Twin Domains in Organometallic Halide Perovskite Thin-Films
Abstract
:1. Introduction
2. Twin Domains in Inorganic Perovskite BaTiO3
3. Subgrain Twin Domains Observed in MAPbI3
- According to the classical understanding, twin domains in ferroelastic or ferroelectric materials originate from the minimum energy evolution of the grain, with the expense of domain wall energy [16]. Therefore, the occurrence of twin domain related to the grain size g. A semi-quantitative model based on typical BaTiO3 twin domains revealed that the total elastic energy due to homogenous strain of phase transformation increased with g3 while the domain wall energy increased with g2. Research on BaTiO3 ceramics concluded that the domain width obeyed the g1/2 rule while g > 1 μm and the domain structure would no longer be unique with g < 1 μm [16]. Small twin structure of ~30 nm are observed in small grains of 300 nm [16].
- In ferroic material rhombohedral perovskite LSMO, it was revealed that the domain period followed the t1/2 rule in ultrathin films which implied high density of twin patterns at small film thickness and was, in turn, more apparent [74].
4. Effect of the Subgrain Microstructure on Perovskite Solar Cells
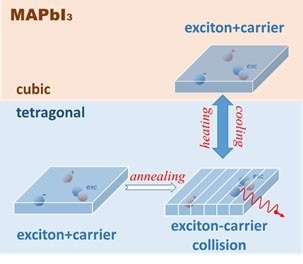
5. Optical Method to Observe the Broad Existence of Subgrain Twin Domains
6. Conclusions and Outlook
Acknowledgments
Conflicts of Interest
References
- Perovskite Mineral Data. Available online: http://webmineral.com/data/Perovskite.shtml#.Wqd1auhubIU (accessed on 13 March 2018).
- Bhalla, A.S.; Guo, R.; Roy, R. The perovskite structure—A review of its role in ceramic science and technology. Mater. Res. Innov. 2000, 4, 3–26. [Google Scholar] [CrossRef]
- Kim, H.-S.; Im, S.H.; Park, N.-G. Organolead Halide Perovskite: New Horizons in Solar Cell Research. J. Phys. Chem. C 2014, 118, 5615–5625. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Efficiency-Chart. Available online: Https://www.nrel.gov/pv/assets/images/efficiency-chart.png (accessed on 10 March 2018).
- Liu, P.; He, X.; Ren, J.; Liao, Q.; Yao, J.; Fu, H. Organic–Inorganic Hybrid Perovskite Nanowire Laser Arrays. ACS Nano 2017, 11, 5766–5773. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Fu, Y.; Meng, F.; Wu, X.; Gong, Z.; Ding, Q.; Gustafsson, M.V.; Trinh, M.T.; Jin, S.; Zhu, X.-Y. Lead halide perovskite nanowire lasers with low lasing thresholds and high quality factors. Nat. Mater. 2015, 14, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Dong, H.; Hu, W. Green light-emitting diode from bromine based organic-inorganic halide perovskite. Sci. China Mater. 2015, 58, 186–191. [Google Scholar] [CrossRef]
- Wang, N.; Cheng, L.; Ge, R.; Zhang, S.; Miao, Y.; Zou, W.; Yi, C.; Sun, Y.; Cao, Y.; Yang, R.; et al. Perovskite light-emitting diodes based on solution-processed self-organized multiple quantum wells. Nat. Photonics 2016, 10, 699–704. [Google Scholar] [CrossRef]
- Dou, L.; Yang, Y.; You, J.; Hong, Z.; Chang, W.-H.; Li, G.; Yang, Y. Solution-processed hybrid perovskite photodetectors with high detectivity. Nat. Commun. 2014, 5, 5404. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Bertolotti, F.; Protesescu, L.; Kovalenko, M.V.; Yakunin, S.; Cervellino, A.; Billinge, S.J.L.; Terban, M.W.; Pedersen, J.S.; Masciocchi, N.; Guagliardi, A. Coherent Nanotwins and Dynamic Disorder in Cesium Lead Halide Perovskite Nanocrystals. ACS Nano 2017, 11, 3819–3831. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.T.; Weber, O.J.; Henry, P.F.; Pumpo, A.M.D.; Hansen, T.C. Complete structure and cation orientation in the perovskite photovoltaic methylammonium lead iodide between 100 and 352 K. Chem. Commun. 2015, 51, 4180–4183. [Google Scholar] [CrossRef] [PubMed]
- Poglitsch, A.; Weber, D. Dynamic Disorder in Methylammoniumtrihalogenoplumbates(II) Observed by Millimeter-wave Spectroscopy. J. Chem. Phys. 1987, 87, 6373–6378. [Google Scholar] [CrossRef]
- Arlt, G. Twinning in ferroelectric and ferroelastic ceramics: Stress relief. J. Mater. Sci. 1990, 25, 2655–2666. [Google Scholar] [CrossRef]
- Snaith, H.J.; Abate, A.; Ball, J.M.; Eperon, G.E.; Leijtens, T.; Noel, N.K.; Stranks, S.D.; Wang, J.T.-W.; Wojciechowski, K.; Zhang, W. Anomalous Hysteresis in Perovskite Solar Cells. J. Phys. Chem. Lett. 2014, 5, 1511–1515. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-W.; Sakai, N.; Ikegami, M.; Miyasaka, T. Emergence of Hysteresis and Transient Ferroelectric Response in Organo-Lead Halide Perovskite Solar Cells. J. Phys. Chem. Lett. 2015, 6, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Frost, J.M.; Butler, K.T.; Walsh, A. Molecular ferroelectric contributions to anomalous hysteresis in hybrid perovskite solar cells. APL Mater. 2014, 2, 081506. [Google Scholar] [CrossRef] [Green Version]
- Frost, J.M.; Butler, K.T.; Brivio, F.; Hendon, C.H.; van Schilfgaarde, M.; Walsh, A. Atomistic Origins of High-Performance in Hybrid Halide Perovskite Solar Cells. Nano Lett. 2014, 14, 2584–2590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothmann, M.U.; Li, W.; Zhu, Y.; Bach, U.; Spiccia, L.; Etheridge, J.; Cheng, Y.-B. Direct observation of intrinsic twin domains in tetragonal CH3NH3PbI3. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Janovec, V.; Hahn, T.; Klapper, H. Twinning and domain structures. In International Tables for Crystallography Volume D: Physical Properties of Crystals; International Tables for Crystallography; Springer: Dordrecht, The Netherlands, 2006; pp. 377–392. ISBN 978-1-4020-0714-9. [Google Scholar]
- Hahn, T.; Klapper, H. Twinning of crystals. In International Tables for Crystallography Volume D: Physical Properties of Crystals; Springer: Dordrecht, The Netherlands, 2006; pp. 393–448. [Google Scholar]
- Buerger, M.J. The genesis of twin crystals. Am. Mineral. 1945, 30, 469–482. [Google Scholar]
- Nespolo, M.; Ferraris, G. The oriented attachment mechanism in the formation of twins-a survey. Eur. J. Mineral. 2004, 16, 401–406. [Google Scholar] [CrossRef]
- Kotrbová, M.; Kadečková, S.; Novák, J.; Brádler, J.; Smirnov, G.V.; Shvydko, Y.V. Growth and perfection of flux grown FeBO3 and 57FeBO3 crystals. J. Cryst. Growth 1985, 71, 607–614. [Google Scholar] [CrossRef]
- Ng, H.N.; Calvo, C. Refinement of the Crystal Structure of the Low-quartz Modification of Ferric Phosphate. Can. J. Chem. 1975, 53, 2064–2067. [Google Scholar] [CrossRef]
- Engel, G.; Klapper, H.; Krempl, P.; Mang, H. Growth twinning in quartz-homeotypic gallium orthophosphate crystals. J. Cryst. Growth 1989, 94, 597–606. [Google Scholar] [CrossRef]
- Fang, X.-S.; Ye, C.-H.; Zhang, L.-D.; Xie, T. Twinning-Mediated Growth of Al2O3 Nanobelts and Their Enhanced Dielectric Responses. Adv. Mater. 2005, 17, 1661–1665. [Google Scholar] [CrossRef]
- Song, S.G.; Gray, G.T. Structural interpretation of the nucleation and growth of deformation twins in Zr and Ti—II. Tem study of twin morphology and defect reactions during twinning. Acta Metall. Mater. 1995, 43, 2339–2350. [Google Scholar] [CrossRef]
- Clayton, J.D. Mechanical Twinning in Crystal Plasticity. In Nonlinear Mechanics of Crystals; Solid Mechanics and Its Applications; Springer: Dordrecht, The Netherlands, 2011; pp. 379–421. ISBN 978-94-007-0349-0. [Google Scholar]
- Van Houtte, P. Simulation of the rolling and shear texture of brass by the Taylor theory adapted for mechanical twinning. Acta Metall. 1978, 26, 591–604. [Google Scholar] [CrossRef]
- Hanada, S.; Izumi, O. Transmission electron microscopic observations of mechanical twinning in metastable beta titanium alloys. Metall. Trans. A 1986, 17, 1409–1420. [Google Scholar] [CrossRef]
- Allain, S.; Chateau, J.-P.; Dahmoun, D.; Bouaziz, O. Modeling of mechanical twinning in a high manganese content austenitic steel. Mater. Sci. Eng. A 2004, 387–389, 272–276. [Google Scholar] [CrossRef]
- Březina, B.; Fousek, J. Twinning in Crystals. In Crystal Growth in Science and Technology; NATO ASI Series; Springer: Boston, MA, USA, 1989; pp. 185–195. ISBN 978-1-4612-7861-0. [Google Scholar]
- Chan, C.-J.; Lange, F.F.; Rühle, M.; Jue, J.-F.; Virkar, A.V. Ferroelastic Domain Switching in Tetragonal Zirconia Single Crystals—Microstructural Aspects. J. Am. Ceram. Soc. 1991, 74, 807–813. [Google Scholar] [CrossRef]
- Lim, A.R.; Choh, S.H.; Jang, M.S. Prominent ferroelastic domain walls in BiVO4 crystal. J. Phys. Condens. Matter 1995, 7, 7309. [Google Scholar] [CrossRef]
- Meschke, F.; Kolleck, A.; Schneider, G.A. R-curve behaviour of BaTiO3 due to stress-induced ferroelastic domain switching. J. Eur. Ceram. Soc. 1997, 17, 1143–1149. [Google Scholar] [CrossRef]
- Cahn, R.W. Twinned crystals. Adv. Phys. 1954, 3, 363–445. [Google Scholar] [CrossRef]
- Cook, W.R. Domain Twinning in Barium Titanate Ceramics. J. Am. Ceram. Soc. 1956, 39, 17–19. [Google Scholar] [CrossRef]
- Forsbergh, P.W. Domain Structures and Phase Transitions in Barium Titanate. Phys. Rev. 1949, 76, 1187–1201. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Ho, N.-J.; Lu, H.-Y. Transformation-Induced Twinning: The 90° and 180° Ferroelectric Domains in Tetragonal Barium Titanate. J. Am. Ceram. Soc. 2006, 89, 2177–2187. [Google Scholar] [CrossRef]
- Hooton, J.A.; Merz, W.J. Etch Patterns and Ferroelectric Domains in BaTiO3 Single Crystals. Phys. Rev. 1955, 98, 409–413. [Google Scholar] [CrossRef]
- Merz, W.J. Domain Formation and Domain Wall Motions in Ferroelectric BaTiO3 Single Crystals. Phys. Rev. 1954, 95, 690–698. [Google Scholar] [CrossRef]
- Chou, J.-F.; Lin, M.-H.; Lu, H.-Y. Ferroelectric domains in pressureless-sintered barium titanate. Acta Mater. 2000, 48, 3569–3579. [Google Scholar] [CrossRef]
- Klassen-Neklyudova, M.V. Mechanical Twinning of Crystals; Springer: Boston, MA, USA, 1964; ISBN 978-1-4684-1541-4. [Google Scholar]
- Nielsen, J.W.; Linares, R.C.; Koonce, S.E. Genesis of the Barium Titanate Butterfly Twin. J. Am. Ceram. Soc. 1962, 45, 12–17. [Google Scholar] [CrossRef]
- De Vries, R.C. Observations on Growth of BaTiO3, Crystals from KF Solutions. J. Am. Ceram. Soc. 1959, 42, 547–558. [Google Scholar] [CrossRef]
- Choi, J.J.; Yang, X.; Norman, Z.M.; Billinge, S.J.L.; Owen, J.S. Structure of Methylammonium Lead Iodide Within Mesoporous Titanium Dioxide: Active Material in High-Performance Perovskite Solar Cells. Nano Lett. 2014, 14, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Grancini, G.; Marras, S.; Prato, M.; Giannini, C.; Quarti, C.; De Angelis, F.; De Bastiani, M.; Eperon, G.E.; Snaith, H.J.; Manna, L.; et al. The Impact of the Crystallization Processes on the Structural and Optical Properties of Hybrid Perovskite Films for Photovoltaics. J. Phys. Chem. Lett. 2014, 5, 3836–3842. [Google Scholar] [CrossRef] [PubMed]
- De Bastiani, M.; D’Innocenzo, V.; Stranks, S.D.; Snaith, H.J.; Petrozza, A. Role of the crystallization substrate on the photoluminescence properties of organo-lead mixed halides perovskites. APL Mater. 2014, 2, 081509. [Google Scholar] [CrossRef]
- D’Innocenzo, V.; Srimath Kandada, A.R.; De Bastiani, M.; Gandini, M.; Petrozza, A. Tuning the Light Emission Properties by Band Gap Engineering in Hybrid Lead Halide Perovskite. J. Am. Chem. Soc. 2014, 136, 17730–17733. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Tsai, H.; Asadpour, R.; Blancon, J.-C.; Neukirch, A.J.; Gupta, G.; Crochet, J.J.; Chhowalla, M.; Tretiak, S.; Alam, M.A.; et al. High-efficiency solution-processed perovskite solar cells with millimeter-scale grains. Science 2015, 347, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Yamada, T.; Phuong, L.Q.; Maruyama, N.; Nishimura, H.; Wakamiya, A.; Murata, Y.; Kanemitsu, Y. Dynamic Optical Properties of CH3NH3PbI3 Single Crystals as Revealed by One- and Two-Photon Excited Photoluminescence Measurements. J. Am. Chem. Soc. 2015, 137, 10456–10459. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Hutter, E.M.; Fang, Y.; Dong, Q.; Huang, J.; Savenije, T.J. Charge Carrier Lifetimes Exceeding 15 μs in Methylammonium Lead Iodide Single Crystals. J. Phys. Chem. Lett. 2016, 7, 923–928. [Google Scholar] [CrossRef] [PubMed]
- De Quilettes, D.W.; Vorpahl, S.M.; Stranks, S.D.; Nagaoka, H.; Eperon, G.E.; Ziffer, M.E.; Snaith, H.J.; Ginger, D.S. Impact of microstructure on local carrier lifetime in perovskite solar cells. Science 2015, 348, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.S.; Ho-Baillie, A.; Huang, S.; Woo, S.H.; Heo, Y.; Seidel, J.; Huang, F.; Cheng, Y.-B.; Green, M.A. Benefit of Grain Boundaries in Organic–Inorganic Halide Planar Perovskite Solar Cells. J. Phys. Chem. Lett. 2015, 6, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.S.; Seidel, J.; Kim, J.; Soufiani, A.M.; Huang, S.; Lau, J.; Jeon, N.J.; Seok, S.I.; Green, M.A.; Ho-Baillie, A. Critical Role of Grain Boundaries for Ion Migration in Formamidinium and Methylammonium Lead Halide Perovskite Solar Cells. Adv. Energy Mater. 2016, 6. [Google Scholar] [CrossRef]
- Yuan, Y.; Huang, J. Ion Migration in Organometal Trihalide Perovskite and Its Impact on Photovoltaic Efficiency and Stability. Acc. Chem. Res. 2016, 49, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Ponseca, C.S.; Savenije, T.J.; Abdellah, M.; Zheng, K.; Yartsev, A.; Pascher, T.; Harlang, T.; Chabera, P.; Pullerits, T.; Stepanov, A.; et al. Organometal Halide Perovskite Solar Cell Materials Rationalized: Ultrafast Charge Generation, High and Microsecond-Long Balanced Mobilities, and Slow Recombination. J. Am. Chem. Soc. 2014, 136, 5189–5192. [Google Scholar] [CrossRef] [PubMed]
- Hermes, I.M.; Bretschneider, S.A.; Bergmann, V.W.; Li, D.; Klasen, A.; Mars, J.; Tremel, W.; Laquai, F.; Butt, H.-J.; Mezger, M.; et al. Ferroelastic Fingerprints in Methylammonium Lead Iodide Perovskite. J. Phys. Chem. C 2016, 120, 5724–5731. [Google Scholar] [CrossRef]
- Strelcov, E.; Dong, Q.; Li, T.; Chae, J.; Shao, Y.; Deng, Y.; Gruverman, A.; Huang, J.; Centrone, A. CH3NH3PbI3 perovskites: Ferroelasticity revealed. Sci. Adv. 2017, 3, e1602165. [Google Scholar] [CrossRef] [PubMed]
- Roehm, H.; Leonhard, T.; Hoffmannbc, M.J.; Colsmann, A. Ferroelectric domains in methylammonium lead iodide perovskite thin-films. Energy Environ. Sci. 2017, 10, 950–955. [Google Scholar] [CrossRef]
- Kim, T.W.; Uchida, S.; Matsushita, T.; Cojocaru, L.; Jono, R.; Kimura, K.; Matsubara, D.; Shirai, M.; Ito, K.; Matsumoto, H.; et al. Self-Organized Superlattice and Phase Coexistence inside Thin Film Organometal Halide Perovskite. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef] [PubMed]
- Rothmann, M.U.; Li, W.; Etheridge, J.; Cheng, Y.-B. Microstructural Characterisations of Perovskite Solar Cells—From Grains to Interfaces: Techniques, Features, and Challenges. Adv. Energy Mater. 2017, 7, 1700912. [Google Scholar] [CrossRef]
- Kutes, Y.; Ye, L.; Zhou, Y.; Pang, S.; Huey, B.D.; Padture, N.P. Direct Observation of Ferroelectric Domains in Solution-Processed CH3NH3PbI3 Perovskite Thin Films. J. Phys. Chem. Lett. 2014, 5, 3335–3339. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhao, Y.; Li, H.; Li, G.; Pan, J.; Xu, D.; Zhao, Q.; Yu, D. Hysteresis Analysis Based on the Ferroelectric Effect in Hybrid Perovskite Solar Cells. J. Phys. Chem. Lett. 2014, 5, 3937–3945. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Yang, M.; Priya, S.; Zhu, K. Origin of J–V Hysteresis in Perovskite Solar Cells. J. Phys. Chem. Lett. 2016, 7, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Xiao, J.; Sun, K.; Chen, L.; Hu, Y.; Ouyang, J.; Ong, K.P.; Zeng, K.; Wang, J. Ferroelectricity of CH3NH3PbI3 Perovskite. J. Phys. Chem. Lett. 2015, 6, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Pratibha Mahale, S.G.; Mahale, P.; Kore, B.P.; Mukherjee, S.; Pavan, M.S.; De, C.; Ghara, S.; Sundaresan, A.; Pandey, A.; Guru Row, T.N.; et al. Is CH3NH3PbI3 Polar? J. Phys. Chem. Lett. 2016, 7, 2412–2419. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Dang, T.-V.; Choi, H.-J.; Park, B.-J.; Eom, J.-H.; Song, H.-A.; Seol, D.; Kim, Y.; Shin, S.-H.; Nah, J.; et al. Piezoelectric properties of CH3NH3PbI3 perovskite thin films and their applications in piezoelectric generators. J. Mater. Chem. A 2016, 4, 756–763. [Google Scholar] [CrossRef]
- Rakita, Y.; Bar-Elli, O.; Meirzadeh, E.; Kaslasi, H.; Peleg, Y.; Hodes, G.; Lubomirsky, I.; Oron, D.; Ehre, D.; Cahen, D. Tetragonal CH3NH3PbI3 is ferroelectric. Proc. Natl. Acad. Sci. USA 2017, 114, E5504–E5512. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.N.F.; Yang, M.; Li, Z.; Islam, N.; Pan, X.; Zhu, K.; Fan, Z. Polarization and Dielectric Study of Methylammonium Lead Iodide Thin Film to Reveal its Nonferroelectric Nature under Solar Cell Operating Conditions. ACS Energy Lett. 2016, 1, 142–149. [Google Scholar] [CrossRef]
- Santiso, J.; Balcells, L.; Konstantinovic, Z.; Roqueta, J.; Ferrer, P.; Pomar, A.; Martínez, B.; Sandiumenge, F. Thickness evolution of the twin structure and shear strain in LSMO films. CrystEngComm 2013, 15, 3908–3918. [Google Scholar] [CrossRef]
- Zhao, P.; Xu, J.; Ma, C.; Ren, W.; Wang, L.; Bian, L.; Chang, A. Spontaneous polarization behaviors in hybrid halide perovskite film. Scr. Mater. 2015, 102, 51–54. [Google Scholar] [CrossRef]
- Yin, W.-J.; Yang, J.-H.; Kang, J.; Yan, Y.; Wei, S.-H. Halide perovskite materials for solar cells: A theoretical review. J. Mater. Chem. A 2015, 3, 8926–8942. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, F.; Koocher, N.Z.; Takenaka, H.; Wang, F.; Rappe, A.M. Ferroelectric Domain Wall Induced Band Gap Reduction and Charge Separation in Organometal Halide Perovskites. J. Phys. Chem. Lett. 2015, 6, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Rashkeev, S.N.; El-Mellouhi, F.; Kais, S.; Alharbi, F.H. Domain Walls Conductivity in Hybrid Organometallic Perovskites and Their Essential Role in CH3NH3PbI3 Solar Cell High Performance. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Bi, F.; Markov, S.; Wang, R.; Kwok, Y.; Zhou, W.; Liu, L.; Zheng, X.; Chen, G.; Yam, C. Enhanced Photovoltaic Properties Induced by Ferroelectric Domain Structures in Organometallic Halide Perovskites. J. Phys. Chem. C 2017, 121, 11151–11158. [Google Scholar] [CrossRef]
- Rossi, D.; Pecchia, A.; der Maur, M.A.; Leonhard, T.; Röhm, H.; Hoffmann, M.J.; Colsmann, A.; Carlo, A.D. On the importance of ferroelectric domains for the performance of perovskite solar cells. Nano Energy 2018, 48, 20–26. [Google Scholar] [CrossRef]
- Tsai, H.; Asadpour, R.; Blancon, J.-C.; Stoumpos, C.C.; Durand, O.; Strzalka, J.W.; Chen, B.; Verduzco, R.; Ajayan, P.M.; Tretiak, S.; et al. Light-induced lattice expansion leads to high-efficiency perovskite solar cells. Science 2018, 360, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Gratia, P.; Grancini, G.; Audinot, J.-N.; Jeanbourquin, X.; Mosconi, E.; Zimmermann, I.; Dowsett, D.; Lee, Y.; Grätzel, M.; De Angelis, F.; et al. Intrinsic Halide Segregation at Nanometer Scale Determines the High Efficiency of Mixed Cation/Mixed Halide Perovskite Solar Cells. J. Am. Chem. Soc. 2016, 138, 15821–15824. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Jain, A.; Voznyy, O.; Lan, X.; de Arquer, F.P.G.; Fan, J.Z.; Quintero-Bermudez, R.; Yuan, M.; Zhang, B.; Zhao, Y.; et al. Efficient and stable solution-processed planar perovskite solar cells via contact passivation. Science 2017, eaai9081. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Leyden, M.R.; Qiu, L.; Wang, S.; Ono, L.K.; Wu, Z.; Juarez-Perez, E.J.; Qi, Y. Combination of Hybrid CVD and Cation Exchange for Upscaling Cs-Substituted Mixed Cation Perovskite Solar Cells with High Efficiency and Stability. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Wang, X.; Liu, Y.; Lv, Y.; Wang, S.; Wang, K.; Shi, Y.; Xiao, L.; Chen, Z.; et al. Interplay between Exciton and Free Carriers in Organolead Perovskite Films. Sci. Rep. 2017, 7, 14760. [Google Scholar] [CrossRef] [PubMed]
- Telfah, H.; Jamhawi, A.; Teunis, M.B.; Sardar, R.; Liu, J. Ultrafast Exciton Dynamics in Shape-Controlled Methylammonium Lead Bromide Perovskite Nanostructures: Effect of Quantum Confinement on Charge Carrier Recombination. J. Phys. Chem. C 2017. [Google Scholar] [CrossRef]
- Makarov, N.S.; Guo, S.; Isaienko, O.; Liu, W.; Robel, I.; Klimov, V.I. Spectral and Dynamical Properties of Single Excitons, Biexcitons, and Trions in Cesium–Lead-Halide Perovskite Quantum Dots. Nano Lett. 2016, 16, 2349–2362. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Y.; Wang, X.; Lv, Y.; Wang, S.; Wang, K.; Shi, Y.; Xiao, L.; Chen, Z.; Gong, Q. Density-dependent dynamical coexistence of excitons and free carriers in the organolead perovskite CH3NH3PbI3. Phys. Rev. B 2016, 94, 140302. [Google Scholar] [CrossRef]
- D’Innocenzo, V.; Grancini, G.; Alcocer, M.J.P.; Kandada, A.R.S.; Stranks, S.D.; Lee, M.M.; Lanzani, G.; Snaith, H.J.; Petrozza, A. Excitons versus free charges in organo-lead tri-halide perovskites. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]











| Sample | Main Method | Phenomenon | Result | Reference |
|---|---|---|---|---|
| β CH3NH3PbI3 film | PFM | 180° phase change after DC poling | ferroelectricity | [66] |
| CH3NH3PbI3(Cl) film | PFM | Striped twin domain without ferroelectric switching | ferroelasticity | [61] |
| CH3NH3PbI3 device | PFM | P-E hysteresis loops without switching | Non-ferroelectricity | [69] |
| Powder and single crystal of CH3NH3PbI3 | SHG XRD P-E loop | a. SHG efficiency below any detectable limit b. XRD result showed the centrosymmetric space group c. Approximately linear P-E loop | Non-ferroelectricity | [70] |
| CH3NH3PbI3 film | P-E loop/PFM | Ferroelectric properties and switchable polarization | ferroelectricity | [71] |
| CH3NH3PbI3 single crystal | P-E loop/SHG | P-E hysteresis loops in tetragonal phase and SHG response | ferroelectricity | [72] |
| CH3NH3PbI3 film | P-E loop | No clear P-E switching | Non-ferroelectricity | [73] |
| Material | Preparation Method | Thickness | Grain Size | Annealing Process | Reference |
|---|---|---|---|---|---|
| MAPbI3(Cl) polycrystalline films | One-step solution-processed | / | Up to 10 μm | 140 °C, 20 min | [61] |
| MAPbI3 polycrystalline films | Doctor blade-coating | 600 nm | 0.5~2 μm | 100 °C, 30 min | [62] |
| MAPbI3 polycrystalline films | Two-step spin-coating | / | 0.5~2 μm | 100 °C, 10 min | [62] |
| MAPbI3(Cl) polycrystalline films | Two-step solution-processed | 300 nm | Several micrometers | 100 °C, 20 min | [63] |
| MAPbI3 polycrystalline films | One-step solution-processed | 300 nm | ~1 μm | 100 °C, 10 min | [21] |
| MAPbI3 polycrystalline films | One-step solution-processed | / | / | 100 °C, 30 min | [64] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Liu, Y.; Wang, J.; Wu, C.; Liu, C.; Xiao, L.; Chen, Z.; Wang, S.; Gong, Q. Twin Domains in Organometallic Halide Perovskite Thin-Films. Crystals 2018, 8, 216. https://doi.org/10.3390/cryst8050216
Liu W, Liu Y, Wang J, Wu C, Liu C, Xiao L, Chen Z, Wang S, Gong Q. Twin Domains in Organometallic Halide Perovskite Thin-Films. Crystals. 2018; 8(5):216. https://doi.org/10.3390/cryst8050216
Chicago/Turabian StyleLiu, Wei, Yang Liu, Ju Wang, Cuncun Wu, Congyue Liu, Lixin Xiao, Zhijian Chen, Shufeng Wang, and Qihuang Gong. 2018. "Twin Domains in Organometallic Halide Perovskite Thin-Films" Crystals 8, no. 5: 216. https://doi.org/10.3390/cryst8050216