High-Pressure Elastic, Vibrational and Structural Study of Monazite-Type GdPO4 from Ab Initio Simulations
Abstract
:1. Introduction
2. Simulations Details
3. Results and Discussion
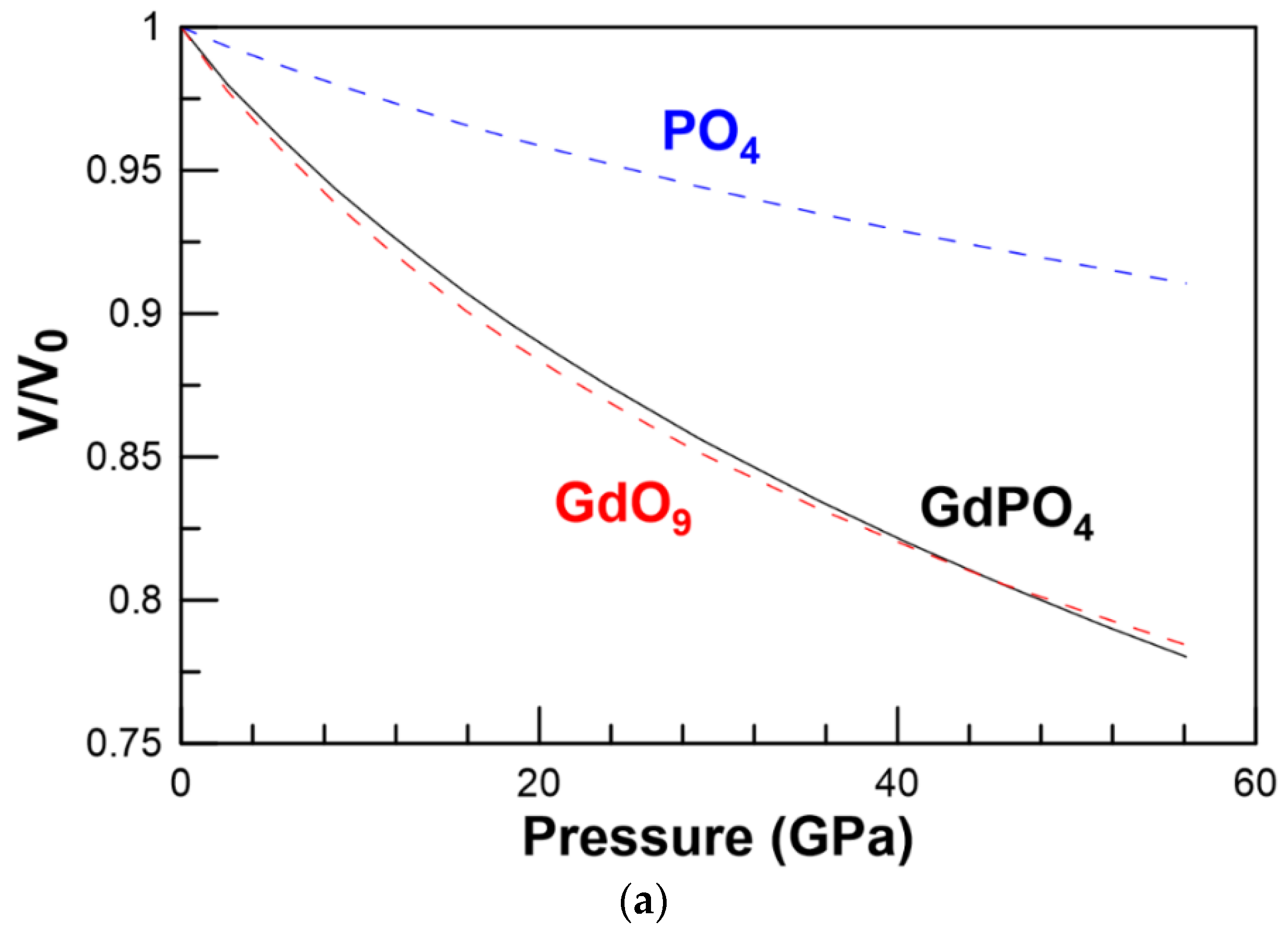
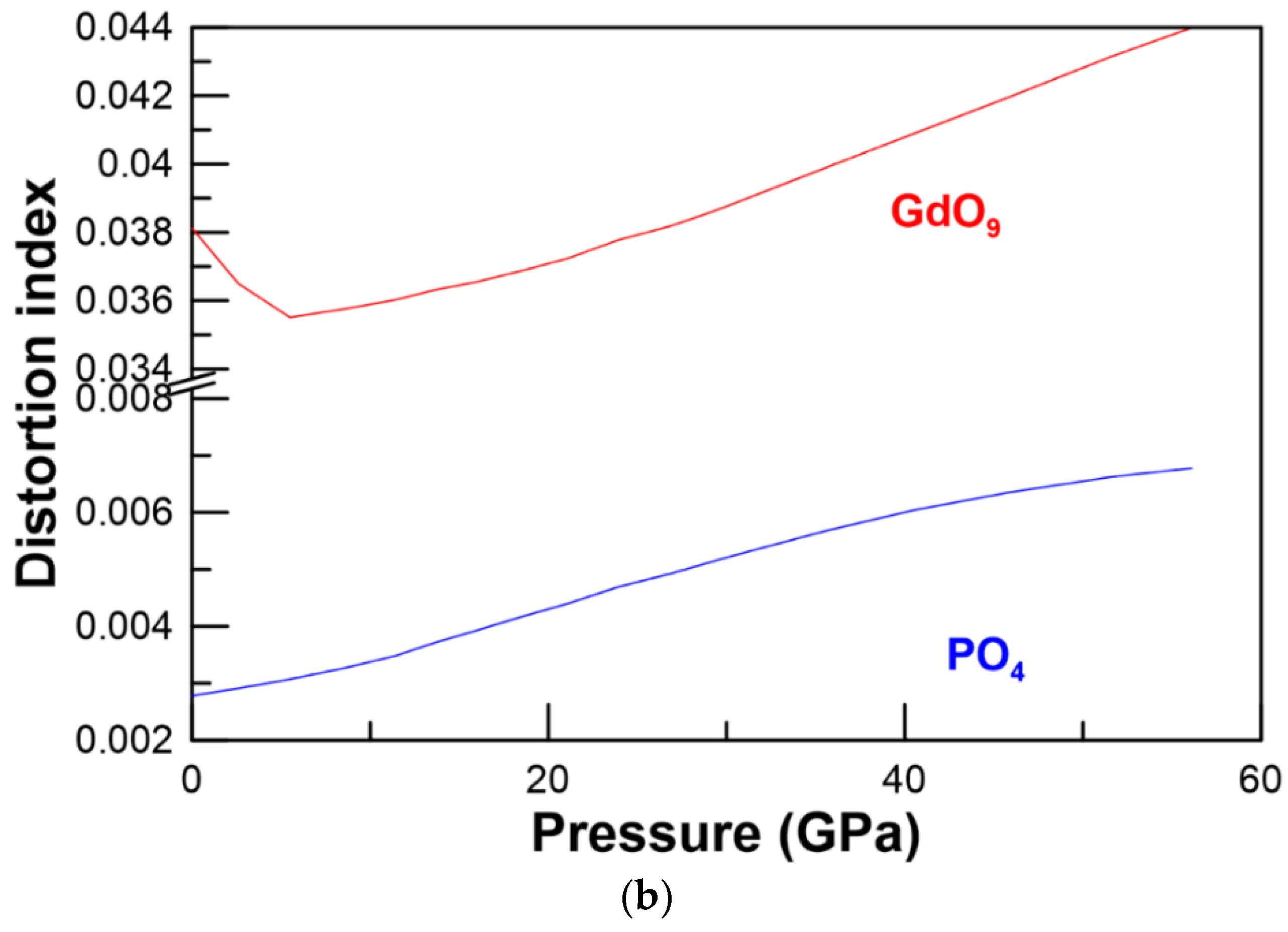
3.1. Structural Properties
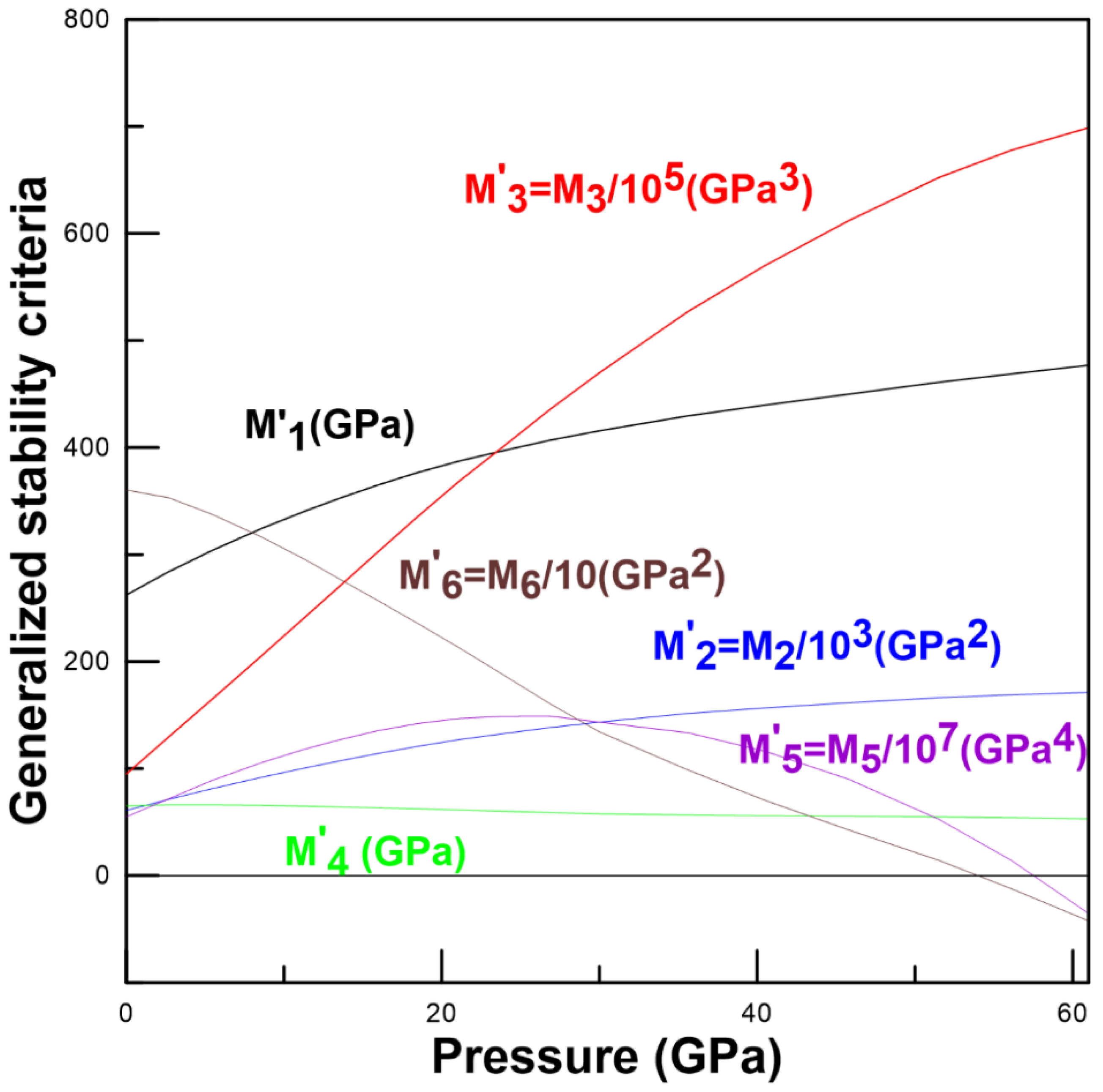
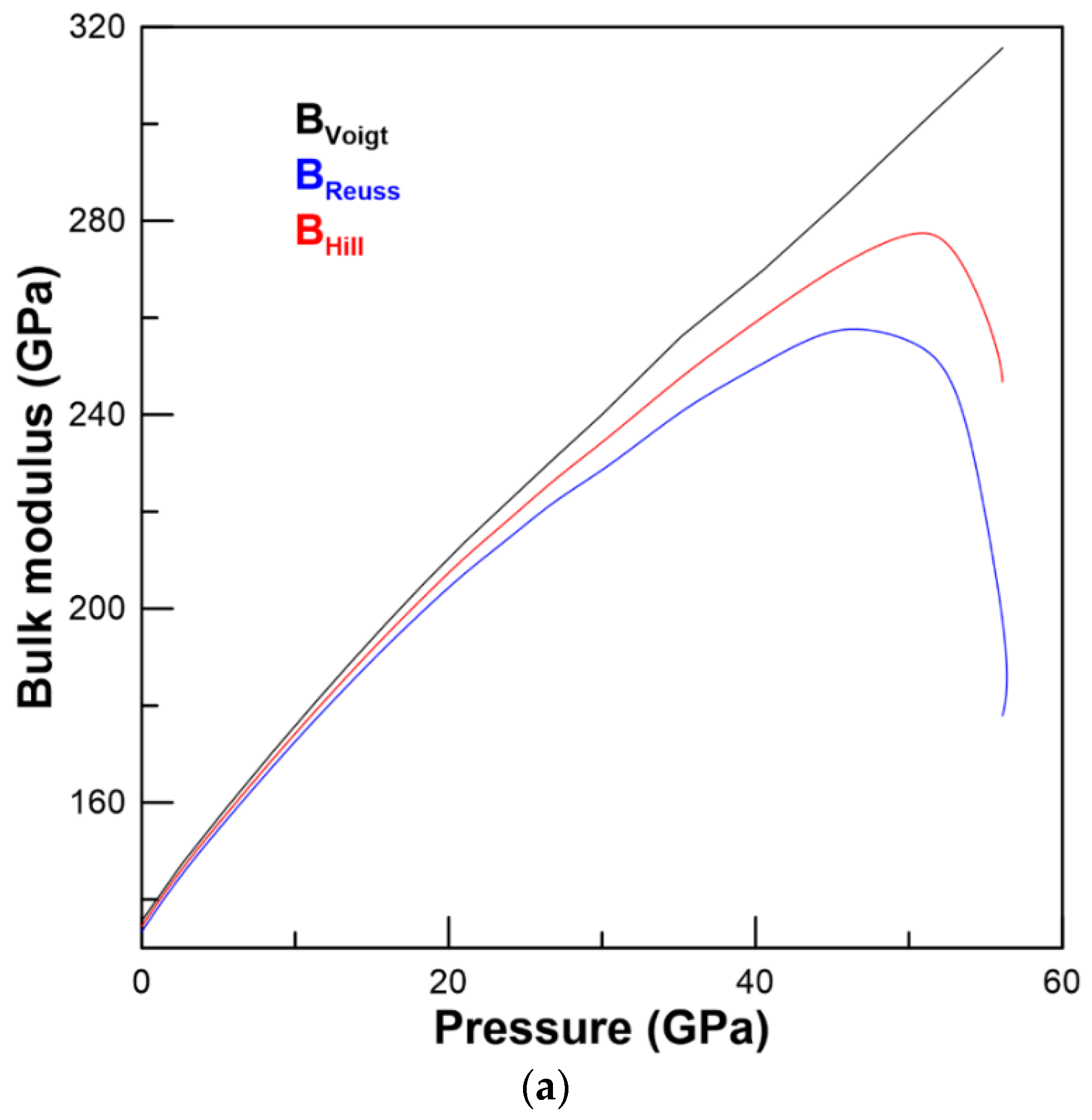
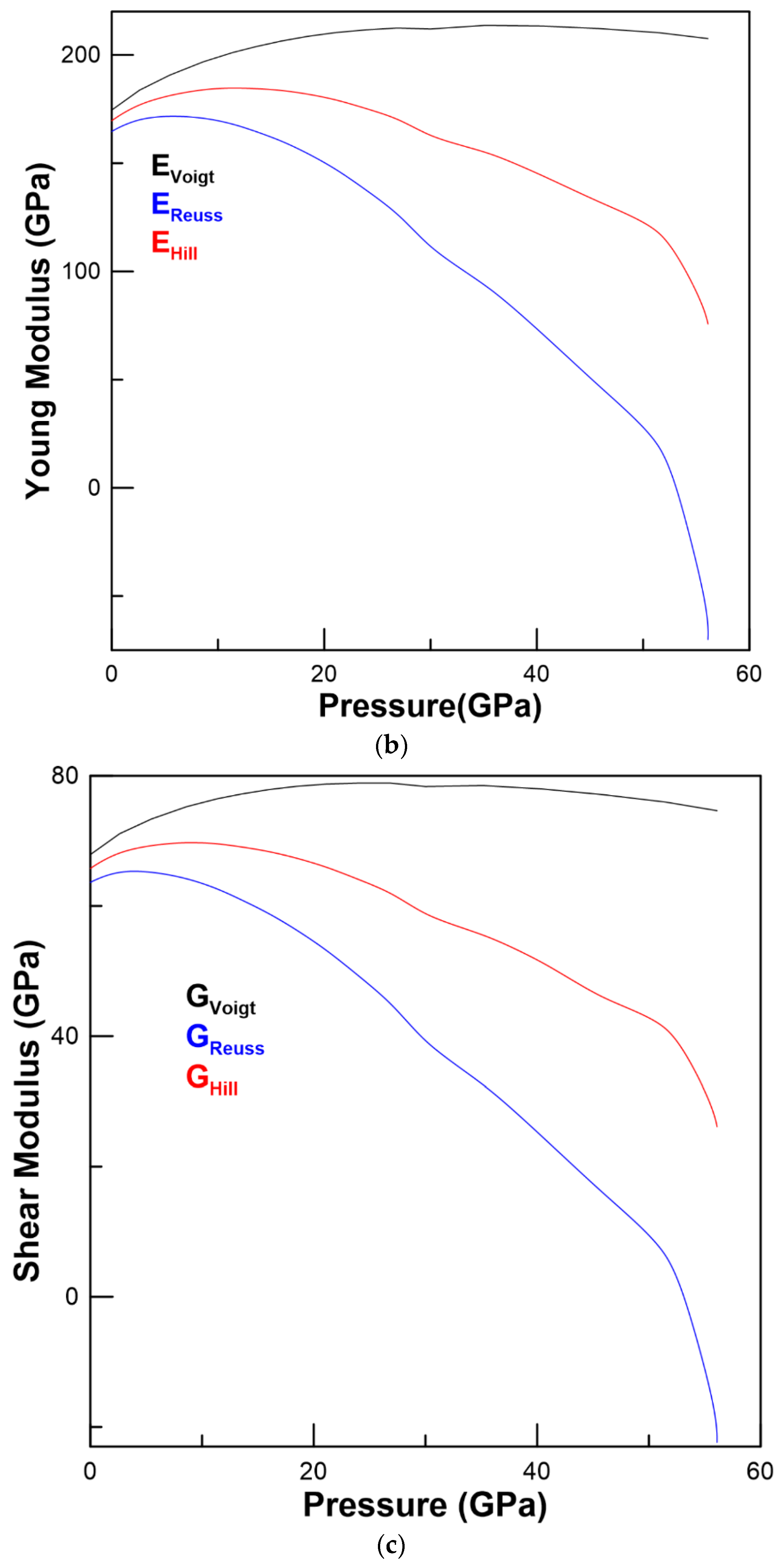
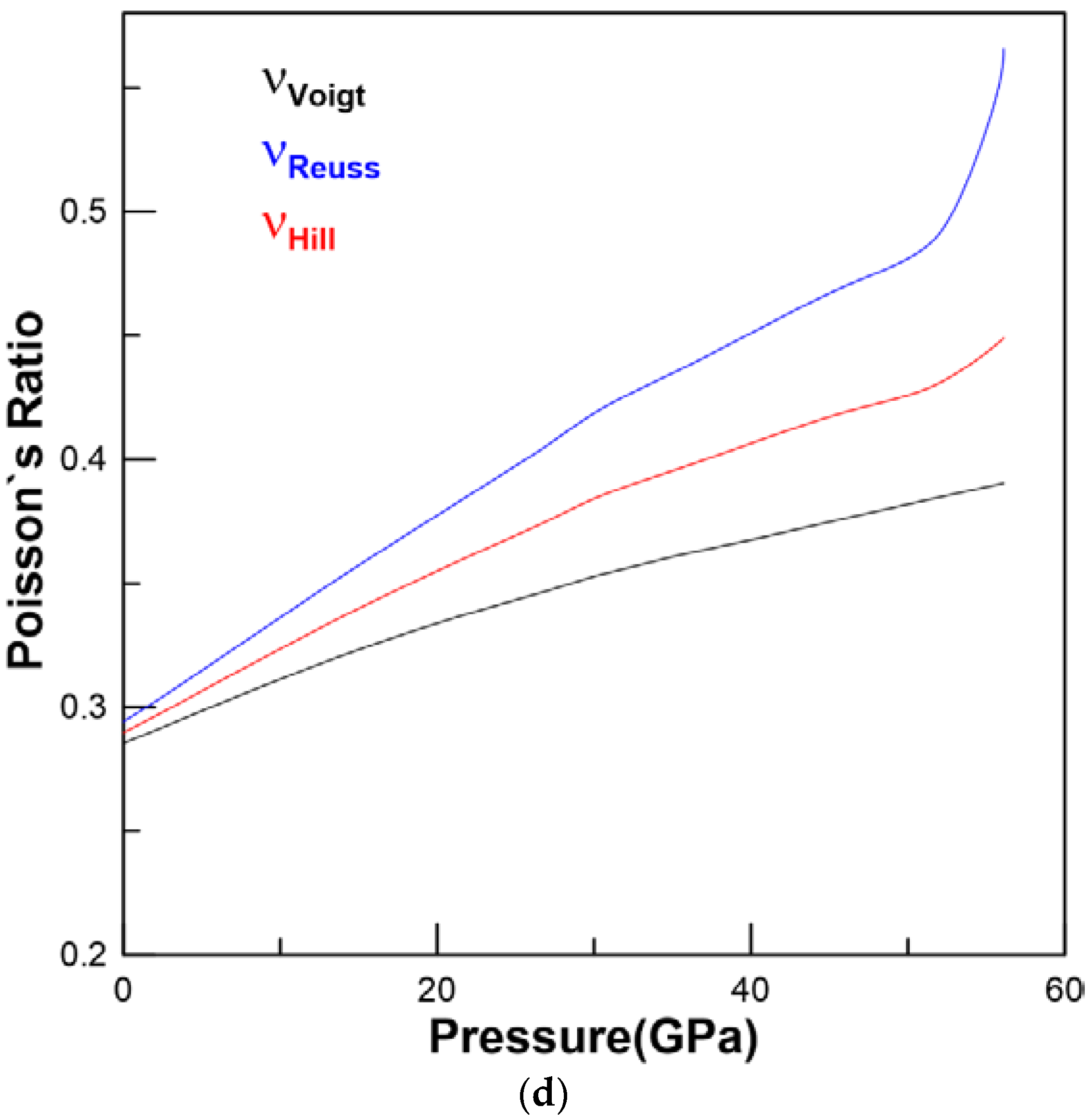
3.2. Elastic Properties
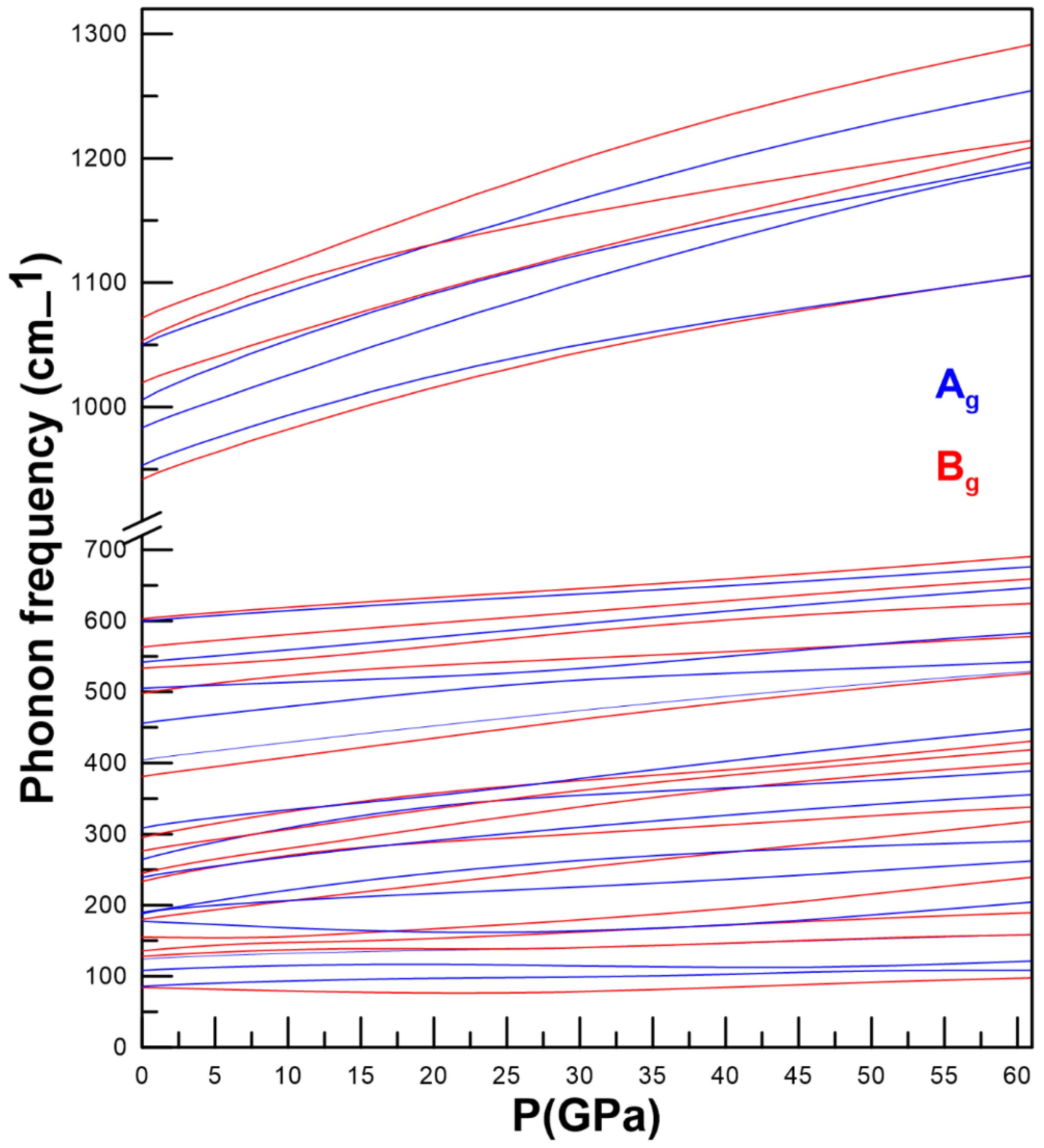
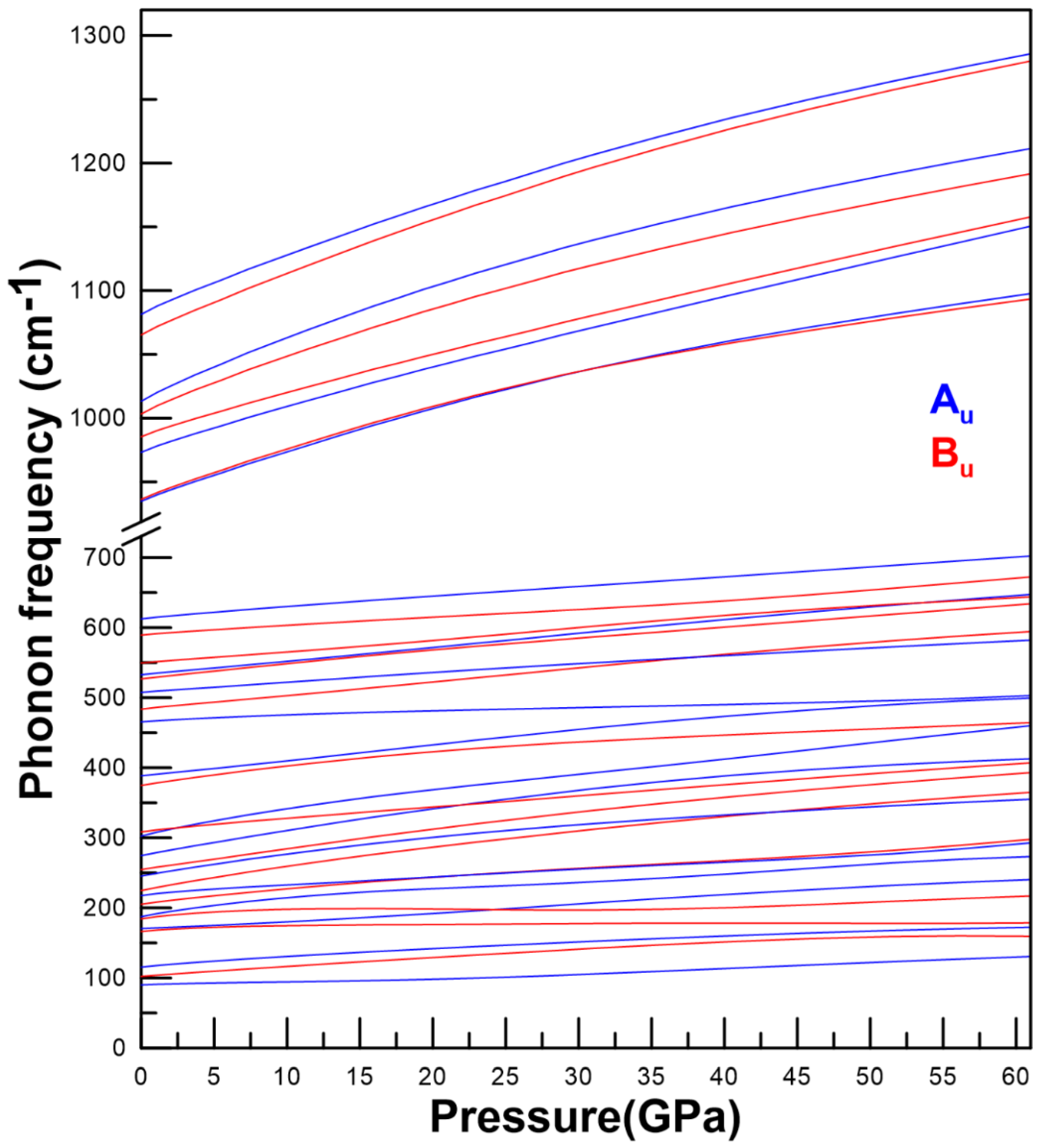
3.3. Vibrational Properties
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ni, Y.X.; Hughes, J.M.; Mariano, A.N. Crystal-chemistry of the monazite and xenotime structures. Am. Mineral. 1995, 80, 21–26. [Google Scholar] [CrossRef]
- Morgan, P.E.D.; Marshall, D.B. Ceramic compounds of monazite and alumina. J. Am. Ceram. Soc. 1995, 78, 1553–1563. [Google Scholar] [CrossRef]
- Kolitsch, U.; Holtstam, D. Crystal chemistry of REEXO4 compounds (X = P, As, V). II. Review of REEXO4 compounds and their stability fields. Eur. J. Mineral. 2004, 16, 117–126. [Google Scholar] [CrossRef]
- Kaminskii, A.A.; Bettinelli, M.; Speghini, A.; Rhee, H.; Eichler, H.J.; Mariotto, G. Tetragonal YPO4—A novel SRS-active crystal. Laser Phys. Lett. 2008, 5, 367–374. [Google Scholar] [CrossRef]
- Clavier, N.; Podor, R.; Dacheux, N. Crystal chemistry of the monazite structure. J. Eur. Ceram. Soc. 2011, 31, 941–976. [Google Scholar] [CrossRef]
- Errandonea, D. High-pressure phase transitions and properties of MTO4 compounds with the monazite-type structure. Phys. Status Solidi B Basic Solid State Phys. 2017, 254, 1700016. [Google Scholar] [CrossRef]
- Rubatto, D.; Hermann, J.; Buick, I.S. Temperature and bulk composition control on the growth of monazite and zircon during low-pressure anataxis (Mount Stafford, central Australia). J. Petrol. 2006, 47, 1973–1996. [Google Scholar] [CrossRef]
- Grove, M.; Harrison, T.M. Monazite Th-Pb age depth profiling. Geology 1999, 27, 487–490. [Google Scholar] [CrossRef]
- Meldrum, A.; Boatner, L.A.; Ewing, R.C. Displacive radiation effects in the monazite- and zircon-structure orthophosphates. Phys. Rev. B 1997, 56, 13805–13814. [Google Scholar] [CrossRef]
- Ewing, R.C. The design and evaluation of nuclear-waste forms: Clues from mineralogy. Can. Mineral. 2001, 39, 697–715. [Google Scholar] [CrossRef]
- Mullica, D.F.; Grossie, D.A.; Boatner, L.A. Coordination geometry and structural determinations of SmPO4, EuPO4 and GdPO4. Inorg. Chim. Acta F 1985, 109, 105–110. [Google Scholar] [CrossRef]
- Neumeier, S.; Arinicheva, Y.; Ji, Y.; Heuser, J.M.; Kowlaski, P.M.; Kegler, P.; Sclenz, H.; Bosbach, D.; Deissmann, G. New insights into phosphate based material for the immobilization of actinides. Radiochim. Acta 2017, 105, 961–984. [Google Scholar]
- Lessing, P.A.; Erickson, A.W. Synthesis and characterization of gadolinium phosphate neutron absorber. J. Eur. Ceram. Soc. 2003, 23, 3049–3057. [Google Scholar] [CrossRef]
- Lacomba-Perales, R.; Errandonea, D.; Meng, Y.; Bettinelli, M. High-pressure stability and compressibility of APO(4) (A = La, Nd, Eu, Gd, Er, and Y) orthophosphates: An x-ray diffraction study using synchrotron radiation. Phys. Rev. B 2010, 81, 064113. [Google Scholar] [CrossRef]
- Errandonea, D.; Gomis, O.; Santamaria-Perez, D.; Garcia-Domene, B.; Munoz, A.; Rodriguez-Hernandez, P.; Achary, S.N.; Tyagi, A.K.; Popescu, C. Exploring the high-pressure behavior of the three known polymorphs of BiPO4: Discovery of a new polymorph. J. Appl. Phys. 2015, 117, 105902. [Google Scholar] [CrossRef]
- Achary, S.N.; Bevara, S.; Tyagi, A.K. Recent progress on synthesis and structural aspects of rare-earth phosphates. Coord. Chem. Rev. 2017, 340, 266–297. [Google Scholar] [CrossRef]
- Errandonea, D.; Gomis, O.; Rodriguez-Hernandez, P.; Munoz, A.; Ruiz-Fuertes, J.; Gupta, M.; Achary, S.N.; Hirsch, A.; Manjon, F.J.; Peters, L.; et al. High-pressure structural and vibrational properties of monazite-type BiPO4, LaPO4, CePO4, and PrPO4. J. Phys. Condens. Matter. 2018, 30, 065401. [Google Scholar] [CrossRef] [PubMed]
- Heffernan Karina, M.; Ross, N.L.; Spencer, E.C.; Boatner, L.A. The structural response of gadolinium phosphate to pressure. J. Solid State Chem. 2016, 241, 180–186. [Google Scholar] [CrossRef]
- Wilkinson, T.M.; Wu, D.; Musselman, M.A.; Li, N.; Mara, N.; Packard, C.E. Mechanical behavior of rare-earth orthophosphates near the monazite/xenotime boundary characterized by nanoindentation. Mater. Sci. Eng. A 2017, 691, 203–210. [Google Scholar] [CrossRef]
- Feng, J.; Xiao, B.; Zhou, R.; Pan, W. Anisotropy in elasticity and thermal conductivity of monazite-type REPO4 (RE = La, Ce, Nd, Sm, Eu and Gd) from first-principles calculations. Acta Mater. 2013, 61, 7364–7383. [Google Scholar] [CrossRef]
- Rustad, J.R. Density functional calculation of enthalpies of formation of rare-earth orthophosphates. Am. Mineral. 2012, 97, 791–799. [Google Scholar] [CrossRef]
- Li, Y.; Kowalski, P.M.; Blanca-Romero, A.; Vinograd, V.; Bosbach, D. Ab initio calculation of excess properties of La1−x (Ln, An)xPO4 solid solutions. J. Sol. State Chem. 2014, 220, 137–141. [Google Scholar] [CrossRef]
- Kowalski, P.M.; Beridze, G.; Vinograd, V.L.; Bosbach, D. Heat capacities of lantanides and actinide monazite-type ceramics. J. Nucl. Mater. 2015, 464, 147–154. [Google Scholar] [CrossRef]
- Kowalski, P.M.; Li, Y. Relationship between the thermodynamic excess properties of mixing and the elastic moduli in the monazite-type ceramics. J. Eur. Ceram. Soc. 2016, 36, 2093–2096. [Google Scholar] [CrossRef]
- Gomis, O.; Lavina, B.; Rodriguez-Hernandez, P.; Munoz, A.; Errandonea, R.; Errandonea, D.; Bettinelli, M. High-pressure structural, elastic, and thermodynamic properties of zircon-type HoPO4 and TmPO4. J. Phys. Condens. Matter. 2017, 29, 095401. [Google Scholar] [CrossRef] [PubMed]
- Errandonea, D.; Pellicer-Porres, J.; Martinez-Garcia, D.; Ruiz-Fuertes, J.; Friedrich, A.; Morgenroth, W.; Popescu, C.; Rodriguez-Hernandez, P.; Munoz, A.; Bettinelli, M. Phase Stability of Lanthanum Orthovanadate at High Pressure. J. Phys. Chem. C 2016, 120, 13749–13762. [Google Scholar] [CrossRef]
- Errandonea, D.; Munoz, A.; Rodriguez-Hernandez, P.; Gomis, O.; Achary, S.N.; Popescu, C.; Patwe, S.J.; Tyagi, A.K. High-Pressure Crystal Structure, Lattice Vibrations, and Band Structure of BiSbO4. Inorg. Chem. 2016, 55, 4958–4969. [Google Scholar] [CrossRef] [PubMed]
- Errandonea, D.; Garg, A.B. Recent progress on the characterization of the high-pressure behaviour of AVO4 orthovanadates. Prog. Mater. Sci. 2018, 97, 123–169. [Google Scholar] [CrossRef]
- Achary, S.N.; Errandonea, D.; Munoz, A.; Rodriguez-Hernandez, P.; Manjon, F.J.; Krishna, P.S.R.; Patwe, S.J.; Grover, V.; Tyagi, A.K. Experimental and theoretical investigations on the polymorphism and metastability of BiPO4. Dalton Trans. 2013, 42, 14999–15015. [Google Scholar] [CrossRef] [PubMed]
- Errandonea, D.; Munoz, A.; Rodriguez-Hernandez, P.; Proctor, J.E.; Sapina, F.; Bettinelli, M. Theoretical and experimental study of the crystal structures, lattice vibrations, and band structures of monazite-type PbCrO4, PbSeO4, SrCrO4, and SrSeO4. Inorg. Chem. 2015, 54, 7524–7535. [Google Scholar] [CrossRef] [PubMed]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. B 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar]
- Kresse, G.; Hafner, J. Ab-Initio molecular-dynamics simulation of the liquid-metal amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics for liquid-metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Gleissner, J.; Errandonea, D.; Segura, A.; Pellicer-Porres, J.; Hakeem, M.A.; Proctor, J.E.; Raju, S.V.; Kumar, R.S.; Rodriguez-Hernandez, P.; Munoz, A.; et al. Monazite-type SrCrO4 under compression. Phys. Rev. B 2016, 94, 134108. [Google Scholar] [CrossRef]
- Perdew, J.P.; Ruzsinszky, A.; Csonka, G.I.; Vydrov, O.A.; Scuseria, G.E.; Constantin Lucian, A.; Zhou, X.; Burke, K. Restoring the density-gradient expansion for exchange in solids and surfaces. Phys. Rev. Lett. 2008, 100, 136406. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Mujica, A.; Rubio, A.; Munoz, A.; Needs, R.J. High-pressure phases of group-IV, III-V, and II-VI compounds. Rev. Mod. Phys. 2013, 75, 863–912. [Google Scholar] [CrossRef]
- Nielsen, O.H.; Martin, R.M. Quantum-mechanical theory of stress and force. Phys. Rev. B 1985, 32, 3780–3791. [Google Scholar] [CrossRef]
- Chetty, N.; Munoz, A.; Martin, R.M. 1st-principles calculation of the elastic-constants of AlAs. Phys. Rev. B 1989, 40, 11934–11936. [Google Scholar] [CrossRef]
- Le Page, Y.; Saxe, P. Symmetry-general least-squares extraction of elastic data for strained materials from ab initio calculations of stress. Phys. Rev. B 2002, 65, 104104. [Google Scholar] [CrossRef]
- Parlinski, K. Computer Code Phonon. Available online: http://www.computingformaterials.com/ (accessed on 14 July 2014).
- Blanca-Romero, A.; Kowalski, P.M.; Beridze, G.; Schlenz, H.; Bosbach, D. Performance of DFT plus U Method for Prediction of Structural and Thermodynamic Parameters of Monazite-Type Ceramics. J. Comput. Chem. 2014, 35, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Birch, F. Finite elastic strain of cubic crystals. Phys. Rev. 1947, 71, 809–824. [Google Scholar] [CrossRef]
- Du, A.; Wan, C.; Qu, Z.; Pan, W. Thermal Conductivity of Monazite-Type REPO4 (RE = La, Ce, Nd, Sm, Eu, Gd). J. Am. Ceram. Soc. 2009, 92, 2687–2692. [Google Scholar] [CrossRef]
- Li, H.; Zhang, S.; Zhou, S.; Cao, X. Bonding Characteristics, Thermal Expansibility, and Compressibility of RXO4 (R = Rare Earths, X = P, As) within Monazite and Zircon Structures. Inorg. Chem. 2009, 48, 4542–4548. [Google Scholar] [CrossRef] [PubMed]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Nye, J.F. Physical Properties of Crystals. Their Representation by Tensor and Matrices; Oxford University Press: Oxford, UK, 1957. [Google Scholar]
- Wallace, D.C. Thermodynamics of Crystals; Dover Publications: New York, NY, USA, 1998. [Google Scholar]
- Grimvall, G.; Magyari-Koepe, B.; Ozolins, V.; Persson, K.A. Lattice instabilities in metallic elements. Rev. Mod. Phys. 2012, 84, 945–986. [Google Scholar] [CrossRef]
- Born, M.; Huang, K. Dynamical Theory of Crystal Lattices; Clarendon Press: London, UK, 1954. [Google Scholar]
- Wallace, D.C. Thermoelasticity of stressed materials and comparison of various elastic constants. Phys. Rev. 1967, 162, 776–789. [Google Scholar] [CrossRef]
- Voigt, W. Lehrbuch der Kristallphysik (mit Ausschluss der Kristalloptik); B.G. Teubner: Leipzig/Berlin, Germany, 1928. [Google Scholar]
- Reuss, A. Berechnung der Fließgrenze von Mischkristallen auf Grund der Plastizitätsbedingung für Einkristalle. J. Appl. Math. Mech. 1929, 9, 49–58. [Google Scholar] [CrossRef]
- Hill, R. The elastic behaviour of a crystalline aggregate. Proc. Phys. Soc. Lond. 1952, 65, 349–355. [Google Scholar] [CrossRef]
- Zhao, X.S.; Shang, S.L.; Liu, Z.K.; Shen, J.Y. Elastic properties of cubic, tetragonal and monoclinic ZrO2 from first-principles calculations. J. Nucl. Mater. 2011, 415, 13–17. [Google Scholar] [CrossRef]
- Caracas, R.; Ballaran, T.B. Elasticity of (K, Na)AlSi3O8 hollandite from lattice dynamic calculations. Phys. Earth Planet. Inter. 2010, 181, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Brazhkin, V.V.; Lyapin, A.G.; Hemley, R.J. Harder than diamond: Dreams and reality. Philos. Mag. A 2002, 82, 231–253. [Google Scholar] [CrossRef]
- Greaves, G.N.; Greer, A.L.; Lakes, R.S.; Rouxel, T. Poisson’s ratio and modern materials. Nat. Mater. 2011, 10, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Pugh, S.F. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Philos. Mag. 1954, 45, 823–843. [Google Scholar] [CrossRef]
- Silva, E.N.; Ayala, A.P.; Guedes, I.; Paschoal, C.W.A.; Moreira, R.L.; Loong, C.K.; Boatner, L.A. Vibrational spectra of monazite-type rare-earth orthophosphates. Opt. Mater. 2006, 29, 224–230. [Google Scholar] [CrossRef]
- Begun, G.M.; Beall, G.W.; Boatner, L.A.; Gregor, W.J. Raman-spectra of the rare-earth ortho-phosphates. J. RAMAN Spectrosc. 1981, 11, 273–278. [Google Scholar] [CrossRef]
- Ruschel, K.; Nasdala, L.; Kronz, A.; Hanchar, J.M.; Toebbens, D.M.; Skoda, R.; Finger, F.; Moeller, A. A Raman spectroscopic study on the structural disorder of monazite-(Ce). Mineral. Petrol. 2012, 105, 41–55. [Google Scholar] [CrossRef]
- Huang, T.; Lee, J.S.; Kung, J.; Lin, C.M. Study of monazite under high pressure. Solid State Commun. 2010, 150, 1845–1850. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, X.; Zuo, J.A.; Ding, Z.J. Pressure effect on optical properties and structure stability of LaPO4:Eu3+ Microspheres. J. Nanosci. Nanotechnol. 2010, 10, 7791–7794. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zuo, J.A.; Ding, Z.J. Pressure effect on optical properties and structure stability of LaPO4:Eu3+ hollow spheres. J. Rare Earths 2010, 28, 254–257. [Google Scholar] [CrossRef]
- Grüneisen, E. Concerning the thermic expansion of metals. Ann. Phys. 1910, 33, 33–64. [Google Scholar] [CrossRef]
- Ruiz-Fuertes, J.; Errandonea, D.; Lopez-Moreno, S.; Gonzalez, J.; Gomis, O.; Vilaplana, R.; Manjon, F.J.; Munoz, A.; Rodriguez-Hernandez, P.; Friedrich, A.; et al. High-pressure Raman spectroscopy and lattice-dynamics calculations on scintillating MgWO4: Comparison with isomorphic compounds. Phys. Rev. B 2011, 83, 214112. [Google Scholar] [CrossRef]






| This Study | Experiments | Theory | |
|---|---|---|---|
| a (Å) | 6.6276 | 6.623 a 6.6516(3) b 6.62(2) c | 6.4152 d 6.713 e |
| b (Å) | 6.8145 | 6.829 a 6.84840(7) b 6.823(2) c | 6.6103 d 6.887 e |
| c (Å) | 6.2930 | 6.335 a 6.33571(12) b 6.319(2) c | 6.0953 d 6.358 e |
| β (°) | 104.18° | 103.80 a 104.023(2) b 104.16(2) c | 104.6 d 104.2 e |
| Atom | Site | x | y | z |
|---|---|---|---|---|
| Gd | 4e | 0.28394 | 0.14513 | 0.08817 |
| P | 4e | 0.29933 | 0.16215 | 0.61291 |
| O1 | 4e | 0.2510 | 0.0000 | 0.4330 |
| O2 | 4e | 0.3850 | 0.3381 | 0.5017 |
| O3 | 4e | 0.4735 | 0.1010 | 0.8184 |
| O4 | 4e | 0.1182 | 0.2100 | 0.7148 |
| This Work | Experiments | Theory | |
|---|---|---|---|
| V (Å3) | 275.55 | 279.1 a 280.008(4) b 276.4(4) c | 250.20 e 284.96 f |
| B0 (GPa) | 138.3 | 160 a 128.1(8) b 137 d | 149 g 121.0 h |
| B′0 | 4.07 | 5.8(2) b |
| C11 | C22 | C33 | C44 | C55 | C66 | C12 | C13 | C15 | C23 | C25 | C25 | C46 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 262.19 | 251.61 | 205.01 | 65.19 | 60.32 | 58.16 | 71.41 | 90.99 | 5.28 | 88.50 | −18.17 | −18.17 | −13.70 |
| BH (GPa) | EH (GPa) | GH (GPa) | BH/GH | ν |
|---|---|---|---|---|
| 134.47 a | 169.62 a | 65.575 a | 2.05 a | 0.289 a |
| 137 b | 172 b | 67 b | 2.045 b | 0.290 b |
| 121.0 c | 165.2 c | 64.9 c | 1.86 c |
| Mode | (cm−1) | (cm−1/GPa) | (cm−1/GPa2) | γ |
|---|---|---|---|---|
| Bg | 84.78 | 0.762 | 0.0180 | 1.24 |
| Ag | 86.33 | 0.807 | 0.0128 | 1.29 |
| Ag | 108.61 | 0.846 | 0.0216 | 1.07 |
| Ag | 124.99 | 0.765 | 0.008 | 0.84 |
| Bg | 129.32 | 0.830 | 0.016 | 0.88 |
| Bg | 137.84 | 0.852 | 0.002 | 0.85 |
| Bg | 153.93 | 0.059 | 0.027 | 0.05 |
| Ag | 178.30 | 1.354 | 0.029 | 1.05 |
| Bg | 180.54 | 2.610 | 0.007 | 1.99 |
| Ag | 190.93 | 1.648 | 0.016 | 1.19 |
| Ag | 189.67 | 3.477 | 0.0343 | 2.53 |
| Bg | 235.29 | 3.887 | 0.057 | 2.28 |
| Ag | 240.14 | 3.014 | 0.0233 | 1.736 |
| Bg | 246.86 | 3.411 | 0.012 | 1.911 |
| Ag | 264.93 | 5.089 | 0.069 | 2.656 |
| Bg | 276.56 | 3.176 | 0.011 | 1.588 |
| Bg | 295.93 | 3.982 | 0.044 | 1.861 |
| Ag | 310.90 | 2.209 | 0.000 | 0.982 |
| Bg | 381.29 | 2.722 | 0.001 | 0.987 |
| Ag | 404.71 | 2.524 | 0.007 | 0.862 |
| Ag | 455.84 | 2.592 | 0.018 | 0.786 |
| Bg | 499.31 | 2.653 | 0.035 | 0.734 |
| Ag | 506.02 | 0.589 | 0.010 | 0.161 |
| Bg | 533.07 | 1.190 | 0.017 | 0.308 |
| Ag | 542.17 | 1.677 | 0.003 | 0.427 |
| Bg | 563.55 | 1.750 | 0.004 | 0.429 |
| Ag | 600.11 | 1.482 | 0.007 | 0.341 |
| Bg | 603.45 | 1.624 | 0.007 | 0.372 |
| Bg | 942.72 | 4.215 | 0.028 | 0.618 |
| Ag | 954.05 | 4.277 | 0.036 | 0.620 |
| A | 984.01 | 4.300 | 0.013 | 0.604 |
| Ag | 1007.23 | 5.005 | 0.039 | 0.687 |
| Bg | 1020.45 | 3.972 | 0.016 | 0.538 |
| A | 1051.30 | 4.269 | 0.014 | 0.561 |
| Bg | 1055.21 | 4.837 | 0.050 | 0.633 |
| Bg | 1072.33 | 4.480 | 0.008 | 0.577 |
| Mode | (cm−1) | (cm−1/GPa) | (cm−1/GPa2) | γ |
|---|---|---|---|---|
| Au | 91.05 | 0.219 | 0.007 | 0.332 |
| Bu | 102.50 | 1.442 | 0.005 | 1.946 |
| Au | 116.32 | 1.541 | 0.0125 | 1.832 |
| Bu | 168.18 | 0.722 | 0.013 | 0.594 |
| Au | 170.55 | 0.866 | 0.010 | 0.702 |
| Bu | 187.21 | 1.270 | 0.032 | 0.938 |
| Au | 189.765 | 2.761 | 0.041 | 2.012 |
| Bu | 205.99 | 2.328 | 0.021 | 1.563 |
| Au | 219.28 | 1.386 | 0.006 | 0.874 |
| Bu | 225.70 | 3.603 | 0.026 | 2.207 |
| Au | 245.96 | 3.397 | 0.032 | 1.910 |
| Bu | 254.49 | 3.153 | 0.013 | 1.713 |
| Au | 274.99 | 3.789 | 0.0231 | 1.905 |
| Au | 304.38 | 4.034 | 0.039 | 1.832 |
| Bu | 309.61 | 1.867 | 0.006 | 0.834 |
| Bu | 375.28 | 3.061 | 0.033 | 1.128 |
| Au | 388.32 | 2.186 | 0.0011 | 0.778 |
| Au | 466.32 | 1.013 | 0.0122 | 0.300 |
| Bu | 484.23 | 1.851 | 0.003 | 0.528 |
| Au | 507.73 | 1.501 | 0.0044 | 0.408 |
| Bu | 526.89 | 2.3165 | 0.0124 | 0.608 |
| Au | 533.09 | 1.864 | 0.0033 | 0.483 |
| Bu | 550.13 | 1.428 | 0.007 | 0.359 |
| Bu | 590.07 | 1.384 | 0.006 | 0.324 |
| Au | 613.26 | 1.765 | 0.008 | 0.398 |
| Au | 935.32 | 4.083 | 0.023 | 0.603 |
| Bu | 936.86 | 4.205 | 0.029 | 0.620 |
| Au | 974.23 | 3.643 | 0.017 | 0.517 |
| Bu | 986.50 | 3.483 | 0.014 | 0.488 |
| Bu | 1004.66 | 4.674 | 0.030 | 0.643 |
| Au | 1014.56 | 5.209 | 0.038 | 0.710 |
| Bu | 1066.28 | 4.956 | 0.024 | 0.642 |
| Au | 1082.61 | 4.763 | 0.024 | 0.608 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz, A.; Rodríguez-Hernández, P. High-Pressure Elastic, Vibrational and Structural Study of Monazite-Type GdPO4 from Ab Initio Simulations. Crystals 2018, 8, 209. https://doi.org/10.3390/cryst8050209
Muñoz A, Rodríguez-Hernández P. High-Pressure Elastic, Vibrational and Structural Study of Monazite-Type GdPO4 from Ab Initio Simulations. Crystals. 2018; 8(5):209. https://doi.org/10.3390/cryst8050209
Chicago/Turabian StyleMuñoz, Alfonso, and Placida Rodríguez-Hernández. 2018. "High-Pressure Elastic, Vibrational and Structural Study of Monazite-Type GdPO4 from Ab Initio Simulations" Crystals 8, no. 5: 209. https://doi.org/10.3390/cryst8050209





