L.; Jiang, Z.G.; Wang, Q.M. Cluster linker approach: preparation of a luminescent porous framework with NbO topology by linking silver ions with gold(I) clusters. Angew. Chem. Int. Ed. 2014, 53, 12771–12775. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; He, W.; Lu, Y.; Fielden, J.; Xiang, X.; Yan, D. Assembly of ruthenium-based complex into metal-organic framework with tunable area-selected luminescence and enhanced photon-to-electron conversion efficiency. J. Phys. Chem. C 2014, 118, 25365–25373. [Google Scholar] [CrossRef]
- Guo, Z.; Song, X.; Lei, H.; Wang, H.; Su, S.; Xu, H.; Qian, G.; Zhang, H.; Chen, B. A ketone functionalized luminescent terbium metal-organic framework for sensing of small molecules. Chem. Commun. 2015, 51, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, S.; Song, D. A luminescent metal-organic framework as a turn-on sensor for DMF vapor. Angew. Chem. Int. Ed. 2013, 52, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J. Luminescent metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Yue, Q.; Jia, Q.-X.; Lemercier, G.; Gao, E.-Q. Lanthanide metal-organic frameworks based on octahedral secondary building units: rare net topology and luminescence. Cryst. Growth Des. 2009, 9, 2984–2987. [Google Scholar] [CrossRef]
- Wei, Y.; Yu, Y.; Wu, K. Highly stable five-coordinated Mn(II) polymer [Mn(Hbidc)]n (Hbidc=1H-Benzimidazole-5,6-dicarboxylate): Crystal structure, antiferromagnetic property, and strong long-lived luminescence. Cryst. Growth Des. 2008, 8, 2087–2089. [Google Scholar] [CrossRef]
- Bo, Q.B.; Wang, H.Y.; Wang, D.Q.; Znang, Z.W.; Miao, J.L.; Sun, G.X. Structure and photoluminescence tuning features of Mn(2+)- and Ln(3+)-activated Zn-based heterometal-organic frameworks (MOFs) with a single 5-methylisophthalic acid ligand. Inorg. Chem. 2011, 50, 10163–10177. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Wu, Y.; Leong, C.F.; D’Alessandro, D.M.; Zuo, J.L. Crystal structures, magnetic properties, and electrochemical properties of coordination polymers based on the tetra(4-pyridyl)-tetrathiafulvalene ligand. Inorg. Chem. 2015, 54, 10766–10775. [Google Scholar] [CrossRef] [PubMed]
- Aijaz, A.; Sanudo, E.C.; Bharadwaj, P.K. Construction of coordination polymers with a bifurcating ligand: Synthesis, structure, photoluminescence, and magnetic studies. Cryst. Growth Des. 2011, 11, 1122–1134. [Google Scholar] [CrossRef]
- Cai, S.L.; Zheng, S.R.; Wen, Z.Z.; Fan, J.; Wang, N.; Zhang, W.G. Two types of new three-dimensional d-f heterometallic coordination polymers based on 2-(pyridin-3-yl)-1 H-imidazole-4,5-dicarboxylate and oxalate ligands: Syntheses, structures, luminescence, and magnetic properties. Cryst. Growth Des. 2012, 12, 4441–4449. [Google Scholar] [CrossRef]
- Calahorro, A.J.; Salinas-Castillo, A.; Seco, J.M.; Zuñiga, J.; Colacio, E.; Rodríguez-Diéguez, A. Luminescence and magnetic properties of three metal–organic frameworks based on the 5-(1H-tetrazol-5-yl)isophthalic acid ligand. CrystEngComm 2013, 15, 7636–7639. [Google Scholar] [CrossRef]
- Gao, W.; Li, P.; Liu, F.; Zhang, X.-M.; Liua, J.-P. Four metal–organic frameworks based on the 5-(1H-tetrazol-5-yl)isophthalic acid ligand: luminescence and magnetic properties. CrystEngComm 2016, 18, 1523–1531. [Google Scholar] [CrossRef]
- Fang, Z.-L.; Yu, R.-M.; He, J.-G.; Zhang, Q.-S.; Zhao, Z.-G.; Lu, C.-Z. Structural, Luminescent, and Magnetic Properties of Three Novel Three-Dimensional Metal-Organic Frameworks Based on Hexadentate N,N′-bis(4-picolinoyl)hydrazine. Inorg. Chem. 2009, 48, 7691–7697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-B.; Peng, Y.; Shan, X.-C.; Tian, C.-B.; Ping-Lin; Du, S.-W. Lanthanide metal organic frameworks based on octahedral secondary building units: Structural, luminescent, and magnetic properties. Inorg. Chem. Commun. 2011, 14, 1165–1169. [Google Scholar] [CrossRef]
- Dey, C.; Kundu, T.; Biswal, B.P.; Mallick, A.; Banerjee, R. Crystalline metal-organic frameworks (MOFs): Synthesis, structure and function. Acta Crystallogr. 2014, B70, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.; Mínguez Espallargas, G. Dynamic magnetic MOFs. Chem. Soc. Rev. 2013, 42, 1525–1539. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Batten, S.R.; Qi, Y.; Zheng, J.M. Two 3-D cluster-based frameworks: highly eight-connected molecular topology and magnetism. Cryst. Growth Des. 2009, 9, 2756–2761. [Google Scholar] [CrossRef]
- Ouellette, W.; Prosvirin, A.V.; Whitenack, K.; Dunbar, K.R.; Zubieta, J. A Thermally and Hydrolytically Stable Microporous Framework Exhibiting Single-Chain Magnetism: Structure and Properties of [Co2(H0.67bdt)3]·20H2O. Angew. Chem. Int. Ed. 2009, 48, 2140–2143. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-W.; Liu, S.-J.; Hu, T.-L.; Bua, X.-H. Doping cobalt into a [Zn7] cluster-based MOF to tune magnetic behaviour and induce fluorescence signal mutation. Dalton Trans. 2014, 43, 11470–11473. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.A.; Timofeeva, T.V.; Settersten, T.B.; Patterson, B.D.; Liu, V.H.; Simmons, B.A.; Allendorf, M.D. Influence of Connectivity and Porosity on Ligand-Based Luminescence in Zinc Metal-Organic Frameworks. J. Am. Chem. Soc. 2007, 129, 7136–7144. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.; Abdel-Fattah, A.I.; Xu, H.; Burrell, A.K.; Larson, T.E.; McCleskey, T.M.; Wei, Q.; Janicke, M.T.; Hickmott, D.D.; Timofeeva, T.V.; Zhao, Y. Porous Metal–Organic Frameworks Containing Alkali-Bridged Two-Fold Interpenetration: Synthesis, Gas Adsorption, and Fluorescence Properties. Cryst. Growth Des. 2010, 10, 1301–1306. [Google Scholar] [CrossRef]
- Zou, R.; Zhong, R.; Han, S.; Xu, H.; Burrell, A.K.; Henson, N.; Cape, J.L.; Hickmott, D.D.; Timofeeva, T.V.; Larson, T.E.; et al. A Porous Metal–Organic Replica of α-PbO2 for Capture of Nerve Agent Surrogate. J. Am. Chem. Soc. 2010, 132, 17996–17999. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Yang, D.; Larson, T.E.; Kinnibrugh, T.L.; Zou, R.; Henson, N.J.; Timofeeva, T.; Xu, H.; Zhao, Y.; Mattes, B.R. Kinetic hysteresis in gas adsorption behavior for a rigid MOF arising from zig-zag channel structures. J. Mater. Chem. 2012, 22, 10166–10171. [Google Scholar] [CrossRef]
- Ordonez, C.; Kinnibrugh, T.L.; Xu, H.; Lindline, J.; Timofeeva, T.; Wei, Q. Synthesis of Framework Isomer MOFs Containing Zinc and 4-Tetrazolyl Benzenecarboxylic Acid via a Structure Directing Solvothermal Approach. Crystals 2015, 5, 193–205. [Google Scholar] [CrossRef]
- Ordonez, C.; Fonari, M.S.; Wei, Q.; Timofeeva, T.V. Crystal structure of poly[bis(ammonium) [bis(μ4-benzene-1,3,5-tricarboxylato)dizincate] 1-methylpyrrolidin-2-one disolvate]. Acta Crystallogr. 2016, E72, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, C.; Fonari, M.; Lindline, J.; Wei, Q.; Timofeeva, T.V. How Structure-directing cations tune the fluorescence of metal–organic frameworks. Cryst. Growth Des. 2014, 14, 5452–5465. [Google Scholar] [CrossRef]
- Xie, L.H.; Liu, S.X.; Gao, B.; Zhang, C.D.; Sun, C.Y.; Li, D.H.; Su, Z.M. A three-dimensional porous metal–organic framework with the rutile topology constructed from triangular and distorted octahedral building blocks. Chem. Commun. 2005, 2402–2404. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.H.; Lin, J.B.; Liu, X.M.; Xue, W.; Zhang, W.X.; Liu, S.X.; Zhang, J.P.; Chen, X.M. Organic ammonium ion-occluded flexible coordination polymers: Thermal activation, structure transformation and proton transfer. Sci. China. Chem. 2010, 53, 2144–2151. [Google Scholar] [CrossRef]
- Zhao, X.J.; Tao, J. A three-dimensional zinc trimesate framework: [(CH3)2NH2][Zn(C9H3O6)]·(C3H7NO). Appl. Organomet. Chem. 2005, 19, 694–695. [Google Scholar] [CrossRef]
- Verwey, E.J.W. Electronic Conduction of Magnetite (Fe3O4) and its Transition Point at Low Temperatures. Nature 1939, 144, 327–328. [Google Scholar] [CrossRef]
- Chen, W.; Wang, J.Y.; Chen, C.; Yue, Q.; Yuan, H.-M.; Chen, J.-S.; Wang, S.-N. Photoluminescent metal-organic polymer constructed from trimetallic clusters and mixed carboxylates. Inorg. Chem. 2003, 42, 944–946. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Zhu, G.; Xue, M.; Sun, J.; Sun, F.; Qiu, S. Structure, luminescence, and adsorption properties of two chiral microporous metal-organic frameworks. Inorg. Chem. 2006, 45, 3582–3587. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.-C.; Wu, X.-T.; Fu, Z.-Y.; Cui, C.-P.; Hu, S.-M.; Du, W.-X.; Wu, L.-M.; Zhang, H.-H.; Sun, R.-Q. Synthesis, structure, and fluorescence of the novel cadmium(II)–trimesate coordination polymers with different coordination architectures. Inorg. Chem. 2002, 41, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, D.; Özdemir, Ö. Rock Magnetism: Fundamentals and Frontiers; Cambridge University Press: New York, NY, USA, 1997; p. 573. ISBN 0521325145. [Google Scholar]
- Moskowitz, B.M.; Jackson, M.; Kissel, C. Low-temperature magnetic behavior of titanomagnetites. Earth Planet. Sci. Lett. 1998, 157, 141–149. [Google Scholar] [CrossRef]
- Bowles, J.; Jackson, M.; Chen, A.; Solheid, P. Interpretation of Low-Temperature Data Part 1: Superparamagnetism and Paramagnetism. IRM Q. 2009, 19, 7–11. [Google Scholar]
- Moskowitz, B.M.; Frankel, R.B.; Bazylinski, D.A. Rock magnetic criteria for the detection of biogenic magnetite. Earth Planet. Sci. Lett. 1993, 120, 283–300. [Google Scholar] [CrossRef]
- Smirnov, A.V. Grain size dependence of low-temperature remanent magnetization in natural and synthetic magnetite: Experimental study. Earth Planets Space 2009, 61, 119–124. [Google Scholar] [CrossRef]
- Verwey, E.J.; Haayman, P.W.; Romeijn, F.C. Physical properties and cation arrangements of oxides with spinel structure II. Electronic conductivity. J. Chem. Phys. 1947, 15, 181–187. [Google Scholar] [CrossRef]
- Xu, X.; Xu, C.; Lin, Y.; Li, J.; Hu, J. Comparison on Photoluminescence and Magnetism between Two Kinds of Undoped ZnO Nanorods. J. Phys. Chem. C 2013, 117, 24549–24553. [Google Scholar] [CrossRef]
- Ganguli, N.; Dasgupta, I.; Sanyal, B. Electronic structure and magnetism of transition metal doped Zn12O12 clusters: Role of defects. J. Appl. Phys. 2010, 108, 123911. [Google Scholar] [CrossRef]


| Metal Dopant | Zn(NO3)2·6H2O (mmol) | H3BTC (mmol) | Metal Dopant Salt (mmol) | DMF (mL) |
|---|---|---|---|---|
| Co | 2.4 | 2.5 | Co(NO3)2·6H2O 0.13 | 50 |
| Cu | 0.47 | 0.5 | Cu(NO3)2·2.5H2O 0.025 | 10 |
| Ni | 0.47 | 0.5 | Ni(NO3)2·6H2O 0.024 | 10 |
| Mn | 0.47 | 0.5 | Mn(NO3)2·4H2O 0.024 | 10 |
| Ca | 2.4 | 2.5 | Ca(NO3)2·4H2O 0.13 | 50 |
| Mg | 0.47 | 0.5 | Mg(NO3)2·6H2O 0.024 | 10 |
| Cd | 0.47 | 0.5 | Cd(NO3)2·4H2O 0.026 | 10 |
| Metal Dopant | Zn(NO3)2·6H2O (mmol) | H3BTC (mmol) | Metal Dopant Salt (mmol) | DMF (mL) |
|---|---|---|---|---|
| Co, Ni | 2.38 | 2.5 | Co(NO3)2·6H2O 0.075 Ni(NO3)2·6H2O 0.075 | 50 |
| Cu, Ni | 2.38 | 2.5 | Cu(NO3)2·2.5H2O 0.075 Ni(NO3)2·6H2O 0.075 | 50 |
| Cu, Co | 2.38 | 2.5 | Cu(NO3)2·2.5H2O 0.075 Co(NO3)2·6H2O 0.075 | 50 |
| a (Å) | b (Å) | c (Å) | β (°) | Volume (Å3) | |
|---|---|---|---|---|---|
| no doping | 9.473(5) | 16.023(9) | 11.107(6) | 98.335(8) | 1668(3) |
| Cd | 9.481(3) | 16.013(4) | 11.098(3) | 98.351(3) | 1667(1) |
| Cu | 9.466(6) | 15.998(11) | 11.100(7) | 98.237(8) | 1663(3) |
| Mg | 9.499(5) | 16.057(8) | 11.124(6) | 98.692(7) | 1677(3) |
| Ca | 9.474(3) | 16.013(5) | 11.110(4) | 98.260(4) | 1668(2) |
| Co | 9.473(4) | 16.018(7) | 11.128(5) | 98.251(5) | 1671(2) |
| Ni | 9.482(4) | 16.028(7) | 11.129(5) | 98.198(6) | 1674(2) |
| Mn | 9.458(4) | 16.067(7) | 11.099(5) | 98.838(6) | 1667(2) |
| Ni, Co | 9.476(3) | 16.017(6) | 11.107(4) | 98.311(5) | 1668(2) |
| Cu, Ni | 9.498(7) | 16.069(10) | 11.158(8) | 98.281(8) | 1685(3) |
| Cu, Co | 9.499(4) | 16.057(8) | 11.154(5) | 98.221(6) | 1684(2) |
| Sample | RTSIRM–LTSIRM Magnetic Order | Within Field ZFC-FC Magnetic Order |
|---|---|---|
| Zn | SP | FM #,* |
| Zn Co | SP | Para |
| Zn Cu Co | SP * | FM $ |
| Zn Cu Ni | SP | FM $ |
| Zn Mg | FM #,* | |
| Zn Mn | Para | |
| Zn Ni Co | Para | |
| Zn Ni | FM # |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, T.; Wei, Q.; Ordonez, C.; Lindline, J.; Petronis, M.; Fonari, M.S.; Timofeeva, T. Tuning of Luminescent and Magnetic Properties via Metal Doping of Zn-BTC Systems. Crystals 2018, 8, 162. https://doi.org/10.3390/cryst8040162
Qu T, Wei Q, Ordonez C, Lindline J, Petronis M, Fonari MS, Timofeeva T. Tuning of Luminescent and Magnetic Properties via Metal Doping of Zn-BTC Systems. Crystals. 2018; 8(4):162. https://doi.org/10.3390/cryst8040162
Chicago/Turabian StyleQu, Taoguang, Qiang Wei, Carlos Ordonez, Jennifer Lindline, Michael Petronis, Marina S. Fonari, and Tatiana Timofeeva. 2018. "Tuning of Luminescent and Magnetic Properties via Metal Doping of Zn-BTC Systems" Crystals 8, no. 4: 162. https://doi.org/10.3390/cryst8040162





