Exploring the Topological Landscape Exhibited by Binary Zinc-triad 1,1-dithiolates
Abstract
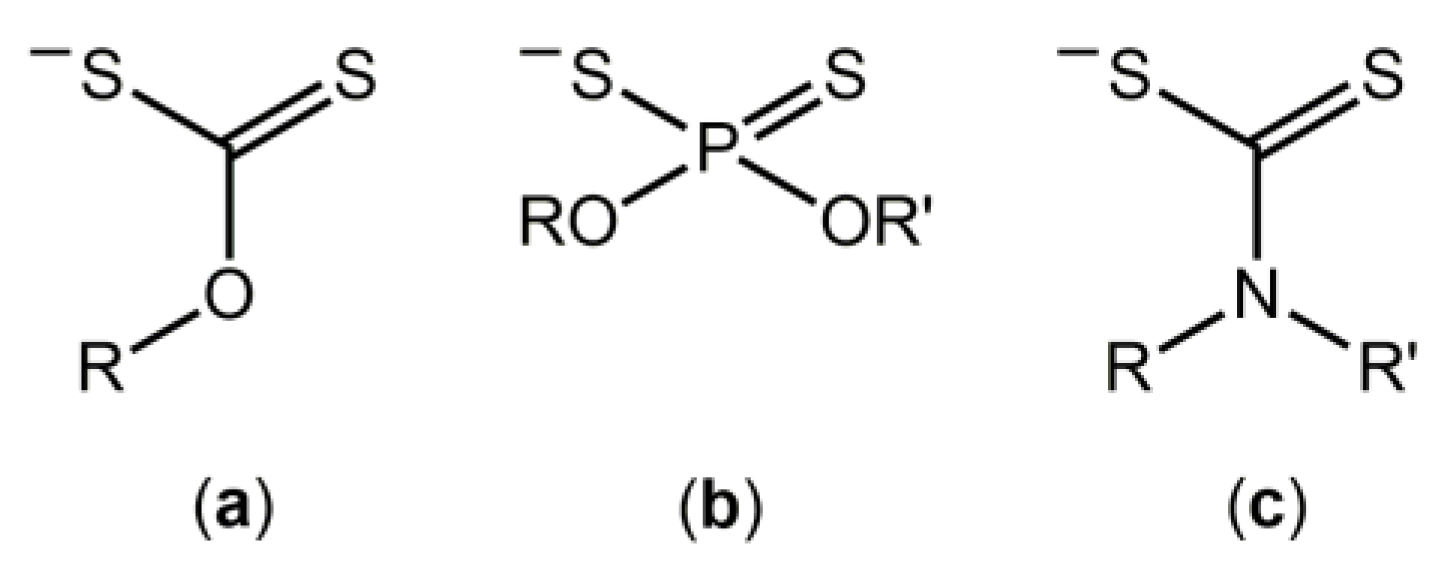
:1. Introduction
2. Methodology and Organisation
3. Discussion
3.1. Zinc-Triad Binary Xanthate Structures
3.1.1. Zinc Xanthates
3.1.2. Cadmium Xanthates
3.1.3. Mercury Xanthates
3.2. Zinc-Triad Binary Dithiophosphate Structures
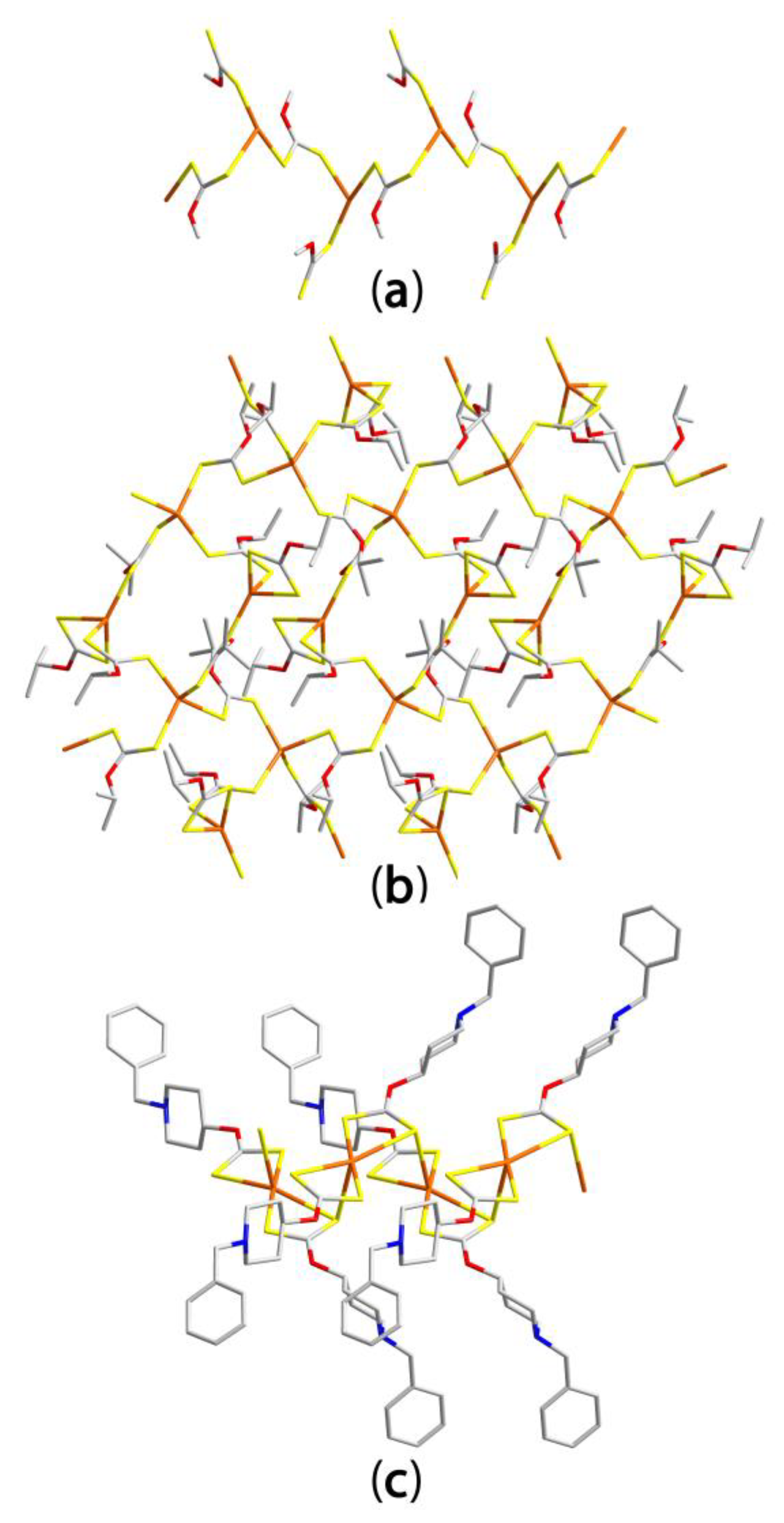
3.2.1. Zinc Dithiophosphates
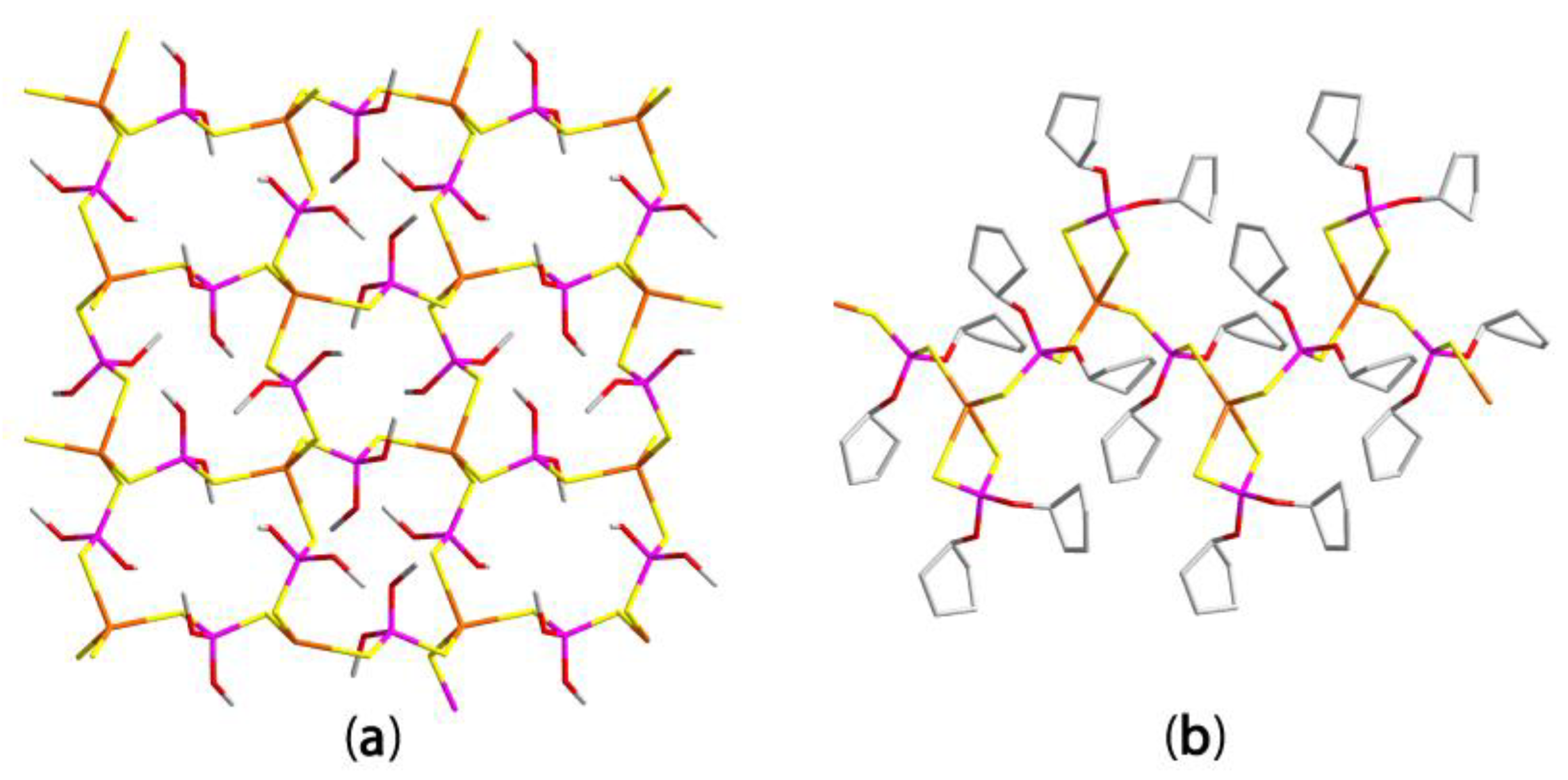
3.2.2. Cadmium Dithiophosphates
3.2.3. Mercury Dithiophosphates
3.3. Zinc-Triad Binary Dithiocarbamate Structures
3.3.1. Zinc Dithiocarbamates
3.3.2. Cadmium Dithiocarbamates
3.3.3. Mercury Dithiocarbamates
4. Overview and Conclusions
Acknowledgments
Conflicts of Interest
References and Notes
- Haiduc, I. 1,1-Dithiolato ligands and related selenium and tellurium compounds. In Handbook of Chalcogen Chemistry; Devillanova, F.A., Ed.; Royal Society of Chemistry: Cambridge, UK, 2007; pp. 593–643. ISBN 978-0-85404-366-8. [Google Scholar]
- Zeise, W.C. Recueil de Memoires del’ Acad. Roy. Des Sciences de Copenhagen 1815, 1, 1. [Google Scholar]
- Pishchimuka, P.S. Action of phosphorus pentasulfide on alcohol. J. Russ. Phys. Chem. Soc. 1925, 56, 11–14. [Google Scholar]
- Johnson, D.W.; Hils, J.E. Phosphate esters, thiophosphate esters and metal thiophosphates as lubricant additives. Lubricants 2013, 1, 132–148. [Google Scholar] [CrossRef]
- Hogarth, G. Transition metal dithiocarbamates: 1978–2003. Prog. Inorg. Chem. 2005, 53, 71–561. [Google Scholar] [CrossRef]
- Dobus, H. Ueber die Verbindungen der Sulfocarbaminsäure. Justus Liebigs Annalen der Chemie 1850, 73, 26–34. [Google Scholar] [CrossRef]
- Delépine, M. Metallic salts of dithiocarbamic acids; preparation of isothiocyanates in the aliphatic series. Compt. Rend. 1907, 144, 1125–1127. [Google Scholar]
- Coucouvanis, D. The chemistry of the dithioacid and 1,1-dithiolate complexes. Prog. Inorg. Chem. 1970, 11, 234–371. [Google Scholar] [CrossRef]
- Eisenberg, R. Structural systematics of 1,1- and 1,2-dithiolato chelates. Prog. Inorg. Chem. 1970, 12, 295–369. [Google Scholar] [CrossRef]
- Coucouvanis, D. The chemistry of the dithioacid and 1,1-dithiolate complexes, 1968–1977. Prog. Inorg. Chem. 1979, 26, 301–469. [Google Scholar] [CrossRef]
- Winter, G. Inorganic xanthates. Rev. Inorg. Chem. 1980, 2, 253–342. [Google Scholar]
- Tiekink, E.R.T.; Winter, G. Inorganic xanthates: A structural perspective. Rev. Inorg. Chem. 1992, 12, 183–302. [Google Scholar] [CrossRef]
- Tiekink, E.R.T.; Haiduc, I. Stereochemical aspects of metal xanthate complexes: Molecular structures and supramolecular self-assembly. Prog. Inorg. Chem. 2005, 54, 127–319. [Google Scholar] [CrossRef]
- Haiduc, I.; Sowerby, D.B. Stereochemical aspects of phosphor-1,1-dithiolato metal complexes: Coordination patterns, molecular structures and supramolecular associations in dithiophosphinates and related compounds. Polyhedron 1996, 15, 2469–2521. [Google Scholar] [CrossRef]
- Heard, P.J. Main group dithiocarbamate complexes. Prog. Inorg. Chem. 2005, 53, 1–69. [Google Scholar] [CrossRef]
- Cox, M.J.; Tiekink, E.R.T. The diverse coordination patterns in the structures of zinc, cadmium and mercury bis(1,1-dithiolates). Rev. Inorg. Chem. 1997, 17, 1–23. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Molecular architecture and supramolecular association in the zinc-triad 1,1-dithiolates. Steric control as a design element in crystal engineering? CrystEngComm 2003, 5, 101–113. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Perplexing coordination behaviour of potentially bridging bipyridyl-type ligands in the coordination chemistry of zinc and cadmium 1,1-dithiolate compounds. Crystals 2018, 8, 18. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. D Biol. Cryst. 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DIAMOND, version 3.2k; K. Brandenburg & M. Berndt GbR: Bonn, Germany, 2006.
- Ikeda, T.; Hagihara, H. The crystal structure of zinc ethylxanthate. Acta Crystallogr. 1966, 21, 919–927. [Google Scholar] [CrossRef]
- Lai, C.S.; Lim, Y.X.; Yap, T.C.; Tiekink, E.R.T. Molecular paving with zinc thiolates. CrystEngComm 2002, 4, 596–600. [Google Scholar] [CrossRef]
- Ito, T. The crystal structure of zinc isopropylxanthate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1972, 28, 1697–1704. [Google Scholar] [CrossRef] [Green Version]
- Cox, M.J.; Tiekink, E.R.T. Structural features of zinc(II) bis(O-alkyldithiocarbonate) and zinc(II) bis(N,N-dialkyldithiocarbamate) compounds. Z. Kristallogr. 1999, 214, 184–190. [Google Scholar] [CrossRef]
- Young, V.G., Jr.; Tiekink, E.R.T. Bis(O-methyldithiocarbonato)cadmium(II). Acta Crystallogr. Sect. E Struct. Rep. Online 2002, 58, m537–m539. [Google Scholar] [CrossRef]
- Iimura, Y.; Ito, T.; Hagihara, H. The crystal structure of cadmium ethylxanthate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1972, 28, 2271–2279. [Google Scholar] [CrossRef] [Green Version]
- Macreadie, L.K.; Maynard-Casely, H.E.; Batten, S.R.; Turner, D.R.; Chesman, A.S.R. Soluble xanthate compounds for the solution deposition of metal sulfide thin films. ChemPlusChem 2015, 80, 107–118. [Google Scholar] [CrossRef]
- Tomlin, D.W.; Cooper, T.M.; Zelmon, D.E.; Gebeyehu, Z.; Hughes, J.M. Cadmium isopropylxanthate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1999, 55, 717–719. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. On the structure of cadmium isopropylxanthate. Corrigendum. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1999, 55, 1176. [Google Scholar] [CrossRef]
- Rietveld, H.M.; Maslen, E.N. The crystal structure of cadmium n-butyl xanthate. Acta Crystallogr. 1965, 18, 429–436. [Google Scholar] [CrossRef] [Green Version]
- Abrahams, B.F.; Hoskins, B.F.; Tiekink, E.R.T.; Winter, G. Investigation of a new xanthate ligand. The crystal and molecular structures of nickel and cadmium (methoxyethyl)xanthates. Aust. J. Chem. 1988, 41, 1117–1122. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Bis(O-methyldithiocarbonato)mercury(II). Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1987, 43, 448–450. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, Y. Mercury ethylxanthate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1977, 33, 3566–3568. [Google Scholar] [CrossRef] [Green Version]
- Chieh, C.; Moynihan, K.J. Xanthate and dithiocarbamate complexes of group IIb elements, and an interesting relationship between two mercury(II) ethylxanthate phases. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1980, 36, 1367–1371. [Google Scholar] [CrossRef] [Green Version]
- Hounslow, A.M.; Tiekink, E.R.T. Correlations between nuclear magnetic resonance spectra and crystal structure. III. A 13C nuclear magnetic resonance study in the solid state of bis(xanthato) complexes of mercury(II); The crystal and molecular structure of bis(n-propyl-dithiocarbonato)mercury(II). J. Crystallogr. Spectrosc. Res. 1991, 21, 133–137. [Google Scholar] [CrossRef]
- Watanabe, Y. The structure of mercury(II) isopropylxanthate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1981, 37, 553–556. [Google Scholar] [CrossRef] [Green Version]
- Cox, M.J.; Tiekink, E.R.T. Structural diversity in mercury(II) bis(O-alkyldithiocarbonate) compounds. Z. Kristallogr. 1999, 214, 486–491. [Google Scholar] [CrossRef]
- Rajput, G.; Yadav, M.K.; Thakur, T.S.; Drew, M.G.B.; Singh, N. Versatile coordination environment and interplay of metal assisted secondary interactions in the organization of supramolecular motifs in new Hg(II)/PhHg(II) dithiolates. Polyhedron 2014, 69, 225–233. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Aggregation patterns in the crystal structures of organometallic Group XV 1,1-dithiolat.es: The influence of the Lewis acidity of the central atom, metal- and ligand-bound steric bulk, and coordination potential of the 1,1-dithiolate ligands upon supramolecular architecture. CrystEngComm 2006, 8, 104–118. [Google Scholar] [CrossRef]
- Chen, D.; Lai, C.S.; Tiekink, E.R.T. Supramolecular aggregation in diimine adducts of zinc(II) dithiophosphates: Controlling the formation of monomeric, dimeric, polymeric (zig-zag and helical), and 2-D motifs. CrystEngComm 2006, 8, 51–58. [Google Scholar] [CrossRef]
- Kang, J.-G.; Shin, J.-S.; Cho, D.-H.; Jeong, Y.-K.; Park, C.; Soh, S.F.; Lai, C.S.; Tiekink, E.R.T. Steric control over supramolecular polymer formation in trans-1,2-bis(4-pyridyl)ethylene adducts of zinc xanthates: Implications for luminescence. Cryst. Growth Des. 2010, 10, 1247–1256. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Structural chemistry of organotin carboxylates: A review of the crystallographic literature. Appl. Organomet. Chem. 1991, 5, 1–23. [Google Scholar] [CrossRef]
- Willem, R.; Verbruggen, I.; Gielen, M.; Biesemans, M.; Mahieu, B.; Basu Baul, T.S.; Tiekink, E.R.T. Correlating Mössbauer and solution- and solid-state 117Sn NMR data with X-ray diffraction structural data of triorganotin 2-[(E)-2-(2-hydroxy-5-methylphenyl)-1-diazenyl]benzoates. Organometallics 1998, 17, 5758–5766. [Google Scholar] [CrossRef]
- Dakternieks, D.; Duthie, A.; Smyth, D.R.; Stapleton, C.P.D.; Tiekink, E.R.T. Steric control over molecular structure and supramolecular association exerted by tin- and ligand-bound groups in diorganotin carboxylates. Organometallics 2003, 22, 4599–4603. [Google Scholar] [CrossRef]
- Alcock, N.W. Secondary bonding to nonmetallic elements. Adv. Inorg. Chem. Radiochem. 1972, 15, 1–58. [Google Scholar] [CrossRef]
- Pyykkö, P. Strong closed-shell interactions in inorganic chemistry. Chem. Rev. 1997, 97, 597–636. [Google Scholar] [CrossRef] [PubMed]
- Haiduc, I. Supramolecular associations, secondary bonds, quasi-cyclic structures and heterogeometrism in metal derivatives of phosphorus- and arsenic-based thioacids and oxo analogs. Coord. Chem. Rev. 1997, 158, 325–358. [Google Scholar] [CrossRef]
- Buntine, M.A.; Cox, M.J.; Lim, Y.X.; Yap, T.C.; Tiekink, E.R.T. Crystal structure of anhydrous potassium O-n-propyldithiocarbonate. Theoretical calculations of O-alkyl dithiocarbonates. Z. Kristallogr. 2003, 218, 56–61. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen-sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Ito, T.; Igarashi, T.; Hagihara, H. The crystal structure of metal diethyldithiophosphates. I. Zinc diethyldithiophosphate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1969, 25, 2303–2309. [Google Scholar] [CrossRef] [Green Version]
- Lawton, S.L.; Kokotailo, G.T. Crystal and molecular structures of zinc and cadmium O,O-diisopropyl phosphorodithioates. Inorg. Chem. 1969, 8, 2410–2421. [Google Scholar] [CrossRef]
- Menzer, S.; Phillips, J.R.; Slawin, A.M.Z.; Williams, D.J.; Woollins, J.D. Structural characterisation of basic zinc O,O’-dialkyl dithiophosphosphate and two isomeric examples of zinc monothiophosphates. J. Chem. Soc. Dalton Trans. 2000, 3269–3273. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Antzutkin, O.N.; Larsson, A.-C.; Kritikos, M.; Forsling, W. Polycrystalline and surface O,O′-dialkyldithiophosphate zinc(II) complexes: Preparation, 31P CP/MAS NMR and single-crystal X-ray diffraction studies. Inorg. Chim. Acta 2001, 315, 26–35. [Google Scholar] [CrossRef]
- Ito, T.; Otake, M. Linear-chain structure of bis(O,O’-dimethyldithiophosphato)cadmium(II). Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1996, 52, 3024–3025. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Gerasimenko, A.V.; Antzutkin, O.N.; Forsling, W. The unique alternation of conformationally different (‘chair’-‘saddle’) eight-membered metallocycles [Cd2S4P2] in the chains of cadmium dialkyldithiophosphates: 13C, 31P, 113Cd CP/MAS NMR and single-crystal X-ray diffraction studies. Inorg. Chim Acta 2005, 358, 2585–2594. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Antzutkin, O.N.; Forsling, W.; Bostrom, D.; Yegao, Y.; Rodionova, N.A. Structural organization and spectral properties of cadmium and nickel(II) O,O’-di-iso-butyl phosphorodithioate complexes as probed by single-crystal X-ray diffraction and CP/MAS NMR (C-13, P-31, Cd-113). Dok. Phys. Chem. 2002, 387, 299–303. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Loseva, O.V.; Ivanov, M.A.; Konfederatov, V.A.; Gerasimenko, A.V.; Antzutkin, O.N.; Forsling, W. Crystalline cadmium dialkyl phosphorodithioate complexes: Synthesis and structural organization as probed by multinuclear C-13, P-31, and Cd-113 CP/MAS NMR and single-crystal X-ray diffraction. Russ. J. Inorg. Chem. 2007, 52, 1595–1602. [Google Scholar] [CrossRef]
- Casas, J.S.; Castiñeiras, A.; Garcia-Tasende, M.S.; Sánchez, A.; Sordo, J.; Vázquez-López, E.M. The X-ray crystal structure of bis(dicyclohexyldithiophosphato)cadmium(II). Polyhedron 1995, 14, 2055–2058. [Google Scholar] [CrossRef]
- Ito, T. Poly[bis(µ2-O,O’-dimethyldithiophosphato)mercury(II)]. Acta Crystallogr. E Cryst. Comm. 2006, 62, m1666–m1667. [Google Scholar] [CrossRef]
- Lawton, S.L. Crystal and molecular structure of the polymeric complex mercuric O,O’-diisopropyl- phosphorodithioate, Hg[i-C3H7O)2PS2]2. Inorg. Chem. 1971, 10, 328–335. [Google Scholar] [CrossRef]
- Casas, J.S.; Castellano, E.E.; Ellena, J.; Haiduc, I.; Sánchez, A.; Sordo, J. The crystal and molecular structure of mercury(II) bis(isopropyl)dithiophosphate, Hg[S2P(OPri)2]2, revisited: New comments about its supramolecular self-organization. J. Chem. Crystallogr. 1999, 29, 831–836. [Google Scholar] [CrossRef]
- Murat Taş, M.; Yağan, M.; Bati, H.; Bati, B.; Büyükgüngör, O. Supramolecular self-organization in mercury(II) dicyclopentyldithiophosphate polynuclear complex. Phosphorus. Sulfur. Silicon Rel. Elements 2009, 185, 242–248. [Google Scholar] [CrossRef]
- Hogarth, G.; Rainford-Brent, E.-J.C.-R.C.R.; Richards, I. Functionalised dithiocarbamate complexes: Synthesis and molecular structures of bis(2-methoxyethyl)dithiocarbamate complexes [M{S2CN(CH2CH2OMe)2}2] (M = Ni, Cu, Zn) and [Cu{S2CN(CH2CH2OMe)2}2][ClO4]. Inorg. Chim. Acta 2009, 362, 1361–1364. [Google Scholar] [CrossRef]
- Decken, A.; Gossage, R.A.; Chan, M.Y.; Lai, C.S.; Tiekink, E.R.T. Crystallographic report: Bis(N,N-dibenzyldithiocarbamato)zinc(II). Appl. Organomet. Chem. 2004, 18, 101–102. [Google Scholar] [CrossRef]
- Chan, M.Y.; Lai, C.S.; Tiekink, E.R.T. Crystallographic report: Bis(N-cyclohexyl,N-methyldithio-carbamato)zinc(II). Appl. Organomet. Chem. 2004, 18, 298. [Google Scholar] [CrossRef]
- Awang, N.; Baba, I.; Yamin, B.M.; Ng, S.W. Bis(N-isobutyl-N-propyldithiocarbamato-k2S,S')zinc(II). Acta Crystallogr. Sect. E Struct. Rep. Online 2010, 66, m215. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, N.; Thirumaran, S. Synthesis of ZnS nanoparticles from pyridine adducts of zinc(II) dithiocarbamates. Compt. Rend. Chim. 2014, 17, 964–970. [Google Scholar] [CrossRef]
- Reck, G.; Becker, R. Bis[N-n-butyl-N-(3,5-di-tert-butyl-2-hydroxybenzyl)dithiocarbamato-k2S,S’]zinc(II). Bis(N-isobutyl-N-propyldithiocarbamato-k2S,S’)zinc(II). Acta Crystallogr. Sect. E Struct. Rep. Online 2010, 66, m234–m235. [Google Scholar] [CrossRef]
- Yadav, R.; Trivedi, M.; Kociok-Kohn, G.; Chauhan, R.; Kumar, A.; Gosavi, S.W. Ferrocenyl dithiocarbamate based d10 transition-metal complexes as potential co-sensitizers in dye-sensitized solar cells. Eur. J. Inorg. Chem. 2016, 1013–1021. [Google Scholar] [CrossRef]
- Reck, G. Pers. Commun. Camb. Str. Database, Refcode: NEQJEZ, 2006.
- Singh, V.; Kumar, V.; Gupta, A.N.; Drew, M.G.B.; Singh, N. Effect of pyridyl substituents leading to the formation of green luminescent mercury(II) coordination polymers, zinc(II) dimers and a monomer. New J. Chem. 2014, 38, 3737–3748. [Google Scholar] [CrossRef]
- Gomathi, G.; Sathiyaraj, E.; Thirumaran, S.; Ciattini, S. Effect of functionlization of N,N-dibenzyldithiocarbamate: Synthesis, spectral and structural studies on bis(N-benzyl-N-(4-methoxybenzyl)dithiocarbamato-S,S′)zinc(II) and bis(N-benzyl-N-(4-cholrobenzyl)dithiocarbamato-S,S′)cadmium(II) and their use for the preparation of MS (M = Zn, Cd). J. Sulfur Chem. 2016, 37, 23–36. [Google Scholar] [CrossRef]
- Manar, K.; Yadav, C.; Tiwari, N.; Singh, R.; Yadav, A.; Drew, M.G.B.; Singh, N. Effect of functionalities on the crystal structures of new zinc(II) dithiocarbamates: A combined anti-leishmanial and thermal decomposition study. CrystEngComm 2017, 19, 2660–2672. [Google Scholar] [CrossRef]
- Sathiyaraj, E.; Tamilvanan, S.; Thirumaran, S.; Ciattini, S. Effect of functionalization of N-bound organic moiety in zinc(II) dithiocarbamate complexes on structure, biological properties and morphology of zinc sulfide nanoparticles. Polyhedron 2017, 128, 133–144. [Google Scholar] [CrossRef]
- Klug, H.P. The crystal structure of zinc dimethyldithiocarbamate. Acta Crystallogr. 1966, 21, 536–546. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Ivakhnenko, A.V.; Gerasimenko, E.V.; Forshling, W. A comparative study of the structural organization of zinc complexes with dialkyl-substituted and cyclic dithiocarbamate ligands: Synthesis, single-crystal X-ray diffraction, and CP/MAS C-13 and N-15 NMR. Russ. J. Inorg. Chem. 2003, 48, 45–54. [Google Scholar]
- Paz, F.A.A.; Neves, M.C.; Trindade, T.; Klinowski, J. The first dinuclear zinc(II) dithiocarbamate complex with butyl substituent groups. Acta Crystallogr. Sect. E Struct. Rep. Online 2003, 59, m1067–m1069. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Redetermination of the crystal structure of dimeric bis(N,N’-diethyldithiocarbamato)zinc, [Zn(S2CNEt2)2]2. Z. Kristallogr. New Cryst. Struct. 2000, 215, 445–446. [Google Scholar] [CrossRef]
- Sreehari, N.; Varghese, B.; Manoharan, P.T. Crystal and molecular structure of dimeric bis[N,N-di-n-propyldithiocarbamato]zinc(II) and the study of exchange-coupled copper(II)-copper(II) pairs in its lattice. Inorg. Chem. 1990, 29, 4011–4015. [Google Scholar] [CrossRef]
- Miyamae, H.; Ito, M.; Iwasaki, H. The structure of zinc(II) N,N-diisopropyldithiocarbamate {bis[m-(N,N-diisopropyldithiocarbamato)-m-S,S']-bis(N,N-diisopropyldithiocarbamato)dizinc(II)}. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1979, 35, 1480–1482. [Google Scholar] [CrossRef]
- Kellö, E.; Vrabel, V.; Kettmann, V.; Garaj, J. The crystal and molecular-structure of the complex of zinc(ii) with di-mu-diallyldithiocarbamate-bis(diallyldithiocarbamate). Collect. Czech. Chem. Commun. 1983, 48, 1272–1280. [Google Scholar] [CrossRef]
- Benson, R.E.; Ellis, C.A.; Lewis, C.E.; Tiekink, E.R.T. 3D-, 2D- and 1D-supramolecular structures of {Zn[S2CN(CH2CH2OH)R]2}2 and their {Zn[S2CN(CH2CH2OH)R]2}2(4,4′-bipyridine) adducts for R = CH2CH2OH, Me or Et: Polymorphism and pseudo-polymorphism. CrystEngComm 2007, 9, 930–940. [Google Scholar] [CrossRef]
- Thirumaran, S.; Venkatachalam, V.; Manohar, A.; Ramalingam, G.; Bocelli, G.; Cantoni, A. Synthesis and characterization of bis(N-methyl-N-ethanol-dithiocarbamato)M(II) (M = Zn, Cd, Hg) and bis(N,N(iminodiethylene)-bisphthalimidedithiocarbamato)M(II) (M = Zn, Cd, Hg) complexes. Single crystal X-ray structure of bis(di(2-hydroxyethyl)dithiocarbamato)zinc(II). J. Coord. Chem. 1998, 44, 281–288. [Google Scholar] [CrossRef]
- Poplaukhin, P.; Tiekink, E.R.T. A triclinic polymorph of bis(μ-N,N-bis(2-hydroxyethyl)-dithiocarbamato-κ3S,S′:S′) bis(N,N-bis(2-hydroxyethyl)-κ2S:S′)zinc(II), C20H40N4O8S8Zn2. Z. Kristallogr. New Cryst. Struct. 2018, 233, 529–531. [Google Scholar] [CrossRef]
- Shahdid, M.; Ruffer, T.; Lang, H.; Awan, S.A.; Ahmad, S. Synthesis and crystal structure of a dinuclear zinc(II)-dithiocarbamate complex, bis{[(μ2-pyrrolidinedithiocarbamato-S,S′)(pyrrolidinedithio- carbamato-S,S′)zinc(II)]}. J. Coord. Chem. 2009, 62, 440–445. [Google Scholar] [CrossRef]
- Shaheen, F.; Gieck, C.; Badshah, A.; Khosa, M.K. Bis(μ-piperidine-1-dithiocarboxylato-κ2S:S')[bis(piperidine-1-dithiocarboxylato-κ2S,S')zinc(II)]. Acta Crystallogr. Sect. E Struct. Rep. Online 2006, 62, m1186–m1187. [Google Scholar] [CrossRef]
- Agre, V.M.; Shugam, E.A. Structures of inner complex compounds with M‒S bonds. 9. Crystal and molecular structures of zinc hexamethylenedithiocarbamate. J. Struct. Chem. 1972, 13, 614–618. [Google Scholar] [CrossRef]
- Shaheen, F.; Gieck, C.; Badshah, A. Bis(μ-4-methylpiperidine-1-dithiocarboxylato-κ2S:S')-[bis(4-methylpiperidine-1-dithiocarboxylato-S:S')zinc(II)]. Acta Crystallogr. Sect. E Struct. Rep. Online 2006, 62, m16–m17. [Google Scholar] [CrossRef]
- Lecina, J.; Carrer, A.; Alvarez-Larena, A.; Mazzi, U.; Melendez-Alafort, L.; Suades, J. New bioconjugated rhenium carbonyls by transmetalation reaction with zinc derivatives. Organometallics 2012, 31, 5884–5893. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, L.-H.; Lu, L.-D. Crystal structure and characterization of Bis[di(N’-ethyl-N-piperazinly-carbodithioato-S,S’)zinc(II)] complexes: [Zn2(S2CNC4H8NC2H5)4]. Chin. J. Inorg. Chem. 2006, 22, 1728–1732. [Google Scholar]
- Bharti, A.; Bharati, P.; Chaudhari, U.K.; Singh, A.; Kushawaha, S.K.; Singh, N.K.; Bharty, M.K. Syntheses, crystal structures and photoluminescent properties of new homoleptic and heteroleptic zinc(II) dithiocarbamato complexes. Polyhedron 2015, 85, 712–719. [Google Scholar] [CrossRef]
- Zia-ur-Rehman; Ibrahim, S.; Khan, A.; Imran, M.; Naseer, M.M.; Khanb, I.; Shah, A.; Tahir, M.N.; Muneeb-ur-Rahman; Awan, I.Z. Homobimetallic zinc(II) dithiocarbamates: Synthesis, characterization and in vivo antihyperglycemic activity. J. Coord. Chem. 2016, 69, 551–561. [Google Scholar] [CrossRef]
- Motevalli, M.; O’Brien, P.; Walsh, J.R.; Watson, I.M. Synthesis, characterization and x-ray crystal structures of asymmetric bis(dialkyldithiocarbamates) of zinc: Potential precursors for ZnS deposition. Polyhedron 1996, 15, 2801–2808. [Google Scholar] [CrossRef]
- Baba, I.; Lee, L.H.; Farina, Y.; Othman, A.H.; Ibrahim, A.R.; Usman, A.; Fun, H.-K.; Ng, S.W. Bis(µ-N-methyl-N-phenyldithiocarbamato)[bis(N-methyl-N-phenyldithiocarbamato)zinc(II)]. Acta Crystallogr. Sect. E Struct. Rep. Online 2002, 58, m744–m745. [Google Scholar] [CrossRef]
- Tan, Y.S.; Ooi, K.K.; Ang, K.P.; Akim, A.M.; Cheah, Y.-K.; Halim, S.N.A.; Seng, H.-L.; Tiekink, E.R.T. Molecular mechanisms of apoptosis and cell selectivity of zinc dithiocarbamates functionalized with hydroxyethyl substituents. J. Inorg. Biochem. 2015, 150, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Nagy, E.M.; Sitran, S.; Montopoli, M.; Favaro, M.; Marchio, L.; Ronconi, L.; Caparrotta, L.; Fregona, D. Zinc(II) complexes with dithiocarbamato derivatives: Structural characterisation and biological assays on cancerous cell lines. J. Inorg. Biochem. 2012, 117, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.P.; de Lima, G.M.; Paniago, E.B.; Pinheiro, C.B.; Wardell, J.L.; Wardell, S.M.S.V. Study of metal dithiocarbamate complexes, Part V. Metal complexes of [S2CN(CH2CH(OMe)2]: A standard dimeric zinc dithiocarbamate structural motive, a rare cadmium dithiocarbamate coordination polymer, and a hydrated sodium dithiocarbarmate complex, with a [Na2O2] core and chain. Inorg. Chim. Acta 2016, 441, 137–145. [Google Scholar] [CrossRef]
- Baba, I.; Farina, Y.; Othman, A.H.; Razak, I.A.; Fun, H.-K.; Ng, S.W. Bis(µ-N-ethyl-N-isopropyldithiocarbamato-S:S’)bis[(N-ethyl-N-isopropyldithiocarbamato-S,S’)zinc(II)]. Acta Crystallogr. Sect. E Struct. Rep. Online 2001, 57, m51–m52. [Google Scholar] [CrossRef]
- Baba, I.; Farina, Y.; Kassim, K.; Othman, A.H.; Razak, I.A.; Fun, H.-K.; Ng, S.W. Bis(µ-N-butyl-N-ethyldithiocarbamato-S:S’)bis[(N-butyl-N-ethyldithiocarbamato)zinc(II)]. Acta Crystallogr. Sect. E Struct. Rep. Online 2001, 57, m55–m56. [Google Scholar] [CrossRef]
- Ajibade, P.A.; Mbese, J.Z.; Omondi, B. Group 12 dithiocarbamate complexes: Synthesis, characterization, and X-ray crystal structures of Zn(II) and Hg(II) complexes and their use as precursors for metal sulfide nanoparticles. Inorg. Nano-Met. Chem. 2017, 47, 202–212. [Google Scholar] [CrossRef]
- Gossage, R.A.; Jenkins, H.A. The crystal structure of bis(μ-N-ethyl-N-phenyldithiocarbamato-S,S’)-bis[(N-ethyl-N-phenyldithiocarbamato-k2S,S’)zinc(II)]. Acta Chim. Slov. 2009, 56, 329–333. [Google Scholar]
- Tan, Y.S.; Tiekink, E.R.T. Crystal structure of bis(μ-N-i-propyl-N-n-propyldithiocarbamato-κ2S:S’) bis(N-i-propyl-N-n-propyldithiocarbamato-κ2S,S’)dizinc(II), C28H56N4S8Zn2. Z. Kristallogr. New Cryst. Struct. 2018, 233, 477–479. [Google Scholar] [CrossRef]
- Sathiyaraj, E.; Perumal, M.V.; Nagarajan, E.R.; Ramalingan, C. Functionalized zinc(II) dithiocarbamate complexes: Synthesis, spectral and molecular structures of bis(N-cyclopropyl-N-4-methoxybenzyldithiocarbamato-S,S′)zinc(II) and (2,2′-bipyridine)bis(N-cyclopropyl-N-4-methoxybenzyldithiocarbamato-S,S′)zinc(II). J. Saudi Chem. Soc. 2017, in press. [Google Scholar] [CrossRef]
- Ajibade, P.A.; Onwudiwe, D.C. Synthesis and characterization of group 12 complexes of N,N-methyl phenyl-N,N-butyl phenyl dithiocarbamate. J. Coord. Chem. 2011, 64, 2963–2973. [Google Scholar] [CrossRef]
- Jia, C.-M.; Yuan, W.-B.; Lin, Q.; Zhang, Q.; Pei, J. Bis(μ-N-benzyl-N-tetradecyldithiocarbamato-κ2S:S’)bis[(N-benzyl-N-tetradecyldithiocarbamato-κ2S,S’)zinc(II)]. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, m471. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.V.; Ivakhnenko, E.V.; Forsling, W.; Gerasimenko, A.V. Single-crystal X-ray diffraction and CP/MAS 13C and 15N NMR study of zinc N,N-di-iso-butyldithiocarbamate Ccomplex: A unique structural organization. Doklady Chem. 2003, 390, 162–167. [Google Scholar] [CrossRef]
- Halim, S.N.A. Pers. Commun. Camb. Str. Database, 2015. Refcode: QUQTED.
- Kumar, V.; Manar, K.K.; Gupta, A.N.; Singh, V.; Drew, M.G.B.; Singh, N. Impact of ferrocenyl and pyridyl groups attached to dithiocarbamate moieties on crystal structures and luminescent characteristics of group 12 metal complexes. J. Organomet. Chem. 2016, 820, 62–69. [Google Scholar] [CrossRef]
- Poplaukhin, P.; Arman, H.D.; Tiekink, E.R.T. A one-dimensional coordination polymer, catena-poly[[[[N-ethyl-N-(pyridin-4-ylmethyl)dithiocarbamato-κ2S,S′]zinc(II)]-μ2-N-ethyl-N-(pyridin-4-ylmethyl)dithiocarbamato-κ3S,S′:N] 4-methylpyridine hemisolvate]. Acta Crystallogr. Sect. E Cryst. Commun. 2017, 73, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Casas, J.S.; Sanchez, A.; Bravo, J.; Garcia-Fontan, S.; Castellano, E.E.; Jones, M.M. Cadmium coordination chemistry related to chelate therapy. Inorg. Chim. Acta 1989, 158, 119–126. [Google Scholar] [CrossRef]
- Domenicano, A.; Torelli, L.; Vaciago, A.; Zambonelli, L. Structural studies of metal dithiocarbamates. Part IV. The crystal and molecular structure of cadmium(II)NN-diethyldithiocarbamate. J. Chem. Soc. A 1968, 1351–1361. [Google Scholar] [CrossRef]
- Dee, C.M.; Tiekink, E.R.T. Refinement of the crystal structure of dimeric bis(N,N-diethyldithiocarbamato) cadmium(II), [Cd(S2CNEt2)2]2. Z. Kristallogr. New Cryst. Struct. 2002, 217, 85–86. [Google Scholar] [CrossRef]
- Jian, F.-F.; Wang, Z.-X.; Bai, Z.-P.; You, X.-Z.; Fun, H.-K.; Chinnakali, K. Structure of bis(dipropyldithiocarbamate) cadmium(II), [Cd2(n-Pr2dtc)4] (dtc = dithiocarbamate). J. Chem. Crystallogr. 1999, 29, 227–231. [Google Scholar] [CrossRef]
- Jian, F.-F.; Wang, Z.-X.; Fun, H.-K.; Bai, Z.-P.; You, X.-Z. A binuclear cadmium(II) complex: Bis[bis(N,N-diisopropyldithiocarbamato)cadmium(II)]. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1999, 55, 174–176. [Google Scholar] [CrossRef]
- Onwudiwe, D.C.; Hrubaru, M.; Hosten, E.C.; Arderne, C. Bis(μ-N,N-diallyldithiocarbamato)bis[(N,N-diallyldithiocarbamato)cadmium]. Acta Crystallogr. Sect. E Cryst. Commun. 2017, 73, 1353–1356. [Google Scholar] [CrossRef] [PubMed]
- Glinskaya, L.A.; Zemskova, S.M.; Klevtsova, R.F. Molecular and crystal, structure of cadmium(II) diisobutyldithiocarbamate {Cd[(i-C4H9)2NCS2]2}2. J. Struct. Chem. 1999, 40, 979–983. [Google Scholar] [CrossRef]
- Cox, M.J.; Tiekink, E.R.T. Structural characteristics of cadmium(II) bis(N,N-dialkyldithiocarbamate) compounds. Z. Kristallogr. 1999, 214, 670–676. [Google Scholar] [CrossRef]
- Yin, X.; Zhang, W.; Zhang, Q.; Fan, J.; Lai, C.S.; Tiekink, E.R.T. Crystallographic report: Bis[bis(N,N-dibenzyldithiocarbamato)cadmium(II)]. Appl. Organomet. Chem. 2004, 18, 139–140. [Google Scholar] [CrossRef]
- Saravanan, M.; Ramalingam, K.; Bocelli, G.; Olla, R. Refinement of the crystal structure of dimeric bis(N,N-diethyldithiocarbamato) cadmium(II), [Cd(S2CNEt2)2]2. Appl. Organomet. Chem. 2004, 18, 103. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, W.; Fan, J.; Tan, M.; Lai, C.S.; Tiekink, E.R.T. Bisµ-N,N-bis(2-hydroxyethyl)dithiocarbamato]-1:2κ3S,S’:S’;2:1κ3S,S’:S’-bis{[N,N-bis(2-hydroxyethyl)dithiocarbamato-κ2S,S’]cadmium(II)}. Acta Crystallogr. Sect. E Struct. Rep. Online 2004, 60, m1633–m1635. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Gerasimenko, A.V.; Konzelko, A.A.; Ivanov, M.A.; Antzutkin, O.N.; Forsling, W. Conformational isomerism of the binuclear N,N-pentamethylenedithiocarbamate cadmium(II) complex, [Cd2{S2CN(CH2)5}4] on multinuclear (15N, 113Cd) CP/MAS NMR and single-crystal X-ray diffraction data. Inorg. Chim. Acta 2006, 359, 3855–3864. [Google Scholar] [CrossRef]
- Manohar, A.; Ramalingam, K.; Bocelli, G.; Cantoni, A. Synthesis and spectral studies on 2:1 adducts involving cadmium dithiocarbamates and 4,4’-bipyridine. Single crystal X-ray structural studies on bis(piperidinecarbodithioato-S,S’)cadmium(II) benzene solvate. Pol. J. Chem. 2005, 79, 671–678. [Google Scholar]
- Thirumaran, S.; Srinivasan, N.; Sharma, V.; Gupta, V.K. Rajnikant, Crystal Structure of Bis(μ-4-methylpiperidine-1-carbodithioato-1:2κ3S,S′:S′;2:1κ3S,S′:S′)bis[(4-methylpiperidine-1-carbodithioato-κ2S,S′)cadmium(II)]. X-ray Struct. Anal. Online 2012, 28, 21–22. [Google Scholar] [CrossRef]
- Zaeva, A.S.; Ivanov, A.V.; Gerasimenko, A.V. Adduct formation of cadmium(II) N,N-cyclo-hexamethylenedithiocarbamate, [Cd2{S2CN(CH2)6}4], with morpholine: Synthesis, molecular structure, and thermal behavior of a crystalline adduct cis-[Cd{NH(CH2)4)O}2{S2CN(CH2)6}2]. Russ. J. Coord. Chem. 2014, 40, 34–42. [Google Scholar] [CrossRef]
- Suwardi, S.A.N.; Lee, S.M.; Lo, K.M.; Jotani, M.M.; Tiekink, E.R.T. Bis(µ2-N-methyl-N-phenyldithiocarbamato)-κ3S,S':S;κ3S:S,S'-bis[(N-methyl-N-phenyldithiocarbamato-κ2S,S’)cadmium]: Crystal structure and Hirshfeld surface analysis. Acta Crystallogr. Sect. E Cryst. Commun. 2017, 73, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.S.; Tiekink, E.R.T. Crystal structure of bis(μ-N-i-propyl-N-n-propyldithiocarbamato-κ2S:S′) bis(N-i-propyl-N-n-propyldithiocarbamato-κ2S,S′)dicadmium(II), C28H56Cd2N4S8. Z. Kristallogr. New Cryst. Struct. 2018, 233, 481–483. [Google Scholar] [CrossRef]
- Tan, Y.S.; Sudlow, A.L.; Molloy, K.C.; Morishima, Y.; Fujisawa, K.; Jackson, W.J.; Henderson, W.; Halim, S.N.Bt.A.; Ng, S.W.; Tiekink, E.R.T. Supramolecular isomerism in a cadmium bis(N-hydroxyethyl, N-isopropyldithiocarbamate) compound: Physiochemical characterization of ball (n = 2) and chain (n = ∞) forms of {Cd[S2CN(iPr)CH2CH2OH]2·solvent}n. Cryst. Growth Des. 2013, 13, 3046–3056. [Google Scholar] [CrossRef]
- Tan, Y.S.; Halim, S.N.A.; Tiekink, E.R.T. Exploring the crystallization landscape of cadmium bis(N-hydroxyethyl, N-isopropyl-dithiocarbamate), Cd[S2CN(iPr)CH2CH2OH]2. Z. Kristallogr. 2016, 231, 113–126. [Google Scholar] [CrossRef]
- Kant, R.; Gupta, V.K.; Kapoor, K.; Valarmathi, P.; Thirumaran, S. Bis(μ-N-benzyl-N-furfuryldithiocarbamato)-1:2κ3S,S’:S’;2:1κ3S,S’:S’-bis[(N-benzyl-N-furfuryldithiocarbamato-κ2S,S’)cadmium]. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68, m12–m13. [Google Scholar] [CrossRef] [PubMed]
- Manar, K.K.; Yadav, M.K.; Anamika; Drew, M.G.B.; Singh, N. Influence of functionalities over polymer, trimer, dimer formation and optical properties of cadmium dithiocarbamates. Polyhedron 2016, 117, 592–599. [Google Scholar] [CrossRef]
- O’Brien, P.; Walsh, J.R.; Watson, I.M.; Motevalli, M.; Henriksen, L. Novel dithio- and diseleno-carbamates of zinc and cadmium as single-molecule precursors for low-pressure metal-organic chemical vapour deposition. J. Chem. Soc. Dalton Trans. 1996, 2491–2496. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, V.; Gupta, A.N.; Manar, K.K.; Drew, M.G.B.; Singh, N. Influence of ligand environments on the structures and luminescence properties of homoleptic cadmium(II) pyridyl functionalized dithiocarbamates. CrystEngComm 2014, 16, 6765–6774. [Google Scholar] [CrossRef]
- Clegg, W.; Coxall, R.A. Pers. Commun. Camb. Str. Database, 2016. Refcode: OJEQUR.
- Arman, H.D.; Poplaukhin, P.; Tiekink, E.R.T. A two-dimensional coordination polymer: Poly[[bis[μ2-N-ethyl-N-(pyridin-4-ylmethyl)dithiocarbamato-κ3N:S,S′]cadmium(II)] 3-methylpyridine monosolvate]. Acta Crystallogr. Sect. E Cryst. Commun. 2017, 73, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Bing, Y.; Li, X.; Zha, M.; Lu, Y. catena-Poly[cadmium-bis(µ-N,N-dimethyldithiocarbamato-κ3S,S’:S)]. Acta Crystallogr. Sect. E Struct. Rep. Online 2010, 66, m1500. [Google Scholar] [CrossRef] [PubMed]
- Moulton, B.; Zaworotko, M.J. From molecules to crystal engineering: supramolecular isomerism and polymorphism in network solids. Chem. Rev. 2001, 101, 1629–1658. [Google Scholar] [CrossRef] [PubMed]
- Tiekink, E.R.T. Supramolecular assembly based on “emerging” intermolecular interactions of particular interest to coordination chemists. Coord. Chem. Rev. 2017, 345, 209–228. [Google Scholar] [CrossRef] [Green Version]
- Ito, M.; Iwasaki, H. The structure of the monomeric form of mercury(II) N,N-diisopropyldithiocarbamate [bis(N,N-diisopropyldithiocarbamato)mercury(II)]. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1979, 35, 2720–2721. [Google Scholar] [CrossRef] [Green Version]
- Cox, M.J.; Tiekink, E.R.T. The crystal structure of monomeric bis(dicyclohexyldithiocarbamato)mercury(II). Main Group Met. Chem. 2000, 23, 793–794. [Google Scholar] [CrossRef]
- Lai, C.S.; Tiekink, E.R.T. Bis(N,N-dibenzyldithiocarbamato)mercury(II). Appl. Organomet. Chem. 2004, 18, 104. [Google Scholar] [CrossRef]
- Lai, C.S.; Tiekink, E.R.T. Bis(pyrrolinedithiocarbamato)mercury(II). Appl. Organomet. Chem. 2003, 17, 143. [Google Scholar] [CrossRef]
- Cox, M.J.; Tiekink, E.R.T. Structural diversity in the mercury(II) bis(N,N-dialkyldithiocarbamate) compounds: An example of the importance of considering crystal structure when rationalising molecular structure. Z. Kristallogr. 1999, 214, 571–579. [Google Scholar] [CrossRef]
- Marimuthu, G.; Ramalingam, K.; Rizzoli, C. Predominant ionic interactions in CdS4N2 and HgS4 coordination environments. J. Coord. Chem. 2013, 66, 699–711. [Google Scholar] [CrossRef]
- Srinivasan, N.; Thirumaran, S.; Ciattini, S. Effect of co-crystallization of ethanol, pyridine and 2,2′-bipyridine on molecular aggregation in bis(1,2,3,4-tetrahydroquinolinedithiocarbamato- S,S′)mercury(II) and synthesis of HgS nanoparticles. RSC Adv. 2014, 4, 22971–22979. [Google Scholar] [CrossRef]
- Yadav, M.K.; Rajput, G.; Gupta, A.N.; Kumar, V.; Drew, M.G.B.; Singh, N. Exploring the coordinative behaviour and molecular architecture of new PhHg(II)/Hg(II) dithiocarbamate complexes. Inorg. Chim. Acta 2014, 421, 210–217. [Google Scholar] [CrossRef]
- Kumar, A.; Chauhan, R.; Molloy, K.C.; Kociok-Kohn, G.; Bahadur, L.; Singh, N. Synthesis, structure and light-harvesting properties of some new transition-metal dithiocarbamates involving ferrocene. Chem.-Eur. J. 2010, 16, 4307–4314. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.J.; Tiekink, E.R.T. Structural variations in the mercury(II) bis(1,1-dithiolate)s: The crystal and molecular structure of [Hg(S2CNMe2)2]. Z. Kristallogr. 1997, 212, 542–544. [Google Scholar] [CrossRef]
- Iwasaki, H. The crystal structure of dimeric and monomeric forms of mercury(II) N,N-diethyldithiocarbamate, Hg2(S2CNEt2)4 and Hg(S2CNEt2)2. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1973, 29, 2115–2124. [Google Scholar] [CrossRef]
- Singh, V.; Kumar, A.; Prasad, R.; Rajput, G.; Drew, M.G.B.; Singh, N. The interplay of secondary Hg⋯S, Hg⋯N and Hg⋯π bonding interactions in supramolecular structures of phenylmercury(II) dithiocarbamates. CrystEngComm 2011, 13, 6817–6826. [Google Scholar] [CrossRef]
- Howie, R.A.; Tiekink, E.R.T.; Wardell, J.L.; Wardell, S.M.S.V. Complementary supramolecular aggregation via O–H···O hydrogen-bonding and Hg···S interactions in bis[N,N′-di(2-hydroxyethyl)-dithiocarbamato-S,S′]mercury(II): Hg[S2CN(CH2CH2OH)2]2. J. Chem. Crystallogr. 2009, 39, 293–298. [Google Scholar] [CrossRef]
- Gurumoorthy, G.; Thirumaran, S.; Ciattini, S. Unusual octahedral Hg(II) dithiocarbamate: Synthesis, spectral and structural studies on Hg(II) complexes with pyrrole based dithiocarbamates and their utility for the preparation of α- and β-HgS. Polyhedron 2016, 118, 143–153. [Google Scholar] [CrossRef]
- Lai, C.S.; Tiekink, E.R.T. Refinement of the crystal structure of bis[bis(N,N-diethyldithiocarbamato) mercury(II)], [Hg(S2CNEt2)2]2. Z. Kristallogr. New Cryst. Struct. 2002, 217, 593–594. [Google Scholar] [CrossRef]
- Loseva, O.V.; Rodina, T.A.; Smolentsev, A.I.; Ivanov, A.V. A new polymorphic modification and chemisorption activity of mercury(II) N,N-di-iso-propyldithiocarbamate: Synthesis and characterisation of the heteronuclear double complex of ([Au{S2CN(iso-C3H7)2}2]2[Hg2Cl6]·OC(CH3)2)n. Polyhedron 2017, 134, 238–245. [Google Scholar] [CrossRef]
- Altaf, M.; Stoeckli-Evans, H.; Batool, S.S.; Isab, A.A.; Ahmad, S.; Saleem, M.; Awan, S.A.; Shaheen, M.A. Mercury(II) complexes of pyrrolidinedithiocarbamate, crystal structure of bis{[μ2-(pyrrolidinedithiocarbamato-S,S′)(pyrrolidinedithiocarbamato-S,S′)mercury(II)]}. J. Coord. Chem. 2010, 63, 1176–1185. [Google Scholar] [CrossRef]
- Benedetti, A.; Fabretti, A.C.; Preti, C. Structure, IR, and NMR spectra of tetrakis(4-methyl piperidinedithiocarbamate) dimercury(II). J. Crystallogr. Spectrosc. Res. 1988, 18, 685–692. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Korneeva, E.V.; Bukvetskii, B.V.; Goryan, A.S.; Antsutkin, O.N.; Forshling, W. Structural organization of mercury(II) and copper(II) dithiocarbamates from EPR and C-13 and N-15 MAS NMR spectra and X-ray diffraction analysis. Russ. J. Coord. Chem. 2008, 34, 59–69. [Google Scholar] [CrossRef]
- Gomathi, G.; Dar, S.H.; Thirumaran, S.; Ciattini, S.; Selvanayagam, S. Bis(N-benzyl-N-furfuryldithiocarbamato-S,S′)mercury(II) as a precursor for the preparation of mercury sulfide nanoparticles. Compt. Rend. Chim. 2015, 18, 499–510. [Google Scholar] [CrossRef]
- Onwudiwe, D.C.; Ajibade, P. Synthesis, characterization and thermal studies of Zn(II), Cd(II) and Hg(II) complexes of N-methyl-N-phenyldithiocarbamate: The single crystal structure of [(C6H5)(CH3)NCS2]4Hg2. Int. J. Mol. Sci. 2011, 12, 1964–1978. [Google Scholar] [CrossRef] [PubMed]
- Green, M.; Prince, P.; Gardener, M.; Steed, J. Mercury(II) N,N′-methyl-phenylethyl-dithiocarbamate and its use as a precursor for the room-temperature solution deposition of β-HgS thin films. Adv. Mater. 2004, 16, 994–996. [Google Scholar] [CrossRef]
- Ondrušová, D.; Pajtášová, M.; Jóna, E.; Koman, M. Structural properties of Co(III), Hg(II) and Pb(II) N-ethyl-N-phenyldithiocarbamates and their application in the rubber industry. Solid State Phenom. 2003, 90–91, 383–388. [Google Scholar] [CrossRef]
- Jotani, M.M.; Tan, Y.S.; Tiekink, E.R.T. Bis[bis(N-2-hydroxyethyl, N-isopropyl-dithiocarbamato)mercury(II)]2: Crystal structure and Hirshfeld surface analysis. Z. Kristallogr. 2016, 231, 403–413. [Google Scholar] [CrossRef]
- Dar, S.H.; Thirumaran, S.; Selvanayagam, S. Synthesis, spectral and X-ray structural studies on Hg(II) dithiocarbamate complexes: A new precursor for HgS nanoparticles. Polyhedron 2015, 96, 16–24. [Google Scholar] [CrossRef]




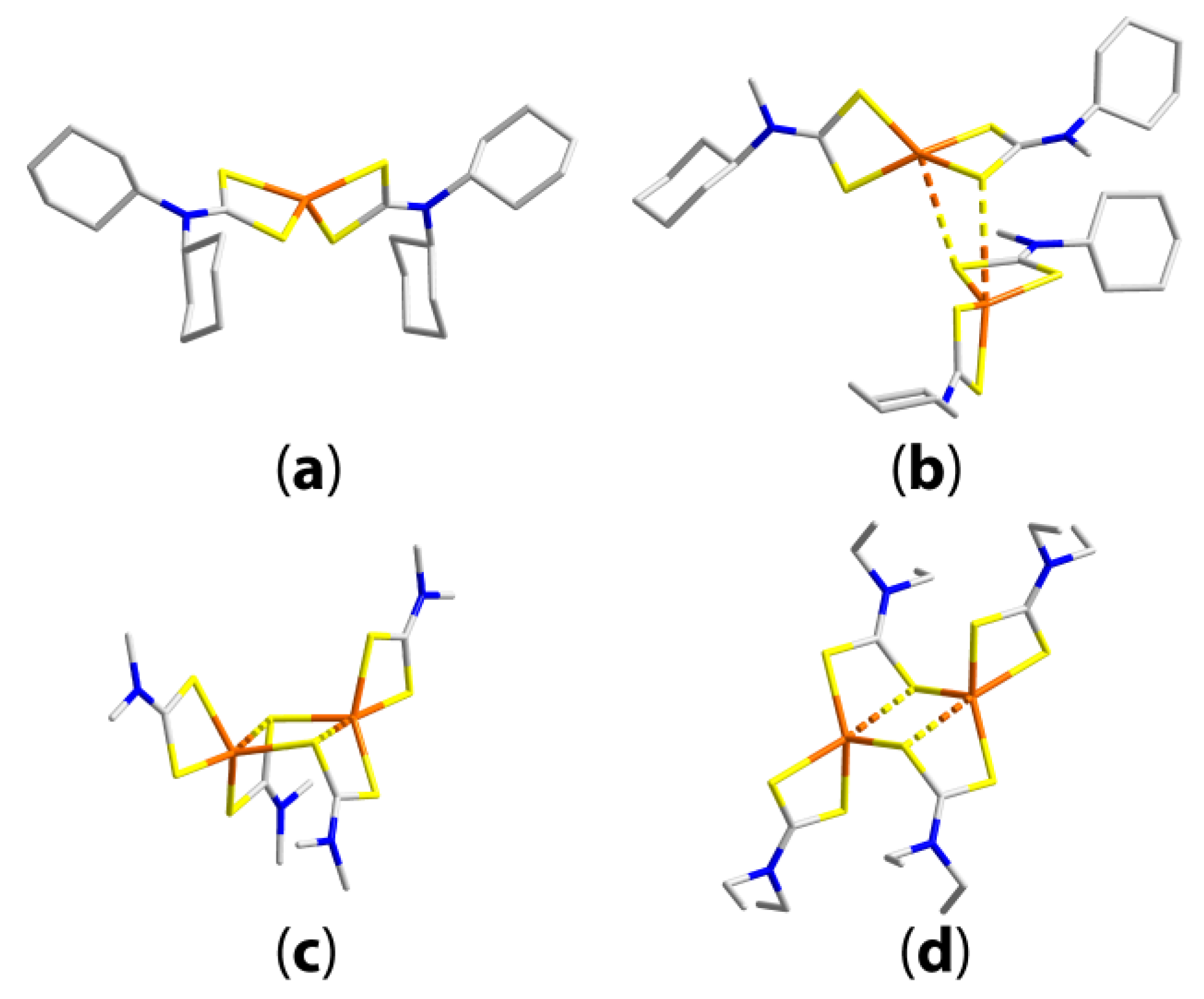
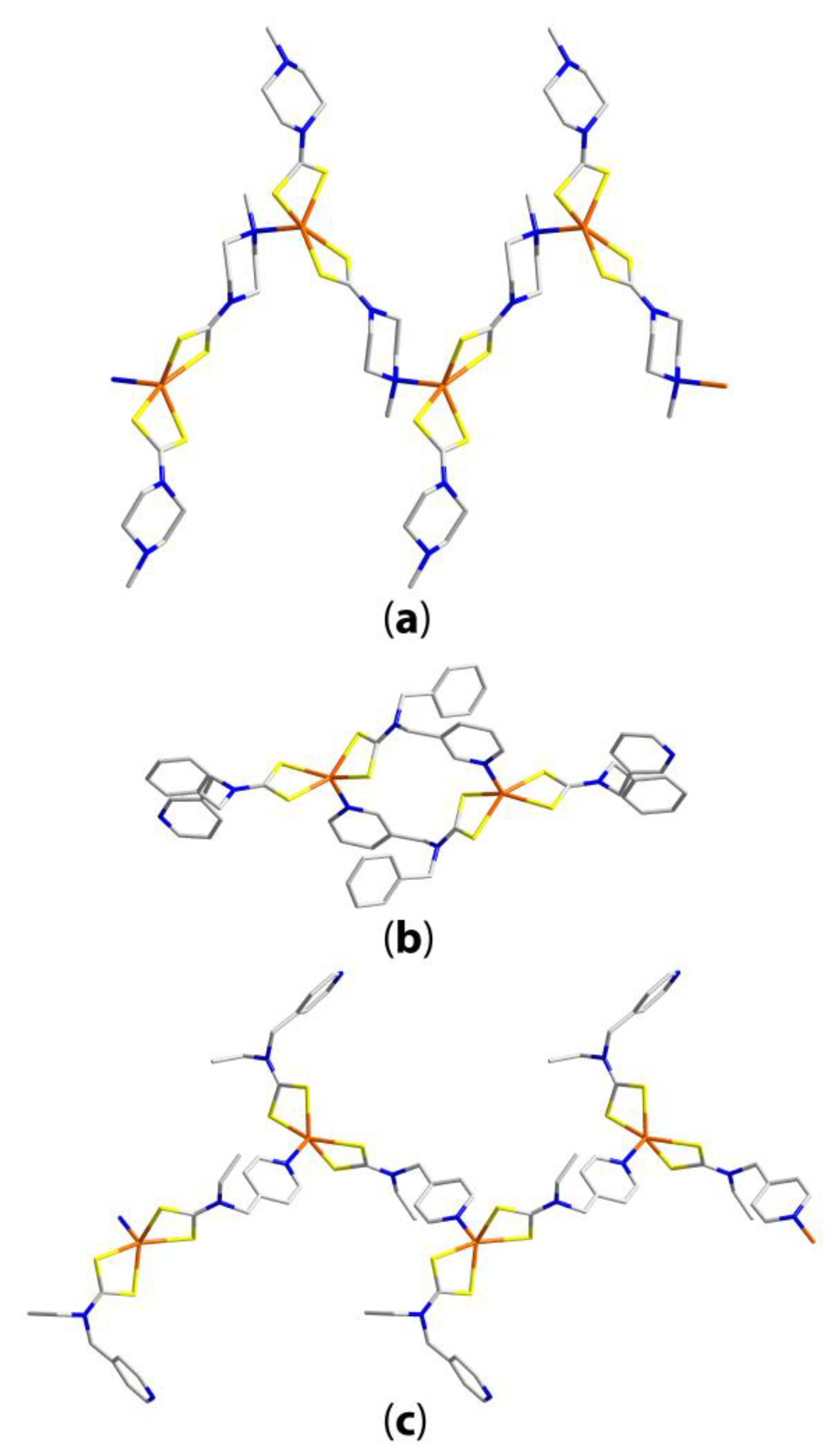
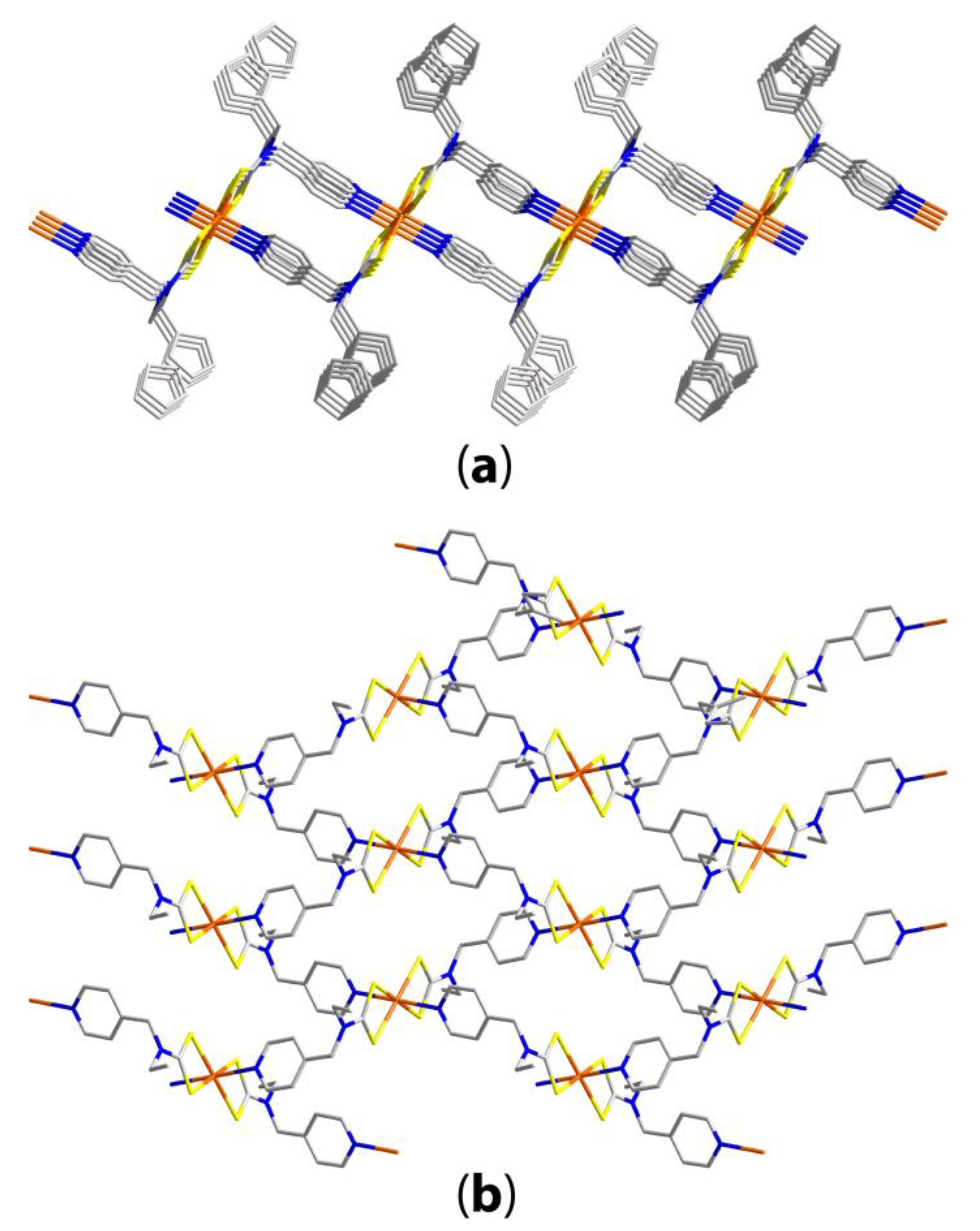
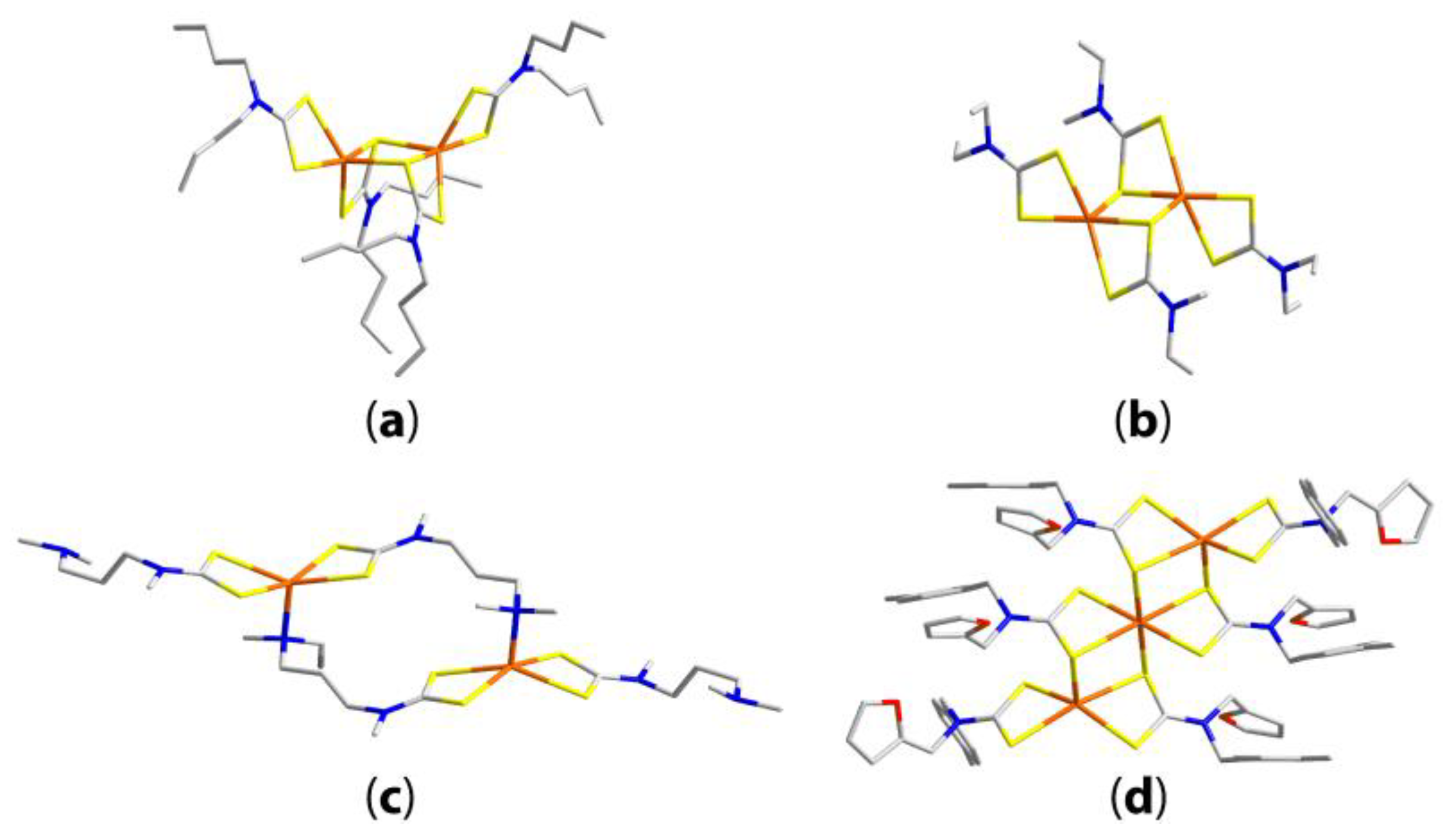

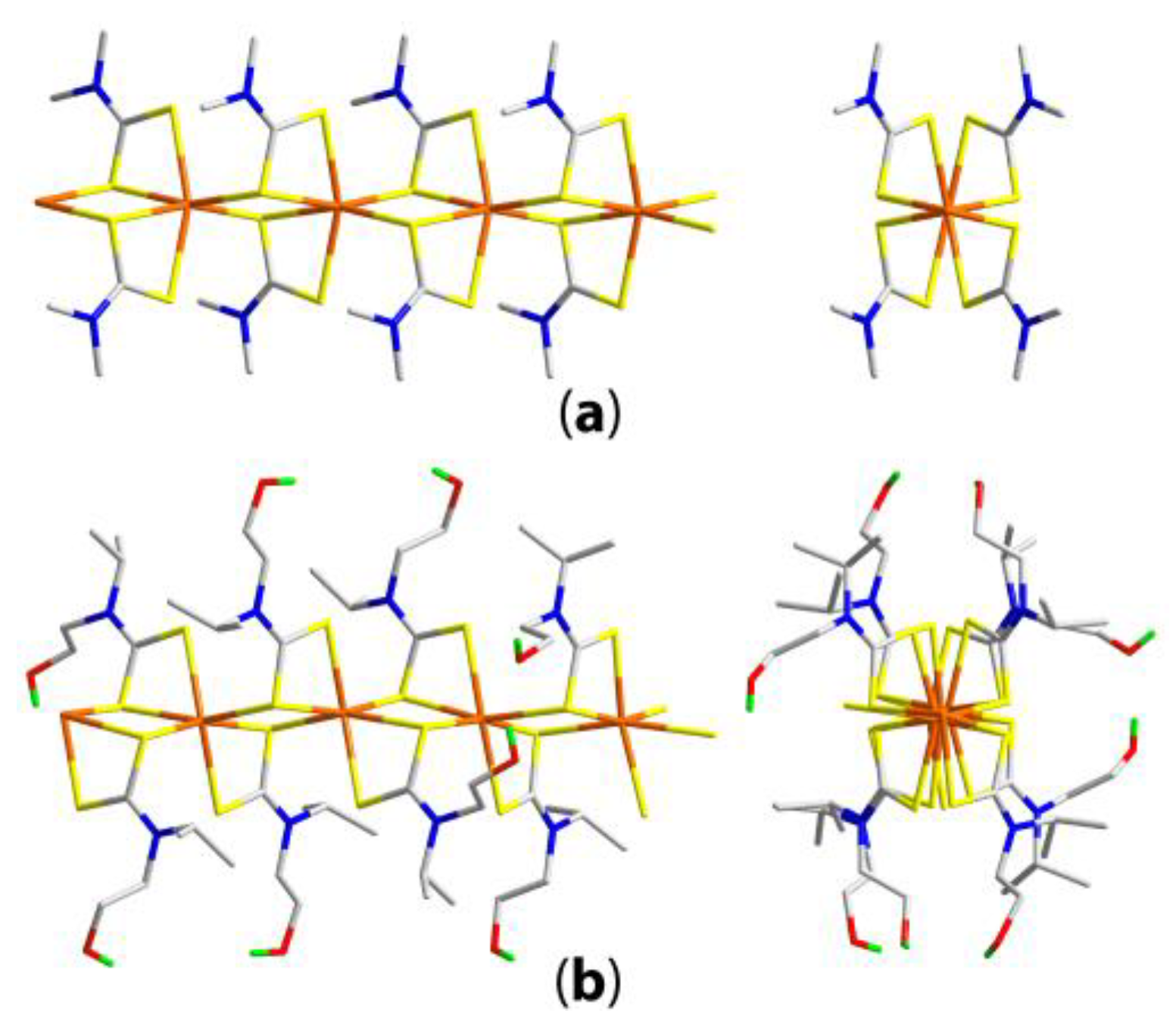


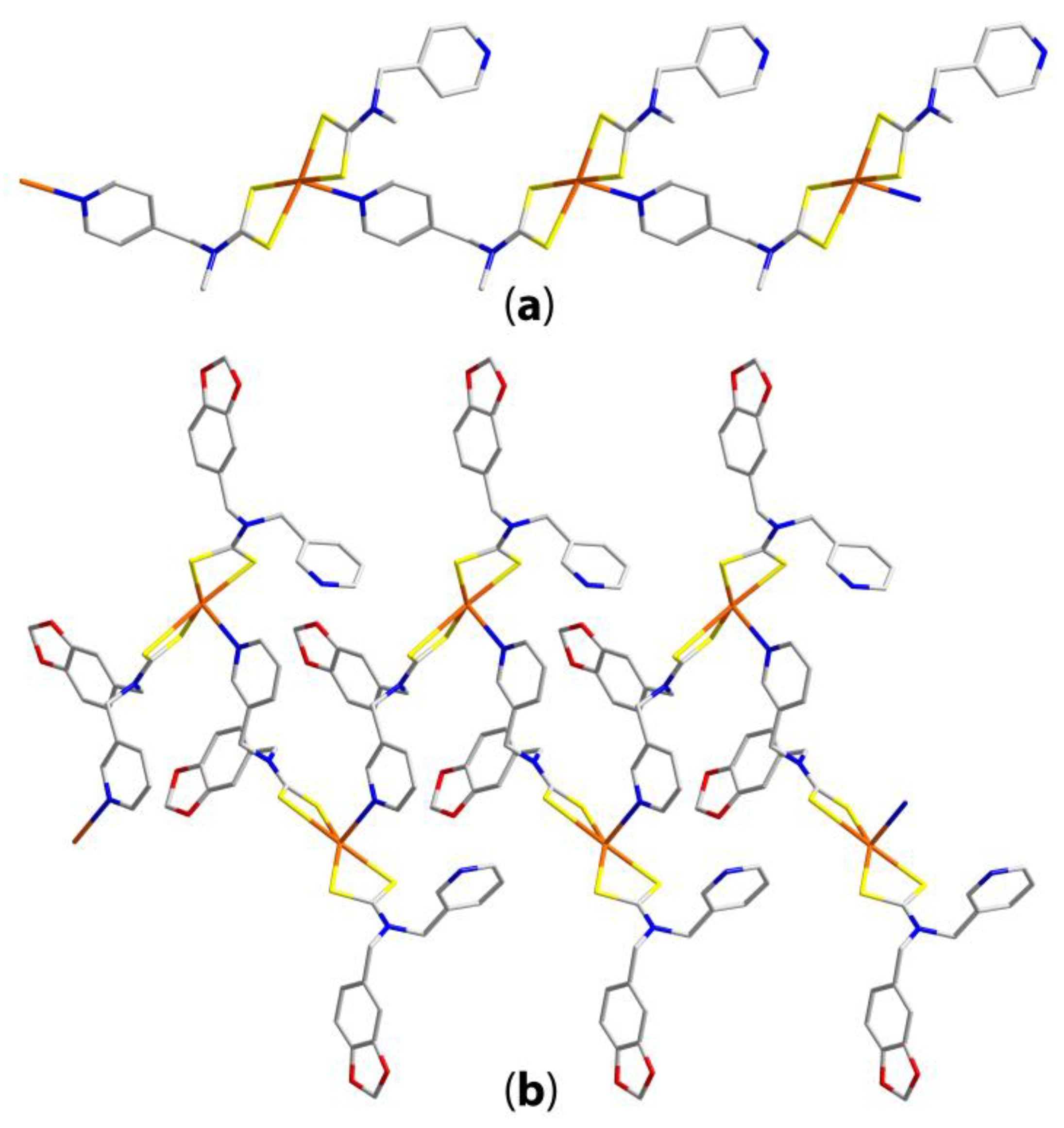
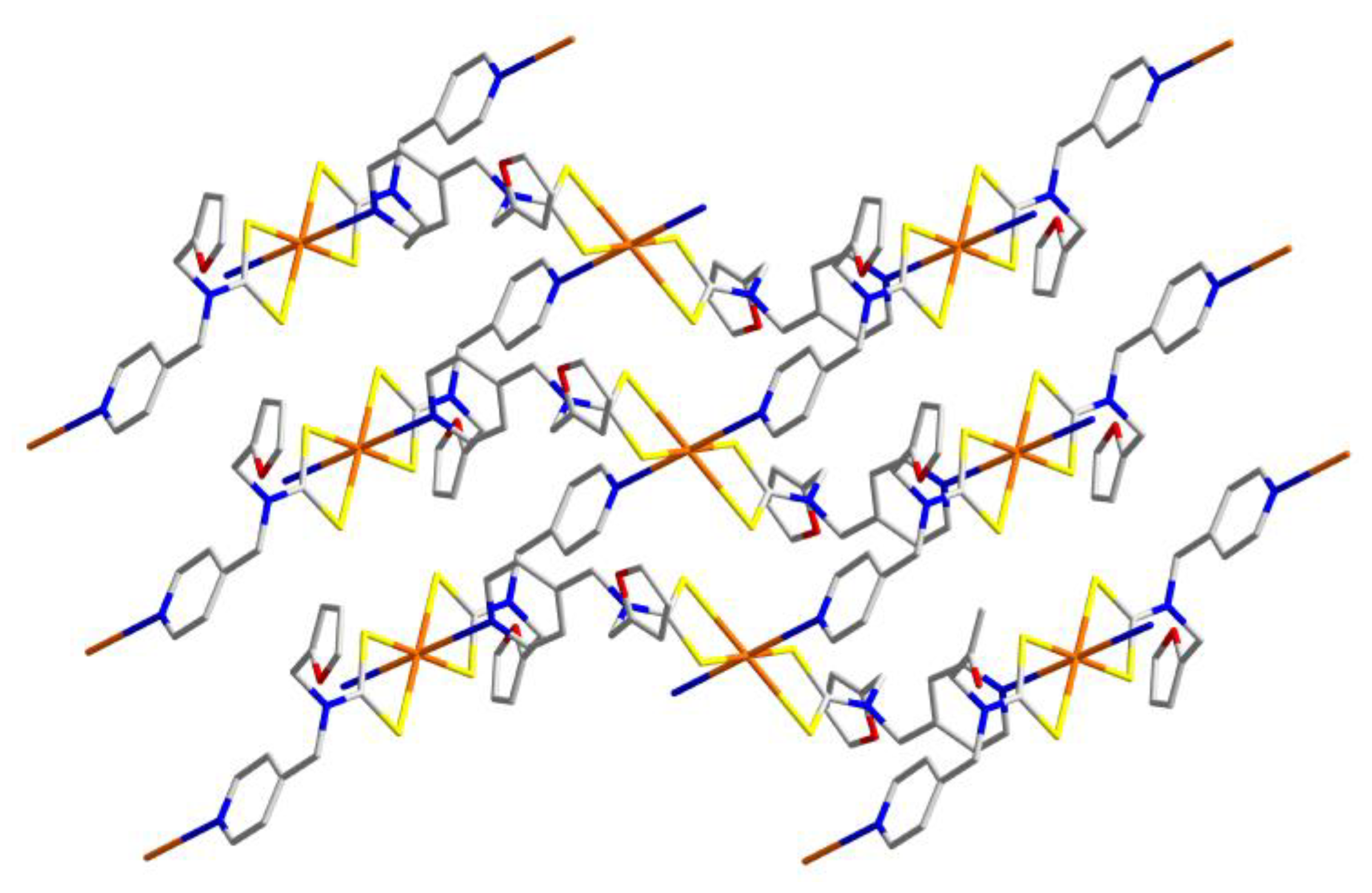
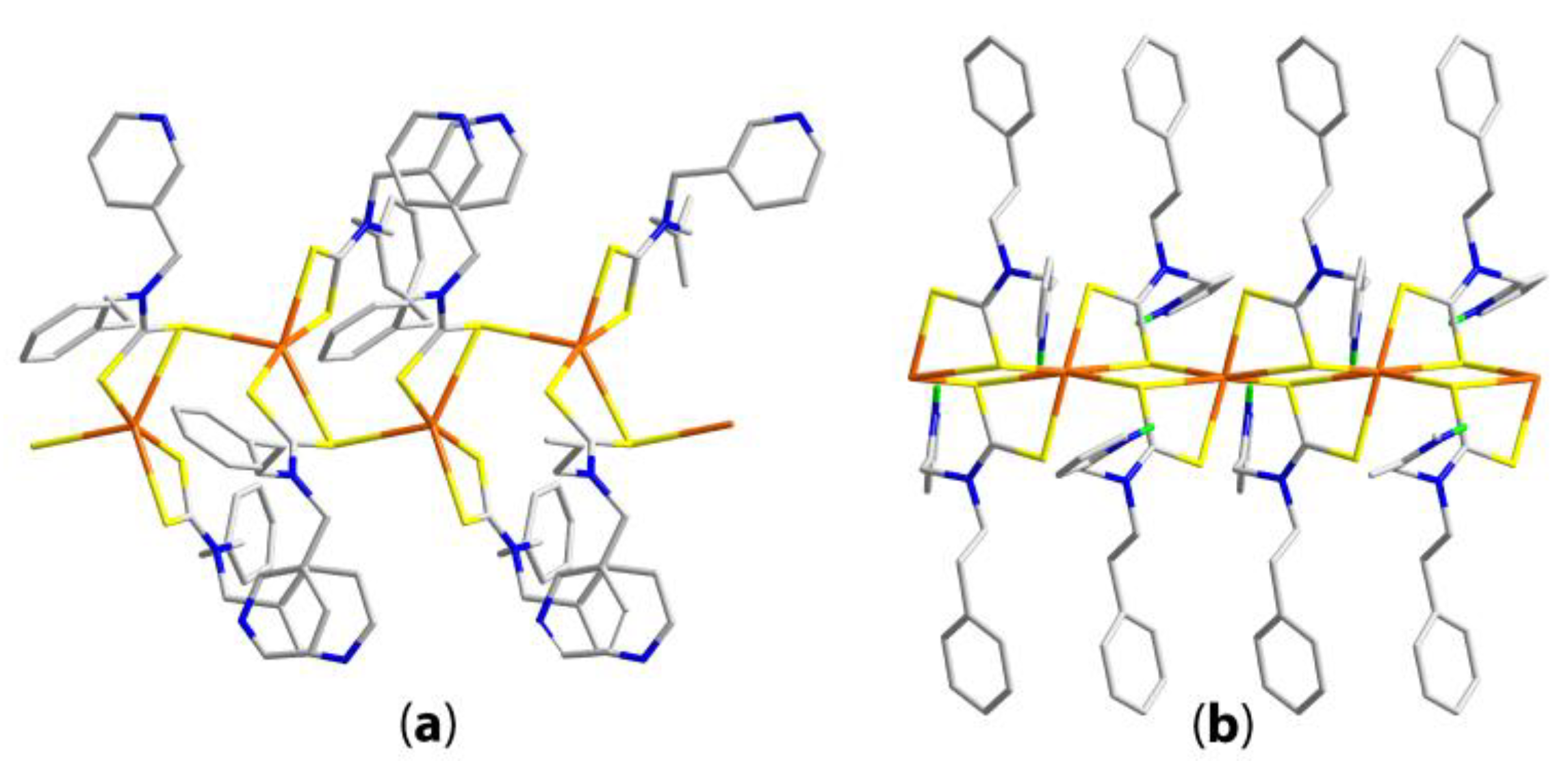
| Compound | R/R’ | Donor set | Motif | Designation | Ref. |
|---|---|---|---|---|---|
| 1 | Et | S4 | layer | A | [22] |
| 2 | n-Pr | S4 | chain | B | [23] |
| 3 | i-Pr | S4 | tetramer | C | [24] |
| 41 | n-Bu | S4 | tetramer | C | [25] |
| 5 | Me | S6 | chain | D | [26] |
| 62 | Et | S4 | layer | A | [27] |
| 73 | Et | S4 | layer | A | [28] |
| 8 | i-Pr | S4 | layer | A | [29,30] |
| 9 | n-Bu | S4 | layer | A | [31] |
| 10 | CH2CH2OMe | S6 | chain | D | [32] |
| 11 | Me | S3 | chain | E | [33] |
| 124 | Et | S4 | layer | A | [34] |
| 133 | Et | S4 | layer | A | [35] |
| 14 | n-Pr | S4 | layer | A | [36] |
| 15 | i-Pr | S4 | layer | F | [37] |
| 16 | n-Bu | S4 | layer | A | [38] |
| 17 | (CH2)2C(H)Me2 | S4 | layer | A | [38] |
| 18 | (CH2)2CMe3 | S4 | layer | A | [38] |
| 19 | Pent | S4 | layer | A | [38] |
| 20 | C(H)[(CH2)2]2NCH2Ph | S5 | chain | G | [39] |
| Compound | R | Donor set | Motif | Designation | Ref. |
|---|---|---|---|---|---|
| 21 | Me | S4 | H | chain | [51] |
| 22 | i-Pr | S4 | I | dimer | [52] |
| 23 | i-Bu | S4 | I | dimer | [53] |
| 24 | Cy | S4 | I | dimer | [54] |
| 25 | Me | S4 | J | chain | [55] |
| 26 | n-Pr | S4 | J | chain | [56] |
| 27 | i-Pr | S4 | I | dimer | [52] |
| 28 | n-Bu | S4 | J | chain | [56] |
| 29 | i-Bu | S4 | J | chain | [57] |
| 30 | s-Bu | S4 | I | dimer | [58] |
| 31 | Cy | S4 | K | dimer | [59] |
| 32 | Me | S4 | A | layer | [60] |
| 331 | i-Pr | S4 | L | chain | [61] |
| 342 | i-Pr | S4 | L | chain | [62] |
| 35 | c-Pentyl | S4 | L | chain | [63] |
| Compound | R/R’ | Donor Set | Motif | Designation | Ref. |
|---|---|---|---|---|---|
| 36 | CH2CH2OMe/CH2CH2OMe | S4 | M | monomer | [64] |
| 37 | Cy/Cy | S4 | M | monomer | [25] |
| 38 | Benzyl/Benzyl | S4 | M | monomer | [65] |
| 39 | Me/Cy | S4 | M | monomer | [66] |
| 40 | n-Pr/CH(Me)Et | S4 | M | monomer | [67] |
| 41 | NRR’ = 3,4-dihydroquinoline | S4 | M | monomer | [68] |
| 42 | n-Bu/5-t-Bu-3-Me-2-OH-benzyl | S4 | M | monomer | [69] |
| 43 | CH2CH2OH/CH2(ferrocenyl) | S4 | M | monomer | [70] |
| 44 | (CH2)3OEt/3,5-di-t-Bu-4-OH-benzyl | S4 | M | monomer | [71] |
| 45 | Benzyl/CH2(1-Me-pyrrol-2-yl) | S4 | M | monomer | [72] |
| 46 | Benzyl/4-OMe-benzyl | S4 | M | monomer | [73] |
| 47 | Benzyl/R1 1 | S4 | M | monomer | [74] |
| 48 | CH2(4-OMe-phenyl)/CH2(2-furyl) | S4 | M | monomer | [75] |
| 49 | Me/Me | S4 + 1 | N | dimer | [76] |
| 502 | n-Bu/n-Bu | S4 + 1 | N | dimer | [77] |
| 513 | n-Bu/n-Bu | S4 + 1 | N | dimer | [78] |
| 52 | Me/CH2(ferrocenyl) | S4 + 1 | N | dimer | [74] |
| 53 | Et/Et | S4 + 1 | O | dimer | [79] |
| 54 | n-Pr/n-Pr | S4 + 1 | O | dimer | [80] |
| 55 | i-Pr/i-Pr | S4 + 1 | O | dimer | [81] |
| 56 | CH2C(H)=CH2/CH2C(H)=CH2 | S4 + 1 | O | dimer | [82] |
| 574 | CH2CH2OH/CH2CH2OH | S4 + 1 | O | dimer | [83] |
| 582 | CH2CH2OH/CH2CH2OH | S4 + 1 | O | dimer | [84] |
| 595 | CH2CH2OH/CH2CH2OH | S4 + 1 | O | dimer | [85] |
| 60 | R + R’ = (CH2)4 | S4 + 1 | O | dimer | [86] |
| 61 | R + R’ = (CH2)5 | S4 + 1 | O | dimer | [87] |
| 62 | R + R’ = (CH2)6 | S4 + 1 | O | dimer | [88] |
| 63 | R + R’ = (CH2)5-4-Me | S4 + 1 | O | dimer | [89] |
| 64 | R + R’ = (CH2)5-4-C(=O)OEt | S4 + 1 | O | dimer | [72] |
| 656 | R + R’ = (CH2)5-4-C(=O)ON[C(=O)CH2]2 | S4 + 1 | O | dimer | [90] |
| 66 | R + R’ = (CH2CH2)2NEt | S4 + 1 | O | dimer | [91] |
| 67 | R + R’ = (CH2CH2)2NPh | S4 + 1 | O | dimer | [92] |
| 68 | R + R’ = (CH2CH2)2NC6H4-3-OMe | S4 + 1 | O | dimer | [93] |
| 69 | R + R’ = (CH2CH2)2NC6H4-4-OMe | S4 + 1 | O | dimer | [93] |
| 70 | Me/Et | S4 + 1 | O | dimer | [94] |
| 71 | Me/n-Pr | S4 + 1 | O | dimer | [94] |
| 72 | Me/i-Pr | S4 + 1 | O | dimer | [94] |
| 73 | Me/n-Bu | S4 + 1 | O | dimer | [94] |
| 74 | Me/Ph | S4 + 1 | O | dimer | [95] |
| 757 | Me/CH2CH2OH | S4 + 1 | O | dimer | [96] |
| 768 | Me/CH2CH2OH | S4 + 1 | O | dimer | [83] |
| 77 | Me/CH2C(=O)OMe | S4 + 1 | O | dimer | [97] |
| 78 | Me/CH2C(H)(OMe)2 | S4 + 1 | O | dimer | [98] |
| 799 | Me/R2 9 | S4 + 1 | O | dimer | [74] |
| 80 | Et/i-Pr | S4 + 1 | O | dimer | [99] |
| 81 | Et/n-Bu | S4 + 1 | O | dimer | [100] |
| 82 | Et/Cy | S4 + 1 | O | dimer | [25] |
| 833 | Et/Ph | S4 + 1 | O | dimer | [101] |
| 845 | Et/Ph | S4 + 1 | O | dimer | [102] |
| 85 | Et/CH2CH2OH | S4 + 1 | O | dimer | [83] |
| 86 | n-Pr/i-Pr | S4 + 1 | O | dimer | [103] |
| 8710 | i-Pr/CH2CH2OH | S4 + 1 | O | dimer | [96] |
| 88 | c-Pr/CH2C6H4-4-OMe | S4 + 1 | O | dimer | [104] |
| 89 | n-Bu/Ph | S4 + 1 | O | dimer | [105] |
| 90 | Benzyl/(CH2)13Me | S4 + 1 | O | dimer | [106] |
| 919 | Benzyl/R2 9 | S4 + 1 | O | dimer | [74] |
| 92 | CH2(2-furyl)/CH2C6H4-4-Cl | S4 + 1 | O | dimer | [75] |
| 93 | CH2(2-furyl)/R2 9 | S4 + 1 | O | dimer | [74] |
| 94 | CH2C6H4-4-OMe/(CH2)2N(CH2CH2)2O | S4 + 1 | O | dimer | [74] |
| 95 | i-Bu/i-Bu | S4 | M | monomer | [107] |
| S4 + 1 | O | ||||
| 96 | R + R’ = (CH2)4NMe | NS4 | P | polymer | [108] |
| 9711 | Benzyl/CH2(3-py) | NS4 | Q | dimer | [72] |
| 98 | CH2(ferrocenyl)/CH2(3-py) | NS4 | Q | dimer | [109] |
| 9912 | Et/CH2(4-py) | NS4 | P | polymer | [110] |
| 10013 | CH2(ferrocenyl)/CH2(4-py) | N2S4 | R | layer | [109] |
| Compound | R/R’ | Donor set | Motif | Designation | Ref. |
|---|---|---|---|---|---|
| 101 | n-Bu/n-Bu | S5 | N | dimer | [111] |
| 102 | Et/Et | S5 | O | dimer | [112,113] |
| 103 | n-Pr/n-Pr | S5 | O | dimer | [114] |
| 104 | i-Pr/i-Pr | S5 | O | dimer | [115] |
| 105 | CH2C(H)=CH2/CH2C(H)=CH2 | S5 | O | dimer | [116] |
| 106 | i-Bu/i-Bu | S5 | O | dimer | [117] |
| 1071 | Cy/Cy | S5 | O | dimer | [118] |
| 1082 | Benzyl/Benzyl | S5 | O | dimer | [119] |
| 1093 | Benzyl/Benzyl | S5 | O | dimer | [120] |
| 110 | CH2CH2OH/CH2CH2OH | S5 | O | dimer | [121] |
| 111 | R + R’ = (CH2)5 | S5 | O | dimer | [122] |
| 112 4 | R + R’ = (CH2)5 | S5 | O | dimer | [123] |
| 113 | R + R’ = (CH2)5Me | S5 | O | dimer | [124] |
| 114 | R + R’ = (CH2)6 | S5 | O | dimer | [125] |
| 115 | Me/Ph | S5 | O | dimer | [126] |
| 116 | Et/Cy | S5 | O | dimer | [118] |
| 117 | n-Pr/i-Pr | S5 | O | dimer | [127] |
| 1185 | i-Pr/CH2CH2OH | S5 | O | dimer | [128] |
| 1196 | i-Pr/CH2CH2OH | S5 | O | dimer | [129] |
| 1207 | i-Pr/CH2CH2OH | S5 | O | dimer | [129] |
| 121 | Benzyl/CH2(2-furyl) | S5 | O | dimer | [130] |
| 122 | Benzyl/CH2C6H4-3-Cl | S5 | O | dimer | [131] |
| 123 | Benzyl/CH2C6H4-4-Cl | S5 | O | dimer | [73] |
| 124 | Me/(CH2)3NMe2 | NS4 | Q | dimer | [132] |
| 125 | CH2(3-py)/R1 8 | NS4 | Q | dimer | [133] |
| 126 | CH2C6H4-4-Cl/CH2(furyl) | 2 × S5 + S6 | S | trimer | [131] |
| 127 | CH2C6H4-4-Me/CH2(furyl) | 2 × S5 + S6 | S | trimer | [133] |
| 128 | Me/(CH2)2NMe2 | NS4 | T | polymer | [134] |
| 1299 | Et/CH2(4-py) | N2S4 | U | double-layer | [135] |
| 130 | Benzyl/CH2(3-py) | N2S4 | R | layer | [133] |
| 131 | CH2(furyl)/CH2(3-py) | N2S4 | R | layer | [133] |
| 132 | CH2(thiophen-2-yl)/CH2(3-py) | N2S4 | R | layer | [133] |
| 133 | CH2(ferrocenyl)/CH2(3-py) | N2S4 | R | layer | [109] |
| 134 | CH2(3-py)/CH2(3-py) | N2S4 | R | layer | [133] |
| 135 | Me/Me | S6 | V | polymer | [136] |
| 13610 | i-Pr/CH2CH2OH | S6 | V | polymer | [129] |
| 137 | Benzyl/R2 11 | S6 | V | polymer | [131] |
| 138 | CH2(furyl)/CH2C6H4-4-F | S6 | V | polymer | [131] |
| 139 | CH2(furyl)/CH2C6H4-4-NO2 | S6 | V | polymer | [131] |
| 140 | Me/CH2C(H)(OMe)2 | S6 | W | polymer | [98] |
| 14112 | i-Pr/CH2CH2OH | S6 | W | polymer | [128] |
| Compound | R/R’ | Donor Set | Motif | Designation | Ref. |
|---|---|---|---|---|---|
| 142 | i-Pr/i-Pr | S4 | M | monomer | [139] |
| 143 | Cy/Cy | S4 | M | monomer | [140] |
| 144 | Benzyl/Benzyl | S4 | M | monomer | [141] |
| 145 | R + R’ = (CH2)4 | S4 | M | monomer | [142] |
| 146 | i-Bu/i-Bu | S4 | K | monomer | [143] |
| 147 | R + R’ = (CH2)4NCH2C(H)=C(H)Ph | S4 | M | monomer | [144] |
| 148 | N(RR’) = R1 1 | S4 | M | monomer | [145] |
| 1492 | Et/Ph | S4 | M | monomer | [101] |
| 150 | i-Pr/Cy | S4 | M | monomer | [143] |
| 1513 | Me/Ph; n-Bu/Ph | S4 | M | monomer | [105] |
| 152 | Benzyl/CH2(1-Me-1H-pyrrol-2-yl) | S4 | M | monomer | [146] |
| 153 | Benzyl/CH2(ferrocenyl) | S4 | M | monomer | [147] |
| 154 | CH2(3-py)/CH2(1-Me-1H-pyrrol-2-yl) | S4 | M | monomer | [39] |
| 155 | CH2CH2OH/CH2(ferrocenyl) | S4 | M | monomer | [70] |
| 156 | Me/Me | S4 | X | monomer | [148] |
| 157 | Et/Et | S4 | X | monomer | [149] |
| 158 | Benzyl/CH2(3-py) | S4 | X | monomer | [72] |
| 159 | Benzyl/CH2(4-py) | S4 | X | monomer | [150] |
| 160 | CH2CH2OH/CH2CH2OH | S4 | X | monomer | [151] |
| 161 | n-Bu/CH2(1H-pyrrol-2-yl) | S4 | Y | monomer | [152] |
| 162 | CH2(4-py)/CH2(1H-pyrrol-2-yl) | S4 | Z | monomer | [146] |
| 163 | Et/Et | S4 + 1 | O | dimer | [149,153] |
| 164 | i-Pr/i-Pr | S4 + 1 | O | dimer | [154] |
| 165 | n-Bu/n-Bu | S4 + 1 | N | dimer | [143] |
| 166 | R + R’ = (CH2)4 | S4 + 1 | O | dimer | [155] |
| 167 | R + R’ = (CH2)5Me | S4 + 1 | O | dimer | [156] |
| 168 | R + R’ = (CH2)6 | S4 + 1 | O | dimer | [157] |
| 169 | CH2(2-furyl)/CH2(2-furyl) | S4 + 1 | O | dimer | [158] |
| 170 | Me/Ph | S4 + 1 | O | dimer | [159] |
| 171 | Me/(CH2)2Ph | S4 + 1 | O | dimer | [160] |
| 172 | Et/Cy | S4 + 1 | O | dimer | [143] |
| 173 | Et/Ph | S4 + 1 | O | dimer | [161] |
| 174 | i-Pr/CH2CH2OH | S4 + 1 | O | dimer | [162] |
| 175 | Benzyl/CH2(2-furyl) | S4 + 1 | O | dimer | [158] |
| 176 | (CH2)2Ph/CH2(2-furyl) | S4 + 1 | O | dimer | [163] |
| 177 | (CH2)2Ph/CH2CH2(thiophen-2-yl) | S4 + 1 | O | dimer | [163] |
| 178 | CH2(3-py)/CH2(ferrocenyl) | S4 + 1 | O | dimer | [109] |
| 1793 | CH2(3-py)/CH2(1-naphthyl) | NS4 | Q | dimer | [39] |
| 1804 | NRR’ = R2 5 | 2 × S5 + S6 | S | trimer | [145] |
| 181 | Me/CH2(4-py) | NS4 | Z | polymer | [72] |
| 182 | CH2(3-py)/CH2(1,3-benzodioxo-5-yl) | NS4 | P | polymer | [72] |
| 183 | CH2(4-py)/CH2(2-furyl) | N2S4 | R | layer | [72] |
| 184 | (CH2)2Ph/CH2(3-py) | NS5 | AA | polymer | [72] |
| 185 | (CH2)2Ph/CH143(1H-pyrrol-2-yl) | S6 | V | polymer | [152] |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiekink, E.R.T. Exploring the Topological Landscape Exhibited by Binary Zinc-triad 1,1-dithiolates. Crystals 2018, 8, 292. https://doi.org/10.3390/cryst8070292
Tiekink ERT. Exploring the Topological Landscape Exhibited by Binary Zinc-triad 1,1-dithiolates. Crystals. 2018; 8(7):292. https://doi.org/10.3390/cryst8070292
Chicago/Turabian StyleTiekink, Edward R.T. 2018. "Exploring the Topological Landscape Exhibited by Binary Zinc-triad 1,1-dithiolates" Crystals 8, no. 7: 292. https://doi.org/10.3390/cryst8070292