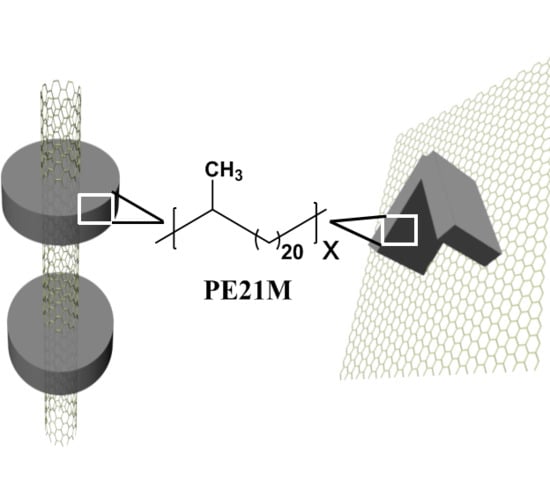
Epitaxial Crystallization of Precisely Methyl-Substituted Polyethylene Induced by Carbon Nanotubes and Graphene
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
3. Results and Discussion
3.1. Morphologies of PE21M/Nanofiller Composites
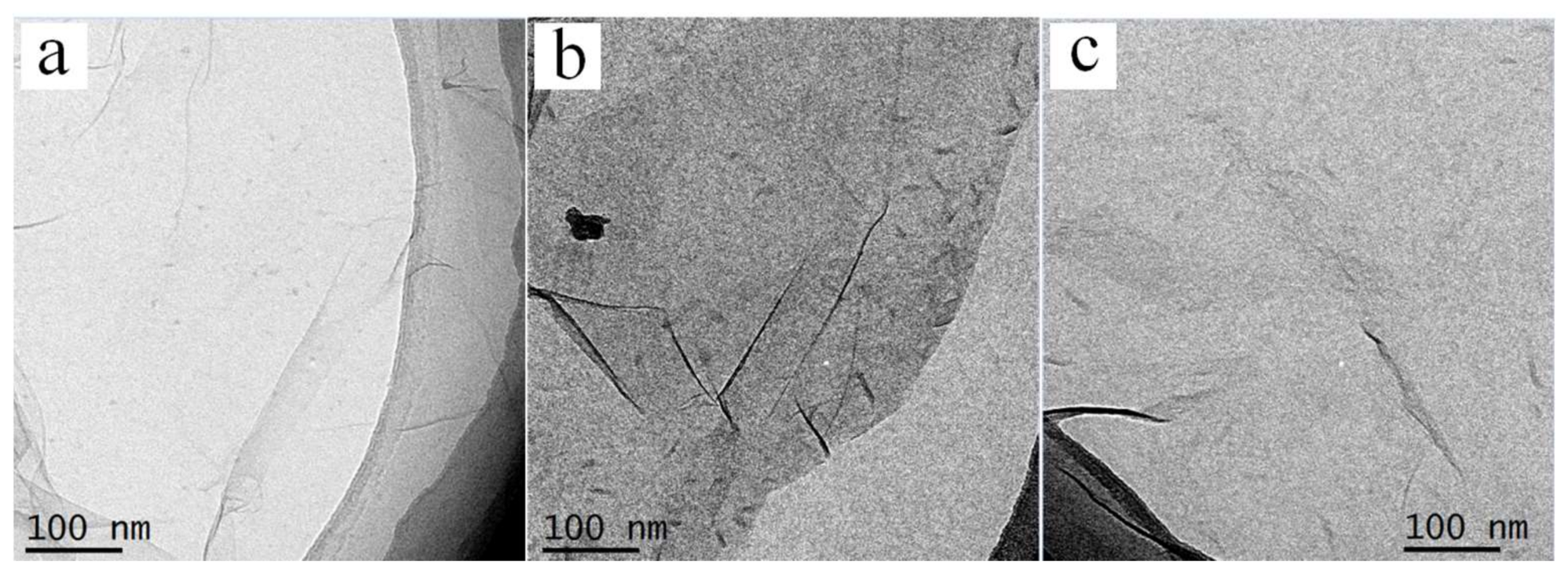
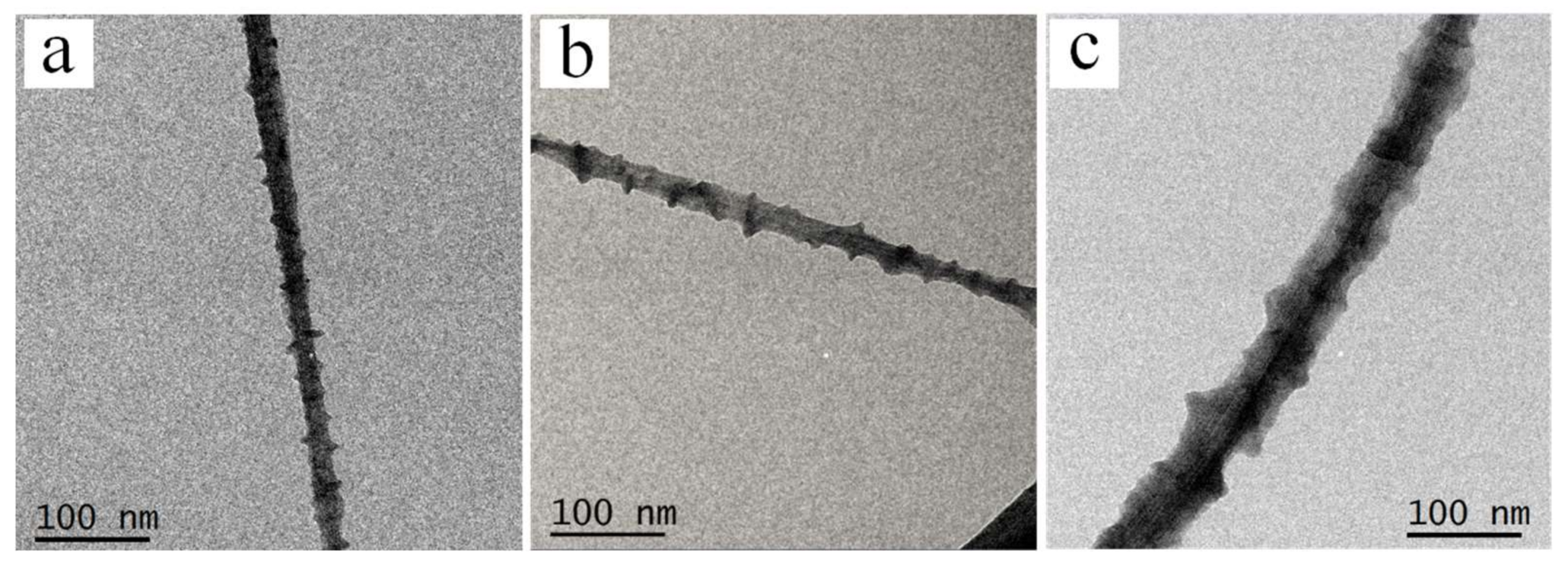
3.1.1. Morphologies of PE21M/CNT Kebab-Like Crystals
3.1.2. Morphologies of PE21M/RGO Rod-Like Crystals
3.1.3. PE21M/Nanofiller Composites Prepared with the Assistance of Supercritical CO2
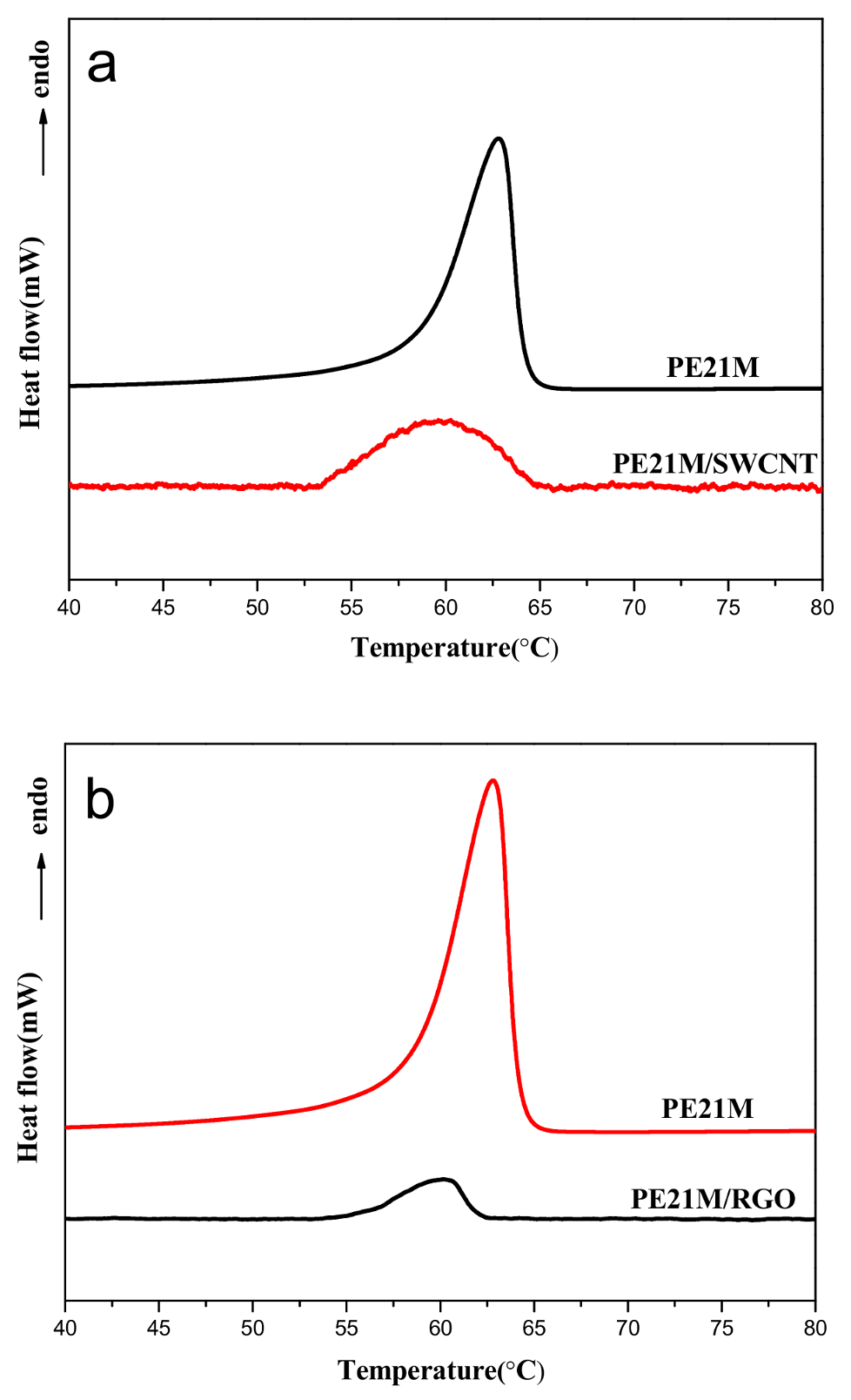
3.2. Thermal Behavior of PE21M/Nanofiller Composites
3.3. Crystalline Structure of PE21M/Nanofiller Composites
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wittmann, J.C.; Lotz, B. Epitaxial crystallization of polymers on organic and polymeric substrates. Prog. Polym. Sci. 1990, 15, 909–948. [Google Scholar] [CrossRef]
- Chang, H.; Zhang, J.; Li, L.; Wang, Z.; Yang, C.; Takahashi, I.; Ozaki, Y.; Yan, S. A Study on the Epitaxial Ordering Process of the Polycaprolactone on the Highly Oriented Polyethylene Substrate. Macromolecules 2010, 43, 362–366. [Google Scholar] [CrossRef]
- Wellinghoff, S.; Rybnikar, F.; Baer, E. Epitaxial crystallization of polyethylene. J. Macromol. Sci. Phys. 1974, 10, 1–39. [Google Scholar] [CrossRef]
- Furuheim, K.M.; Axelson, D.E.; Antonsen, H.W.; Helle, T. Phase structural analyses of polyethylene extrusion coatings on high-density papers. I. Monoclinic crystallinity. J. Appl. Polym. Sci. 2010, 91, 218–225. [Google Scholar] [CrossRef]
- Mittal, G.; Rhee, K.Y.; Park, S.J. The Effects of Cryomilling CNTs on the Thermal and Electrical Properties of CNT/PMMA Composites. Polymers 2016, 8, 169. [Google Scholar] [CrossRef]
- Flores, A.; Poeppel, A.; Riekel, C.; Schulte, K. Evidence of a transcrystalline interphase in fiber PE homocomposites as revealed by microdiffraction experiments using synchrotron radiation. J. Macromol. Sci. Phys. 2001, 40, 749–761. [Google Scholar] [CrossRef]
- Xu, J.Z.; Zhong, G.J.; Hsiao, B.S.; Fu, Q.; Li, Z.M. Low-dimensional carbonaceous nanofiller induced polymer crystallization. Prog. Polym. Sci. 2014, 39, 555–593. [Google Scholar] [CrossRef]
- Yang, S.; Meng, D.; Sun, J.; Hou, W.; Ding, Y.; Jiang, S.; Huang, Y.; Geng, J. Enhanced electrochemical response for mercury ion detection based on poly(3-hexylthiophene) hybridized with multi-walled carbon nanotubes. RSC Adv. 2014, 4, 25051–25056. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, S.; Li, B.; Hou, W.; Li, G.; Memon, M.A.; Huang, Y.; Geng, J. Graphene Oxide: A Versatile Agent for Polyimide Foams with Improved Foaming Capability and Enhanced Flexibility. Chem. Mater. 2015, 27, 4358–4367. [Google Scholar] [CrossRef]
- Zhu, W.; Yang, O.; Sun, J.; Memon, J.; Wang, C.; Geng, J.; Huang, Y. Scalable preparation of three-dimensional porous structures of reduced graphene oxide/cellulose composites and their application in supercapacitors. Carbon 2013, 62, 501–509. [Google Scholar]
- Ning, N.; Fu, S.; Zhang, W.; Chen, F.; Wang, K.; Deng, H.; Zhang, Q.; Fu, Q. Metal-catalyst-free growth of carbon nanotubes/carbon nanofibers on carbon blacks using chemical vapor deposition. Prog. Polym. Sci. 2012, 37, 1425–1455. [Google Scholar] [CrossRef]
- Mai, F.; Wang, K.; Yao, M.; Deng, H.; Chen, F.; Fu, Q. Superior Reinforcement in Melt-Spun Polyethylene/Multiwalled Carbon Nanotube Fiber through Formation of a Shish-Kebab Structure. J. Phys. Chem. B 2010, 114, 10693–10702. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Zhang, C.; Zhang, Y.F. Thermal Conductivity of Graphene-Polymer Composites: Mechanisms, Properties, and Applications. Polymers 2017, 9, 437–454. [Google Scholar]
- Deng, H.; Skipa, T.; Zhang, R.; Lellinger, D.; Bilotti, E.; Alig, I.; Peijs, T. Effect of melting and crystallization on the conductive network in conductive polymer composites. Polymer 2009, 50, 3747–3754. [Google Scholar] [CrossRef]
- Deng, H.; Skipa, T.; Bilotti, E.; Zhang, R.; Lellinger, D.; Mezzo, L.; Fu, Q.; Alig, I.; Peijs, T. Preparation of High-Performance Conductive Polymer Fibers through Morphological Control of Networks Formed by Nanofillers. Adv. Funct. Mater. 2010, 20, 1424–1432. [Google Scholar] [CrossRef]
- Kodjie, S.L.; Li, L.; Li, B.; Cai, W.; Li, C.Y.; Keating, M. Morphology and Crystallization Behavior of HDPE/CNT Nanocomposite. J. Macromol. Sci. Phys. 2006, 45, 231–245. [Google Scholar] [CrossRef]
- Li, C.Y.; Li, L.; Cai, W.; Kodjie, S.L.; Tenneti, K.K. Nano hybrid shish-kebab: Periodically functionalize carbon nanotubes. Adv. Mater. 2005, 17, 1198–1202. [Google Scholar] [CrossRef]
- Li, L.; Li, C.Y.; Ni, C. Polymer Crystallization-Driven, Periodic Patterning on Carbon Nanotubes. J. Am. Chem. Soc. 2006, 128, 1692–1699. [Google Scholar] [CrossRef] [PubMed]
- Tracz, A.; Jeszka, J.K.; Kucinska, I.; Chapel, J.P.; Boiteux, G.; Kryszewski, M. Influence of the crystallization conditions on the morphology of the contact layer of polyethylene crystallized on graphite: Atomic force microscopy studies. J. Appl. Polym. Sci. 2002, 86, 1329–1336. [Google Scholar] [CrossRef]
- Tracz, A.; Kucinska, I.; Jeszka, J.K. Formation of Highly Ordered, Unusually Broad Polyethylene Lamellae in Contact with Atomically Flat Solid Surfaces. Macromolecules 2003, 36, 10130–10132. [Google Scholar] [CrossRef]
- Tracz, A.; Kucinska, I.; Jeszka, J.K. Unusual crystallization of polyethylene at melt/atomically flat interface: Lamellar thickening growth under normal pressure. Polymer 2006, 47, 7251–7258. [Google Scholar] [CrossRef]
- Takenaka, Y.; Miyaji, H.; Hoshino, A.; Tracz, A.; Jeszka, J.K.; Kucinska, I. Interface Structure of Epitaxial Polyethylene Crystal Grown on HOPG and MoS2 Substrates. Macromolecules 2004, 37, 9667–9669. [Google Scholar] [CrossRef]
- Vadlamudi, M.; Alamo, R.G.; Fiscus, D.M.; Varma-Nair, M.J. Inter and intra-molecular branching distribution of tailored LLDPEs inferred by melting and crystallization behavior of narrow fractions. J. Therm. Anal. Calorim. 2009, 96, 697–704. [Google Scholar] [CrossRef]
- Vadlamudi, M.; Subramanian, G.; Shanbhag, S.; Alamo, R.G.; Fiscus, D.M.; Brown, G.M.; Lu, C.; Ruff, C.J. Molecular Weight and Branching Distribution of a High Performance Metallocene Ethylene 1-Hexene Copolymer Film-Grade Resin. Macromol. Symp. 2009, 282, 1–13. [Google Scholar] [CrossRef]
- Smith, J.A.; Brzezinska, K.R.; Valenti, D.J.; Wagener, K.B. Precisely Controlled Methyl Branching in Polyethylene via Acyclic Diene Metathesis (ADMET) Polymerization. Macromolecules 2000, 33, 3781–3794. [Google Scholar] [CrossRef]
- Sworen, J.C.; Smith, J.A.; Berg, J.M.; Wagener, K.B. Modeling Branched Polyethylene: Copolymers Possessing Precisely Placed Ethyl Branches. J. Am. Chem. Soc. 2004, 126, 11238–11246. [Google Scholar] [CrossRef] [PubMed]
- Boz, E.; Wagener, K.B.; Ghosal, A.; Fu, R.; Alamo, R.G. Synthesis and Crystallization of Precision ADMET Polyolefins Containing Halogens. Macromolecules 2006, 39, 4437–4447. [Google Scholar] [CrossRef]
- Sworen, J.C.; Wagener, K.B. Linear Low-Density Polyethylene Containing Precisely Placed Hexyl Branches. Macromolecules 2007, 40, 4414–4423. [Google Scholar] [CrossRef]
- Boz, E.; Ghiviriga, I.; Nemeth, A.J.; Jeon, K.; Alamo, R.G.; Wagener, K.B. Random, Defect-Free Ethylene/Vinyl Halide Model Copolymers via Condensation Polymerization. Macromolecules 2008, 41, 25–30. [Google Scholar] [CrossRef]
- Boz, E.; Nemeth, A.J.; Wagener, K.B.; Jeon, K.; Smith, R.; Nazirov, F.; Bockstaller, M.R.; Alamo, R.G. Well-Defined Precision Ethylene/Vinyl Fluoride Polymers: Synthesis and Crystalline Properties. Macromolecules 2008, 41, 1647–1653. [Google Scholar] [CrossRef]
- Rojas, G.; Inci, B.; Wei, Y.; Wagener, K.B. Precision polyethylene: Changes in morphology as a function of alkyl branch size. J. Am. Chem. Soc. 2009, 131, 17376–17386. [Google Scholar] [CrossRef] [PubMed]
- Inci, B.; Lieberwirth, I.; Steffen, W.; Mezger, M.; Graf, R.; Landfester, K.; Wagener, K.B. Decreasing the Alkyl Branch Frequency in Precision Polyethylene: Effect of Alkyl Branch Size on Nanoscale Morphology. Macromolecules 2012, 45, 3367–3376. [Google Scholar] [CrossRef]
- Gaines, T.W.; Nakano, T.; Chujo, Y.; Trigg, E.B.; Winey, K.I.; Wagener, K.B. Precise Sulfite Functionalization of Polyolefins via ADMET Polymerization. ACS Macro Lett. 2015, 4, 624–627. [Google Scholar] [CrossRef]
- Tasaki, M.; Yamamoto, H.; Hanesaka, M.; Tashiro, K.; Boz, E.; Wagener, K.B.; Ruiz-Orta, C.; Alamo, R.G. Polymorphism and Phase Transitions of Precisely Halogen-Substituted Polyethylene. (1) Crystal Structures of Various Crystalline Modifications of Bromine-Substituted Polyethylene on Every 21st Backbone Carbon. Macromolecules 2014, 47, 4738–4749. [Google Scholar] [CrossRef]
- Kaner, P.; Ruiz-Orta, C.; Boz, E.; Wagener, K.B.; Tasaki, M.; Tashiro, K.; Alamo, R.G. Kinetic Control of Chlorine Packing in Crystals of a Precisely Substituted Polyethylene. Toward Advanced Polyolefin Materials. Macromolecules 2014, 47, 236–245. [Google Scholar] [CrossRef]
- Miao, W.; Lv, Y.; Zheng, W.; Wang, Z.; Chen, Z.R. Epitaxial crystallization of precisely fluorine substituted polyethylene induced by carbon nanotube and reduced graphene oxide. Polymer 2016, 83, 205–213. [Google Scholar] [CrossRef]
- Miao, W.; Wang, Z.; Li, Z.; Zheng, W.; Chen, Z.R. Epitaxial crystallization of precisely chlorine-substituted polyethylene induced by carbon nanotube and graphene. Polymer 2016, 94, 53–61. [Google Scholar] [CrossRef]
- Miao, W.; Wang, B.; Li, Y.; Zheng, W.; Chen, H.; Zhang, L.; Wang, Z. Epitaxial crystallization of precisely brominesubstituted polyethylene induced by carbon nanotubes and graphene. RSC Adv. 2017, 7, 17640–17649. [Google Scholar] [CrossRef]
- Wang, B.; Li, H.; Li, Z.; Chen, P.; Wang, Z.; Gu, Q. Electrostatic adsorption method for preparing electrically conducting ultrahigh molecular weight polyethylene/graphene nanosheets composites with a segregated network. Compos. Sci. Technol. 2013, 89, 180–185. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, Q.; Chen, Z.; Yue, Z. Nanohybrid Shish-Kebabs: Supercritical CO2-Induced PE Epitaxy on Carbon Nanotubes. Macromolecules 2008, 41, 2868–2873. [Google Scholar] [CrossRef]
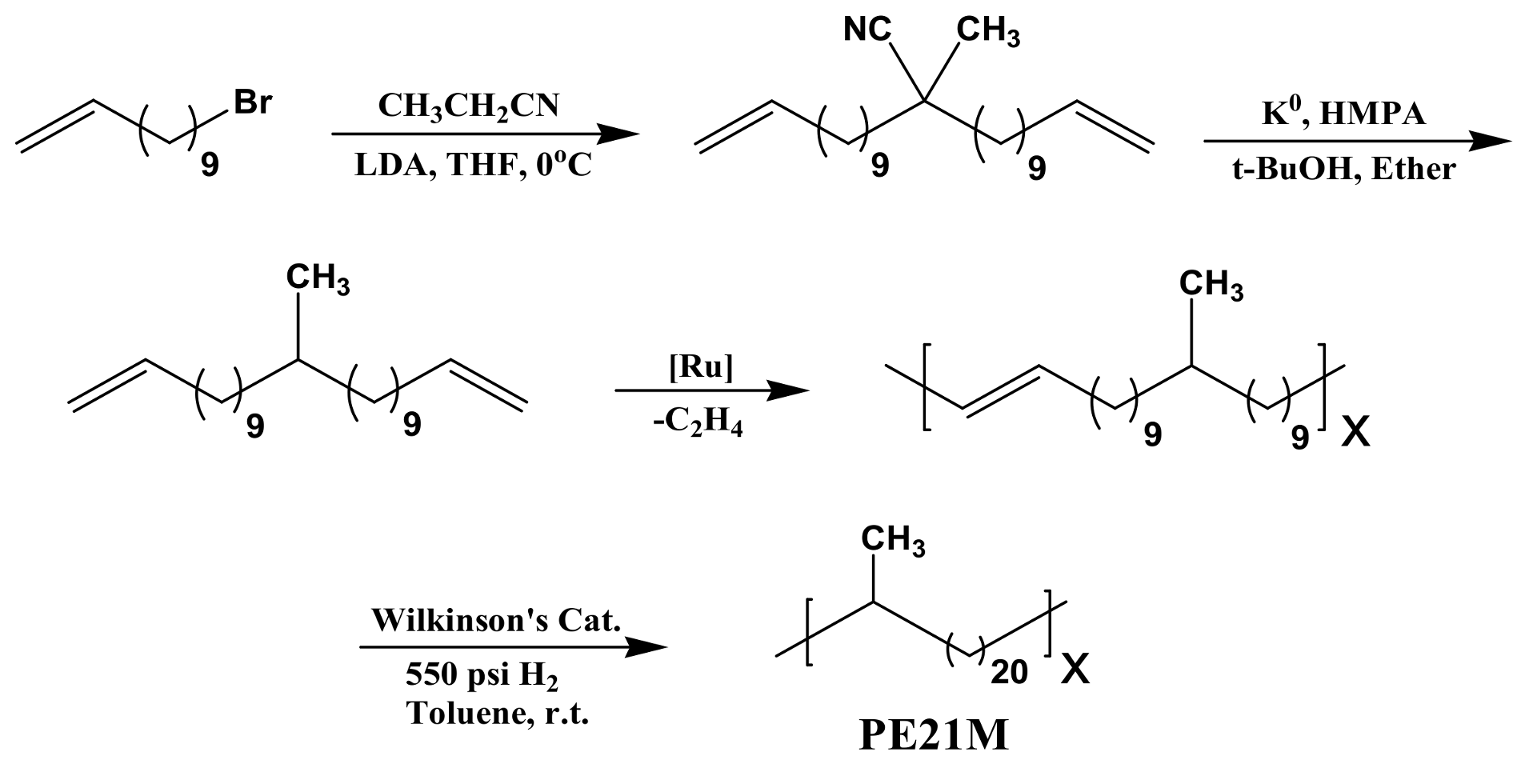
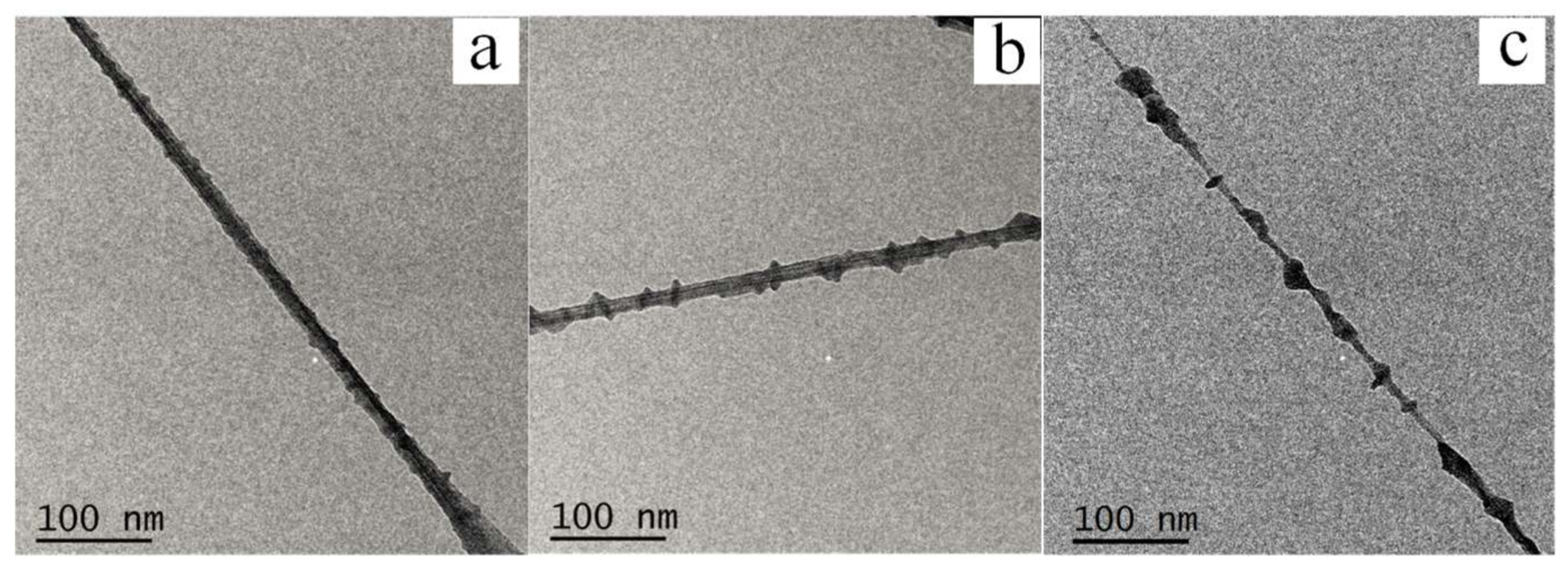
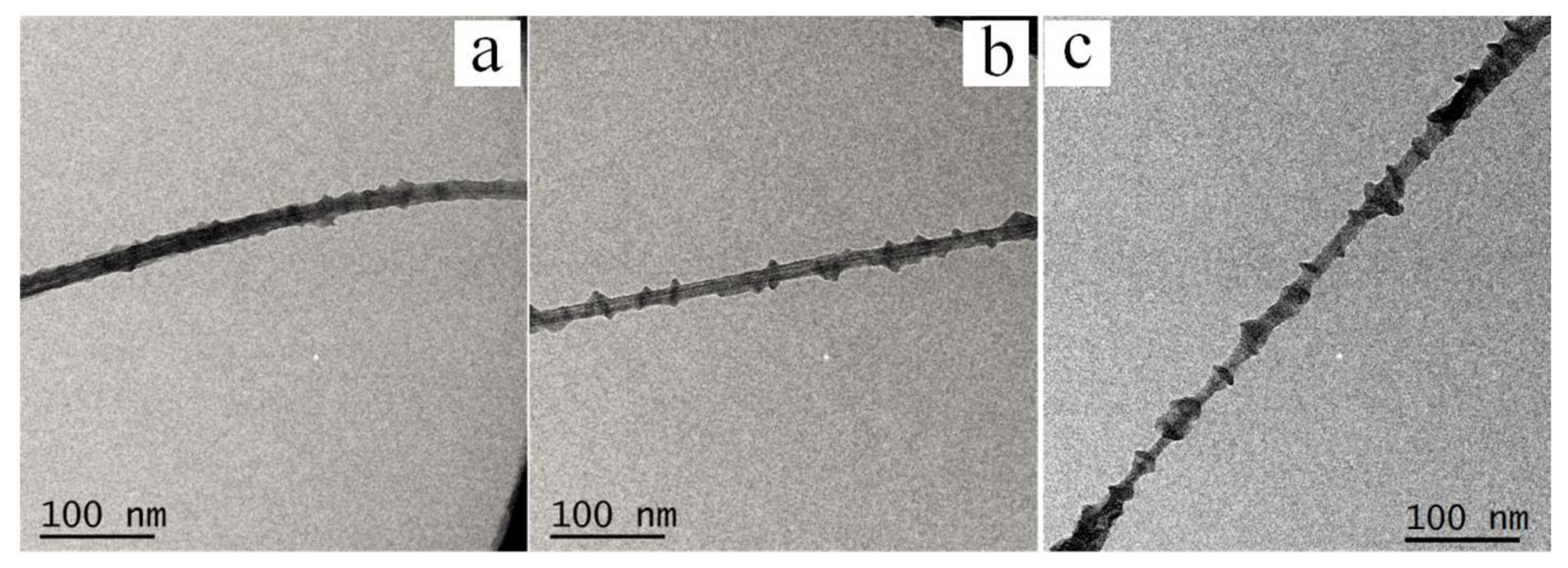



| Sample (Crystallization Temperature) | Diameter of Kebab (nm) | Thickness of Kebab (nm) | Interval of Kebab (nm) |
|---|---|---|---|
| PE21M/SWCNT (40 °C) | 16.3 ± 1.0 | 5.1 ± 0.3 | 10.0 ± 0.8 |
| PE21M/SWCNT (50 °C) | 20.2 ± 1.1 | 5.4 ± 0.4 | 16.3 ± 1.0 |
| PE21M/SWCNT (60 °C) | 18.4 ± 0.9 | 5.6 ± 0.4 | 20.5 ± 1.1 |
| Crystallization Time | Diameter of Kebab (nm) | Thickness of Kebab (nm) | Interval of Kebab (nm) |
|---|---|---|---|
| 3 h | 10.3 ± 0.8 | 5.2 ± 0.3 | 15.8 ± 0.7 |
| 6 h | 20.2 ± 1.1 | 5.4 ± 0.4 | 16.3 ± 1.0 |
| 12 h | 22.4 ± 0.9 | 5.4 ± 0.4 | 15.5 ± 0.8 |
| Sample (Crystallization Temperature) | Size of Lamellae (nm) | Thickness of Lamellae (nm) | Density (number/0.01 μm2) |
|---|---|---|---|
| PE21M/RGO (40 °C) | 5.0 ± 0.2 | 3.0 ± 0.3 | 7.0 |
| PE21M/RGO (50 °C) | 18.0 ± 1.2 | 5.3 ± 0.4 | 8.0 |
| PE21M/RGO (60 °C) | 16.0 ± 1.0 | 5.5 ± 0.3 | 5.0 |
| Sample (Pressure) | Diameter of Kebab (nm) | Thickness of Kebab (nm) | Interval of Kebab (nm) |
|---|---|---|---|
| PE21M/SWCNT (10 MPa) | 23.5 ± 1.0 | 5.4 ± 0.6 | 18.9 ± 0.8 |
| PE21M/SWCNT (15 MPa) | 28.3 ± 1.1 | 5.5 ± 0.5 | 20.6 ± 0.9 |
| PE21M/SWCNT (20 MPa) | 20.5 ± 1.1 | 5.4 ± 0.7 | 16.5 ± 0.8 |
| Sample (Pressure) | Size of Lamellae (nm) | Thickness of Lamellae (nm) | Density (number/0.01 μm2) |
|---|---|---|---|
| PE21M/RGO (10 MPa) | 20.0 ± 1.3 | 5.4 ± 0.6 | 11 |
| PE21M/RGO (15 MPa) | 28.0 ± 1.3 | 5.6 ± 0.4 | 15 |
| PE21M/RGO (20 MPa) | 30.0 ± 1.5 | 5.6 ± 0.6 | 18 |
| Sample | Tm (°C) Peak | Tm (°C) Onset | Onset-End (°C) | ΔH (J/g) |
|---|---|---|---|---|
| PE21M | 62.8 | 58.0 | 6.8 | 18 |
| PE21M/SWCNT | 60.2 | 54.0 | 10.5 | 0.52 |
| PE21M/RGO | 60.5 | 56.2 | 5.7 | 0.16 |
| Sample | Tm (°C) Peak | Tm (°C) Onset | Onset-End (°C) | ΔH (J/g) |
|---|---|---|---|---|
| PE21M | 62.8 | 58.0 | 6.8 | 18 |
| PE21M/SWCNT | 60.5 | 50.7 | 13.5 | 0.71 |
| PE21M/RGO | 60.7 | 52.5 | 12.2 | 0.34 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, W.; Li, Y.; Jiang, L.; Wu, F.; Zhu, H.; Chen, H.; Wang, Z. Epitaxial Crystallization of Precisely Methyl-Substituted Polyethylene Induced by Carbon Nanotubes and Graphene. Crystals 2018, 8, 168. https://doi.org/10.3390/cryst8040168
Miao W, Li Y, Jiang L, Wu F, Zhu H, Chen H, Wang Z. Epitaxial Crystallization of Precisely Methyl-Substituted Polyethylene Induced by Carbon Nanotubes and Graphene. Crystals. 2018; 8(4):168. https://doi.org/10.3390/cryst8040168
Chicago/Turabian StyleMiao, Weijun, Yiguo Li, Libin Jiang, Feng Wu, Hao Zhu, Hongbing Chen, and Zongbao Wang. 2018. "Epitaxial Crystallization of Precisely Methyl-Substituted Polyethylene Induced by Carbon Nanotubes and Graphene" Crystals 8, no. 4: 168. https://doi.org/10.3390/cryst8040168